Abstract
Background: The health-related quality of life (HRQOL) in subjects with chronic thoracic aortic disease (TAD) not scheduled for intervention has not been previously reported. Such information may aid counseling, management, and clinical decision-making. We report HRQOL in TAD, its main subtypes (aneurysm versus dissection and proximal versus distal), compare it to a reference group from the general population, and explore independent predictors. Methods: The short-form 36-item (SF-36) questionnaire was used, as part of a self-reporting health survey, to measure HRQOL in eight domains and a physical component summary (PCS) and a mental component summary (MCS) score. Median differences (Δ) between the component summary scores and a sex- and age-matched reference group from the general population were the primary outcome measures. Multivariable techniques were used to evaluate independent predictors. Results: In 178 TAD subjects, the HRQOL was reduced (versus the reference group) in the PCS, Δ −6.4 [95% confidence limits −8.8, −4.0] and in three out of eight SF-36 domains: physical functioning (PF), Δ −10 [−15, −4.5]; physical role (RP), Δ −25 [−34, −16]; general health (GH), Δ −5.0 [−9.7, −0.27]. There were no statistically significant differences in HRQOL scores in type (aneurysm versus dissection) or location (proximal versus distal) of TAD. Multivariable analyses identified symptoms of exertional dyspnea, exertional calf pain, joint pain, and angina pectoris as predictors of lower SF-36 component summary scores. Conclusions: The HRQOL in subjects with chronic TAD is reduced compared to a matched reference group. Differences, however, are comparably small and limited to physical domains. There were no differences according to type or location of TAD. Present symptoms and conditions were influential on the physical and mental component summary scores. HRQOL could be a useful part of thoracic aortic disease surveillance and could help guide interventional decision-making.
Keywords: Thoracic aorta, Quality of life, SF-36
Introduction
The health-related quality of life (HRQOL) in patients operated for thoracic aortic disease (TAD)—predominantly aneurysm and dissection—has been reported in a few clinical series [1–6]. However, a substantial proportion of individuals with TAD will be subjected to long-lasting surveillance. A proportion will, in fact, never undergo surgical or endovascular repair. Thus, TAD can often be experienced as a comparably unobtrusive chronic condition—yet a potentially lethal one. Recognizing its increasing prevalence [7], it becomes more important to understand the different aspects of TAD, and specifically its impact on HRQOL. Apart from a limited study in subjects with chronic Type B aortic dissection [8], HRQOL in subjects with chronic TAD not scheduled for intervention has not been previously reported. We report (1) the HRQOL in such TAD subjects, as measured by the Medical Outcomes Study Short-Form 36-item (SF-36) questionnaire, (2) the HRQOL in TAD compared to an age- and sex-matched reference group from the general population, (3) the HRQOL in the principal TAD subgroups: aneurysm versus dissection and proximal versus distal disease, and (4) clinical variables acting as predictors independently related to HRQOL in TAD.
Patients and Methods
Study Cohort
Subjects (n = 756) enlisted for regular clinical and radiological follow-up at a referral aortic outpatient clinic (sole provider of service in a county of approximately 2 million inhabitants) were identified in administrative hospital databases. Six individuals followed for increased risk (family history) of TAD but confirmed free of TAD were excluded, as were 96 deceased patients, yielding 654 eligible subjects invited to participate in a study comprising blood pressure measurement, blood sampling, health-survey questionnaires, and medical records data retrieval. Of these, 417 (64%) returned a completed SF-36 HRQOL questionnaire, of which 178 were from individuals with TAD who had not undergone surgical or endovascular repair, forming the study group. The demographic and clinical characteristics of the study group are displayed in Table 1, and a delineation of the TAD distribution is shown in Table 2. The regional research ethics committee approved the study. Written informed consent was obtained from all participants.
Table 1.
Demographic and Clinical Characteristics of Subjects with Thoracic Aortic Disease (n = 178)
| Variable | n (%) |
|---|---|
| Male | 117 (66) |
| Age, median (IQR) | 64 (14) |
| Comorbid or health-related conditions | |
| Obesity | 3 (1.7) |
| BMI, median (IQR) | 25.7 (4.7) |
| Hypertension | 117 (66) |
| Ischemic heart disease | 53 (30) |
| Prior myocardial infarction | 28 |
| Angina | 25 |
| Coronary intervention | 28 (16) |
| Coronary artery bypass graft | 12 |
| Percutaneous intervention | 16 |
| Peripheral vascular intervention | 11 (6.2) |
| Claudication | 7 (3.9) |
| Venous thromboembolic disease | 18 (10) |
| Chronic obstructive pulmonary disease | 5 (2.8) |
| Smoking | 71 (40) |
| Diabetes | 8 (4.5) |
| Renal failure | 21 (12) |
| Cerebrovascular insult | 16 (9.0) |
| Coumadin treatment | 12 (6.7) |
| Marfan syndrome | 3 (1.7) |
| Current symptoms or complaints | |
| Exertional dyspnea | 56 (31) |
| Exertional chest discomfort | 15 (8.4) |
| Exertional calf discomfort | 22 (12) |
| Joint pain | 48 (27) |
| Echocardiographic findings | |
| Bicuspid aortic valve | 9 (5.1) |
| Aortic regurgitation | 50 (28) |
| Aortic stenosis | 8 (4.5) |
IQR indicates interquartile range; BMI, body mass index.
