Abstract
We review the results from the most common animal models of arterial aneurysm, including recent findings from our novel, laparoscopy-based pig model of abdominal aortic aneurysm, that contribute important insights into early pathogenesis. We emphasize the relevance of these findings for evaluation of treatment protocols and novel device prototypes for mechanism-based prevention of progression and rupture.
Keywords: Abdominal aortic aneurysm, Arterial aneurysm, Animal models, Periarterial calcium chloride
Introduction
Interventional treatment regimens for abdominal aortic aneurysm (AAA) have focused on either open abdomen surgical repair/grafting or endovascular stenting when the maximum anterior-posterior diameter exceeds 5 or 5.5 cm or when the growth rate is unacceptably accelerated. However, since the 2005 report of the US Preventive Services Task Force recommendations that all males between the ages of 65 and 74, who have ever smoked, undergo ultrasound screening for AAA [1], we are seeing many more small, developing aneurysms than before [2–4]. This has emphasized the need to develop effective, noninvasive treatment options that target pathogenetic mechanisms responsible for aneurysm progression [5] in order to avoid these procedures, and their frequently serious consequences, in the future [6,7].
Unfortunately, but understandably, most histopathological studies of human abdominal aortic aneurysms have been performed on severely advanced aneurysms that were excised at autopsy or resected at surgery after exceeding 5-6 cm maximum diameter. Such specimens are frequently complicated by mural thrombus, calcific deposits, intramural hemorrhage, and necrotic pultaceous debris that severely obscure the initial structural changes in the arterial wall at the beginning of aneurysm formation.
In this review we present the findings from the most common animal models, including some very recent findings from our new pig model, that contribute important insights related to the early pathogenesis of AAA (and possibly aneurysms in other arterial beds as well) that are important for evaluation of novel, mechanism-based treatment approaches.
Experimental Studies in Small Mammals
Periarterial Calcium Chloride Model
Experimental studies of aneurysm formation in small mammals have been conducted primarily in mice, rats, and rabbits. The periarterial calcium chloride model of arterial aneurysm in the rabbit common carotid artery seems to be the earliest of these models. This model was originally developed in our laboratory in Jerusalem in the late 1980s [8]—actually by mistake. At that time we were looking for a model of Prinzmetal's angina to understand the pathogenesis of myocardial infarction with normal coronary arteries. We used calcium chloride as a spasmogenic agent that would let us study the short- and long-term effects of vasospasm on the arterial wall itself. We sought to show that, as with cerebral vasospasm, spasm of any artery, including the coronaries, even if it resolves, is not an innocent event; rather, it may have profound effects on the artery itself in the short and long term, even, and perhaps particularly, when the associated reduction in luminal diameter is insufficient to substantially alter distal arterial flow [9].
It was shown that the mechanical effects of severe arterial constriction [10], or alterations in the magnitude or directionality of shear forces at the site of partial constriction [11], can cause endothelial damage and/or desquamation followed by platelet deposition and thrombus formation leading to partial or total luminal occlusion at that site or embolization and occlusion of a smaller artery distally—as has been reported in cases of cerebral vasospasm 2-3 weeks after subarachnoid hemorrhage [12,13]. We further hypothesized that the damage done to the vessel at the site of spasm could result in reactive fibromuscular hyperplasia possibly progressing to a fixed stenosis [9,11], as has been reported in some cases of Prinzmetal's angina where the first catheterization showed a spasm that resolved after nitroglycerin, but months later there was a fixed stenosis at the same site [14].
When we looked at the common carotid arteries of rabbits 3 days after periarterial application of CaCl2 [15], indeed, we found all of the expected changes at the site of spasm including endothelial desquamation, platelet attachment on exposed subendothelial tissues, and thrombus formation. However, in long-term studies designed to show that a single periarterial application of calcium chloride can progress to a fixed stenosis, surprisingly, we found aneurysmal dilatation (Fig. 1) [8].
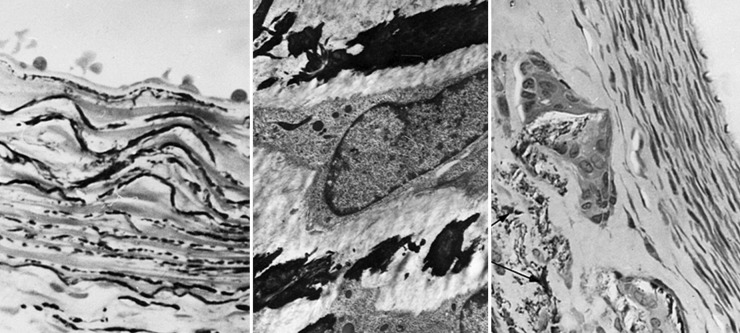
Figure 1.
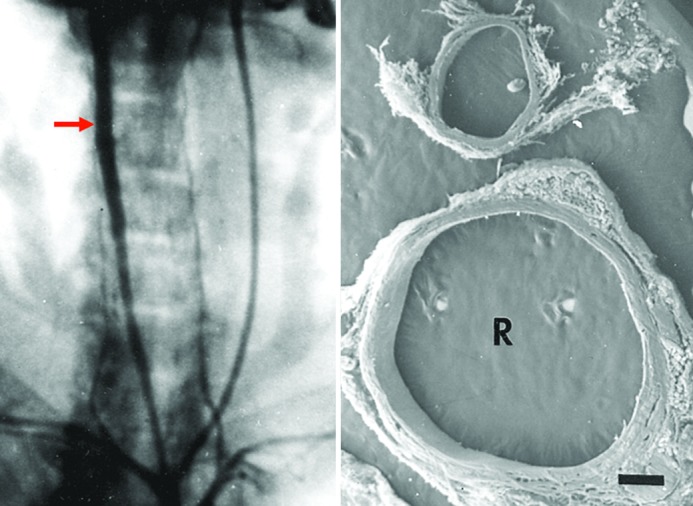
Periarterial calcium chloride. Left, Anteroposterior angiographic projection 3 weeks after a single, periarterial application of CaCl2 to the right common carotid artery of a rabbit. Note aneurysmal dilatation (arrow). Right, Scanning electron micrograph of cross-sections of the common carotid arteries of a rabbit 6 weeks after periarterial application of calcium chloride to the right vessel (R). Note marked dilatation compared with the contralateral, control vessel to which NaCl was applied (profile above) (15×). Reprinted with permission from Gertz et al., J Clin Invest. 1988;81:649-656.
