Abstract
The toxicity of pesticides used in agriculture towards non-targeted organisms and especially pollinators has recently drawn the attention from a broad scientific community. Increased honeybee mortality observed worldwide certainly contributes to this interest. The potential role of several neurotoxic insecticides in triggering or potentiating honeybee mortality was considered, in particular phenylpyrazoles and neonicotinoids, given that they are widely used and highly toxic for insects. Along with their ability to kill insects at lethal doses, they can compromise survival at sublethal doses by producing subtle deleterious effects. In this study, we compared the bee’s locomotor ability, which is crucial for many tasks within the hive (e.g. cleaning brood cells, feeding larvae…), before and after an acute sublethal exposure to one insecticide belonging to the two insecticide classes, fipronil and thiamethoxam. Additionally, we examined the locomotor ability after exposure to pyrethroids, an older chemical insecticide class still widely used and known to be highly toxic to bees as well. Our study focused on young bees (day 1 after emergence) since (i) few studies are available on locomotion at this stage and (ii) in recent years, pesticides have been reported to accumulate in different hive matrices, where young bees undergo their early development. At sublethal doses (SLD48h, i.e. causing no mortality at 48h), three pyrethroids, namely cypermethrin (2.5 ng/bee), tetramethrin (70 ng/bee), tau-fluvalinate (33 ng/bee) and the neonicotinoid thiamethoxam (3.8 ng/bee) caused a locomotor deficit in honeybees. While the SLD48h of fipronil (a phenylpyrazole, 0.5 ng/bee) had no measurable effect on locomotion, we observed high mortality several days after exposure, an effect that was not observed with the other insecticides. Although locomotor deficits observed in the sublethal range of pyrethroids and thiamethoxam would suggest deleterious effects in the field, the case of fipronil demonstrates that toxicity evaluation requires information on multiple endpoints (e.g. long term survival) to fully address pesticides risks for honeybees. Pyrethroid-induced locomotor deficits are discussed in light of recent advances regarding their mode of action on honeybee ion channels and current structure-function studies.
Introduction
Pollinators play a crucial role in maintaining vegetal biodiversity but also participate in improving agricultural production. Therefore, a number of managed honeybee colonies are periodically moved in the vicinity of agricultural fields, not only to increase honey production but to improve crop pollination as well. As a consequence, insecticide exposure of honeybee colonies does not only occur when individuals are foraging, but also happens directly in the hive, as demonstrated by measurements of in-hive pesticide residues [1, 2]. In the last few decades, an increase in honeybee colony mortalities has been reported around the world and focused the attention of a broad scientific community on the potential consequences of pesticide misuse on pollinator survival [3–5]. These studies have been especially focused on two families of insecticides, neonicotinoids and phenylpyrazoles, owing to their use as systemic insecticides in seed treatment [6]. Recently, the sublethal toxicity of neonicotinoids towards honeybees has been demonstrated in real-world environments and led the European Union to restrict the use of three members of this class for two years [7–9]. Similarly, fipronil, a phenylpyrazole highly toxic to bees even at sublethal levels (by impairing memory and synergistically enhancing sensitivity to the pathogen Nosema [10, 11]) has also been banned as an agrochemical product in France and more recently in other countries of the European Union, although it is still widely used elsewhere, like the neonicotinoids [12]. It is worth mentioning that these temporary restrictions apply for seed coating only, whereas other agrochemical formulations are still authorized (Official Journal of the European Union OJ L219/22–15.8.2013 and OJ L139/12–25.5.2013). Besides neonicotinoids and phenylpyrazoles, pyrethroid insecticides constitute a large insecticide family produced through chemical synthesis, with a limited number of compounds (e.g. deltamethrin, cypermethrin, λ-cyalothrin, permethrin) accounting for the majority of sales [13]. The restrictions imposed on neonicotinoids and phenylpyrazoles may lead to an increase in pyrethroid use. Many pyrethroids are also highly toxic towards honeybees [14], and very few studies have compared the sublethal toxicities of pyrethroids, neonicotinoids and phenylpyrazoles in honeybees [15, 16]. These insecticides all target ion channels involved in the function of a variety of tissues (including the nervous and the muscular systems), and it is known that their primary mode of action is to interfere with the normal function of voltage-gated sodium channels (for pyrethroids), nicotinic acetylcholine receptors (for neonicotinoids) and glutamate and GABA receptors (for phenylpyrazoles).
The effects of sublethal doses of insecticides on the neuromuscular system of honeybees are not easy to analyze. Methods for evaluating the ability of bees to fly back to the hive after exposure to a sublethal dose of insecticide (the ‘homing flight assay’) have been recently developed [7, 17, 18]. Besides the importance of flight for bees, efficient ambulation (walking) inside the hive is required for many tasks, including cell construction and cleaning, larval feeding and social interactions in general [19]. Muscle contraction, allowing physical movements, also produces heat [20] and thus participates in maintaining proper temperature levels around the brood. In feral colonies and in managed hives, the combs, built vertically, add an additional physical challenge by requiring vertical displacements. Experimentally, evaluation of locomotor abilities inside the hive is challenging and requires special observation hives with glass sides. Locomotion assays in laboratory conditions (open-field arena) are easier to set-up and produce a simple standardized and reproducible test to evaluate the effect of sublethal doses of insecticides [21]. Sublethal doses of neonicotinoids can (acetamiprid 0.1 μg/bee) or cannot (thiamethoxam 1 ng/bee) modify walking locomotion [22]. Whereas chronic exposure to thiamethoxam or imidacloprid sublethal concentrations (24h, 10 nM) did not modify the walking behavior, the righting reflex was affected [23]. Imidacloprid sublethal doses reduce waggle dancing 24 h after ingestion [24]. Low doses of phenylpyrazole (fipronil 1 ng/bee) modify behavior (impaired olfactory learning and decreased sucrose sensitivity) without any effect on locomotion [10], while locomotion is affected by sublethal doses of one of the least toxic pyrethroids (tau-fluvalinate at a doses causing 10% mortality, LD10 [25]). Although these studies suggest that the sublethal effects of most insecticides are molecule-specific and cannot simply be extrapolated directly from the LD50 values, none of these molecules have been tested together in parallel tests in the same experimental conditions. Owing to the prevalence of pesticides in the vicinity of hives but also inside the hive’s matrices, locomotor deficits may affect multiple developmental stages of bees, from foragers (after contact with treated plants) to larvae and recently emerged, young bees (through contact with contaminated matrices). However, the current testing protocols only impose toxicological tests on either young larvae or unselected adults (samples shall be collected without regard to the age of the bees). While the exploration of specific toxicological effects on newly emerged bees are not yet required, this early stage of imaginal development may show a different sensitivity compared to older adults [26, 27].
