Abstract
Gonorrhea occurs at high incidence throughout the world and significantly impacts reproductive health and the spread of human immunodeficiency virus. Current control measures are inadequate and seriously threatened by the rapid emergence of antibiotic resistance. Progress on gonorrhea vaccines has been slow; however, recent advances justify significant effort in this area. Conserved vaccine antigens have been identified that elicit bactericidal antibodies and, or play key roles in pathogenesis that could be targeted by a vaccine-induced response. A murine genital tract infection model is available for systematic testing of antigens, immunization routes and adjuvants, and transgenic mice exist to relieve some host restrictions. Furthermore, mechanisms by which N. gonorrhoeae avoids inducing a protective adaptive response are being elucidated using human cells and the mouse model. Induction of a Th1 response in mice clears infection and induces a memory response, which suggests Th1-inducing adjuvants may be key in vaccine-induced protection. Continued research in this area should include human testing and clinical studies to confirm or negate findings from experimental systems and to define protective host factors.
Keywords: immunity, T cells, antibodies, immune regulation, innate defenses, gonococcal antigens
1.0 Introduction
1.1. Epidemiology
Gonorrhea is a sexually transmitted bacterial infection caused by the Gram-negative diplococcus Neisseria gonorrhoeae (Gc). Gonorrhea is one of the most common infectious diseases worldwide, with significant immediate and long-term morbidity and mortality. In sexually active adolescents and adults Gc causes clinically inapparent mucosal infections (most common in women), symptomatic urethritis and cervicitis, upper urogenital tract infections, and pelvic inflammatory disease. Extra-genital rectal and pharyngeal infections occur frequently and coinfections with other sexually transmitted pathogens are common. Systemic or disseminated gonococcal infections (DGI) are infrequent (0.5–3%), occur mainly in women, and include a characteristic gonococcal arthritis-dermatitis syndrome, suppurative arthritis, and rarely endocarditis, meningitis or other localized infections. Neonates exposed during birth may develop ophthalmia neonatorum, skin infections, or, rarely, disseminated disease.
Complications from Gc infections are frequent, debilitating, and disproportionately affect women. Untreated cervical infections commonly progress to the upper reproductive tract, which contributes to pelvic inflammatory disease (PID), infertility, life-threatening ectopic pregnancy, and chronic pain. Infertility rates following PID are high, at >10% following a single episode and >50% following three or more episodes [1]. In men 10–30% of untreated urethritis cases may progress to epididymitis, a common cause of male infertility in some regions [2]. During pregnancy, Gc causes chorioamnionitis complicated by septic abortion in up to 13% of women, preterm delivery in 23% of women, and premature rupture of membranes in 29% of women [3]. Neonatal conjunctival infections are destructive, leading to corneal scarring and blindness. Gonorrhea also dramatically increases the acquisition and transmission of human immunodeficiency virus (HIV) [4].
An estimated 106 million Gc infections occur annually, worldwide [5]. Diagnostic capabilities and surveillance systems vary between nations, and thus, infection is greatly underreported and prevalence is often highest among economically or socially disadvantaged populations. Microbiologic culture is diagnostic, but syndromic management alone is standard for many regions of the world. Rapid DNA-based tests have improved sensitivity, especially for asymptomatic disease, but are not available in all countries. In all situations, treatment is empiric at the initial point of care to eliminate further transmission.
Antimicrobial resistance patterns guide treatment recommendations, the goal of which is to effectively treat ≥95% of infections at first presentation. Antibiotic resistance is widespread and has developed rapidly with each successive treatment regimen. Alarmingly, with the advent of resistance to extended-spectrum cephalosporins, we have now reached the point where untreatable disease can be anticipated in the near future [6]. Although rapid effective treatment of gonorrhea decreases long-term sequelae and can eliminate the effect on HIV transmission [7], expansion of multi-drug resistant Gc is a global threat to public health and amplifies the urgent need for novel prevention methods.