In trying to understand how this happened, by studying Von Kossa-stained sections (for calcium precipitates) with correlative transmission electron microscopy using pyroantimonate staining (for calcium) and correlative energy dispersive x-ray elemental microanalysis (EDS), we found that the periadventitially applied CaCl2 diffused into the wall and bound preferentially to the internal elastic lamina and the elastic lamellae between the layers of smooth muscle cells of the media (Fig. 2). This calcium–elastic tissue complex apparently changed the antigenicity of the elastic tissue, attracting inflammatory cells including monocytes and macrophages that appeared to “eat up” this calcium–elastic tissue complex and disrupt the normal lamellar unit of the arterial media, resulting in progressive luminal dilatation.
Figure 2.
Periarterial calcium chloride. Left, Light microscopic section through the rabbit right common carotid artery (RCCA) 3 days after periarterial CaCl2 showing calcium precipitation within the internal elastic lamina and elastic layers of the media (Von Kossa, 640×). Middle, Transmission electron micrograph (pyroantimonate stain) of a CCA 1 week after CaCl2 showing calcium deposits (black) within the medial elastica (4200×). Right, RCCA 6 weeks after CaCl2 application showing calcium precipitation within the media with giant cells showing central vacuolization and engulfment of fragmented calcium-elastica beneath an area of intimal proliferation (Von Kossa, 350×). Reprinted with permission from Gertz et al., J Clin Invest. 1988;81:649-656.
The periarterial CaCl2 model has since been used by several other groups to induce experimental abdominal and thoracic aortic aneurysm in mice and rats [16–20].
Intraluminal Elastase Model
The role of disruption of the elastic lamellae in aneurysm formation was also shown in the elastase model introduced first by Anidjar et al. in 1990 [21,22]. In that model, pancreatic elastase was infused into an isolated segment of rat aorta resulting in marked degradation of the medial elastic lamellae with a very robust inflammatory response followed by aneurysmal dilatation. Subsequent studies by others have shown similar results in mice [23], rats [24], and rabbits, whether the elastase was delivered intraluminally [25] or applied perivascularly to the adventitial surface [26,27]. Similar results were also obtained with combined intra-aortic elastase and periaortic CaCl2 in rats [28] and rabbits [29].
In the studies of Pyo et al. [23], elastase infusion into the mouse aorta was associated with increased local production of several elastolytic matrix metalloproteinases (MMPs). When treating these mice with the systemic, nonselective MMP inhibitor, doxycycline, or using MMP-9-deficient mice, they found attenuation of the aneurysmal degeneration. Of interest, local, controlled release of doxycycline from biodegradable fibers decreased AAA progression in mice [30], but human clinical studies with doxycycline have shown conflicting results [4,31].
Angiotensin-II-Infused Apolipoprotein E-Deficient Mouse Model
One major disadvantage of the initial small animal models is that the degree of aneurysmal dilatation is sometimes too subtle to permit evaluation of treatment regimens without using a large number of animals. This disadvantage was basically eliminated with the development of the apolipoprotein e-deficient (apo-e−/−) mouse model of AAA by Alan Daugherty's group in Kentucky [32–37]. Infusion of angiotensin-II into these apo-e−/− mice results in very impressive aneurysms in the suprarenal portion of the aorta within 2-3 weeks in 30-85% of cases (Fig. 3). Because of the robustness of these aneurysms, testing the efficacy of any treatment modality in this model requires far fewer animals to reach statistical separation between the treatment groups.
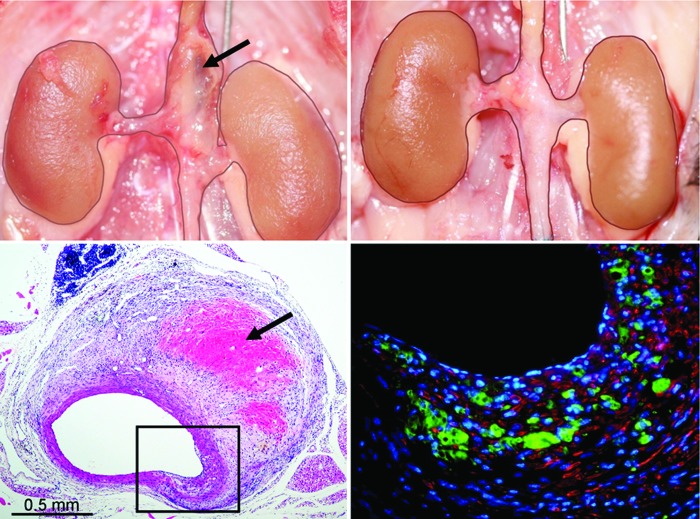
Figure 3.
Suprarenal AAA 4 weeks after angiotensin-II (Ang-II) infusion in the apo-e−/− mouse (top left) not present in a similar mouse treated with low-level laser irradiation (LLLI) (top right). Needle standard is 0.5 mm. Note hemorrhage into the wall in the macroscopic (upper left, arrow) and H&E section (lower left; arrow) of the suprarenal segment of a nontreated Ang-II-infused mouse. The immunofluorescent stained section (lower right) of the boxed-in area of transmural disruption shows marked infiltration of MAC-2+ macrophages (green) in the area of the fragmented media (red = α-actin positive smooth muscle cells). Reprinted with permission from Gavish et al., Lasers in Surgery and Medicine. 2012;44:664–674.