The aim of this study was therefore to compare the deleterious effects of sublethal doses of several pyrethroids, one neonicotinoid and one phenylpyrazole on the locomotion of honeybees in their first day of adult life. This study shows for the first time that an acute contact exposure to sublethal doses of pyrethroid or neonicotinoid insecticides induces serious locomotor deficits in young bees that can be quantified several hours after exposure.
Materials and Methods
Honeybees
Newly emerged bees (Apis mellifera) were obtained during the spring season from two hives (with sister queens) maintained in the experimental apiary of the Abeilles & Environnement Research Department on the Avignon INRA PACA campus. Colonies received a treatment against Varroa in October (ApivarTM, active ingredient amitraze) and were healthy, without any obvious symptoms of disease. Thiamethoxam experiments were performed on colony 1 (summer 2013), all other experiments were performed on colony 2 (summer 2014). To collect bees, frames of developing brood were gently brushed to get rid of adult bees and placed into an incubator (30°C, high humidity) overnight in order to harvest newly emerged bees the next morning (upon emergence, bees were fed on food stored in combs).
Exposure to insecticides
Technical-grade insecticides (the active ingredients) were purchased from EhrenStorfer GmbH (cypermethrin, tetramethrin, tau-fluvalinate, fipronil and thiamethoxam 96, 98, 94, 97 and 98% pure, respectively). Molecules (whose molecular structure are given in Fig 1) were dissolved in acetone and final concentrations were obtained by successive dilutions in amber glass vials thoroughly vortexed at each step. Exposure to insecticides was performed between 9 and 10 am. Honeybees were anaesthetized with CO2 (batches of bees were exposed to a controlled volume of CO2 (final concentration 50%) for 30 seconds in an anesthesia induction chamber). They were placed on ice while 1 μl solution was applied to the dorsal part of the thorax with a Hamilton syringe mounted in a repeating dispenser. Full acetone evaporation was allowed and bees were placed in standard plastic cages (10.5 cm x 7.5 cm x 11.5 cm, modified from [28]) and provided with water and sugar paste (Apifonda, Ickowicz–sucrose 85%, glucose 5%, fructose 3%, water) in a ventilated incubator (29°C, 40% humidity, dark). Mortality tests were performed for all tested insecticides prior to the locomotion assay in order to determine each insecticide’s sublethal dose (SLD). A minimum of two replicates of 30 bees was used at each dose (S1 Table), which is twice the number of bees required in registration tests [26, 27]. The sublethal range was defined as doses producing a mortality level not statistically different from the control 48 hours after exposure (S1 Table). Moreover, for each insecticide, a dose two fold higher than the SLD48h caused a mortality significantly higher than the control 48 hours after exposure (S1 Table). The sublethal range for each insecticide was validated on a minimum of four replicates of 30 bees (up to eight, S1 Table). Selected sublethal doses (SLD48h) were 2.5, 33 and 70 ng/bee for the pyrethroids cypermethrin, tau-fluvalinate and tetramethrin respectively. Selected SLD48h were 0.5 and 3.8 ng/bee for fipronil and thiamethoxam, respectively. In control modalities (acetone only), mortality at 48 h did not exceed 2.5% (see Results). Control mortality was measured from a minimum of six replicates of 30 bees (up to eight). A long-term survival test (up to 5 days after exposure) was performed as well, to quantify mortality over durations longer than 48 h. If any, dead bees were removed daily from cages. Mortality at 48 h and long-term survival (at 120 h) were assessed on different bees from those assayed for locomotion.
Fig 1. Synthetic insecticides from three classes.
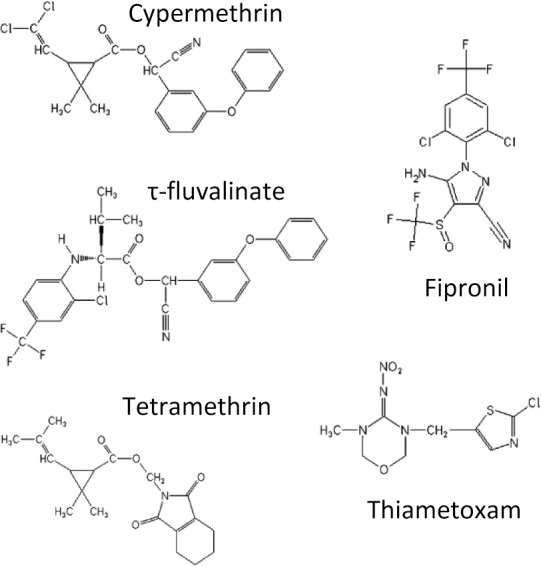
Chemical structure of 3 pyrethroids (cypermethrin, tau-fluvalinate, tetramethrin), a phenylpyrazole (fipronil) and a neonicotinoid (thiamethoxam).
Video tracking analysis
Locomotor activity was monitored for 3 minutes using a webcam controlled with VirtualDub (GNU free software, acquisition frequency 1 Hz, http://sourceforge.net/projects/virtualdub/files/). The arena set up allows video tracking one bee at a time, every 5 minutes (3 minutes of effective video tracking and 2 additional minutes to transfer the bee from its cage to the arena and to allow for short time acclimation, and to transfer it back to a cage at the end of the tracking). Video tracking was performed between 2 and 6 pm and bees were allowed to recover from anesthesia during several hours in a ventilated incubator (29°C, 40% humidity, dark). For a single insecticide, control and insecticide-exposed bees were alternatively assayed and the total series duration was thus >200 minutes. Three minutes of video tracking were sufficient to characterize a distance covered at a nearly steady-state speed. The three minute duration also prevented speed adaptation (S3 Fig) that has been shown to arise quickly in some arena systems (e.g. a significant speed decrease ~10 minutes after placing the bee in the arena [29]). The vertical observation arena, inspired from existing arenas [10], measured 30 x 30 x 4 cm (height: width: depth), was illuminated from above and placed in a dark chamber to avoid any variation due to daylight. The light source consisted of two parallel flicker free LED ramps (length 10 inches, 9 LED each), for a total of 0.72 W, 70 lumens of cold light (StarLED sticks, Starlicht, Germany). Experiments were done at room temperature (22–24°C). For each insecticide, bees were taken alternatively from control and exposed groups (random selection in each case) and introduced into the arena through a hole at the bottom with entomological forceps. Videos were semi-automatically analyzed using Image J (open source, Rasband WS, National Institutes of Health, Bethesda, http://imagej.nih.gov/ij/) with available filters and plugins in order to obtain a series of x,y coordinates for each bee. Individual paths were analyzed with Excel and Origin softwares (OriginLab) and the total distance covered by insecticide-exposed bees was expressed relative to the respective mean value obtained in control bees for each pesticide.