1.2. Modeling vaccine impact
Development of an effective gonorrhea vaccine is likely to have significant benefits given the impact of gonorrhea on human health. Ebrahim et al. estimated 1,326 disability-adjusted life years (DALYs) are attributable to 321,300 Gc infections. Applied to WHO global estimates of new Gc infections, this translates to 440,000 DALYs per year [8, 9]. The benefits of effective treatment to women also have been estimated: treatment of 100 women with gonorrhea, of which 25% are pregnant, would prevent 25 cases of PID, one ectopic pregnancy, 6 cases of infertility, and 7 cases of neonatal ophthalmia. Additionally, treatment of 100 high-frequency transmitters of Gc could prevent 425 new HIV infections over 10 years [10]. This projection is supported by experience in Mwanza, Tanzania where HIV infection was several times greater among individuals with gonorrhea [11]. Given the increases in duration of infection, transmission rates, and complications that can be anticipated with rising antibiotic resistance, there is an urgent need for expanded efforts to develop preventive vaccines.
Modeling studies are needed to assess the impact of various vaccination strategies. While an ideal vaccine would eliminate Gc from all mucosal surfaces, as observed with Haemophilus influenzae B conjugate vaccines [12], this vaccine outcome may not be achievable for Gc. Estimates of the impact of gonorrhea vaccines that decrease extension of disease, decrease transmissibility, or eliminate only complicated disease are needed and may support multiple early approaches. In one model, focused treatment of core groups results in collapse of disease transmission. However, when antibiotic resistance is added to the model, there is rebound and increased dissemination of disease [13]. Similar studies should investigate whether vaccination of only women, core groups, or all individuals who present with a sexually transmitted infection STI) would be adequate, or whether broader vaccination strategies are needed.
2. Pathogenesis
Gc is a human-specific pathogen with no animal or environmental reservoir. Initial adherence to epithelial cells is mediated by type 4 colonization pili, which are multifunctional appendages that also mediate genetic exchange, twitching motility, bacterial aggregation, and cell signaling [14]. Gc also has an intracellular niche; invasion of urethral cells occurs through the binding of the lacto-N-neotetraose (LNT) species of lipooligosaccharide (LOS) to the asialoglycoprotein receptor. Gc also invade epithelial cells of the female genital tract, and the best characterized pathways are uptake through complement receptor 3 (CR3) on cervical cells due to binding of a complex formed by LOS, porin (PorB) and host C3b molecules [15], and interactions between Gc opacity (Opa) proteins and human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) on cervical or endometrial cells [16]. PorB1a-mediated invasion of epithelial cells occurs through the scavenger receptor SREC [17] and may explain in part the strong association between PorB1a strains and DGI.
Gc is also well adapted to evade host innate defenses. Gc circumvents iron sequestration on host mucosal surfaces by expressing receptors for hemoglobin, human transferrin (Tf) and human lactoferrin [18]. The MtrC-MtrD-MtrE active efflux pump system protects Gc by actively expeling hydrophobic antimicrobial substances (e.g. fatty acids, bile salts, progesterone, antimicrobial peptides). Similarly, the FarA-FarB-MtrE pump protects Gc from long fecal lipids found in rectal mucosae [19]. Gc has several mechanisms for evading complement-mediated defenses. Sialylation of the lacto-neotetraose species of LOS increases resistance to the bactericidal activity of human serum and significantly reduces opsonophagocytosis by polymorphonuclear leukocytes (PMNs) [20]. Serum-resistant strains down-regulate complement activation on their surface by expressing PorB molecules that bind C4b-binding protein or factor H [21]. Phase variation of glycosyltransferase genes can cause production of LOS species that are more resistant to bactericidal antibodies [22]. Survival of Gc within PMNs may prolong infection and increase dissemination and transmission and occurs by mechanisms not yet fully elucidated [23].