In this model, we find the aneurysms to be associated with transmural disruptions of the media that appear to occur exclusively in the vicinity of branch orifices where alterations in magnitude and directionality of hemodynamic shear forces are the greatest (Figs. 3 and 4) [38]. In general, these transmedial defects are accompanied by extensive inflammatory infiltrates at sites of disrupted elastic lamellae and damaged smooth muscle cells with reactive fibromuscular hyperplasia contiguous proximally or distally with the areas of media-adventitial dissection typically found in this model. As with human AAA, the fragmentation of the elastic lamellae with damage and loss of smooth muscle cells is associated with variable luminal and wall expansion with frequent intramural hemorrhage and thrombus formation.
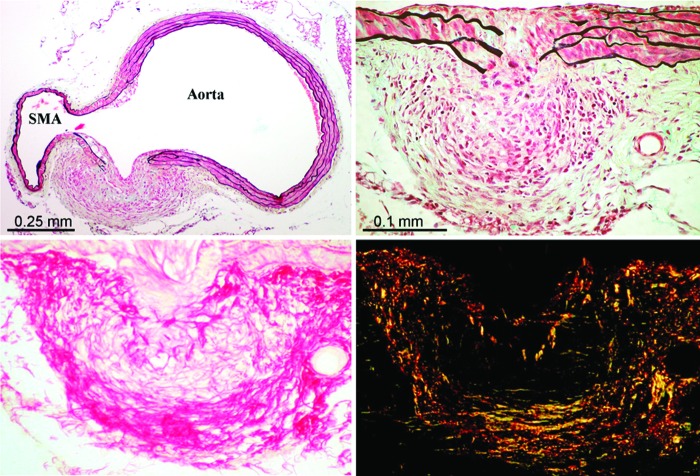
Figure 4.
Suprarenal abdominal aorta of LLL-treated Ang-II-infused apo-e−/− mouse showing marked transmural disruption in the vicinity of the orifice of the superior mesenteric artery (SMA) with reactive fibromuscular hyperplasia appearing to wall off the medial defect preventing significant aneurysmal dilatation (top left and right; Movat pentachrome stain). Top right is a section immediately adjacent to that shown in the top left. Note the marked infiltration of inflammatory cells in the area of the disrupted media with macrophages and giant cells (arrow) at the site of fragmented elastic lamellae and injured smooth muscle cells. Lower panel shows the extensive collagen matrix formation in this reactive, “walling off” area (lower left = picrosirius red [PSR] stain for collagen in nonpolarized light; lower right = PSR stain viewed with polarized light). Reprinted with permission from Gavish et al., Lasers in Surgery and Medicine 2012;44:664–674.
Genetically Driven Mouse Models of Arterial Aneurysm
Inherited human arterial aneurysms have been shown to be associated with several specific mutations in genes that code, for example, for extracellular matrix proteins such as fibrillin-1 in Marfan syndrome or collagen-1 or -3 in Ehlers-Danlos syndrome, specific transmembrane proteins such as transforming growth factor (TGF)-β receptors-1 and -2 in Loeys-Dietz syndrome, or polycistin-1 and -2 in polycystic kidney disease, as well as mutations in a variety of cytoplasmic and nuclear proteins [39]. In a comprehensive review of mouse models of such single-gene-driven syndromes, Lindsay and Dietz [39] have presented compelling evidence in favor of a prominent role for the TGF-β pathway in the pathogenesis of aneurysm at the molecular level.
Other Models of Aortic Aneurysm
Two additional models that should be mentioned are the Broad-Breasted White (BBW) turkey model and the Blotchy Mouse model. In the former, administration of β-aminopropionitrile (BAPN) from Lathyrus odoratus seeds results in dissection and rupture of the abdominal aorta by interfering with lysyl oxidase activity necessary for cross-linking in collagen and elastin [40–42]. In the Blotchy Mouse, an abnormality on the X chromosome results in decreased copper-dependent lysyl oxidase activity, also reducing elastin and collagen cross-linking, resulting in a high percentage of aneurysms of the ascending aorta [43]. Subsequent studies by Brophy et al. [43–45] showed that the β-blocker propranolol, which prevented aneurysm formation in the BBW turkey, also does so in the Blotchy Mouse—not by its effect on heart rate or blood pressure, but by stimulating the cross-linking of elastin and collagen, further emphasizing the central importance of the integrity of the matrix in aneurysm progression and rupture.
Experimental Studies in Large Animals
Although the small animal models have provided very important insights concerning the pathogenesis of arterial aneurysm, those models do not permit evaluation of novel medical devices where an aortic diameter similar to that of humans is deemed necessary. On the other hand, most of the existing large animal models of aneurysm have serious limitations in terms of relevance. We can summarize the currently available large animal models of AAA as follows.
First, there is the enzymatic approach which, as in the small animal models, involves injecting pancreatic elastase into isolated, clamped segments of the porcine abdominal aorta alone [46], or with collagenase, or with mechanical balloon dilation [47,48] or with the addition of periaortic calcium chloride [49]. The mechanical restriction model involves periaortic application of a polymeric cuff causing coarctation leading to turbulent blood flow that induces poststenotic dilatation [50,51]. The surgical models include creating an aortic dilatation by interposition of either an autologous gastric serosa patch [52] or a Dacron patch [53]. A variety of other combinations of the above have been performed, such as enzymatic and mechanical restriction or mechanical restriction together with surgical Dacron patch insertion [52].
Clamping the aorta, physically dilating the vessel, or creating an artificial aneurysmal sac by surgical interposition of a patch can hardly be viewed as simulating the actual cellular physiology underlying the disease process. Moreover, none of these surgical/mechanical models has shown further significant growth of the aorta after the initial procedure. Thus, although these swine models of AAA may be useful for device procedural training, they are of very limited use for determining the effect of novel technologies on the cellular mechanisms of aneurysmal dilatation.
Novel Laparoscopic Porcine Model of AAA
In our laboratory, we are developing a more physiological porcine model of AAA that is based on laparoscopic delivery of CaCl2 to the periadventitial surface of the aorta combined with angiotensin-II infusion in the setting of a 1-month high fat diet. We present this model here, briefly, for the first time (except for in abstract form).