Statistics
Distances are expressed as mean ± S.E.M. The absolute total distance (in meters) covered by individuals during the 3-min time slots was compared among trials using a linear mixed model (LMM) framework. To gain statistical robustness, we handled the five control-vs-treated trials (cypermethrin, fipronil, tau-fluvalinate, tetramethrin, thiamethoxam) simultaneously as a part of the same model, followed by post-hoc pairwise comparisons with Bonferroni p-value adjustments for multiple testing. In a preliminary step, we assessed the constancy and stability of the experimental design by comparing monitored distances among the five control groups only (simple linear model LM and Tukey multiple pairwise comparisons). In a second step, we introduced into the model the five treated groups and set the correct matching with their respective control group by specifying the trial identity as a random grouping factor (LMM and Dunnett multiple comparisons with control). We verified that the LMM normality and homoscedasticity assumptions were met by graphically inspecting model residuals and QQ-plots [30]. We further statistically confirmed residual normality (Shapiro-Wilk test, w = 0.98, p = 0.15) and variance homogeneity among all trials (Bartlett test, K² = 1.84, df = 4, p = 0.76) and all treatments (K² = 11.17, df = 9, p = 0.26). Statistical analyses were performed with the R software for statistical computing [31]. Fisher exact tests were performed with the JMP software (SAS) to compare mortality rates, assuming significant differences for P<0.01.
Results
Determination of sublethal doses
Sublethal doses (SLD48h) were determined from mortality assays preceding the locomotion tests. Two criteria were mandatory in our experiments to select experimental SLD48h: i) a dose producing a mortality level not statistically different from the control was considered as a SLD48h and ii) twice the chosen dose (SLD48h) had to produce a mortality level significantly higher than the control. SLD48h for each insecticide are given in S1 Table, with results of the statistical analysis on mortality assays (p-values from exact Fisher tests). SLD48h were 2.5, 33 and 70 ng for the three pyrethroids cypermethrin, tau-fluvalinate and tetramethrin respectively. SLD48h were 3.8 and 0.5 ng for thiamethoxam and fipronil respectively. Mortality levels after insecticide exposure were not corrected for control mortality levels [26], which were low in all series (0–2.5%).
Locomotion in control bees
Locomotor function and deficits produced after exposure to an insecticide were evaluated by video tracking bees placed in a closed vertical arena. Individual honeybees subjected to this assay displayed variable trajectories, as illustrated for four individuals (first day after emergence) monitored for 3 minutes at a frequency of 1 Hz (Fig 2A). During 3 minutes, each bee can explore only a fraction of the arena. However, overall, bees visited all parts of the arena, although sides were visited more often, possibly owing to a positive thigmotaxis phenomenon (Fig 2B, superimposed trajectories of 80 control bees). The total distance covered was chosen as a proxy for the bee’s locomotor ability. In laboratory conditions, a bee’s locomotor ability only slightly increased with age as shown by distances measured from bees kept in a cage for 6 days (Fig 2C). Mean distance covered by bees increased from 3.2 ± 1.0 to 3.8 ± 1.6 m between day 1 and 2 (Mann-Whitney U = 3119, n1 = 138, n2 = 63, p<0.01) and did not significantly further increase at day 6 after emergence (4.0 ± 2.5 m, n = 38). Bees already showed good locomotion skills at day one after emergence, making this age suitable for the following locomotion assays on insecticide-treated bees.
Fig 2. Video tracking of bees using a vertical arena.
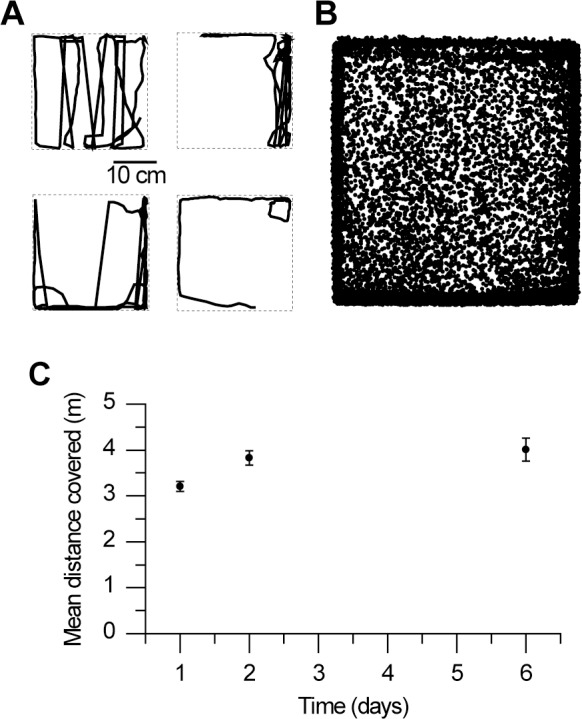
A, Examples of paths followed during 3 minutes by four individual young bees (day 1 after emergence). B, Superimposed paths followed by eighty individual bees. Overall, arena sides were more frequently visited. C, Locomotor ability measured at day 1, 2 and 6 after emergence (bees kept in an incubator). Mean distance (± S.E.M) covered by bees slightly increased from 3.2 to 3.8 meters between day 1 and 2 (p<0.01, n = 138 and 63 respectively) and did not significantly further increase as shown at 6 days after emergence (n = 38).
Locomotion in bees exposed to a SLD48h
Average distances covered by young bees (day 1 after emergence) were measured after exposure to an SLD48h of one of the three pyrethroids: cypermethrin (2.5 ng/bee), tau-fluvalinate (33 ng/bee) and tetramethrin (70 ng/bee). For ease of comparison, distances covered by exposed bees were standardized to the average distance covered by corresponding control bees, set at 1 (Fig 3, relative control distances in black). S1 Fig also reports individual actual distances in meters (S1 Fig). All the five control groups delivered statistically identical locomotion properties, with no significant distance variation in any pairwise combination of trial (S2 Table, S2 Fig). Given the experimental stability of control groups, we could readily assess the five treatments as a part of a single LMM model. The distance covered by individuals was significantly lower in all treated groups compared to control, except for the fipronil trial (S2 Table, S2 Fig). In bees exposed to an SLD48h of cypermethrin, the mean covered distance was significantly decreased by 71 ± 9% relative to control bees (Fig 3, S1 Fig, S2 Table). Tau-fluvalinate was also very potent at the SLD48h, and the covered distance was significantly diminished by 58 ± 10% (Fig 3, S1 Fig, S2 Table), while tetramethrin (70 ng/bee) significantly decreased the distance covered by 48 ± 7% (Fig 3, S1 Fig, S2 Table). At the chosen SLD48h, all 3 pyrethroids appear qualitatively equally potent. Exposure to an SLD48h of the neonicotinoid thiamethoxam (3.8 ng/bee) resulted in a similar significant decrease by 58 ± 8% of the distance covered (Fig 3, S1 Fig, S2 Table). Exposure to an SLD48h of fipronil (0.5 ng/bee) did not produce any significant effect on locomotion (Fig 3, S1 Fig, S2 Table).