Table 2.
Characteristics of Thoracic Aortic Disease Distribution in the Study Group (n = 178)
| Variable | n (%) |
|---|---|
| Thoracic aortic aneurysm∗ | 109 (61) |
| Aortic root | 3 |
| Ascending aorta | 85 |
| Aortic arch | 8 |
| Descending or thoraco-abdominal aorta | 32 |
| Chronic aortic dissection | 60 (34) |
| Type A | 2 |
| Type B | 58 |
| Intramural hematoma | 5 (2.8) |
| Other thoracic aortic disease | 4 (2.2) |
Anatomical locations of thoracic aortic aneurysms not mutually exclusive.
SF-36 HRQOL Questionnaire
The Medical Outcomes Study SF-36 is a well-established self-administered questionnaire quantifying HRQOL. The Swedish translation has been validated and a normative population (n = 8930) characterized [9]. From this normative (general) population, a study-specific age- and sex-matched reference group (1:1 ratio, age matching within 5-year limits) was created by an independent external facility (HRQL-gruppen®, Sahlgrenska Akademin, Gothenburg, Sweden) blinded to the study material. There was no matching on other characteristics, assuming a normal distribution of medical conditions and lifestyle factors in the large general population sample. SF-36 measures HRQOL in eight domains by 2-10 items per domain: physical functioning (PF), bodily pain (BP), role physical (RP), mental health (MH), social functioning (SF), role emotional (RE), vitality (VT), and general health (GH). Raw scores in each domain are summarized and transformed to a 0-100 scale as described in the SF-36 users' manual [9], the higher score, the better. Summary scores for the combined physical (PCS, physical component summary) and mental (MCS, mental component summary) domains can be constructed and have been validated in separate studies [9,10]. The difference (Δ) in median PCS and MCS from the age- and sex-adjusted reference group served as the primary outcome measure. The PCS and MCS were analyzed and interpreted in conjunction with the individual domain scores as appropriate [9,10]. In addition to SF-36, data from a self-reporting questionnaire covering comorbidity (specific conditions and their definitions are given below); smoking, drinking, and physical exercise habits; family history of aortic, ischemic heart or valve disease; and current medications were accrued.
Data Collection
Together with other health-survey material, an informed consent form and an invitation to an outpatient visit with a study nurse for blood pressure control, measurement of height and weight, and blood sampling, respectively, the SF-36 HRQOL was mailed to eligible individuals, enclosing a self-addressed return envelope. Within one month, nonresponders were reminded by a second identical mailing. Those still not responding within another month were contacted by telephone on one or more occasions. Individuals declining the in-house nurse visit were offered a nurse home-visit. Whenever possible, the reasons for not responding were explored and are given in Table 3.
Table 3.
Differences (Δ) in transformed (0-100 scale) median SF-36 domain scores including PCS and MCS scores in subjects with unoperated TAD compared to an age- and sex-matched reference group from the normal population (n = 178 in both groups)
| SF-36 domain | TAD median transformed score (IQR) n (%) | Reference group median transformed score (IQR) n (%) | Δ Median (TAD-Reference) [95% CL] |
|---|---|---|---|
| PF | 75 (35) | 85 (34) | −10 [−15, −4.5] |
| RP | 75 (75) | 100 (50) | −25 [−34, −16] |
| BP | 74 (49) | 74 (59) | 0 [−5.8, 5.8] |
| GH | 62 (32) | 67 (33) | −5 [−9.7, −0.27] |
| VT | 70 (40) | 70 (35) | 0 [−5.2, 5.2] |
| SF | 100 (25) | 100 (25) | 0 [−5.2, 5.2] |
| RE | 100 (67) | 100 (67) | 0 [−8.2, 8.2] |
| MH | 80 (28) | 84 (28) | −4 [−8.3, 0.3] |
| PCS | 43 (18) | 50 (17) | −6.4 [−8.8, −4.0] |
| MCS | 54 (14) | 54 (12) | −0.37 [−2.8, 2.1] |
PCS indicates physical component summary; MCS, mental component summary; TAD, thoracic aortic disease; IQR, interquartile range; CL, confidence limit; PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health.