Using conventional laparoscopic techniques, the infrarenal portion of the abdominal aorta is exposed and CaCl2 (0.5 M) applied to the periadventitial surface of the central third using a gauze swatch that is left in for 20 minutes (Fig. 5, top). Additional CaCl2 is applied until saturation of the gauze. Angiotensin-II is delivered by osmotic minipump (Alzet, model 2ML4, DURECT Corp., Cupertino, CA) inserted retroperitoneally by laparoscopy just prior to withdrawal of all instruments and closure of access ports (Fig. 5, bottom). The animals are fed a diet of pig chow with addition of 1% cholesterol and 20% beef tallow beginning 1 month before laparoscopy. The aortas are followed serially by ultrasound until sacrifice at 4-6 weeks.
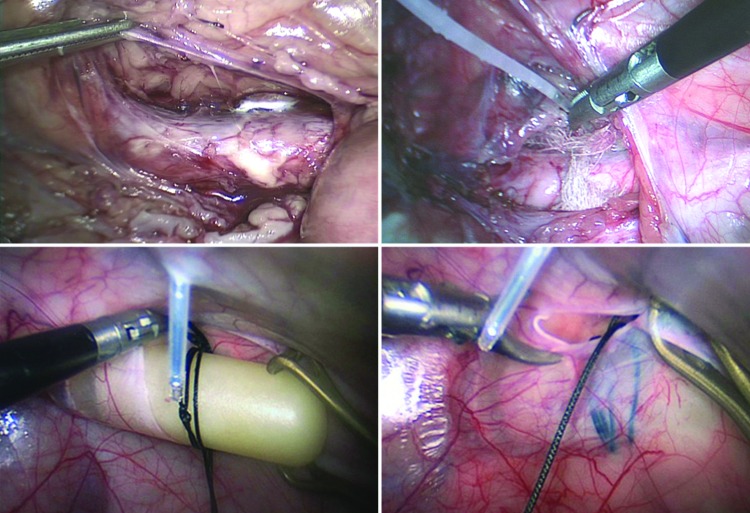
Figure 5.
Images of the porcine model of experimental AAA showing laparoscopic exposure of the infrarenal abdominal aorta (top left), periarterial insertion of gauze and application of CaCl2 (top right), retroperitoneal insertion of angiotensin-II-filled osmotic minipump (bottom left), and closure of the peritoneum with the pump seen behind (bottom right).
Fig. 6 is an ultrasound image at baseline and after aneurysmal dilatation at 4 weeks. This animal died a week later (5 weeks) from a ruptured aneurysm. Postmortem specimens of the abdominal aortas of two pigs are shown in Fig. 7. The aorta on the left was excised at sacrifice at 6 weeks, but the aorta on the right was excised after rupture at 5 weeks.
Figure 6.
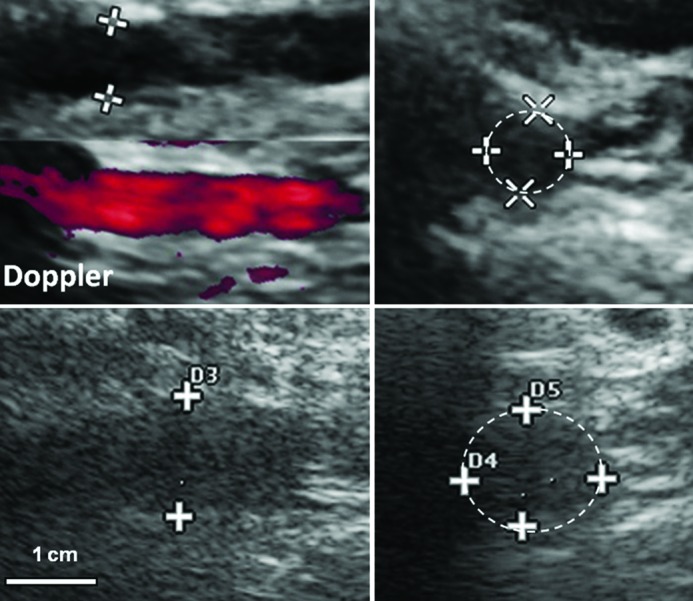
B-mode ultrasound before (top) and 4 weeks after (bottom) laparoscopic induction of AA(A). Left, Longitudinal views. Right, Cross-sections of infrarenal aorta at site of maximum cross-sectional diameter. This pig died from ruptured AAA at 5 weeks. A 5-9 MHz linear-array ultrasonographic probe is shown.
Figure 7.
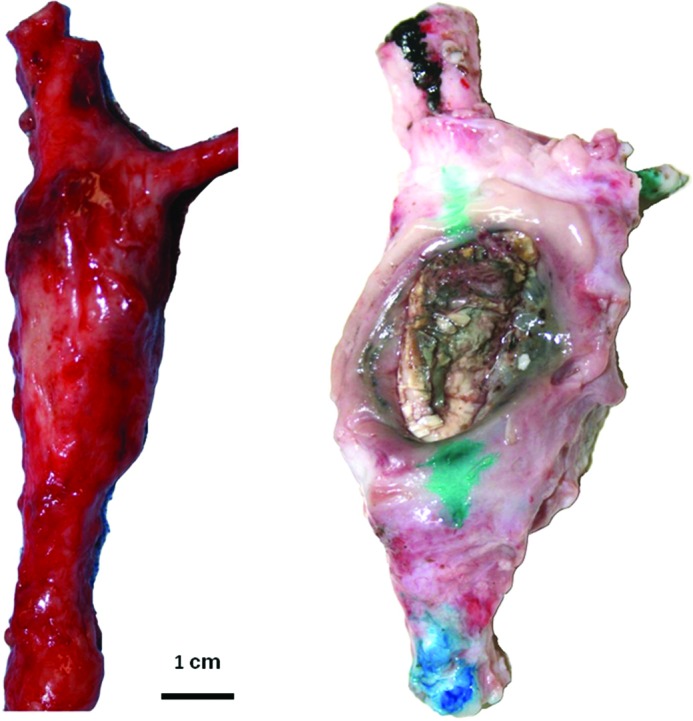
Postmortem photographs of infrarenal abdominal aortic aneurysms in pigs at the site of laparoscopically delivered periaortic calcium chloride. Both pigs had angiotensin-II infusion and were fed an atherogenic diet. The aneurysmatic aorta on the left was excised at 6 weeks. The AAA on the right ruptured at 5 weeks.