Fig 3. Evidence for locomotor deficits after exposure to a sublethal dose (SLD48h) of a pyrethroid or a neonicotinoid but not a phenylpyrazole.
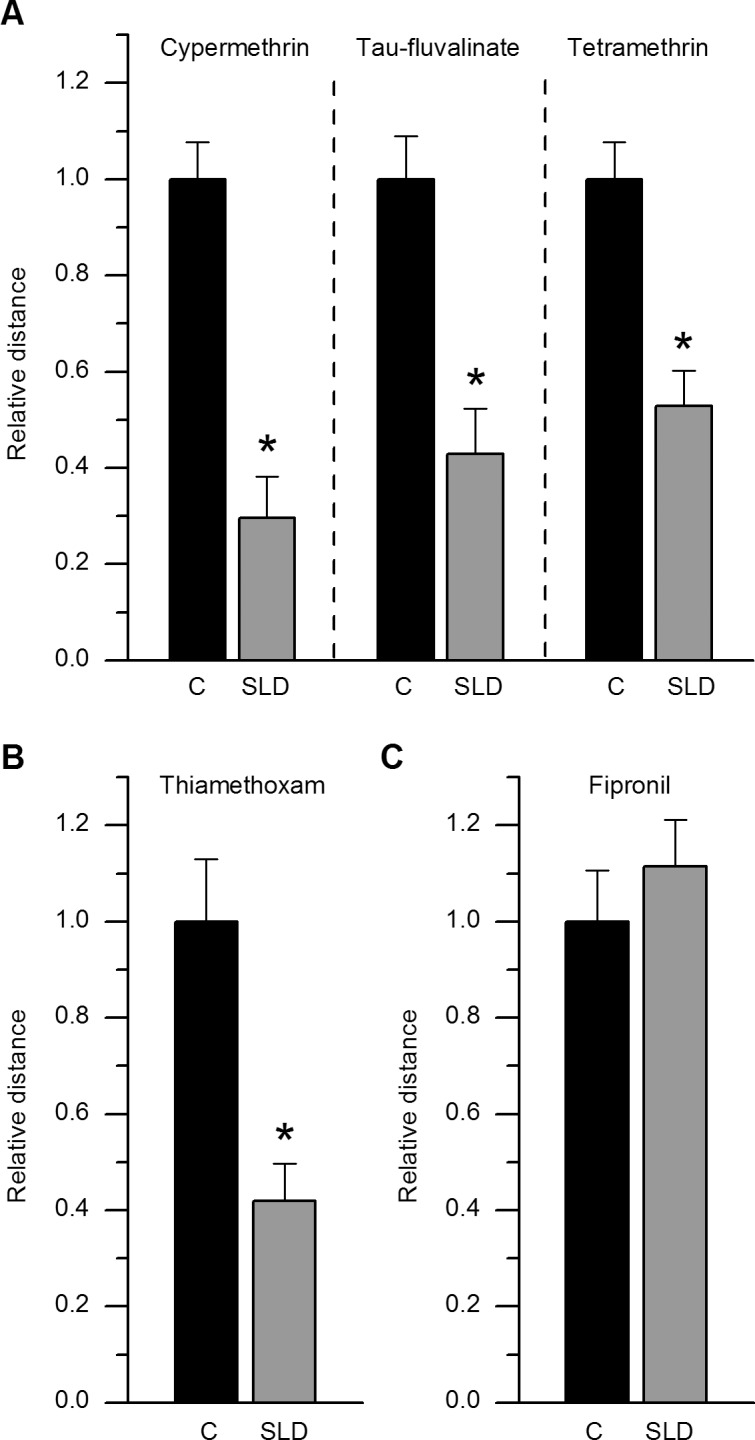
A, The average (± S.E.M) relative distance covered by young bees is significantly decreased 6±2h after exposure to either a SLD48h of cypermethrin (2.5 ng/bee), tau-fluvalinate (33 ng/bee) or tetramethrin (70 ng/bee). B, A significant decrease in distance is observed after exposure to a SLD48h of thiamethoxam (3.8 ng/bee) as well. C, The relative distance covered by bees after exposure to a SLD48h of fipronil (0.5 ng/bee) is similar to the distance covered by control bees. In the case of fipronil, whereas early deleterious effects cannot be evidenced by the locomotion assay, an increased mortality is observed five days after exposure. For cypermethrin, n = 19 control and n = 20 exposed bees respectively. For tau-fluvalinate, n = 12 control and n = 19 exposed bees respectively. For tetramethrin, n = 20 control and n = 20 exposed bees respectively. For thiamethoxam, n = 19 control and n = 19 exposed bees respectively. For fipronil, n = 19 control and n = 20 exposed bees respectively.
Mortality several days after exposure to fipronil in the absence of early locomotor deficits
In parallel with locomotion assays, long-term survival was measured after exposure to the insecticide SLD48h, to explore whether the locomotor deficits that we have measured could induce any mortality several days after exposure in laboratory conditions. The current legislation imposes that acute contact mortality tests have to be routinely performed for 48h [26]. However, if mortality increases by more than 10% between 24 and 48 h, the assay should be extended up to 96 h. Here, for all pyrethroids and the neonicotinoid tested, mortality rates were stable between 24 and 48 h. We found that the SLD48h was sublethal at 120 h as well, indicating that the early locomotor deficit observed does not compromise survival five days after exposure, at least in a controlled laboratory environment. At 120 h, the SLD48h of cypermethrin, tetramethrin, tau-fluvalinate or thiamethoxam did not induce mortality more than their respective controls (Fisher exact tests, P>0.14). Interestingly, whereas fipronil was the only modality in which no locomotor deficits were detected, with a mortality rate stable between 24 and 48 h (1 and 2% respectively, P = 0.6515), the SLD48h of this insecticide started to produce an increased mortality at 72 h (14%, P<0.0001 as compared to 48 h) and a high mortality rate at 120 h after exposure (78%, n = 180 bees) as compared to control bees (1.5%, n = 180 bees, P<0.0001). It is noteworthy to mention that the survival of control bees was stable between 48h and 120h (99.5% and 98.5% respectively, n = 180, P = 0.6229).
