Abstract
Background
About a fourth of acute decompensated heart failure (ADHF) patients develop renal dysfunction during their admission. To date, the association of ADHF treatment with the development of worsening renal function (WRF) remains contentious. Thus, we examined the association of WRF with changes in BNP levels and with mortality.
Methods
We performed retrospective chart review of patients admitted with ADHF who had BNP, eGFR, creatinine and blood urea nitrogen (BUN) values measured both on admission and discharge. Survival analysis was conducted using Cox proportional hazards model and correlation was measured using Spearman's rank correlation test.
Results
358 patients admitted for ADHF were evaluated. WRF was defined as >20% reduction in eGFR from admission to discharge and response to treatment was assessed by ΔBNP. There was a statistically significant reduction in BNP and increase in BUN during the admission. ΔBNP did not correlate with either ΔGFR or ΔBUN. Patients who developed WRF and those who did not, had a similar reduction in BNP. On univariate survival analysis, ΔBUN, but not ΔeGFR, was associated with 1-year mortality. In multivariate Cox proportional hazards model, BUN at discharge was associated with 1-year mortality (HR: 1.02, p<0.001), but ΔeGFR and ΔBUN were not associated with the primary endpoint.
Conclusion
During ADHF treatment, ΔBNP was not associated with changes in renal function. Development of WRF during ADHF treatment was not associated with mortality. Our study suggests that development of WRF should not preclude diuresis in ADHF patients in the absence of volume depletion.
Keywords: BNP, renal function, heart failure
INTRODUCTION
Worsening renal function (WRF) occurs in about one-fourth of the patients admitted for acute decompensated heart failure (ADHF) [1-4]. Till date, the mechanisms underlying WRF are not completely clear; however emerging mechanistic pathways have identified low cardiac output, elevation of central venous pressure, renin-aldosterone-angiotensin axis (RAAS) dysfunction, sympathetic overactivity, oxidative injury, and reduced renal perfusion as important contributing factors [5].
In clinical practice, a common and often perplexing presentation of WRF occurs during hospitalization of patients with acute decompensated heart failure (ADHF) [6]. Given the close association with intravascular volume reduction and WRF, diuresis during treatment for ADHF has been “implied” as the cause of development of WRF [7,8]. However, emerging data regarding intravenous volume reduction argue against this concept [7-9]. Additionally, the long-term prognostic significance of the development of WRF during treatment of ADHF is still debatable [2,10-13]. To date, the association of ADHF treatment with the development of WRF remains contentious. Brain natriuretic peptide (BNP) is an established biomarker for diagnosis and prognosis of HF, and has been shown to decrease in a significant proportion of the patients with common treatment strategies such as diuretics and or vasodilators during hospitalization for ADHF [14-16]. In patients with ADHF, BNP has been shown to be a direct correlate of both thoracic fluid content as well as marker for response to therapy [17]. However, there are lack of data on the relationship between changes in BNP and the development of WRF during hospitalization for ADHF. To this effect, we examined the association between changes in BNP levels during treatment of ADHF, with the development of WRF and clinical outcomes.
METHODS
We reviewed records of patients admitted over the last 10 years with a diagnosis of ADHF (Diagnosis Related Group of Congestive Heart Failure # 127) at the DeBakey VA Medical Center in Houston, TX. Subjects with admissions for dyspnea related specifically to ADHF who fulfilled Framingham criteria for HF and had BNP, eGFR, creatinine and blood urea nitrogen (BUN) values measured both on admission and discharge were included. Exclusion criteria included volume overload due to reasons other than HF, stage V Chronic kidney disease (CKD) [18], illnesses other than HF with a life expectancy <12 months, acute coronary syndrome or a competing admitting diagnosis that was as or more likely than ADHF at index admission and length of index admission < 2 days. Of the 855 patients who were admitted with a diagnosis of ADHF based on our inclusion and exclusion criteria, 358 patients had paired admission and discharge values of BNP, eGFR, creatinine and BUN and were included in the analyses. Overall, there was no difference in age, demographic profile, presence of comorbidities and discharge medications between patients that were included or excluded (Table S1). Although most of the parameters were similar between the 2 groups, patients that were included had more advanced HF as evidenced by lower admission ejection fraction, systolic blood pressure and higher BNP (Table S1). Admission and discharge eGFR were lower among the patients who were included in the study, compared with those who were excluded. Significantly, there was no difference in our main variables of interest i.e. ΔeGFR, Δcreatinine, ΔBUN and ΔBNP, between the 2 populations (Table S1). Data pertaining to the index admission for ADHF, demographics, laboratory study results, medication use and co-morbid medical conditions were extracted from the electronic medical records. Vital status was confirmed using the Social Security Death Index and VA electronic medical records.