The histopathological features of these specimens show striking similarities to human AAA. By electron dispersive spectroscopy, we confirmed that the periarterially applied calcium indeed attached preferentially to the elastic lamella just like in the original rabbit model (Fig. 8). The normal, nonaneurysmatic segments of these aortas showed some intimal hyperplasia that is to be expected with this model which includes angiotensin infusion and a hypercholesterolemic diet even though calcium was not applied; however, the lamellar units of the media were preserved (Fig. 9). On the other hand, at sites of periarterial CaCl2, there were variable degrees of luminal dilation, associated with marked disruption of the media with fragmentation of the elastic lamellae, infiltration of inflammatory cells including giant cells, some of which could be seen engulfing fragmented elastica, injury and depletion of smooth muscle cells with replacement by intercellular matrix, neovascularization with extravasation of red blood cells, and in some cases larger areas of intramural hemorrhage (Fig. 9).
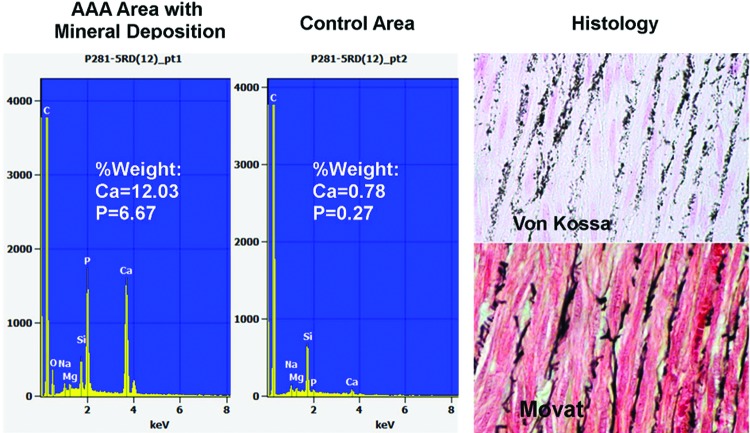
Figure 8.
Left, Elemental analysis of porcine AAA by scanning electron microscopy (SEM) with electron dispersive spectrometery (EDS) showing calcium precipitates in the elastic lamellae. Right, top = Von Kossa-stained section showing calcium precipitates within the medial elastica as additionally confirmed with Movat pentachrome (bottom) that stains elastica black.
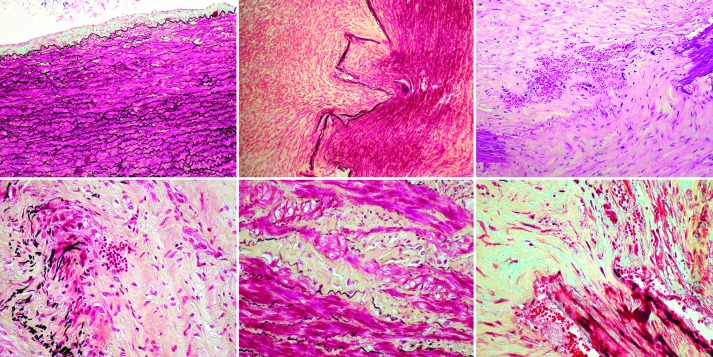
Figure 9.
Infrarenal porcine abdominal aorta 4 weeks after laparoscopically delivered periarterial CaCl2 and angiotensin-II infusion by osmotic minipump inserted retroperitoneally by laparoscopy on the background of a 1-month high cholesterol, high fat diet. Top left, Relatively normal area adjacent to the site of calcium application (except for mild intimal hyperplasia). The remaining sections are from the site of aneurysm formation. Top middle, Marked disruption of the media with extensive fibromuscular hyperplasia (Movat). Top right, Transmedial disruption with fragmented elastica, neovascularization, RBC extravasation (H&E). Bottom left, Marked fragmentation of the elastic lamellae with infiltration of inflammatory cells including giant cells, some of which can be seen engulfing fragmented elastica (Movat). Bottom middle, Injury and depletion of smooth muscle cells with replacement by intercellular matrix (yellow) (Movat). Bottom right, Marked disruption of the media with fibrous tissue proliferation, neovascularization, and intramural extravasation of red blood cells (Movat).
Lessons and Conclusions
We have made very good progress in the development of a novel, robust, physiologically relevant, large animal model of AAA that closely resembles the pathobiology of human aneurysms, but several problems remain. First, the model may be a bit too severe to successfully test the effect of novel pharmacological treatments and new devices that prevent aneurysm progression. We probably need to reduce the level of insult, e.g., by reducing the concentration of calcium chloride (to 0.25 M) or removing one of the other two insults.
But there are other problems for which the solutions are much less obvious. These large animal experiments are very, and sometimes prohibitively, expensive. Each pig, when considering purchase, housing, surgical and laparoscopic supplies, operating room time, veterinary expenses, and tissue preparation, costs, conservatively speaking, nearly $10,000 per pig. This is a huge burden on any laboratory with the average level of funding—particularly in the current era of belt-tightening.
In addition, these experiments are very labor intensive, requiring a large, multidisciplinary staff—not to mention the huge additional expenditure of time that such large animal experiments require.
Thus, our recommended approach for testing new treatments is to do the majority of efficacy studies in the angiotensin-infused apo e-knock-out model of aneurysm. Clearly, as we have shown, there is no small animal model to date that is more robust or more suitable. This should be appropriate for most new pharmacological regimens, and today, with advancements in miniaturization capabilities and three-dimensional printing, this also should be appropriate for a variety of novel device prototypes. The more expensive and labor-intensive pig model should be reserved for the bare minimum of highly focused efficacy studies as well as additional safety studies performed with an up-sized version of the device prototype prior to first-in-man trials.