Definitions
The definition of TAD was all-inclusive, but conditions other than aneurysm or chronic dissection were uncommon. Aortic dissection was subdivided by the Stanford classification (Type A and Type B, respectively). Again, chronic Type A dissection was uncommon (n = 2). Thoracic aortic aneurysm was described according to involved aortic segment(s): aortic root, ascending aorta, aortic arch, and descending and thoraco-abdominal aorta, respectively, with the possibility of several involved segments in a single individual. In statistical analyses, subjects with chronic intramural hematoma (n = 5) were included in the dissection group, whereas subjects with infrequent forms of TAD (penetrating aortic ulcer, mural thrombus, n = 4) were excluded. In comparisons between ascending and descending TAD, disease involving the aortic root, the ascending aorta, or the aortic arch (or combinations thereof) were categorized as proximal TAD. Whenever the descending or thoraco-abdominal aorta was affected, categorization was distal TAD. Self-reported comorbid conditions [ie, myocardial infarction, angina, heart failure, coronary artery bypass surgery, percutaneous coronary intervention, vascular surgery or percutaneous intervention, cerebrovascular incident (stroke or transitory ischemic attack), claudication, deep venous thrombosis with or without pulmonary embolism, hypertension, diabetes, renal failure, systemic inflammatory disease, other serious illness] were considered present when the question “Have you ever been informed by a doctor that you have or have had...?” was answered with a “yes”, and otherwise absent. Parallel to diagnoses, specific symptoms were asked for: exertional dyspnea, exertional chest pain (or discomfort), exertional calf pain (or discomfort), and joint pain, respectively. Additional definitions were: Smoking: earlier or ongoing habitual smoking, ie, distinguishing between ever-smokers and never-smokers; Obesity: BMI > 30; Marfan syndrome: clinically established diagnosis; Bicuspid aortic valve, aortic regurgitation, and aortic stenosis: considered present if affirmed in an echocardiographic report and otherwise absent; and Current medications (yes/no): anticoagulation, anti-platelet drug(s), lipid-lowering drug(s), and antihypertensive drug(s). All variables were analyzed in multivariable modeling as defined.
Statistical Analysis
Data are presented as numbers with percentages and medians, with interquartile range (IQR). Because of non-normal distributions, a nonparametric rank-sum (Mann-Whitney) test was used for group comparisons of transformed SF-36 domain and component summary scores. In consequence, a nonparametric approach was also applied to multivariable modeling: quantile regression on the median was used for multivariable modeling of predictors of the primary outcome variables [ie, differences of PCS and MCS median values (Δ), respectively, from the general population reference group], utilizing a backward stepwise approach (with forced entry of age and sex) to model selection. Avoiding data distribution assumptions, the resulting regression coefficient standard errors were obtained by generation of 1000 bootstrap samples and standardized as β-coefficients (variance = 1) to accommodate differences in variable units. As a subsidiary analysis, standard linear regression on the mean of the primary outcome variables was also performed. To further explore predictors of the primary outcome variables, bootstrap bagging, as described by Blackstone [11], was applied to a large set of variables generated from the health questionnaires. With a stepwise backward approach, models were generated from a random set of cases using observation sampling with replacement (sample size = cohort size = 178) and repeated in 1000 cycles (bootstraps). Variables appearing in > 60% of bootstraps were considered relevant and are reported. The statistical power of the obtained study group (n = 178) to detect a 10% difference (at α = 0.05 level) in the median SF-36 score from that of a reference group from the general population was calculated. Cronbach's α-coefficient [12] was calculated to evaluate internal consistency of each SF-36 domain. Statistical analysis was performed with Stata version 12 (StataCorp LP, College Station, TX).
Results
The SF-36 response rate was 64%. Reasons for not responding were evaluated and, in general, were not considered related to TAD. The distributions of TAD in terms of underlying aortic pathology and anatomy were also very similar in responders and nonresponders.
As detailed in Table 3, the median scores of PCS and MCS were 43(18) and 54(14), respectively. Scores in the eight SF-36 domains varied between 62(32) for GH, and 100(25) and 100(67) for SF and RE, respectively. Overall, scores were equal to (n = 5) or significantly worse than (n = 3) those of the reference group. PCS median score was significantly lower (Δ= −6.4) than that in the reference group, but not MCS (Δ = −0.37). The single largest deviations were found in the PF (physical functioning, Δ = −10) and RP (role physical, Δ = −25) domains. Mean SF-36 scores (Table 4) were not statistically different from those of the reference group. Cronbach's α varied 0.77-0.92, overall on par with that found in the general population [9]. The statistical power to detect a 10% difference varied 0.65-1.00 and was 1.00 for the primary outcome variables PCS and MCS (Table 5).
Table 4.