3. Host Response
3.1 Innate response
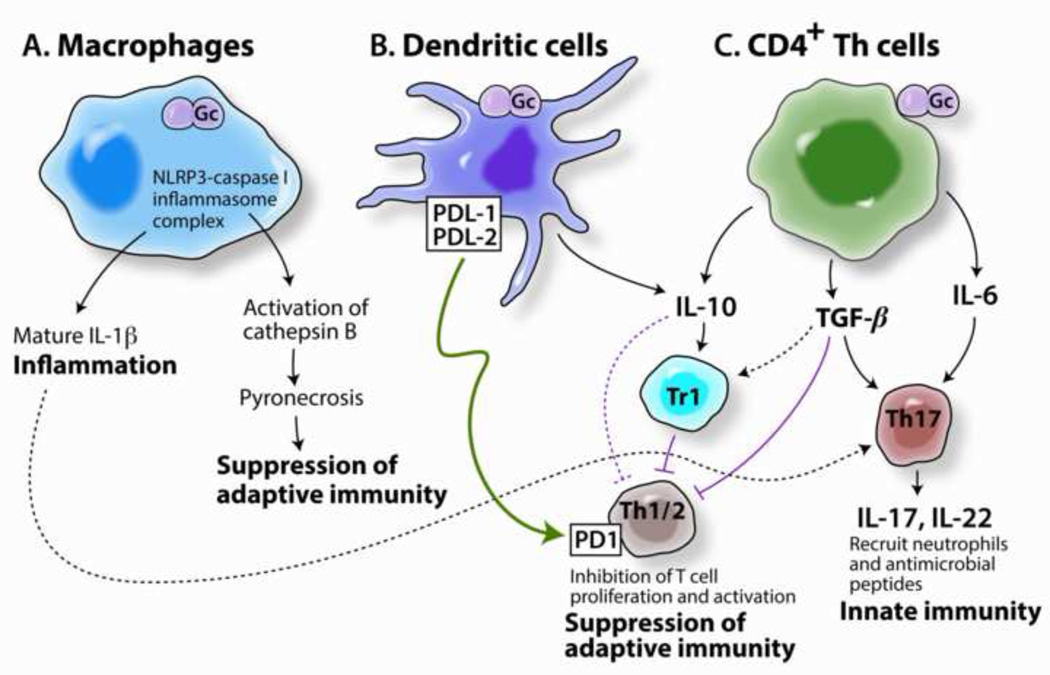
During acute infections, Gc induces a purulent exudate that consists of PMNs, exfoliated epithelial cells, and intracellular and extracellular Gc. The capacity of Gc to evade PMN killing is supported by the observation that Gc colonization levels are similar in BALB/c and C57/BL6 mice despite marked differences in PMN influx [24]. Elevated proinflammatory cytokines and chemokines have been detected in experimentally infected men [25], but not in naturally infected women unless coinfected with another STI pathogen [26]. In the mouse model Gc selectively induces Th17 cells, which leads to the recruitment of innate defense effectors including PMNs and results in faster clearance of infection [27]. Signaling through TLR4 is critical for Th17 responses in vitro [27] and in vivo [28], and colonization load is increased in TLR4-deficient mice [28]. Gonococcal LOS-mediated signaling through lectins such as DC SIGN induces cytokine production [29] and both PorB and the H.8 lipoprotein stimulate TLR2 leading to NF-κB activation, inflammatory cytokine production, and dendritic cell (DC) maturation [30, 31]. Activation of NLRP3 inflammasomes in human monocytic cells or DCs by Gc results in the production of the inflammatory cytokines IL-1β and IL-18 and pyronecrosis of the cells [32, 33] (Fig. 1).
Fig. 1. Mechanisms of interaction of N. gonorrhoeae with cells of the immune system.