Variables used for the analyses are enumerated in Table 1. Variables with a univariate p value < 0.1 were used in the backward elimination Cox proportional hazards (PH) model with removal set at p ≥ 0.1 for all survival analyses. In the presence of significant correlation between variables (r2=0.3) the variable with weaker association with outcome was excluded. CKD was defined as eGFR < 60 mL/min/1.73 m2 [18]. eGFR was calculated using the 4 variable Modification of Diet in Renal Disease (MDRD) formula [19]. WRF was defined as more than 20% decrease in eGFR [20]. For the multivariate survival analysis, missing values of the variables were imputed. All covariates had values for more than 99% of the patients except for history of coronary artery disease (97%), QRS duration (97%), admission hemoglobin level (98%) and EF (94%). Continuous variables were imputed using linear regression, constrained to observed upper and lower range of observed variables. Categorical variables were imputed based on probabilities calculated by logistic regression. Continuous variables are presented as either mean ± standard deviation or median with interquartile range; and categorical variables are presented as percentages. Categorical variables were compared using Fisher's exact test and continuous variables were compared using either 2 tailed t test or Mann-Whitney test as applicable. Correlation was measured using Spearman's rank correlation test. All the analyses were done on SPSS v. 18. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [21].
Table 1.
Baseline variables of the study population
| Variable | Mean±S.D. / Median (IQR) (n=358) |
|---|---|
| Admission variables | |
| Age (years) | 68±11* |
| Male (%) | 99 |
| White (%) | 56 |
| Atrial Fibrillation (%) | 26 |
| Coronary artery Disease (%) | 63* |
| Hypertension (%) | 83 |
| Peripheral vascular Disease (%) | 24* |
| Diabetes mellitus (%) | 48 |
| Valvular abnormality (%) | 28* |
| NSVT (%) | 14* |
| Chronic renal insufficiency (%) | 59* |
| Dementia (%) | 5* |
| EF (%) | 25.3±13* |
| QRS duration (msec) | 123±35* |
| Systolic blood pressure (mm Hg) | 135±26* |
| Diastolic Blood Pressure (mm Hg) | 79±18* |
| Heart Rate (bpm) | 88±20 |
| Sodium (meq/l) | 137±5* |
| Hemoglobin (gm/dl) | 12.3±2.1 |
| eGFR (ml/min/1.73 m2) | 57±23* |
| Creatinine (mg/dl) | 1.6±0.6* |
| BUN (mg/dl) | 28.0±15.1* |
| BNP (pg/ml) | 1267 (773-1475) |
| Discharge variables | |
| Systolic blood pressure (mm Hg) | 121 ± 22 |
| Diastolic Blood Pressure (mm Hg) | 70 ± 13 |
| eGFR at discharge (ml/min/1.73 m2) | 57±23* |
| Creatinine at discharge (mg/dl) | 1.6±0.7* |
| BUN at discharge (mg/dl) | 31.9±17.9* |
| BNP at discharge (pg/ml) | 718 (369-1300)* |
| ACE inhibitors/ ARB (%) | 77* |
| HF specific beta-blocker (%) | 65* |
| Loop diuretic (%) | 93 |
| Diuretic dose (mg) | 80 (60-160) |
| Spironolactone (%) | 17 |
| Amiodarone (%) | 13* |
| Digoxin (%) | 32 |
| ΔSystolic blood pressure | −14 ± 25 |
| Change in renal function and BNP | |
| ΔeGFR (ml/min/1.73 m2) | −0.5±15 |
| ΔCreatinine (mg/dl) | 0.01±0.4 |
| ΔBUN (mg/dl) | 3.9±12.9* |
| ΔBNP (pg/ml) | −285 (−727-0)* |
ACE: Angiotensin Converting Enzyme; ARB: Angiotensin Receptor Blocker; BNP: Brain Natriuretic Peptide; BUN: Blood Urea Nitrogen; EF: Ejection Fraction; eGFR: estimated Glomerular Filtration Rate; HF: Heart Failure; NSVT: Non-sustained Ventricular Tachycardia.
indicates p<0.1 on univariate survival analysis for primary end point of all cause mortality at 1 year.