Mean (with 95% Confidence Limits) Transformed (0-100 scale) SF-36 Domain and Summary Scores in the Study Group (Thoracic Aortic Disease) and Reference Group (Age- and Sex-Matched Individuals from Normal Population): Differences (Δ) in Mean Scores (with 95% Confidence Limits)
| SF-36 domain | TAD mean transformed score [95% CL] | Reference group mean transformed score [95% CL] | Δ Mean (TAD-Reference) [95% CL] |
|---|---|---|---|
| PF | 69 [66-73] | 74 [69-78] | −4.4 [−9.9, 1.1] |
| RP | 63 [56–69] | 69 [63–76] | −6.7 [−15, 1.9] |
| BP | 72 [68–76] | 69 [64–73] | 3.6 [−2.2, 9.4] |
| GH | 62 [59–65] | 65 [61–68] | −2.5 [−7.5, 2.5] |
| VT | 64 [61–68] | 64 [60–68] | 0 [−5.2, 5.2] |
| SF | 82 [79–86] | 85 [81–89] | −2.4 [−7.6, 2.8] |
| RE | 75 [69–81] | 74 [68–80] | 1.1 [−7.1, 9.3] |
| MH | 78 [75–80] | 79 [76–82] | −1.4 [−5.7, 2.9] |
| PCS | 42 [41–44] | 45 [42–46] | −2.2 [−4.6, 0.20] |
| MCS | 49 [48–51] | 50 [48–52] | −0.73 [−3.2, 1.7] |
TAD indicates thoracic aortic disease; CL, confidence limit; PF, physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary.
Table 5.
Cronbach's α-Coefficient for Internal Consistency for Each SF-36 Domain, Compared to a Normal Swedish Population (n = 8930)
| SF-36 domain | Cronbach's alpha (Normal Population) | Power |
|---|---|---|
| PF | 0.91 (0.91) | 0.92 |
| RP | 0.89 (0.88) | 0.65 |
| BP | 0.86 (0.93) | 0.90 |
| GH | 0.77 (0.84) | 0.86 |
| VT | 0.86 (0.85) | 0.87 |
| SF | 0.84 (0.85) | 0.99 |
| RE | 0.92 (0.79) | 0.69 |
| MH | 0.83 (0.87) | 1.00 |
| PCS | n/a | 1.00 |
| MCS | n/a | 1.00 |
Statistical power for each SF-36 domain and summary score was used to detect a 10% difference from the reference group median score. PF indicates physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary.
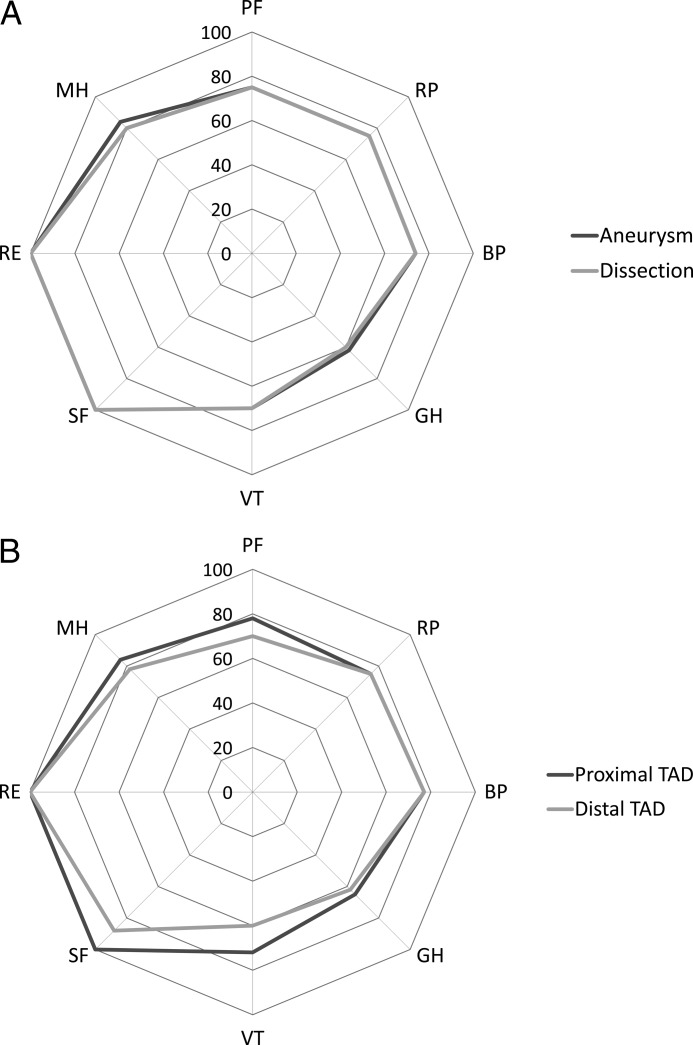
The median SF-36 domain scores (Table 6) did not differ significantly in magnitude or distribution for aneurysm versus dissection or for proximal versus distal TAD, as illustrated in Figure 1A and 1B. PCS was 41(16) versus 45(18) in dissection versus aneurysm, P = 0.38. MCS was 54(12) versus 55(16) in dissection versus aneurysm, P = 0.96. PCS was 46(18) versus 42(18) in proximal versus distal TAD, P = 0.61. MCS was 54(11) versus 54(18) in proximal versus distal TAD, P = 0.65.
Table 6.