Gc can interact with various immune cells to elicit innate inflammatory responses and suppress Th1/Th2-mediated specific immune responses. (A) Phagocytosis by macrophages results in activation of NLRP3 inflammasomes, the production of IL-1 and activation of PMNs, and activation of cathepsin B, which leads to pyronecrosis of APC [33]. (B) Interactions with DCs lead to up-regulation of PDL-1 and PDL-2, which induce apoptosis of cells bearing PD1. This up-regulation also causes release of IL-10 [32], which has immunoregulatory properties and stimulates type 1 regulatory T cells (Tr1). (C) Interaction with CD4+ T helper cells (or B cells) induces secretion of IL-10, TGF-β, and IL-6 [37, 100]. IL-10 and TGF- suppress the activation of Th1 and Th2 cells both directly, and through the activation of Tr1 cells. TGF-β and IL-6 drive the development of Th17 cells which secrete IL-17 and IL-22, leading to the recruitment or induction of innate defenses such as PMNs and anti-microbial peptides. Gc is able to resist destruction by PMNs and anti-microbial peptides while concomitantly suppressing the development of adaptive immune responses such as Gc-specific antibodies that could enhance phagocytosis and intracellular killing by phagocytes and bacteriolysis through the classical complement pathway [101]. Not shown is the expansion of IgD(+),CD27(+) B cells in response to gonococcal infection, which may contribute to a localized non-specific antibody response without immunologic memory in response to N. gonorrhoeae [42], or Opa-mediated B cell suppression[39] or killing [40], which are discussed in the text.
3.2 Adaptive response
The adaptive response to Gc is ineffective as evidenced by the fact that repeat infections are common. The humoral response to uncomplicated Gc infections is poor. Quantitative evaluation of serum and local antibody responses in both female and male subjects presenting with uncomplicated cervicitis or urethritis showed at best only modest responses to antigens expressed by the homologous clinical isolates. Antibody responses were not sustained over the few weeks of follow-up, and there was no discernable memory arising from known prior episodes of infection [26, 34]. These results are consistent with earlier reports by others (reviewed in [35]).
Insights into the mechanisms by which Gc interferes with immune responses are being elucidated (Fig. 1). In mice, Gc suppresses the development of Th1- and Th2-driven adaptive immune responses by mechanisms dependent on TGF-β and IL-10 as well as type 1 regulatory T cells [36, 37] (Liu et al., Mucosal Immunology, in press). This immunosuppression occurs concomitantly with induction of IL-17, resulting in strong inflammatory responses but no adaptive immune response. Consistent with these observations, humans with gonorrhea have elevated serum IL-17 and IL-23 [38], and human monocyte-derived DCs secrete IL-23 and IL-10 upon stimulation with Gc in vitro [27, 37]. Other mechanisms of immunosuppression include induction of apoptosis in antigen presenting cells (APC) through the NLRP3 inflammasome pathway [33] and inhibition of DC-induced proliferation of T cells [32]. Gc Opa proteins that bind CEACAM1 were reported to down-regulate proliferation of activated CD4+ T cells and also B cells [39, 40], although these findings have been questioned by others [41]. Gc also induces a polyconal IgM+ B cell response with poor specificity to the bacteria [42].
Mechanisms to evade specific antibodies include the expression of blocking antigens, production of IgA1 protease, molecular mimicry, retreat into epithelial cells, blebbing of membranes to create a decoy, and changes in the antigenicity of surface molecules due to an extensive capacity for uptake and incorporation of DNA from other neisseriae, or in the case of Gc pili, recombination between the expressed pilin gene and silent loci. Phase variable expression of LOS biosynthesis genes and genes that encode surface molecules, such as opa genes, also contributes to evasion of specific antibodies [43].
4. Challenges and Progress for Gonorrhea Vaccines
Progress on gonorrhea vaccines lags behind that of several other STIs for many reasons. First, repeat infections are common and correlates of protection in humans have not been identified. Second, early vaccine efforts were frustrated by the highly antigenically variable surface of Gc and the lack of a small laboratory animal model for identifying protective responses and for systematic testing of antigens and immunization routes. Finally, there has been a lack of a concerted effort in this area. Only two antigens, killed whole cells and purified pilin, have been tested in clinical trials, which occurred over 30 years ago and were unsuccessful [35]. These failures discouraged research, funding and commercial interest in gonorrhea vaccines. Advances in microbial pathogenesis, immunology, molecular epidemiology, combined with new infection models and the powerful new tools of genomics, proteomics and glycomics justify a renewed and intensified research focus on gonorrhea vaccine development.