Acknowledgments
Acknowledgments
This work is funded in part by the Israel Science Foundation (Grant No. 1298/10), The Rosetrees Trust Fund of the United Kingdom, and Professor Eliyahu Kelman. SDG is The Brandman Foundation Professor of Cardiac and Pulmonary Diseases, Institute for Medical Research—IMRIC, Faculty of Medicine of The Hebrew University and Hadassah, Jerusalem, Israel. This investigation (the newly reported findings in swine) conforms to the Guide for the Care and Use of Laboratory Animals published by the National Research Council, revised 1996. (http://www.nap.edu/catalog/5140.html). Animal care and experimental procedures were approved by the Ethics Committee of the Faculty of Medicine of The Hebrew University, Jerusalem, Israel (MD-13-12282-4).
EDITOR'S COMMENTS
I had the pleasure of hearing Dr. Gertz deliver the lecture that motivated this invited article. It was a privilege to hear his historical and scientific perspectives on animal models of aortic aneurysm—a field to which he has made immense contributions. In this article, he clearly and succinctly presents this important area of research.
Of note, Dr. Jeffrey Jones, Dr. John Ikonomidis, and their team have very recently reported a porcine model of aortic aneurysm. We suggest that interested readers also peruse this seminal work, which is found in the following reference:
Eckhouse SR, Logdon CB, Oelsen JM, Patel Rk, Rice AD, Stroud RE, et al. Reproducible porcine model of thoracic aortic aneurysm. Circulation. 2013;128:S186–S193.
EDITOR'S COMMENTS
M. David Tilson, MD, Columbia University, New York, NY, USA
First, the authors observe that CaCl2 applied to the periadventitia of the rabbit carotid artery becomes bound to the elastic lamellae and the internal elastic lamina, apparently changing the “antigenicity” of the elastica and attracting monocytes and macrophages which “appeared to 'eat up'” the complex, resulting in mechanical failure of the wall and vessel enlargement. This highly prescient observation anticipated by years the growing evidence that the immune system is involved in AAA development [1]. The role of the polymorphonuclear leukocyte is also receiving more attention, and the role of the adventitial fibroblast has been seriously neglected [2,3]. Investigators should note that the CaCl2 did not work in the rat in our hands, although it does work in the mouse. So others are advised not to invest too much effort in the species of rat (personal communication).
Second, in the intraluminal elastase model, which does work in rat, the authors note that a “very robust inflammatory response” is followed by AAA formation. We also found in this model that there is a sequential response in the profile of proteolytic activities that occur over the first week, suggesting that this destruction of the media and adventitia involved a complex course of biochemical as well as inflammatory events [4].
Third, this editorialist believes that there has been a misunderstanding of some of the fundamentals of the Angiotensin-II infused Apolipoprotein E-Deficient Mouse model. Apolipoprotein E deficiency is not required for this model. We have successfully induced aneurysms in retired male breeder C57BL/6 mice that are genetically intact [5]. Additional studies will be needed to determine whether Angiotensin-II alone also works in younger or female mice.
Fourth, the authors make somewhat discouraging but realistic comments about the future of large animal models in the study of aneurysm pathogenesis. They have innovated a successful model in the pig with the trivalent insults of 1) a one-month high fat diet, 2) hypertension induced by Angiotensin-II infusion, and 3) a periadventitial CaCl2 insult applied by laparoscopic means. The unfortunate part is that these experiments quickly become prohibitively expensive, at a cost of approximately $10,000 (USD) per pig. Since we have found that hyperlipidemia is not required in the Angiotensin-II-infused mouse model, elimination of the one-month preoperative lipid-loading period would reduce the boarding costs substantially for further development of the porcine model. Another casualty of the present era of austerity affecting all endeavors is that it may not be possible to repeat the experiment to see whether lipid loading and regression are required for aneurysm development in the gorilla, since aneurysm is a leading cause of death in the gorilla without additional diet-induced insults in captive animals [6,7].
References
- 1. U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: recommendation statement. Ann Intern Med. 2005;142:198–202. 10.7326/0003-4819-142-3-200502010-00011 [DOI] [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. 10.1161/CIR.0b013e31823ac046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2010;8:92–102. 10.1038/nrcardio.2010.180 [DOI] [PubMed] [Google Scholar]
- 4. Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. 10.1161/CIRCULATIONAHA.107.735274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson RW. Reflections on the pathogenesis of abdominal aortic aneurysms. Cardiovasc Surg. 2002;10:389–394. 10.1016/S0967-2109(02)00042-X [DOI] [PubMed] [Google Scholar]
- 6. Drury D, Michaels JA, Jones L, Ayiku L. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005;92:937–946. 10.1002/bjs.5123 [DOI] [PubMed] [Google Scholar]
- 7. Wilt TJ, Lederle FA, Macdonald R, Jonk YC, Rector TS, Kane RL. Comparison of endovascular and open surgical repairs for abdominal aortic aneurysm. Evid Rep Technol Assess (Full Rep). 2006:1–113. [PMC free article] [PubMed] [Google Scholar]