Δ refers to change in variable from admission to discharge.
RESULTS
Baseline Characteristics
358 patients with paired admission and discharge values of BNP and eGFR had an overall follow-up of 998 person-years. 73% patients met their primary end-point of all-cause mortality with 30% dying within the first year. The average age of the patients was 68±11 years; 99% were males and 56% were white representing the Veteran Affairs population (Table 1). Ischemic etiology was the cause of HF in 63% of cases and patients had an average EF of 26±13% (Table 1). There was a high prevalence of comorbidities as evident in Table 1. The median duration of admission was 5 days (IQR: 3 to 9 days).
Overall, during hospitalization, there was a statistically significant reduction in BNP from admission value of 1267 pg/ml (IQR: 773 to 1478 pg/ml) to 719 pg/ml (IQR: 368 to 1300 pg/ml) at discharge (p<0.001). BUN increased significantly from 28±15 mg/dl at admission to 32±18 mg/dl at discharge (p<0.001). In contrast, in the overall cohort, there was no change in creatinine (admission: 1.6±0.6 mg/dl and discharge: 1.6±0.7 mg/dl) or eGFR (admission: 57.0±2.3 ml/min/1.73 m2 and discharge: 56.7±2.3 ml/min//1.73 m2) from admission to discharge. ΔBNP was found not to correlate with ΔeGFR, Δcreatinine or ΔBUN (p=0.65, 0.86 and 0.39, respectively).
In post-hoc analyses, we evaluated whether the degree of renal dysfunction at baseline, influenced the correlation between ΔBNP and ΔeGFR. The results remained unchanged in the subgroup analyses, with no correlation between ΔBNP and ΔeGFR (CKD I, II: n= 207, ρ = 0.04, p = 0.63; and CKD III, IV: n = 151, ρ = 0.01, p = 0.92). In addition to ΔBNP, changes in the serum sodium and hemoglobin concentration also reflect changes in intravascular volume [13]. Thus, to supplement our observation of lack of association between ΔBNP and ΔeGFR, we performed correlation analyses between ΔeGFR, and Δsodium and Δhemoglobin. ΔSodium (ρ = 0.07, p = 0.17) and Δhemoglobin (ρ = −0.02, p = 0.75) did not correlate with ΔeGFR, indicating a lack of association between intravascular volume changes and WRF.
Factors associated with the development of WRF
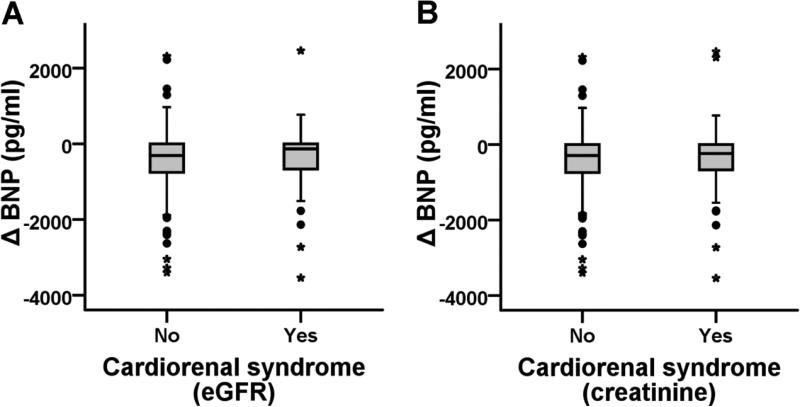
Upon categorization of the data, 17% of the patients (n=60) developed WRF (Table 2). Patients who developed WRF had a significantly higher length of index admission in comparison with patients who did not develop WRF (8 (5 to 14) and 5 (3 to 8), respectively, p<0.001). Patients who developed WRF during the admission had similar baseline BNP levels and a similar ΔBNP during hospital admission, in comparison with patients who did not develop WRF (Table 2, Figure 1). In addition, other markers of volume change like Δsodium and Δhemoglobin showed no difference between the 2 groups (Table 2). Interestingly, these patients had lower prevalence of history of CKD, better renal function at the time of admission but worse renal function at discharge.
Table 2.