4.1 Identification of protective responses
Knowledge of the specific immune mechanisms that protect against Gc infection is severely lacking. An estimated 20–35% of men become infected following a single exposure to an infected woman; the risk for women exposed to an infected man is estimated at 60–90% [44]. Comprehensive studies are needed to identify factors that might explain differential susceptibility to infection (Fig. 2). The lack of evidence that natural infection induces immunity to reinfection also seriously limits our ability to prospectively define the types of immune responses that an effective vaccine must induce. Conventional thinking suggests that for a purulent infection such as gonorrhea, antibody- rather than cell-mediated immunity would be key; however, this has not been experimentally proven, and Th responses are likely required to drive antibody generation and memory. In addition, to the extent that Gc reside intracellularly and thereby escape antibody-mediated defenses, T cell-mediated immunity could have a role that merits exploration. Repeat exposure and bactericidal antibodies were associated with reduced risk of salpingitis [35], however there are few data to support a protective immune response against uncomplicated infections. In one report, repeatedly infected women in Nairobi, Kenya showed partial serovar-specific immunity against the prevalent circulating Gc strain [45], this finding was not replicated in a study of less exposed subjects in a rural setting in the United States [46]. Antibodies against the reduction-modifiable protein (Rmp) block the bactericidal activity of PorB or LOS-specific antibodies, and the relative proportion of blocking and bactericidal antibodies has been proposed to correlate with immunity [47]. Lacking are studies on the effect of high-titer bactericidal antibody, which natural infection does not induce, or cellular immunity in protecting against human infection.
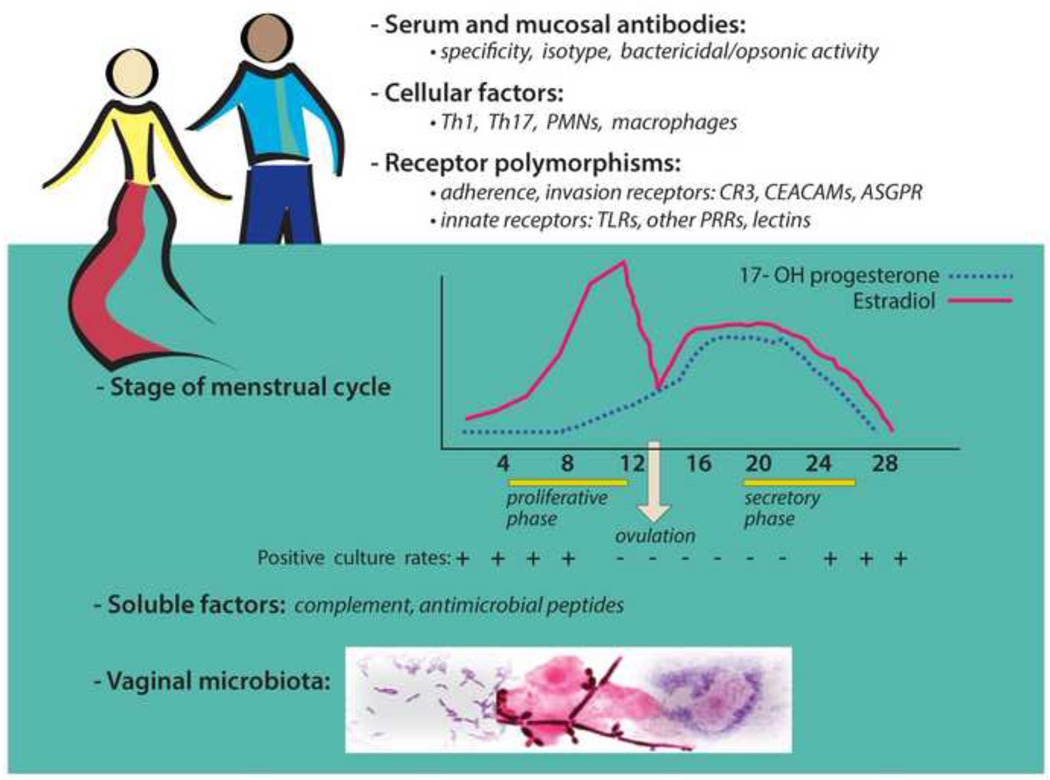
Fig. 2. Factors that may reduce susceptibility to gonorrhea.