Discussion
For pollinators, sublethal effects of insecticides increase toxicological risks and thus should be taken more into account in the methods of risk evaluation [32]. For the first time, we analyzed the sublethal effects of pesticides from three major insecticide classes on young bees (day 1 after emergence) in a standardized honeybee walking locomotion test. Emphasis has been put on pyrethroids that were much less studied than the two other insecticide classes despite 1) their high toxicity towards insects, 2) their pervasive use in agriculture and 3) their prevalence in hives. Specific experimentally-determined sublethal doses were selected for each insecticide (see Methods). In our study, assuming a mean individual bee weight of 0.1 g [1], the SLD48h of pyrethroids were 25 ppb for cypermethrin, 330 ppb for tau-fluvalinate and 700 ppb for tetramethrin. By comparison, quantitatively similar values of 13 pyrethroid residues have been detected in North American hives [1]. For instance, in foundation wax, cypermethrin and fluvalinate were found in 23.8 and 100% of samples at maximal levels of 131 and 10120 ppb respectively (average levels 51.6 and 2006 ppb respectively). Knowing that multiple pyrethroids can be found in the same hive, preimaginal bees and newly emerged bees were thus potentially exposed to cumulated doses [1] that are compatible with SLD48h used in the present study. However, the gaps in the current knowledge on pesticides toxicokinetics (e.g. on the transfer rate of pesticides from hive matrices to the body of a young bee) precludes comparing the level of exposure resulting from contact with contaminated waxes and the level of exposure resulting from a laboratory procedure in which a droplet of contaminated solution is applied on the thorax. Currently, a model that would link these two modes of exposure is unfortunately lacking for a risk evaluation to be accurately performed. For fipronil, our SLD48h was 5 ppb. By comparison, 1.4% of wax samples contained a maximum of 35.9 ppb of fipronil, 0.3% of pollen samples contained a maximum of 28.5 ppb [1]. For thiamethoxam, our SLD48h was 38 ppb. By comparison, 0.3% of pollen samples contained a maximum of 53.3 ppb of thiamethoxam and it was not detected in wax [1].
An SLD48h of all the insecticides tested (except fipronil) triggered serious locomotor deficits. According to available LD50 at 48h values for cypermethrin, that vary from 25 to 121 ng/bee, [33, 34], an SLD48h of cypermethrin 10 to 48 fold lower than the LD50 seriously impairs locomotion. The SLD48h for tau-fluvalinate is between 75 and 600 fold lower than published LD50 values (2.5 μg—20 μg/bee, [34, 35]). In the case of tetramethrin, the effective SLD48h was only two times lower than the available LD50 [36]. Very different maximal ratios between LD50 and SLD48h values for the three tested pyrethroids (2, 48 and 600 for tetramethrin, cypermethrin and tau-fluvalinate, respectively) suggest that within a chemical family, deleterious effects of individual insecticides have to be evaluated separately. A similar locomotor deficit was observed with a SLD48h of tau-fluvalinate and a 13 fold lower SLD48h of cypermethrin. This result is consistent with a 20 fold lower LD50 obtained with cypermethrin than with tau-fluvalinate [34]. Tau-fluvalinate is commonly used against the bee parasite Varroa destructor. The locomotor deficits observed here after exposure to low doses of tau-fluvalinate surely challenge the widespread concept that it can safely be used in hives.
Since honeybees’ skeletal muscles do not express functional voltage-gated Na+ channels, locomotion deficits cannot be explained by a direct action of pyrethroids on such channels in muscle [37, 38]. Locomotor deficits can then be potentially attributed to non-mutually exclusive explanations. First, the locomotor deficits observed in our study could be a consequence of pyrethroid effects on sodium channels located in the central nervous system (in brain and other ganglia) impairing information processing and motor command [39]. Alternatively, impaired electrical activity of sensory neurons housed in the antennae, that are more pyrethroid-sensitive than central neurons, may affect sensory perception and thus impede locomotion [39, 40]. Structural differences between tau-fluvalinate, cypermethrin and tetramethrin in the acidic and alcohol moieties (Fig 1) may produce the different sets of interactions within the channel pore revealed by molecular modeling [41–43], thus giving molecular support for drug-specific modifications in the honeybee sodium channel kinetic parameters [39]. The cloning and expression of the honeybee voltage-dependent sodium channel, AmNav1 [43] together with the analysis of the changes induced by the different pyrethroids using numerical simulation bring a set of important tools that will be useful to fully characterize and understand the binding differences between these pyrethroids and hence their differential toxicity. Deficits could also be related to pyrethroid potency on secondary targets such as voltage-gated calcium channels [44, 45] that are broadly distributed in honeybee tissues [46, 47]. Calcium channels underlie action potentials in muscles of the honeybee or other insects, [37, 38, 48, 49]. Effects on muscle calcium channels would thus not only impair locomotion but thermoregulation or hemolymph circulation as well. A direct effect on bee muscle cells has actually been shown in vitro [37]. Our recent cloning and expression of Apis mellifera calcium channels (AmCav) will allow for more systematic testing of pyrethroids [46, 47].
To our knowledge, the identification of a strong walking deficit in young bees after contact exposure to an SLD48h of thiamethoxam has never been reported before. At 1 ng/bee, no walking deficit was detected [22], whereas the SLD48h used in the present study (3.8 ng/bee) clearly impairs locomotion. At an oral SLD of 1.3 ng/bee (i.e. 25–33 fold lower than the LD50, [34, 50]), a fraction of bees also fail to perform their normal homing flight [7]. The neonicotinoids primary mode of action has been studied in honeybee neurons and is compatible with neurotoxic actions on the nervous cholinergic system [51–54]. Several nicotinic receptor subtypes are involved in complex behaviors and memory processes, and may be differentially altered by sublethal doses of neonicotinoids [55]. Fipronil did not affect locomotion at the SLD48h (see also [10]), but surprisingly induced significant mortality at 5 days after exposure. We did not observe this phenomenon with other insecticides (see also [34] for cypermethrin and thiamethoxam), strongly suggesting that it is insecticide-specific. Whereas SLD48h of all three pyrethroids and the neonicotinoid clearly impair the distance covered by bees, analysis of more subtle behaviors could resolve undetected fipronil-induced deficits. For instance, longer recording durations could reveal subtle alterations in inter-individual interactions, grooming behaviors and time spent near a food source [25, 29, 56].