Median (Interquartile Range) Transformed (0-100 Scale) SF-36 Domain and Summary Scores by Thoracic Aortic Disease Type (Aneurysm versus Dissection) and Location (Proximal versus Distal)
| SF-36 domain | Aneurysm | Dissection | Proximal | Distal |
|---|---|---|---|---|
| PF | 75 (35) | 75 (35) | 78 (32) | 70 (45) |
| RP | 75 (75) | 75 (100) | 75 (75) | 75 (88) |
| BP | 74 (49) | 74 (49) | 77 (44) | 77 (59) |
| GH | 62 (32) | 60 (32) | 65 (32) | 62 (38) |
| VT | 70 (35) | 70 (45) | 72 (35) | 60 (38) |
| SF | 100 (25) | 100 (25) | 100 (25) | 88 (50) |
| RE | 100 (33) | 100 (67) | 100 (33) | 100 (83) |
| MH | 84 (24) | 80 (28) | 84 (24) | 78 (34) |
| PCS | 45 (18) | 41 (16) | 46 (18) | 42 (18) |
| MCS | 54 (12) | 55 (16) | 54 (11) | 54 (18) |
PF indicates physical functioning; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary.
Figure 1.
Polar plots of median transformed scores of the eight SF-36 domains in (A) aneurysm versus dissection and (B) proximal versus distal TAD. No median score difference was statistically significant.
For ΔPCS and ΔMCS, multivariable (median) regression was performed to identify independently related variables. ΔPCS was significantly inversely related to (in order of magnitude): exertional dyspnea, joint pain, angina pectoris, and exertional calf pain, respectively (Table 7). There were no variables independently related to ΔMCS when adjusted for sex and age. Modeling with linear regression on the mean did not return significantly different findings. Results of the bootstrap bagging method of identifying related variables are presented in Table 8. This procedure returned the same variables significantly related to ΔPCS and also identified joint pain, exertional dyspnea, and history of cerebrovascular incident, respectively, as relevant (occurring in > 60% of models) predictors for ΔMCS. The order of magnitude by which these variables outperform age, sex, TAD type, and TAD location, respectively, are evident from Table 8.
Table 7.
Variables Independently Related to the Difference in Medians between the Study Group and the Reference Group for the Physical Component Score (ΔPCS) in Multivariable Median Regression, Adjusted for Sex and Age
| Variable | Coefficient | 95% CL | p-value |
|---|---|---|---|
| Exertional dyspnea | −12 | −15, −8.0 | < 0.0005 |
| Joint pain | −9.2 | −13, −5.0 | < 0.0005 |
| Angina pectoris | −7.1 | −12, −2.2 | 0.003 |
| Exertional calf pain | −6.1 | −10, −2.1 | 0.013 |
Standardized (β) coefficients with bootstrapped (n = 1000 repetitions) standard errors were used for confidence limits. CL indicates confidence limits.
Table 8.
Variables (in Italics) with > 600 Occurrences (> 60%) in Bootstrap Analysis (n = 1000 Bootstrapped Samples with Replacement) of ΔPCS and ΔMCS, respectively
| Variable | Occurrences/1000 bootstraps |
|---|---|
| A) ΔPCS | |
| Exertional dyspnea | 996 |
| Joint pain | 996 |
| Angina pectoris | 766 |
| Exertional calf pain | 714 |
| Male sex | 231 |
| Age | 211 |
| Distal TAD (versus Proximal TAD) | 227 |
| Aortic dissection (versus Aneurysm) | 171 |
| B) ΔMCS | |
| Joint pain | 818 |
| Cerebrovascular insult | 736 |
| Exertional dyspnea | 631 |
| Male sex | 337 |
| Age | 305 |
| Distal TAD (versus Proximal TAD) | 229 |
| Aortic dissection (versus Aneurysm) | 164 |
Age, sex, location of TAD (distal versus proximal), and type of TAD (dissection versus aneurysm) were used for reference. Δ indicates mean difference; TAD, thoracic aortic disease.
Discussion
This is the first and only sizeable (n = 178) study describing HRQOL, as measured by the SF-36 questionnaire, in a cross-sectional population of individuals with chronic TAD not scheduled for (and also not denied) intervention. A significantly reduced HRQOL compared to a sex- and age-matched reference group from the general population was evident in three (PF, RP, and GH) out of eight SF-36 domains and in the PCS, but not in the MCS. There were no statistically significant differences between aneurysm and dissection (including intramural hematoma) forms of TAD, and no statistically significant differences between proximal and distal TAD, in any of the eight SF-36 domains (Fig. 1A and 1B and Table 5), or in PCS or MCS, respectively.
Given the common combination of aortic valve dysfunction (regurgitation or stenosis) and proximal TAD, a discernible negative effect on the physical domains was anticipated. However, there were no statistically significant differences in PCS, MCS, or SF-36 domain scores. Quantitative data on valve dysfunction were limited in this study. Presumably, individuals with symptomatic valve dysfunction underwent operation, leaving individuals with mild dysfunction in the present study cohort.