It is not understood why some individuals do not become infected following exposure to Gc. Differences in humoral (antibody, complement, antimicrobial peptides) or cellular factors (Th1 and Th17 cells, PMNs, macrophages) may affect the host’s ability to kill the organism or block infection. Polymorphisms in adherence or invasion receptors and innate immune receptors could also influence susceptibility to infection. In women, the stage of the reproductive cycle may affect host susceptibility based on well documented associations between positive culture rates and the estrogen-dominant phases of the menstrual cycle in women with gonorrhea [102, 103] and the temporal link between menses and the onset of pelvic inflammatory disease. The impact of reproductive hormones on susceptibility is also supported by differences in the kinetics of Gc colonization of normal and ovariectomized mice [104] and the effect of progesterone on Gc survival within cervical cells [105]. Potential soluble factors include complement components and antimicrobial peptides secreted by genital tract epithelial cells. Some commensal microbes may protect against Gc through competition for nutrients or adherence receptors, and the release of inhibitory factors. Reported associations between vaginal H2O2-producing lactobacilli and a reduced risk of gonorrhea [106] are countered by co-colonization studies in mice [107] and the demonstration that biofilms protect Gc from these commensals (Apicella, et al., 2012 IPNC abstract #0064). The vaginal microbiota of susceptible and uninfected women exposed to Gc could be comprehensively defined using modern genomic technology.
The conventional paradigm in vaccine development of mimicking natural infection to provoke an immune response without actually causing disease, therefore, is not applicable to gonorrhea as recovery does not confer protective immunity against re-infection. This situation could arise either because a specific immune response is ineffective against a continually variable antigenic target like Gc, or because Gc interferes with the normal course of an immune response and suppresses its development. A successful vaccine must demonstrate the ability to protect against all or most known and unknown antigenic types, and novel approaches to address this challenge are needed. In addition, if the mechanisms by which Gc manipulates the host immune responses can be identified, vaccines might be designed to inhibit or sidestep these mechanisms and allow an effective protective immune response to develop
The relative contributions of Th17-driven innate responses and Th1/Th2-driven adaptive responses to protective immunity remain to be elucidated. Gc-induced immunosuppression in mice can be reversed by treatment with blocking antibodies against TGF-β and IL-10, which permit the development of Th1- and Th2-dependent responses with circulating and vaginal anti-Gc antibodies, immunological memory, and protective immunity against reinfection (48) (Liu Muc Immun 2013, in press). However, neutralization of TGF-β also inteferes with Th17 responses (48). Alternatively, intravaginal administration of IL-12 incorporated in sustained-release microspheres enhances Th1-dependent adaptive immune responses including generation of anti-Gc antibodies, accelerated clearance of infection, and protection against reinfection, but without interfering with the induction of Th17 responses (Liu, JID, 2013 in press)
4.2 Models for immunization/challenge studies
Systematic testing of antigens, immunization routes and adjuvants is greatly facilitated by animal models, but the strict human specificity of Gc poses a challenge for animal modeling of Gc infections [48]. Experimental urethral infection of male volunteers has been used to define the innate and humoral responses to infection and reinfection and the importance of selected virulence factors [25, 49–51]. This well-characterized model currently is being conducted at the University of North Carolina [50] and provides a system for early testing of vaccine candidates. However, the human challenge model can only assess immunoprotection against early stages of male urethral infection and might not identify candidates that would be effective in women or prevent complicated infections or DGI. Chimpanzees are less subject to Gc host restrictions than other laboratory animals. Male chimpanzees develop Gc urethritis that is similar to that observed in humans, and natural transmission of gonorrhea from a male chimpanzee to two females was documented. Immunization of chimpanzees with a whole cell vaccine resulted in increased resistance to infection (reviewed in [35]). Chimpanzees are no longer available for gonorrhea research, but the insights gained from these experiments should not be ignored.