- 8. Gertz SD, Kurgan A, Eisenberg D. Aneurysm of the rabbit common carotid artery induced by periarterial application of calcium chloride in vivo. J Clin Invest. 1988;81:649–656. 10.1172/JCI113368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gertz SD. Vascular damage and thrombosis from spasm. N Engl J Med. 1979;300:197 10.1056/NEJM197901253000413 [DOI] [PubMed] [Google Scholar]
- 10. Joris I, Majno G. Endothelial changes induced by arterial spasm. Am J Pathol. 1981;102:346–358. [PMC free article] [PubMed] [Google Scholar]
- 11. Gertz SD, Uretsky G, Wajnberg RS, Navot N, Gotsman MS. Endothelial cell damage and thrombus formation after partial arterial constriction: relevance to the role of coronary artery spasm in the pathogenesis of myocardial infarction. Circulation. 1981;63:476–486. 10.1161/01.CIR.63.3.476 [DOI] [PubMed] [Google Scholar]
- 12. Blaumanis OR, Gertz SD, Grady PA, Nelson E. Thrombosis in acute experimental cerebral vasospasm. The Joint Meeting on Stroke and Cerebral Circulation, Dallas, Texas. Stroke. 1976;7:9–10. [Google Scholar]
- 13. Blaumanis OR, Grady PA and Nelson E. Hemodynamic and morphological aspects of cerebral vasospasm. In: Price TR, Nelson E, editors. Cerebrovascular Diseases. New York: Raven Press; 1979;pp. 283–294. [Google Scholar]
- 14. Marzilli M, Goldstein S, Trivella MG, Palumbo C, Maseri A. Some clinical considerations regarding the relation of coronary vasospasm to coronary atherosclerosis: a hypothetical pathogenesis. Am J Cardiol. 1980;45:882–886. 10.1016/0002-9149(80)90135-6 [DOI] [PubMed] [Google Scholar]
- 15. Kurgan A, Gertz SD, Wajnberg RS. Intimal changes associated with arterial spasm induced by periarterial application of calcium chloride. Exp Mol Pathol. 1983;39:176–193. 10.1016/0014-4800(83)90050-3 [DOI] [PubMed] [Google Scholar]
- 16. Chiou AC, Chiu B, Pearce WH. Murine aortic aneurysm produced by periarterial application of calcium chloride. J Surg Res. 2001;99:371–376. 10.1006/jsre.2001.6207 [DOI] [PubMed] [Google Scholar]
- 17. Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. 10.1172/JCI15334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, et al. A murine model of thoracic aortic aneurysms. J Surg Res. 2003;115:157–163. 10.1016/S0022-4804(03)00193-8 [DOI] [PubMed] [Google Scholar]
- 19. Geng L, Wang W, Chen Y, Cao J, Lu L, Chen Q, et al. Elevation of ADAM10, ADAM17, MMP-2 and MMP-9 expression with media degeneration features CaCl2-induced thoracic aortic aneurysm in a rat model. Exp Mol Pathol. 2010;89:72–81. 10.1016/j.yexmp.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Krishna S, Golledge J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis. 2013;226:29–39. 10.1016/j.atherosclerosis.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 21. Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. 10.1161/01.CIR.82.3.973 [DOI] [PubMed] [Google Scholar]
- 22. Anidjar S, Dobrin PB, Eichorst M, Graham GP, Chejfec G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J Vasc Surg. 1992;16:139–147. 10.1067/mva.1992.35585 [DOI] [PubMed] [Google Scholar]
- 23. Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. 10.1172/JCI8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamaguchi T, Yokokawa M, Suzuki M, Higashide S, Katoh Y, Sugiyama S, et al. Morphologic changes in the aorta during elastase infusion in the rat aneurysm model. J Surg Res. 2001;95:161–166. 10.1006/jsre.2000.6025 [DOI] [PubMed] [Google Scholar]
- 25. Kallmes DF, Fujiwara NH, Berr SS, Helm GA, Cloft HJ. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol. 2002;23:295–298. [PMC free article] [PubMed] [Google Scholar]
- 26. White JV, Mazzacco SL. Formation and growth of aortic aneurysms induced by adventitial elastolysis. Ann N Y Acad Sci. 1996;800:97–120. 10.1111/j.1749-6632.1996.tb33302.x [DOI] [PubMed] [Google Scholar]
- 27. Origuchi N, Shigematsu H, Izumiyama N, Nakamura K, Toku A, Muto T. Aneurysm induced by periarterial application of elastase heals spontaneously. Int Angiol. 1998;17:113–119. [PubMed] [Google Scholar]
- 28. Tanaka A, Hasegawa T, Chen Z, Okita Y, Okada K. A novel rat model of abdominal aortic aneurysm using a combination of intraluminal elastase infusion and extraluminal calcium chloride exposure. J Vasc Surg. 2009;50:1423–1432. 10.1016/j.jvs.2009.08.062 [DOI] [PubMed] [Google Scholar]
- 29. Bi Y, Zhong H, Xu K, Zhang Z, Qi X, Xia Y, et al. Development of a novel rabbit model of abdominal aortic aneurysm via a combination of periaortic calcium chloride and elastase incubation. PLoS One. 2013;8:e68476 10.1371/journal.pone.0068476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamawaki-Ogata A, Hashizume R, Satake M, Kaneko H, Mizutani S, Moritan T, et al. A doxycycline loaded, controlled-release, biodegradable fiber for the treatment of aortic aneurysms. Biomaterials. 2010;31:9554–9564. 10.1016/j.biomaterials.2010.08.069 [DOI] [PubMed] [Google Scholar]
- 31. Dodd BR, Spence RA. Doxycycline inhibition of abdominal aortic aneurysm growth: a systematic review of the literature. Curr Vasc Pharmacol. 2011;9:471–478. 10.2174/157016111796197288 [DOI] [PubMed] [Google Scholar]
- 32. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. 10.1172/JCI7818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. 10.1161/01.ATV.0000085631.76095.64 [DOI] [PubMed] [Google Scholar]
- 34. Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. 10.1161/01.ATV.0000118013.72016.ea [DOI] [PubMed] [Google Scholar]
- 35. Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg. 2006;44:372–376. 10.1016/j.jvs.2006.04.047 [DOI] [PubMed] [Google Scholar]