In contrast, the often longstanding nature of distal TAD, related to a different size criterion for intervention [13] and a generally more conservative approach related to surgical risks, could have produced negative effects on the MCS. Again, no such differences measurably affecting HRQOL were indicated. Chronic TAD, at least when perceived as stable, could entail similar coping mechanisms regardless of underlying pathology or anatomic location. Alternatively, and supported by the findings of this study, factors other than TAD are more important in determining HRQOL as measured by the SF-36. Indeed, in multivariable analyses, neither TAD-related factors nor age and sex (well-established predictors of HRQOL in the general population [9]) were independently related to ΔPCS or ΔMCS. Instead, other current symptoms (angina, exertional dyspnea, exertional calf pain and joint pain) surfaced as predictors of decreased PCS and MCS (Tables 7 and 8). Bootstrap bagging of predictors showed that exertional dyspnea and joint pain were present in nearly 100% of bootstrapped models, TAD subgroups were present in 20-30% (Table 8). Age and sex, in turn, seemed somewhat more influential on MCS than on PCS. Exertional dyspnea appeared as the single most prevalent predictor of both PCS and MCS. Interestingly, putative diagnoses underlying exertional dyspnea (myocardial infarction, heart failure) did not exhibit such a relationship. As previously reported in adult congenital heart disease [14], perceived symptoms, regardless of known associated diagnoses, seemed most important to HRQOL. Other severe chronic conditions, such as chronic obstructive pulmonary disease (COPD), diabetes, hypertension, or Marfan syndrome, did not occur as predictors of HRQOL. They were relatively uncommon [2.8-9.0% (Table 1)] but did not appear in multivariable models even after removing, for example, variables related to musculoskeletal conditions (ie, joint pain, chronic inflammatory disease). Apparently, if SF-36 is used to inform about HRQOL in TAD, simultaneous data on comorbid conditions need to be collected to interpret the findings. Alternatively, a disease-specific HRQOL instrument should be developed and used as a complement to a generic HRQOL questionnaire. To the best of our knowledge, no such instrument has been developed as yet.
Winnerkvist et al. [8], in a cross-sectional study of conservatively managed Type B aortic dissection, used an adapted form of the SF-36 to evaluate HRQOL. The study included 53 subjects with comparably scarce comorbidity: stroke in 7.5% (versus 9.0% in the present study), myocardial infarction or angina pectoris in 9.4% (30%), and renal failure in 1.9% (12%) of subjects. In essence, their findings of a near-to-normal HRQOL largely relates to the beneficial patient group characteristics. Yet, it is an important finding, pointing to the probably limited effect of chronic stable TAD per se on HRQOL in otherwise healthy individuals or, again, to the limited role of generic HRQOL questionnaires to perform adequately in TAD. Immer and colleagues [2,3] have made important contributions to the characterization of HRQOL in patients operated for TAD. Among their interesting findings is that of a low prevalence of exertional dyspnea, 8.9% (vs 31%), at follow-up (mean 3.2 years) after surgery [2]. If surgical treatment of (in their case, proximal) TAD can improve symptoms such as exertional dyspnea, a positive effect on HRQOL could be anticipated. The pattern that factors other than those considered disease-specific are instrumental for HRQOL has been previously recognized [15]. In comparison to other chronic conditions, the HRQOL of subjects with TAD appears favorable. Lyons et al. [16] reported HRQOL in 11 common chronic illnesses. They had in common a pattern of lower SF-36 scores in all or almost all domains and, on crude comparison, often of a more pronounced magnitude than what we found in subjects with TAD. Notably, Lyons et al. [16] report mean rather than median SF-36 values. In the present study group, mean values did not differ in any SF-36 domain from the reference group (Table 4).
The prevalence of TAD is increasing [7]. More individuals will be diagnosed with TAD, regardless of whether they eventually will undergo repair or not. To many, TAD will be regarded and experienced as a chronic condition. Chronic conditions otherwise considered relatively benign, eg, asthma, diabetes, back pain, and anxiety, can profoundly impair HRQOL [16]. TAD, on the other hand, is potentially lethal. Knowledge about how TAD affects HRQOL is important to the understanding of this, and, in perspective, other chronic conditions. On the patient-group level, it is relevant to acknowledge the central findings of this study: HRQOL in TAD is reduced compared to the general population but, arguably, less so than anticipated and of as yet undetermined clinical importance. The reduction is often related to conditions other than TAD. Evaluation of HRQOL in TAD cohorts can help identify unmet medical needs. Deteriorating HRQOL in a TAD cohort may suggest revised criteria for surgical or endovascular intervention, not to thwart possible increase in HRQOL. For the individual, when treatment indication is equivocal, assessing HRQOL could help decision-making: favoring intervention if unexpectedly low or deteriorating over time. It could be prudent to integrate HRQOL measurement as part of a TAD surveillance protocol.