Comparison of variables between patients who developed worsening renal function (WRF) with the rest of the patients
| WRF Patients (n=60) | Patients without WRF (n=298) | p value | |
|---|---|---|---|
| Admission variables | |||
| Age (years) | 69.3±9.8 | 67.4±10.7 | 0.21 |
| Male (%) | 100 | 99 | 1.0 |
| White (%) | 65 | 54 | 0.15 |
| Atrial Fibrillation (%) | 29 | 26 | 0.62 |
| Coronary artery disease (%) | 63 | 68 | 0.45 |
| Hypertension (%) | 77 | 84 | 0.19 |
| Peripheral vascular Disease (%) | 22 | 24 | 0.87 |
| Diabetes mellitus (%) | 52 | 47 | 0.57 |
| Valvular abnormality (%) | 23 | 29 | 0.43 |
| NSVT | 10 | 14 | 0.42 |
| Chronic renal insufficiency (%) | 37 | 63 | <0.001 |
| Dementia (%) | 5 | 5 | 1.0 |
| EF (%) | 27±13 | 25±12 | 0.24 |
| QRS duration (msec) | 121±31 | 124±36 | 0.61 |
| Systolic Blood Pressure (mm Hg) | 138±27 | 134±26 | 0.28 |
| Diastolic Blood Pressure (mm Hg) | 79±17 | 79±18 | 0.88 |
| Heart Rate (bpm) | 88±21 | 88±20 | 0.96 |
| Sodium (meq/l) | 136±6 | 137±5 | 0.07 |
| Hemoglobin (gm/dl) | 12.4±1.9 | 12.3±2.1 | 0.76 |
| eGFR (ml/min/1.73 m2) | 67±24 | 55±22 | <0.001 |
| Creatinine (mg/dl) | 1.4±0.6 | 1.7±0.6 | 0.002 |
| BUN (mg/dl) | 24.1±15.1 | 28.0±15.1 | 0.04 |
| BNP (pg/ml) | 1300 (871-1381) | 1225 (742-1540) | 0.30 |
| Discharge variables | |||
| eGFR at discharge (ml/min/1.73 m2) | 48±20 | 59±23 | 0.001 |
| Creatinine at discharge (mg/dl) | 1.9±1.0 | 1.6±0.6 | <0.001 |
| BUN at discharge (mg/dl) | 43.1±24.1 | 30.0±16.0 | <0.001 |
| BNP at discharge (pg/ml) | 740 (471-1300) | 710 (347-1300) | 0.57 |
| ACE inhibitors/ ARB (%) | 70 | 79 | 0.18 |
| HF specific beta-blocker (%) | 62 | 65 | 0.66 |
| Loop diuretic (%) | 88 | 94 | 0.16 |
| Diuretic dose (mg) | 80 (40-160) | 100 (60-160) | 0.41 |
| Spironolactone (%) | 12 | 18 | 0.26 |
| Amiodarone (%) | 13 | 13 | 0.84 |
| Digoxin (%) | 33 | 32 | 0.88 |
| Change in renal function and BNP | |||
| ΔeGFR (ml/min/1.73 m2) | −19±1 | 4±1 | <0.001 |
| ΔCreatinine (mg/dl) | 0.5±0.4 | −0.1±0.3 | <0.001 |
| ΔBUN (mg/dl) | 19.0±14.0 | 1.1±10.9 | <0.001 |
| ΔSodium (meq/l) | 0.35±5.92 | 0.0±3.96 | 0.66 |
| ΔHemoglobin (gm/dl) | −0.42±1.6 | −0.07±1.5 | 0.13 |
| ΔBNP (pg/ml) | −372 (−663 - 0) | −265 (−741-0) | 0.73 |
ACE: Angiotensin Converting Enzyme; ARB: Angiotensin Receptor Blocker; BNP: Brain Natriuretic Peptide; BUN: Blood Urea Nitrogen; EF: Ejection Fraction; eGFR: estimated Glomerular Filtration Rate; HF: Heart Failure; NSVT: Non-sustained Ventricular Tachycardia. Δ: refers to change in variable from admission to discharge, WRF was defined as >20% reduction in eGFR
Figure 1.
Box plot showing 1) Non-significant difference in change in Brain Natriuretic Peptide (BNP) levels from admission to discharge between patients without cardiorenal syndrome and patients with cardiorenal syndrome defined by A) >20% reduction in eGFR (n=298 and 60, respectively) and B) ≥ 0.3 mg/dl increase in creatinine (n=292 and 66, respectively).