In conclusion, the locomotion test allowed the identification of important deficits in young bees. It revealed that these effects are insecticide-specific and cannot be simply extrapolated from LD50 values. This assay could thus be used as a preliminary analysis before implementing more sophisticated homing-flight experiments or more subtle memory or orientation tests [7, 18, 57]. It is worth noting that such a laboratory locomotion test is formalized, standardized and displays the least sensitivity to seasonal, phenologic, weather and landscape variations [58]. The recent temporary ban of neonicotinoids in Europe, due to their high toxicity towards the honeybee, calls for alternative methods of pest control, which thus become a priority for modern agriculture, but also a societal issue. Pyrethroids, that already represent one fifth of the global pesticides market [59], have already been used as an alternative solution to restricted or banned pesticides. Their toxicity identified using a simple locomotion test suggests that pyrethroids can be as toxic as a neonicotinoid towards bees, and therefore implies that the molecules to be used would need to be carefully selected.
Supporting Information
Individual distances (in meters) covered by control bees and exposed bees are plotted as white and grey dots respectively, for each insecticide. Average distances (± S.E.M) are shown for each modality. Mean distances in control groups were similar (3.14 ± 0.24 m, 3.26 ± 0.29 m, 3.50 ± 0.27 m, 3.22 ± 0.42 m, 3.37 ± 0.35 m for cypermethrin, tau-fluvalinate, tetramethrin, thiamethoxam and fipronil respectively, see S2 Table for statistics). Mean distances after exposure to a SLD48h were 0.93 ± 0.27 m, 1.40 ± 0.31 m, 1.85 ± 0.26 m, 1.35 ± 0.25 m, 3.22 ± 0.33 m for cypermethrin, tau-fluvalinate, tetramethrin, thiamethoxam and fipronil respectively (see Fig 3 for numbers of bees in each group).
(TIF)
Horizontal bars stand for the 95% confidence intervals returned by the post-hoc multiple pairwise comparisons. The vertical dashed line indicates the no-effect level.
(TIF)
The instantaneous speed during the 3-min recording time was measured in a pilot study performed on 80 non-exposed individual bees. The mean instantaneous speed (mm.s-1) was averaged per 10-s slots among the 80 individual bees. We compared the observed averages with the 95% confidence interval (CI) range expected under the hypothesis of steady-state average instantaneous speed. The 95% CI range was obtained from a bootstrapping procedure whereby the speed data were randomly shuffled along the temporal axis. We recomputed 200 random rearrangements of the raw database and then extracted the average speed values at the 2.5% and 97.5% ranks for each 10s step to delineate the 95% CI. Average speed tended to decrease as time lapses, with observed values being closer to (or slightly above) the upper CI boundary during the first minute of recording, and closer to the lower CI boundary during the third minute of recording. At the very last 10s recording slot, average speed fell below the expected steady-state confidence limits. We therefore considered that the 3-min standard recording duration was appropriate to cover statistically steady-state locomotion samples in our control-vs.-treated experiments.
(TIF)
(XLSX)
The post-hoc pairwise comparisons indicate that only the fipronil treatment did not significantly affect distances. See S2 Fig for effect size estimates.
(DOCX)
Acknowledgments
We thank Michel Pélissier for his technical help with the locomotion arena and the laboratory beekeepers who provided domestic honeybees. We thank Dr Cynthia Mc Donnell for carefully reading the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support from the Agence Nationale de la Recherche (ANR Bee-channels ANR-13-BSV7-0010) was received by MC, JCS, PC, CC; and from the European Agricultural Guarantee Fund to MC, AD, CC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One. 2010;5(3):e9754 Epub 2010/03/25. 10.1371/journal.pone.0009754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chauzat MP, Faucon JP. Pesticide residues in beeswax samples collected from honey bee colonies (Apis mellifera L.) in France. Pest Management Science. 2007;63(11):1100–6. [DOI] [PubMed] [Google Scholar]
- 3. Chauzat MP, Cauquil L, Roy L, Franco S, Hendrikx P, Ribiere-Chabert M. Demographics of the European apicultural industry. PLoS One. 2013;8(11):e79018 Epub 2013/11/16. 10.1371/journal.pone.0079018 PONE-D-13-02274 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. VanEngelsdorp D, Speybroeck N, Evans JD, Nguyen BK, Mullin C, Frazier M, et al. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J Econ Entomol. 2010;103(5):1517–23. Epub 2010/11/11. . [DOI] [PubMed] [Google Scholar]
- 5. Vanengelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, et al. Colony collapse disorder: a descriptive study. PLoS One. 2009;4(8):e6481 Epub 2009/08/04. 10.1371/journal.pone.0006481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ Sci Pollut Res Int. 2015;22(1):68–102. Epub 2014/09/17. 10.1007/s11356-014-3471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Henry M, Beguin M, Requier F, Rollin O, Odoux JF, Aupinel P, et al. A common pesticide decreases foraging success and survival in honey bees. Science. 2012;336(6079):348–50. Epub 2012/03/31. 10.1126/science.1215039 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8. Whitehorn PR, O'Connor S, Wackers FL, Goulson D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science. 2012;336(6079):351–2. Epub 2012/03/31. 10.1126/science.1215025 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9. Eisenstein M. Pesticides: Seeking answers amid a toxic debate. Nature. 2015;521(7552):S52–5. Epub 2015/05/21. 10.1038/521S52a [pii]. . [DOI] [PubMed] [Google Scholar]