- 36. Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, et al. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–H1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin II-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. 10.1111/j.1749-6632.2011.06332.x [DOI] [PubMed] [Google Scholar]
- 38. Gavish L, Rubinstein C, Berlatzky Y, Gavish LY, Beeri R, Gilon D, et al. Low level laser arrests abdominal aortic aneurysm by collagen matrix reinforcement in apolipoprotein E-deficient mice. Lasers Surg Med. 2012;44:664–674. 10.1002/lsm.22068 [DOI] [PubMed] [Google Scholar]
- 39. Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. 10.1038/nature10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barnett BD, Bird HR, Lalich JJ, Strong FM. Toxicity of beta-amino-propionitrile for turkey poults. Proc Soc Exp Biol Med. 1957;94:67–70. 10.3181/00379727-94-22857 [DOI] [PubMed] [Google Scholar]
- 41. Simpson CF, Kling JM, Robbins RC, Harms RH. Beta-aminopropionitrile-induced aortic ruptures in turkeys: inhibition by reserpine and enhancement by monoamine oxidase inhibitors. Toxicol Appl Pharmacol. 1968;12:48–59. 10.1016/0041-008X(68)90175-0 [DOI] [PubMed] [Google Scholar]
- 42. Simpson CF, Boucek RJ. The B-aminopropionitrile-fed turkey: a model for detecting potential drug action on arterial tissue. Cardiovasc Res. 1983;17:26–32. 10.1093/cvr/17.1.26 [DOI] [PubMed] [Google Scholar]
- 43. Rowe DW, McGoodwin EB, Martin GR, Grahn D. Decreased lysyl oxidase activity in the aneurysm-prone, mottled mouse. J Biol Chem. 1977;252:939–942. [PubMed] [Google Scholar]
- 44. Brophy C, Tilson JE, Tilson MD. Propranolol delays the formation of aneurysms in the male blotchy mouse. J Surg Res. 1988;44:687–689. 10.1016/0022-4804(88)90101-1 [DOI] [PubMed] [Google Scholar]
- 45. Brophy CM, Tilson JE, Tilson MD. Propranolol stimulates the crosslinking of matrix components in skin from the aneurysm-prone blotchy mouse. J Surg Res. 1989;46:330–332. 10.1016/0022-4804(89)90197-2 [DOI] [PubMed] [Google Scholar]
- 46. Marinov GR, Marois Y, Pâris E, Roby P, Formichi M, Douville Y, et al. Can the infusion of elastase in the abdominal aorta of the Yucatán miniature swine consistently produce experimental aneurysms? J Invest Surg. 1997;10:129–150. 10.3109/08941939709032144 [DOI] [PubMed] [Google Scholar]
- 47. Sadek M, Hynecek RL, Goldenberg S, Kent KC, Marin ML, Faries PL. Gene expression analysis of a porcine native abdominal aortic aneurysm model. Surgery. 2008;144:252–258. 10.1016/j.surg.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hynecek RL, DeRubertis BG, Trocciola SM, Zhang H, Prince MR, Ennis TL, et al. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery. 2007;142:143–149. 10.1016/j.surg.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 49. Czerski A, Bujok J, Gnus J, Hauzer W, Ratajczak K, Nowak M, et al. Experimental methods of abdominal aortic aneurysm creation in swine as a large animal model. J Physiol Pharmacol. 2013;64:185–192. [PubMed] [Google Scholar]
- 50. Molácek J, Treska V, Kobr J, Certík B, Skalický T, Kuntscher V, et al. Optimization of the model of abdominal aortic aneurysm–experiment in an animal model. J Vasc Res. 2009;46:1–5. 10.1159/000135659 [DOI] [PubMed] [Google Scholar]
- 51. Lin PY, Wu YT, Lin GC, Shih YH, Sampilvanjil A, Chen LR, et al. Coarctation-induced degenerative abdominal aortic aneurysm in a porcine model. J Vasc Surg. 2013;57:806–815.e1. 10.1016/j.jvs.2012.08.104 [DOI] [PubMed] [Google Scholar]
- 52. Usón-Gargallo J, Crisóstomo V, Loscertales B, Sun F, Sánchez-Margallo FM, Martín-Cancho MF, et al. A new model of abdominal aortic aneurysm with gastric serosa patch: surgical technique and short-term evaluation. J Invest Surg. 2006;19:97–104. 10.1080/08941930600569415 [DOI] [PubMed] [Google Scholar]
- 53. Diaz S, Uzieblo MR, Desai KM, Talcott MR, Bae KT, Geraghty PJ, et al. Type II endoleak in porcine model of abdominal aortic aneurysm. J Vasc Surg. 2004;40:339–344. 10.1016/j.jvs.2004.04.003 [DOI] [PubMed] [Google Scholar]
References
- 1. Kuivaniemi H, Platsoucas CD, Tilson MD. Aortic aneurysms: an immune disease with a strong genetic component. Circulation 2008;117:242–252. 10.1161/FCIRCULATIONAHA.107.690982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rizas KD, Ippagunta N, Tilson MD. Immune cells and molecular mediators in the pathogenesis of the abdominal aortic aneurysm. Cardiol Rev 2009;17:201–210. 10.1097/CRD.0b013e3181b04698 [DOI] [PubMed] [Google Scholar]
- 3. Suh JH, Yoon JS, Kim HW, Jo KH. Adventitial fibroblast abnormality in thoracic aortic aneurysms and aortic dissections. Korean J Thorac Cardiovasc Surg 2011;44:406–412. 10.5090/kjtcs.2011.44.6.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halpern VJ, Nackman GB, Gandhi RH, Irizarry E, Scholes JV, Ramey WG, et al. The elastase infusion model of experimental aortic aneurysms: synchrony of induction of endogenous proteinases with matrix destruction and inflammatory cell response. J Vasc Surg 1994;20:51–60. 10.1016/0741-5214(94)90175-9 [DOI] [PubMed] [Google Scholar]
- 5. Luo J, Fujikura K, Tyrie LS, Tilson MD, Konofagou EE. Pulse wave imaging of normal and aneurismal abdominal aortas in vivo. IEE Trans Med Imaging 2009;28:477–486. 10.1109/TMI.2008.928179 [DOI] [PubMed] [Google Scholar]
- 6. Gorilla heart disease deaths puzzling zoos. Los Angeles Times. April 9, 2008. [Google Scholar]
- 7. Kenny DE, Cambre RC, Alvarado TP, Prowten AW, Allchurch AF, Marks SK, et al. Aortic dissection: an important cardiovascular disease in captive gorillas (Gorilla gorilla gorilla). J Zoo Wildlife Med 1994;25:561–568. 10.1016/S0967-2109(02)00140-0 [DOI] [Google Scholar]