As a post-hoc analysis we also categorized patients into having WRF or not on the basis of rise in creatinine ≥ 0.3 mg/dl [16]. 66 patients (18%) were found to have WRF based on the creatinine definition. Among the 66 WRF patients based on creatinine definition, 73% (n=48) were also classified as having WRF based on change in GFR, which is similar to a previously published study [20]. Although, the results were mostly similar to the primary definition, the most significant difference was in admission renal function. Patients with WRF based on the latter definition had somewhat poorer renal function (lower eGFR, higher creatinine and higher BUN) at admission in comparison with those without, although it was not significant. Among the patients that were re-classified (n=18 and 12, respectively), there was no difference in age, prevalence of Afro-American race or gender that are used in estimation of eGFR by MDRD formula but 18 patients who were classified as having WRF by increase in creatinine had significantly poorer renal function both at admission and discharge in comparison with patients classified as having WRF based on eGFR definition (p<0.001 for comparisons of eGFR, creatinine and BUN at admission and discharge). Thus, due to this discordance, we used both creatinine and eGFR as continuous variables for further analyses.
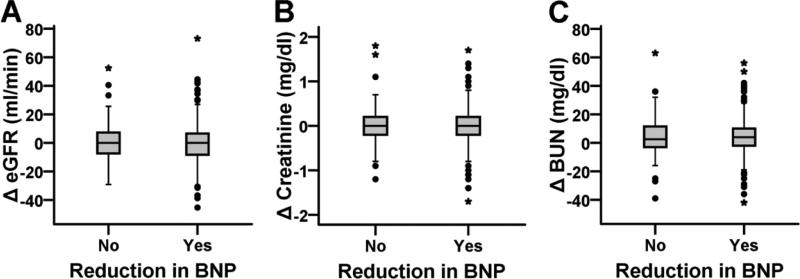
Next, we categorized the database into patients with and without a reduction in BNP. In 71% patients there was a reduction in BNP (n=254) whereas in the rest, there was either no change or increase in BNP (Table 3). Overall, patients who did not have a reduction in BNP were sicker at baseline with lower eGFR, systolic and diastolic blood pressure, higher BUN and creatinine and lower use of ACE inhibitors/ARBs and diuretics, in comparison with patients with a reduction in BNP (Table 3). Overall, change in eGFR, creatinine or BUN from admission to discharge was not different between these two groups (Table 3, Figure 2). 19.2% of the patients without a reduction in BNP and 15.7% of the patients with a reduction in BNP developed WRF (>20% reduction in GFR) during hospitalization (p = 0.44). Also, there were no significant differences in Δcreatinine and ΔeGFR between the quartiles of admission BNP.
Table 3.
Comparison of variables between patients with reduction in Brain Natriuretic Peptide (BNP) levels versus the rest of the patients
| Patients with reduction in BNP (n=254) | Patients with no change or increase in BNP (n=104) | p value | |
|---|---|---|---|
| Admission variables | |||
| Age (years) | 67±11 | 69±11 | 0.24 |
| Male (%) | 99 | 100 | 0.56 |
| White (%) | 57 | 52 | 0.35 |
| Atrial Fibrillation (%) | 25 | 30 | 0.35 |
| Coronary artery disease (%) | 63 | 71 | 0.18 |
| Hypertension (%) | 83 | 81 | 0.54 |
| Peripheral vascular Disease (%) | 23 | 25 | 0.68 |
| Diabetes mellitus (%) | 48 | 49 | 0.82 |
| Valvular abnormality (%) | 26 | 35 | 0.09 |
| NSVT (%) | 15 | 12 | 0.45 |
| Chronic renal insufficiency (%) | 55 | 67 | 0.03 |
| Dementia (%) | 4 | 8 | 0.11 |
| EF (%) | 26±13 | 24±12 | 0.11 |