Female mice are transiently susceptible to Gc during proestrus [52], and administration of 17β-estradiol and antibiotics prolongs colonization with ascending infection occurring in 17–20% of mice. The innate response in mice is similar to that reported for humans; infection of BALB/c mice induces proinflammatory cytokines and chemokines (IL-6, TNFα, KC, and MIP-2) and a vaginal PMN influx. Gc is readily found within mouse PMNs and infection persists during periods of inflammation. Specific serum and vaginal antibodies are low after infection and mice can be reinfected with the same strain. This model has been useful for studying Gc factors that facilitate evasion of innate defenses and for further examining the immune modulation associated with Gc infection [53]. The mouse model has also been used for vaccine studies [54](Gulati et al., 2012 IPNC, Abstract #0118) and was recently standardized in challenge-aged mice for vaccine testing (D. Simon and A.E. Jerse, in preparation). However, numerous host restrictions severely limit the capacity of this model to mimic human gonorrhea, some of which might affect the predictive power of this model for human vaccines. These restrictions include human-specific receptors for adherence and invasion, iron-binding glycoproteins, soluble regulators of the complement cascade (fH, C4BP), and IgA1, the substrate of gonococcal IgA1 protease, whose role in evasion of IgA1 is uncertain. Although Gc IgA1 protease activity is found in the secretions of infected women [55], the typical cleavage fragments of IgA have not been identified [56] and the predominant isotype of IgA in female genital secretions is IgA2 [57], which is totally resistant to this cleavage. IgA1 is predominant in human semen, but whether IgA1 protease shields Gc from IgA1 antibodies in men has not been investigated [49]. In addition, mice lack FcαR (CD89), the opsonophagocytic receptor for IgA. Other host-restricted interactions include the capacity of Gc to avoid complement-mediated killing by binding human but not murine C4BP and fH. The development of hC4BP and fH transgenic mice [58] or administration of purified human fH or C4BP [59] could overcome this restriction. Likewise, the potential protective effects of vaccines against the Gc Tf receptor [60, 61] or specific adherence or invasion ligands that bind to host-restricted receptors might be underestimated in normal mice. Nonetheless, challenge studies in normal mice can provide information on conventional immune responses (agglutination, osponophagocytosis, bactericidal activity, cell-mediated immunity), which can be combined with in vitro studies using human target molecules or cells to better predict the efficacy of candidate vaccines in humans. In addition, severe combined immunodeficient mice engrafted with human lymphocytes to reconstitute a functional human immune system (huSCID mice) [62] might find application in the development of a gonorrhea vaccine.
4.3 Antigen discovery
Gc is a leading paradigm of a pathogen that utilizes antigenic variation to escape specific immune responses as famously illustrated by the failure of a large pilin vaccine trial in Korea [63]. However, several other potentially protective surface molecules have since been identified (Table 1). These antigens include the Tf receptors, TbpA and TbpB, the 2C7 LOS epitope, and PorB, although none has progressed to clinical trial. The Tf receptor was required for experimental urethral infecton of male volunteers by a Gc strain that naturally lacks the Lf receptor [64]. Intranasal immunization of mice with TbpA or TbpB proteins that were genetically fused with the B subunit of cholera toxin elicited specific serum and vaginal IgG and IgA antibodies, which were bactericidal and inhibited Gc growth dependent on human Tf [60, 61]. Antibodies against the 2C7 oligosaccharide (2C7-OS) epitope of Gc LOS [65] or a 2C7-OS peptide mimic [66] are highly bactericidal and promote opsonophagocytic killing of Gc. Intraperitoneal immunization of mice with a multi-antigenic form of the 2C7-OS peptide mimic protected mice from subsequent challenge as did passive delivery of 2C7 monoclonal antibody (Gulati et al., 2012 IPNC, Abstract #0118). Although the 2C7 epitope is phase variable [67], it is expressed by 95% of Gc isolates from clinical samples [65] and could be combined with other antigens to minimize evasion of immune responses.
Table 1.