Study Limitations
The present study population is not indisputably representative of TAD. For instance, the few individuals with aortic root disease, Marfan syndrome, or chronic Type A dissection (Table 1) may indicate a lower threshold of surgical repair in certain TAD conditions. Indeed, a cohort of individuals with non-operated TAD will arguably represent a selection of subjects deemed inoperable and subjects not requiring surgical repair, respectively. However, we would dispute such a view: a cross-sectional study of TAD subjects will probably be representative of a real-world situation given the indolent nature of TAD when chronic and asymptomatic. Furthermore, the cohort is population-based inasmuch as no other aortic referral center operates in the same region. Data on individual aneurysm sizes were not at our disposal, but we believe that aneurysm size per se does not affect HRQOL (assuming the subject remains asymptomatic), and all were given similar information on rupture risk, limitations of strenuous physical exercise, and the importance of blood pressure control. The SF-36 response rate was 64%, comparable to previous similar studies [4] and to the 67% response rate in the normative Swedish population [9]. For purposes of describing general aspects of HRQOL in the study group, we consider the response rate sufficient. Notably, post hoc power calculations verified that the obtained study group was sufficient in size to detect clinically relevant differences in SF-36 scores compared to the reference group. The 10% difference arrived at for this purpose was derived from previous (albeit unrelated to TAD) studies describing a “minimally clinically important difference” in PCS and MCS to 2-5 points [17–18], approximating 10-20% in our study group.
Conclusions
As measured by SF-36, HRQOL in subjects with TAD not scheduled for intervention— ie, chronic stable TAD—is reduced compared to an age- and sex-matched reference group in three out of eight domains and in the physical component summary score (PCS). However, this reduction is comparably small, mainly present in physical domains, and apparently related to conditions other than TAD. There were no statistically significant differences between type of TAD (aneurysm versus dissection) and location of TAD (proximal versus distal). Knowledge about HRQOL is a basic requisite for chronic conditions, aiding in understanding of the disease, resource allocation, and eventually in treatment decisions, especially when endovascular or less invasive treatment options offer repair with perceived lower procedural risks.
Acknowledgments
Acknowledgments
We gratefully acknowledge the participants of this study, to whom this work is dedicated. We wish to thank Camilla Lindell, RN, for dedicated data acquisition and invaluable work with on-site and extramural patient visits. The study was financially supported by a donation from the Mats Kleberg foundation.
Editor's Comments and Questions
Editor's comments:
This study documents for the first time the health-related quality of life in patients with nonoperated thoracic aortic aneurysm. Although there was no overall decrement in quality of life, there were small decrements in parameters of physical functioning. We congratulate the authors for examining and documenting this important baseline information in the literature.
Editor's Questions:
-
Thoracic aortic aneurysm is known to be a predominantly asymptomatic disease. So, the finding of no overall decrement in quality of life is not surprising, correct?
In the case of unknown thoracic aortic aneurysm we would agree. However, we investigated a cohort with known thoracic aortic disease. Many of us have witnessed patients refer to their—asymptomatic—aneurysm as a “ticking bomb” or the like. Therefore, it is also reasonable to assume that a chronic condition eliciting such strong emotions may, in fact, affect the quality of life negatively, in a multitude of possible ways, conscious or subconscious.
-
You find that physical symptoms are “predictive” of lower scores on the physical components of your tests. Does this not represent circular reasoning or, at least, circular testing?
No. If physical symptoms were unconditionally correlated to reduced quality of life, investigating the latter would be redundant. However, as is often exemplified in different forms of disability, quality of life is often more related to perceived symptoms, coping, and similar phenomena. The opposite finding, e.g., lower physical scores in depressed patients, would have been equally plausible. It remains important to (1) sort out which, if any, physical symptoms actually do affect quality of life, (2) quantify this effect, and (3) elucidate possible relation to underlying disease; in this study, thoracic aortic disease.
Statistical Commentary:
Dr. John A. Rizzo, Professor, Stony Brook University, New York, USA
This paper employs health-related quality of life (HRQOL) data using the SF-36 instrument to investigate how thoracic aortic disease (TAD; eg, aneurysm or dissection) affects HRQOL in comparison to an age- and sex-matched cohort without TAD. The results suggest modest differences. But, these differences appear to reflect non-TAD-related factors. The statistical approach used—quantile regression—is nonstandard, and as I read through the paper I wondered why the authors elected this approach in favor of the more standard linear regression model. Quantile regression is typically employed when one believes that the explanatory variables of interest work differently at different levels of the dependent variable. That is, if one believes that explanatory variable X will have a stronger effect on lower values of the dependent variable than on higher ones, quantile regression may be indicated. One may also argue that the median value on which the authors focus is the relevant one to study, which could also justify the quantile regression approach.
The authors have worked hard to unearth significant differences in HRQOL between the TAD and non-TAD cohorts. I suspect that multivariable modeling with linear regression would find no significant effects of TAD on HRQOL. But this, if true, is also an interesting finding. As the authors note, while TAD in medically managed patients is usually asymptomatic prior to complications, a catastrophic complication may occur at any time, albeit with low probability. Given this, one might have anticipated that the TAD cohort would exhibit significant deficits in the mental health dimension of the SF-36, resulting from fear, anxiety, and dread.