| QRS duration (msec) | 123±36 | 125±34 | 0.73 |
| Systolic blood pressure (mm Hg) | 137±27 | 129±25 | 0.005 |
| Diastolic Blood Pressure (mm Hg) | 80±19 | 74±16 | 0.006 |
| Heart Rate (bpm) | 88±20 | 87±19 | 0.39 |
| Sodium (meq/l) | 137±5 | 137±5 | 0.49 |
| Hemoglobin (gm/dl) | 12.4±2.0 | 12.2±2.2 | 0.40 |
| eGFR (ml/min/1.73 m2) | 59±22 | 53±22 | 0.01 |
| Creatinine (mg/dl) | 1.6±0.6 | 1.8±0.7 | 0.007 |
| BUN (mg/dl) | 26.1±14.0 | 31.9±0.1 | 0.002 |
| BNP (pg/ml) | 1263 (807 to 1793) | 1290 (618 to 1300) | 0.05 |
| Discharge variables | |||
| eGFR at discharge (ml/min/1.73 m2) | 58±22 | 53±23 | 0.047 |
| Creatinine at discharge (mg/dl) | 1.6±0.6 | 1.8±0.9 | 0.03 |
| BUN at discharge (mg/dl) | 30.0±16.0 | 36.1±21.0 | 0.005 |
| BNP at discharge (pg/ml) | 562 (297 to 1010) | 1300 (821 to 1300) | <0.001 |
| ACE inhibitors/ ARB (%) | 84 | 60 | <0.001 |
| HF specific beta-blocker (%) | 67 | 61 | 0.33 |
| Loop diuretic (%) | 95 | 88 | 0.04 |
| Diuretic dose (mg) | 80 (60 to 160) | 113 (60 to 160) | 0.94 |
| Spironolactone (%) | 18 | 16 | 0.65 |
| Amiodarone (%) | 13 | 12 | 0.73 |
| Digoxin (%) | 32 | 35 | 0.62 |
| Change in renal function and BNP | |||
| ΔeGFR (ml/min/1.73 m2) | −0.6±14.7 | 0.6±13.3 | 0.31 |
| ΔCreatinine (mg/dl) | 0.1±1.0 | 0.0±0.4 | 0.32 |
| ΔBUN (mg/dl) | 4.2±12.9 | 4.2±14.0 | 0.69 |
| ΔBNP (pg/ml) | −548 (−940 to −195) | 68 (0 to 281) | <0.001 |
ACE: Angiotensin Converting Enzyme; ARB: Angiotensin Receptor Blocker; BUN: Blood Urea Nitrogen; EF: Ejection Fraction; eGFR: estimated Glomerular Filtration Rate; HF: Heart Failure; NSVT: Non-sustained Ventricular Tachycardia; Δ: refers to change in variable from admission to discharge.
Figure 2.
Box plots showing non-significant difference in 1) change in eGFR (ΔeGFR), 2) change in creatinine (Δcreatinine), and 2) change in Blood Urea Nitrogen (ΔBUN) from admission to discharge, between patients with either no change or increase in BNP (n=104) and patients with reduction in BNP levels (n=254).
Prognostic significance of WRF
At the end of 1 year, 30% of the patients had met the primary end point of all-cause mortality. On survival analyses, both admission and discharge renal function were associated with all-cause mortality at 1-year (Table 4). Although, ΔeGFR and Δcreatinine were not associated with the primary endpoint, ΔBUN had a significant association (Table 4). Surprisingly, although discharge BNP levels and change in BNP were associated with the primary endpoint, admission BNP was not associated with 1-year mortality. On multivariate survival analyses, only BUN at discharge was included among the renal parameters and rest were excluded because of high collinearity. BUN at discharge was the most powerful predictor of death with a Χ2 loss of 22 if the variable was removed (Χ2 for the overall model = 105). Similarly, because of strong association of BUN at discharge, BNP at discharge was excluded from the final model because of high correlation with discharge BUN (ρ=0.17, p=0.001). In the presence of BUN at discharge, only ΔBNP had a significant independent association with 1-year mortality.
Table 4.