- 10. El Hassani AK, Dacher M, Gauthier M, Armengaud C. Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol Biochem Behav. 2005;82(1):30–9. Epub 2005/08/17. doi: S0091-3057(05)00241-8 [pii] 10.1016/j.pbb.2005.07.008 . [DOI] [PubMed] [Google Scholar]
- 11. Vidau C, Diogon M, Aufauvre J, Fontbonne R, Vigues B, Brunet JL, et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS One. 2011;6(6):e21550 Epub 2011/07/09. 10.1371/journal.pone.0021550 PONE-D-11-04998 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int. 2015;22(1):5–34. Epub 2014/09/23. 10.1007/s11356-014-3470-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khambay BPS, Jewess PJ. Pyrethroids In: Gilbert LI, editor. Comprehensive Molecular Insect Science. Amsterdam: Elsevier; 2005. p. 1–29. [Google Scholar]
- 14. Hardstone MC, Scott JG. Is Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci. 2010;66(11):1171–80. Epub 2010/07/31. 10.1002/ps.2001 . [DOI] [PubMed] [Google Scholar]
- 15. Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delegue MH. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol Environ Saf. 2004;57(3):410–9. . [DOI] [PubMed] [Google Scholar]
- 16. Decourtye A, Devillers J, Genecque E, Le Menach K, Budzinski H, Cluzeau S, et al. Comparative sublethal toxicity of nine pesticides on olfactory learning performances of the honeybee Apis mellifera. Arch Environ Contam Toxicol. 2005;48(2):242–50. . [DOI] [PubMed] [Google Scholar]
- 17. Decourtye A, Devillers J, Aupinel P, Brun F, Bagnis C, Fourrier J, et al. Honeybee tracking with microchips: a new methodology to measure the effects of pesticides. Ecotoxicology. 2011;20(2):429–37. Epub 2011/01/27. 10.1007/s10646-011-0594-4 . [DOI] [PubMed] [Google Scholar]
- 18. van Dame R, Meled M, Colin M-E, Belzunces LP. Alteration of the homing-flight in the honey bee Apis mellifera L. Exposed to sublethal dose of deltamethrin. Environmental Toxicology and Chemistry. 1995;14(5):855–60. 10.1002/etc.5620140517 [DOI] [Google Scholar]
- 19. Winston ML. The biology of the honey bee: harvard university press; 1987. 281 p. [Google Scholar]
- 20. Carlson FD, Wilkie DR. Muscle Physiology: Prentice-Hall; 1974. 170 p. [Google Scholar]
- 21. Lambin M, Armengaud C, Raymond S, Gauthier M. Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch Insect Biochem Physiol. 2001;48(3):129–34. Epub 2001/10/24. 10.1002/arch.1065 [pii]. . [DOI] [PubMed] [Google Scholar]
- 22. El Hassani A, Dacher M, Gary V, Lambin M, Gauthier M, Armengaud C. Effects of Sublethal Doses of Acetamiprid and Thiamethoxam on the Behavior of the Honeybee (Apis mellifera). Arch Environ Contam Toxicol. 2008;54(4):653–61. 10.1007/s00244-007-9071-8 [DOI] [PubMed] [Google Scholar]
- 23. Williamson SM, Willis SJ, Wright GA. Exposure to neonicotinoids influences the motor function of adult worker honeybees. Ecotoxicology. 2014;23(8):1409–18. Epub 2014/07/12. 10.1007/s10646-014-1283-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eiri DM, Nieh JC. A nicotinic acetylcholine receptor agonist affects honey bee sucrose responsiveness and decreases waggle dancing. J Exp Biol. 2012;215(Pt 12):2022–9. Epub 2012/05/25. 10.1242/jeb.068718 215/12/2022 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25. Teeters BS, Johnson RM, Ellis MD, Siegfried BD. Using video-tracking to assess sublethal effects of pesticides on honey bees (Apis mellifera L.). Environ Toxicol Chem. 2012;31(6):1349–54. Epub 2012/04/11. 10.1002/etc.1830 . [DOI] [PubMed] [Google Scholar]
- 26. OECD . OECD guidelines for the testing of chemicals Number 214, Honeybee acute toxicity tests. 1998;1:7. [Google Scholar]
- 27. OEPP/EPPO. Environmental risk assessment scheme for plant protection products. Side-effects on honeybees. Bull OEPP/EPPO. 2010;40:323–31. [Google Scholar]
- 28. Pain J. Note technique nouveau modèle de cagettes expérimentales pour le maintien d'abeilles et captivité. Ann Abeille. 1966;9(1):71–6. [Google Scholar]
- 29. Sokolowski MBC, Moine M, Naassila M. "Beetrack": A software for 2D open field locomotion analysis in honey bees. J Neurosci Meth. 2012;207(2):211–7. 10.1016/j.jneumeth.2012.03.006 . [DOI] [PubMed] [Google Scholar]
- 30. Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology With R: Springer-Verlag; New York; 2009. [Google Scholar]
- 31.R Development Core Team. R: A Language and Environment for Statistical Computing. Computing RFfS, editor. Vienna, Austria2014.
- 32. Desneux N, Decourtye A, Delpuech JM. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007;52:81–106. . [DOI] [PubMed] [Google Scholar]
- 33. Delabie J, Bos C, Fonta C, Masson C. Toxic and repellent effects of cypermethrin on the honeybee: Laboratory, glasshouse and field experiments. Pesticide Science. 1985;16(4):409–15. 10.1002/ps.2780160417 [DOI] [Google Scholar]
- 34. Poquet Y, Bodin L, Tchamitchian M, Fusellier M, Giroud B, Lafay F, et al. A pragmatic approach to assess the exposure of the honey bee (Apis mellifera) when subjected to pesticide spray. PLoS One. 2014;9(11):e113728 Epub 2014/11/21. 10.1371/journal.pone.0113728 PONE-D-14-36042 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dahlgren L, Johnson RM, Siegfried BD, Ellis MD. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. J Econ Entomol. 2012;105(6):1895–902. Epub 2013/01/30. . [DOI] [PubMed] [Google Scholar]
- 36.U.S. Environmental Protection Agency. ECOTOXicology Database System. Version 4.0.. Available: http:/www.epagov/ecotox/.2015.