This is the first study to examine HRQOL effects due to TAD in a group of patients managed medically. As such, it addresses an interesting and useful topic.
References
- 1. Olsson C, Thelin S. Quality of life in survivors of thoracic aortic surgery. Ann Thorac Surg. 1999;67:1262–1267. 10.1016/S0003-4975(99)00165-4 [DOI] [PubMed] [Google Scholar]
- 2. Immer FF, Krähenbühl ES, Immer-Bansi AS, Berdat PA, Kipfer B, Eckstein FS, et al. Quality of life after interventions on the thoracic aorta with deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2002;21:10–14. 10.1016/S1010-7940(01)01067-3 [DOI] [PubMed] [Google Scholar]
- 3. Immer FF, Lippeck C, Barmettler H, Berdat PA, Eckstein FS, Kipfer B, et al. Improvement of quality of life after surgery on the thoracic aorta. Circulation. 2004;110[suppl II]:II-250–II-255 [DOI] [PubMed] [Google Scholar]
- 4. Crawford RS, Pedraza JD, Chung TK, Corey M, Conrad MF, Cambria RP. Functional outcome after thoracoabdominal aneurysm repair. J Vasc Surg. 2008;48:828–835. 10.1016/j.jvs.2008.05.018 [DOI] [PubMed] [Google Scholar]
- 5. Dick F, Hinder D, Immer FF, Hirzel C, Do DD, Carrel TP, et al. Outcome and quality of life after surgical and endovascular treatment of descending aortic lesions. Ann Thorac Surg. 2008;85:1605–1612. 10.1016/j.athoracsur.2008.01.027 [DOI] [PubMed] [Google Scholar]
- 6. Zierer A, Melby SJ, Lubahn JG, Sicard GA, Damiano RJ Jr, Moon MR. Elective surgery for thoracic aortic aneurysms: late functional status and quality of life. Ann Thorac Surg. 2006;82:573–578. 10.1016/j.athoracsur.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 7. Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. 10.1161/CIRCULATIONAHA.106.630400 [DOI] [PubMed] [Google Scholar]
- 8. Winnerkvist A, Brorsson B, Rådegran K. Quality of life in patients with chronic type B aortic dissection. Eur J Vasc Endovasc Surg. 2006;32:34–37. 10.1016/j.ejvs.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 9. Sullivan M, Karlsson J, Taft C. SF-36 Hälsoenkät: Svensk Manual och Tolkningsguide, 2:a upplagan. (Swedish Manual and Interpretation Guide, 2nd Edition). 2002; Gothenburg: Sahlgrenska University Hospital. [Google Scholar]
- 10. Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10:395–404. 10.1023/A:1012552211996 [DOI] [PubMed] [Google Scholar]
- 11. Blackstone EH. Generating knowledge from information, data, and analyses. In Kouchoukos NT, Blackstone EH, Doty DB, Hanley FL, Karp RB. Kirklin/Barratt-Boyes Cardiac Surgery. Third edition. New York: Churchill Livingstone, 2003;303–305. [Google Scholar]
- 12. Bland JM, Altman DG. Cronbach's alpha. BMJ. 1997;314:572 10.1136/bmj.314.7080.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coady MA, Rizzo JA, Hammond GL, Mandapati D, Darr U, Kopf GS, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–491. 10.1016/S0022-5223(97)70360-X [DOI] [PubMed] [Google Scholar]
- 14. Schoormans D, Sprangers MAG, Budts W, Mulder BJM, Apers S, Moons P. Perceived health is partially associated with the symptomatological profile in patients with benign and severe conditions: the case of congenital heart disease. Qual Life Res. 2012. Published online July 31, 2012 10.1007/s11136-012-0241-4 [DOI] [PubMed] [Google Scholar]
- 15. Diaz-Ledezma C, Lichstein PM, Maltenfort M, Restrepo C, Parvizi J. Pattern of impact of femoroacetabular impingement upon health-related quality of life: the determinant role of extra-articular factors. Qual Life Res. Published online Feb. 8, 2013 10.1007/s11136-013-0359-z [DOI] [PubMed] [Google Scholar]
- 16. Lyons RA, Lo SV, Littlepage BN. Comparative health status of patients with 11 common illnesses in Wales. J Epidemiol Comm Health. 1994;48:388–390. 10.1136/jech.48.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coteur G, Feagan B, Keininger DL, Kosinski M. Evaluation of the meaningfulness of health-related quality of life improvements as assessed by the SF-36 and the EQ-5D VAS in patients with active Crohn's disease. Aliment Pharmcol Ther. 2009;29:1032–1041. 10.1111/j.1365-2036.2009.03966.x [DOI] [PubMed] [Google Scholar]
- 18. Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimally clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8:968–974. 10.1016/j.spinee.2007.11.006 [DOI] [PubMed] [Google Scholar]