Survival analyses for the primary end-point of all-cause mortality at 1 year
| Variable | Univariate HR (95% CI) | Multivariate HR (95% CI) |
|---|---|---|
| Admission variables | ||
| eGFR (ml/min) | 0.987 (0.978 to 0.996)** | |
| Creatinine (mg/dl) | 1.415 (1.100 to 1.820)** | |
| BUN (mg/dl) | 1.027 (1.017 to 1.036)*** | |
| BNP (100 pg/ml) | 1.011 (0.995 to 1.028) | |
| Discharge variables | ||
| eGFR (ml/min) | 0.987 (0.978 to 0.996)** | |
| Creatinine (mg/dl) | 1.442 (1.147 to 1.814)** | |
| BUN (mg/dl) | 1.029 (1.020 to 1.038)*** | 1.024 (1.015 to 1.033)*** |
| BNP (100 pg/ml) | 1.028 (1.011 to 1.044)*** | |
| Change in renal function and BNP | ||
| ΔeGFR (ml/min) | 1.000 (0.987 to 1.013) | |
| ΔCreatinine (mg/dl) | 1.222 (0.738 to 2.024) | |
| ΔBUN (mg/dl) | 1.016 (1.001 to 1.031)* | |
| ΔBNP (100 pg/ml) | 1.032 (1.005 to 1.060)* | 1.036 (1.009 to 1.063)** |
BNP: Brain Natriuretic Peptide; BUN: Blood Urea Nitrogen; CI: Confidence Interval; eGFR: estimated Glomerular Filtration Rate; HR: Hazard Ratio. Multivariate model is based on backward stepwise model with exclusion set at 0.1. Other variables included in the multivariate model are history of peripheral vascular disease and dementia; presence of valvular abnormalities; ejection fraction and QRS interval, and; use of angiotensin converting enzyme inhibitors and beta blockers at discharge.
p<0.05
p<0.01
p<0.001.
DISCUSSION
Our study showed that during hospitalization of ADHF, ΔBNP did not correlate with ΔeGFR, Δcreatinine or ΔBUN. Patients who developed WRF had similar levels of admission BNP and ΔBNP, in comparison with those who did not. Vice versa, patients who had a reduction in BNP levels from admission to discharge had a similar change in renal function, in comparison with patients with either no change or increase in BNP. Our study shows that ΔBUN was associated with higher mortality in the first year on univariate analysis. Among all the renal parameters, discharge BUN had the strongest association with mortality at 1 year. Lastly, BUN at discharge correlated with BNP at discharge and precluded the inclusion of discharge BNP in the final multivariate model.
Previous studies have shown that approximately 21-29% of patients admitted for HF develop WRF based on the definition of increase in creatinine of either more than 0.2 or 0.3 mg/dl [1-4]. Our study showed that, using a definition of >20% reduction in eGFR, 17% of our patients developed WRF. With the use of definition of WRF as ≥ 0.3 mg/dl increase in creatinine, the incidence of WRF in our population remained unchanged. The reason for slightly lower incidence of WRF in our study was probably due to the exclusion of Stage V CKD at baseline.
Previous studies have reported that higher creatinine predicts the development of WRF [2-4,7,9]. Other comorbidities such as diabetes, high systolic blood pressure, poor renal function, and signs of volume overload at baseline have shown to have variable association with development of WRF [2-4,7]. The results of our study and another study by Testani et al show that based on the older definition of WRF as ≥ 0.3 mg/dl, patients have a higher creatinine at baseline [20]. We performed post-hoc analyses to evaluate differences in age, gender, race but found no difference between the two group. This discordance is probably because the definition of ≥ 0.3 mg/dl increase in creatinine does not take into account baseline renal function and thus this metric is influenced by baseline renal function (discussed in detail by Testani et al) [20].
Studies have shown a complex relation between the diuretic dose and volume status and the development of WRF. Butler et al showed that although WRF is associated with higher and escalating diuretic dose during ADHF admission, change in volume status and weight were not associated with the development of WRF [7]. However, one major limitation of the study was missing data in 10-57% of cases. In contrast, Mullens et al showed that diuretic dose was not different between patients who developed WRF and those who did not [9]. Interestingly, patients who had a reduction in CVP < 8 mmHg were less likely to develop WRF. Finally, a post-hoc analyses of ESCAPE trial showed that although the diuretic dose was not associated with change in weight during ADHF admission, it was associated with increase in creatinine from baseline to discharge and a higher dose was associated with increased mortality [8]. Thus, although these 3 studies were discordant in the association of diuretic use with development of WRF, all suggested that volume reduction may not be associated with development of WRF. Lack of association between change in BNP, hemoglobin and sodium levels (that can be used as surrogate markers for change in volume status and response to treatment in HF patients), and development of WRF in our study supports this concept.