- 37. Collet C. Excitation-contraction coupling in skeletal muscle fibers from adult domestic honeybee. Pflugers Arch. 2009;458(3):601–12. Epub 2009/02/10. 10.1007/s00424-009-0642-6 . [DOI] [PubMed] [Google Scholar]
- 38. Collet C, Belzunces L. Excitable properties of adult skeletal muscle fibres from the honeybee Apis mellifera. J Exp Biol. 2007;210(3):454–64. 10.1242/jeb.02667 [DOI] [PubMed] [Google Scholar]
- 39. Kadala A, Charreton M, Jakob I, Cens T, Rousset M, Chahine M, et al. Pyrethroids differentially alter voltage-gated sodium channels from the honeybee central olfactory neurons. PLoS One. 2014;9(11):e112194 Epub 2014/11/13. 10.1371/journal.pone.0112194 PONE-D-14-28292 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kadala A, Charreton M, Jakob I, Le Conte Y, Collet C. A use-dependent sodium current modification induced by type I pyrethroid insecticides in honeybee antennal olfactory receptor neurons. Neurotoxicology. 2011;32(3):320–30. Epub 2011/03/08. 10.1016/j.neuro.2011.02.007 S0161-813X(11)00044-1 [pii]. . [DOI] [PubMed] [Google Scholar]
- 41. Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, et al. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci U S A. 2013;110(29):11785–90. Epub 2013/07/04. 10.1073/pnas.1305118110 1305118110 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA, Davies TG. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J. 2006;396(2):255–63. Epub 2006/02/16. doi: BJ20051925 [pii] 10.1042/BJ20051925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gosselin-Badaroudine P, Moreau A, Delemotte L, Cens T, Collet C, Rousset M, et al. Characterization of the honeybee AmNav1 channel and tools to assess the toxicity of insecticides. Scientific Reports. 2015;in press. [DOI] [PMC free article] [PubMed]
- 44. Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, et al. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171(1):3–59. . [DOI] [PubMed] [Google Scholar]
- 45. Shafer TJ, Meyer DA. Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicology and Applied Pharmacology. 2004;196(2):303–18. [DOI] [PubMed] [Google Scholar]
- 46. Cens T, Rousset M, Collet C, Charreton M, Garnery L, Le Conte Y, et al. Molecular characterization and functional expression of the Apis mellifera voltage-dependent Ca2+ channels. Insect biochemistry and molecular biology. 2015;58:12–27. 10.1016/j.ibmb.2015.01.005 . [DOI] [PubMed] [Google Scholar]
- 47. Cens T, Rousset M, Collet C, Raymond V, Demares F, Quintavalle A, et al. Characterization of the first honeybee Ca2+ channel subunit reveals two novel species- and splicing-specific modes of regulation of channel inactivation. Pflugers Arch. 2013;465(7):985–96. Epub 2013/04/17. 10.1007/s00424-013-1223-2 . [DOI] [PubMed] [Google Scholar]
- 48. Gu G- G, Singh S. Pharmacological analysis of heartbeat in Drosophila. Journal of Neurobiology. 1995;28(3):269–80. 10.1002/neu.480280302 [DOI] [PubMed] [Google Scholar]
- 49. Papaefthimiou C, Theophilidis G. The Cardiotoxic Action of the Pyrethroid Insecticide Deltamethrin, the Azole Fungicide Prochloraz, and Their Synergy on the Semi-Isolated Heart of the Bee Apis mellifera macedonica. Pesticide Biochemistry and Physiology. 2001;69(2):77–91. [Google Scholar]
- 50. Iwasa T, Motoyama N, Ambrose JT, Roe RM. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004;23(5):371–8. 10.1016/j.cropro.2003.08.018 . [DOI] [Google Scholar]
- 51. Barbara GS, Zube C, Rybak J, Gauthier M, Grunewald B. Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis mellifera. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191(9):823–36. . [DOI] [PubMed] [Google Scholar]
- 52. Barbara GS, Grunewald B, Paute S, Gauthier M, Raymond-Delpech V. Study of nicotinic acetylcholine receptors on cultured antennal lobe neurones from adult honeybee brains. Invert Neurosci. 2008;8(1):19–29. . [DOI] [PubMed] [Google Scholar]
- 53. Deglise P, Grunewald B, Gauthier M. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci Lett. 2002;321(1–2):13–6. Epub 2002/03/02. doi: S0304394001024004 [pii]. . [DOI] [PubMed] [Google Scholar]
- 54. Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat Commun. 2013;4:1634 Epub 2013/03/29. 10.1038/ncomms2648 pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gauthier M. State of the art on insect nicotinic acetylcholine receptor function in learning and memory. Adv Exp Med Biol. 2010;683:97–115. Epub 2010/08/27. . [DOI] [PubMed] [Google Scholar]
- 56. Maze IS, Wright GA, Mustard JA. Acute ethanol ingestion produces dose-dependent effects on motor behavior in the honey bee (Apis mellifera). Journal of Insect Physiology. 2006;52(11–12):1243–53. doi: 10.1016/j.jinsphys.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Decourtye A, Devillers J. Ecotoxicity of neonicotinoid insecticides to bees. Adv Exp Med Biol. 2010;683:85–95. Epub 2010/08/27. . [DOI] [PubMed] [Google Scholar]
- 58. Henry M, Bertrand C, Le Feon V, Requier F, Odoux JF, Aupinel P, et al. Pesticide risk assessment in free-ranging bees is weather and landscape dependent. Nat Commun. 2014;5:4359 Epub 2014/07/11. 10.1038/ncomms5359 [pii]. . [DOI] [PubMed] [Google Scholar]
- 59. Kaneko H. Pyrethroids: Mammalian Metabolism and Toxicity. Journal of Agricultural and Food Chemistry. 2011;59(7):2786–91. 10.1021/jf102567z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual distances (in meters) covered by control bees and exposed bees are plotted as white and grey dots respectively, for each insecticide. Average distances (± S.E.M) are shown for each modality. Mean distances in control groups were similar (3.14 ± 0.24 m, 3.26 ± 0.29 m, 3.50 ± 0.27 m, 3.22 ± 0.42 m, 3.37 ± 0.35 m for cypermethrin, tau-fluvalinate, tetramethrin, thiamethoxam and fipronil respectively, see S2 Table for statistics). Mean distances after exposure to a SLD48h were 0.93 ± 0.27 m, 1.40 ± 0.31 m, 1.85 ± 0.26 m, 1.35 ± 0.25 m, 3.22 ± 0.33 m for cypermethrin, tau-fluvalinate, tetramethrin, thiamethoxam and fipronil respectively (see Fig 3 for numbers of bees in each group).
(TIF)
Horizontal bars stand for the 95% confidence intervals returned by the post-hoc multiple pairwise comparisons. The vertical dashed line indicates the no-effect level.
(TIF)
The instantaneous speed during the 3-min recording time was measured in a pilot study performed on 80 non-exposed individual bees. The mean instantaneous speed (mm.s-1) was averaged per 10-s slots among the 80 individual bees. We compared the observed averages with the 95% confidence interval (CI) range expected under the hypothesis of steady-state average instantaneous speed. The 95% CI range was obtained from a bootstrapping procedure whereby the speed data were randomly shuffled along the temporal axis. We recomputed 200 random rearrangements of the raw database and then extracted the average speed values at the 2.5% and 97.5% ranks for each 10s step to delineate the 95% CI. Average speed tended to decrease as time lapses, with observed values being closer to (or slightly above) the upper CI boundary during the first minute of recording, and closer to the lower CI boundary during the third minute of recording. At the very last 10s recording slot, average speed fell below the expected steady-state confidence limits. We therefore considered that the 3-min standard recording duration was appropriate to cover statistically steady-state locomotion samples in our control-vs.-treated experiments.
(TIF)
(XLSX)
The post-hoc pairwise comparisons indicate that only the fipronil treatment did not significantly affect distances. See S2 Fig for effect size estimates.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