Possible mechanisms implicated in the development of WRF include diuretic resistance and overdiuresis leading to volume depletion. Lack of association between ΔBNP and development of WRF in our study and previous results of 2 other studies suggest that diuresis may lead to slight increase in BUN, but does not appear to cause WRF [1,7]. Thus, taken together, results from our study and from the study by Butler et al suggest that patients who have baseline renal insufficiency and who require higher doses of diuretics without volume reduction are predisposed to develop WRF [7]. Thus, withholding or reducing diuretics in the setting of temporary rise in BUN/creatinine, in the absence of volume contraction and dehydration, may not be warranted.
A few previous studies have shown a significant association of short-term mortality [3,4] and long-term mortality [22] with development of WRF during ADHF admission. In contrast, Testani et al showed that patients who had hemoconcentration developed worsening of renal function but still had better 180 day mortality [13]. Another study showed no association between WRF and mortality on univariate analysis, but significant association on multivariate analysis (without factoring EF, NYHA class and BNP levels) [23]. Lastly, two prospective studies and two secondary analyses of prospective trials evaluating association of WRF with mortality at 6 months follow-up showed conflicting results [2,10-12]. Thus, the association of change in renal function with long-term mortality is still conflicting and our study adds to the existing literature that discharge renal function but not the change in renal function is the most important predictor of mortality in the first year.
Our study has limitations inherent of retrospective chart review. The relationship between baseline variables including BNP and renal function with mortality therefore remains an association and not a cause-effect relationship. In addition, although BNP, hemoglobin and sodium levels are very good markers of change in volume status [13,17], we did not have weight parameters during hospitalization that could have further strengthened our study. In addition, although we had data on medications at discharge, we did not have information regarding in-hospital medications particularly loop diuretics. In addition, there were a limited numbers of patients with ADHF with both admission and discharge BNP, eGFR and BUN levels. Similarly these patients may represent a higher risk group requiring frequent profiling of renal function and filling pressures by BNP measurement since the biomarkers were not collected by trial design, but rather clinical necessity.
In summary, our study showed that treatment of ADHF during hospitalization resulting in BNP reduction was not associated with the development of WRF. Our findings imply that response to treatment and changes in intravascular volume are not the major cause of development of worsening renal function. However, since the treatment of ADHF was not carried out in a prospective, randomized, controlled fashion, our study does not answer whether use or overuse of diuretics with reduction in BNP levels results in development of WRF. In addition, we demonstrated that discharge BUN, but not change in BUN and creatinine, had a significant association with long term mortality.
Supplementary Material
ACKNOWLEDGEMENTS
None
Funding sources: S.A. is supported by American Heart Association SCA predoctoral fellowship (2010-2012) and Alkek foundation fellowship (2009-2012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, POSH Investigators Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 3.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM, Chen YT, Vaccarino V, et al. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. doi: 10.1016/s0002-9149(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 5.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 6.Kshatriya S, Kozman H, Siddiqui D, et al. The cardiorenal syndrome in heart failure: an evolving paradigm. Am J Med Sci. 2010;340:33–37. doi: 10.1097/MAJ.0b013e3181e59108. [DOI] [PubMed] [Google Scholar]
- 7.Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hasselblad V, Gattis Stough W, Shah MR, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9:1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akhter MW, Aronson D, Bitar F, et al. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94:957–960. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 12.Smith GL, Vaccarino V, Kosiborod M, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 13.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cataliotti A, Boerrigter G, Costello-Boerrigter LC, et al. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]
- 15.Dhaliwal AS, Deswal A, Pritchett A, et al. Reduction in BNP levels with treatment of decompensated heart failure and future clinical events. J Card Fail. 2009;15:293–299. doi: 10.1016/j.cardfail.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 16.van Kimmenade RR, Januzzi JL, Jr, Baggish AL, et al. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48:1621–1627. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 17.Pimenta J, Paulo C, Mascarenhas J, et al. BNP at discharge in acute heart failure patients: is it all about volemia? A study using impedance cardiography to assess fluid and hemodynamic status. Int J Cardiol. 2010;145:209–214. doi: 10.1016/j.ijcard.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Shah BN, Greaves K. The cardiorenal syndrome: a review. Int J Nephrol. 2010;2011:920195. doi: 10.4061/2011/920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–150. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 22.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Card Fail. 2006;12:257–262. doi: 10.1016/j.cardfail.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Kociol RD, Greiner MA, Hammill BG, et al. Long-term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am J Cardiol. 2010;105:1786–1793. doi: 10.1016/j.amjcard.2010.01.361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.