Potential gonorrhea vaccine antigens
| Antigen | Function | Expression | Variability | Immunogenicity | Reference |
|---|---|---|---|---|---|
| PilC | Pilus-associated adhesin | Phase variable | Variable and conserved regions | [89, 90] | |
| PilQ | Outer membrane channel for pilus extrusion | Stable | Conserved at C-terminus | fPilQ406–770 mouse immunization: surface-binding and bactericidal antibodies | [91] |
| Opa | Adherence, invasion | Phase variable | Variable | Bactericidal antibodies, meningococcal Opa proteins protective in mice | [92, 93] [94] |
| AniA | Nitrite reductase, biofilm formation | Induced by nitrite and low oxygen tension | Conserved, variable glycosylation | Antiserum to truncated non-glycosylated recombinant protein blocked function of AniA | [69] |
| TdfJ | Iron-induced zinc transporter | Regulated | Conserved | Antiserum to the meningococcal homologue ZnuD is bactericidal | [95, 96] |
| PorB | Porin, major OMP, nutrient acquisition, antibiotic and serum resistance, invasion | Stable, essential | Variable | Cyclic loop peptides induce cross-reactive bactericidal antibodies; antibodies block use of Tf as a sole iron source | Garvin et al., 2010 IPNC Abstract #P235 |
| Lst | α-2,3-sialyltransferase; increases serum resistance | Constitutive | Conserved | Antibodies reduce sialylation | [97] |
| TbpB, TbpA | Transferrin receptor | Induced in iron- limiting conditions | TbpA, conserved; TbpB variable | Bactericidal when Tf is sole iron source. Tf required for infection of male volunteers by strains lacking Lf receptor | [50] [60] |
| 2C7 epitope | Monoclonal antibody to lipooligosaccharide (LOS) | Phase variable; expressed by most Gc strains | Common epitope in variable LOS | Antisera to peptide mimic are bactericidal and opsonic; protection in mouse model | [65, 66] (Gulati et al., 2012 IPNC, Abstract #0118 |
| OmpA | Adherence, invasion | Transcriptiona l regulation | Bactericidal | [98] | |
| OpcA | Adherence, invasion | Stable | Conserved | Bactericidal | [99] |
Nitrite reductase (AniA) is also being developed as a gonorrhea vaccine target. Anaerobic growth of Gc occurs by a two-step denitrification process, and AniA, which catalyzes the first step of this process, is a surface-exposed copper-containing glycosylated protein that is anchored to the outer membrane by its lipid-modified N-terminus [68, 69]. Recently, Shewell et al. demonstrated that deletion of the glycosylated immunodominant C-terminus of AniA produced a truncated protein that elicited antibodies that inhibited nitrite reductase activity [69].Vaccine-mediated inhibition of AniA function may be an effective approach because the capacity to grow anaerobically is likely an important adaptation during infection of the genital tract where oxygen tension is reduced. This hypothesis is supported by the detection of AniA-specific antibodies from women with lower or upper genital tract infections and one patient with DGI [70]. AniA is also required for mature biofilm formation, which may protect against innate defenses [71].
The exciting development of group B meningococcal vaccines, which was a formidible challenge for many years, may provide a useful template for developing a gonorrhea vaccine [72–74]. Some of these vaccines contain outer membrane vesicles (OMV) and some are genetically engineered to stabilize the expression of phase variable antigens and increase the range of antigenic specificities. Detergent-treated OMVs or OMVs produced from LOS mutants have been used to diminish endotoxicity. Immunization and challenge studies with Gc OMV have not been reported; a Gc outer membrane protein preparation demonstrated protection in mice when delivered intranasally with CT [54], but this approach was not successful in subsequent studies, possibly due to differences in the protein isolation methods used [35]. The Novartis 4CmenB vaccine consists of OMVs combined with the NadA protein and two fusion proteins, factor H-binding protein (fHbp) and neisserial heparin binding antigen (NHBA) fused to two other conserved antigens [74]. None of the three proteins (fHBP, NHBA and NadA) in the 4CmenB vaccine [74] are predicted to be suitable vaccine targets for Gc [75]; however, gonorrhea research may benefit from the use of proteomics technology and, or genome mining, which have advanced the development of vaccines for group B N. meningitidis.
4.4 Immunization routes
Immunization of the genital tract also challenges gonorrhea vaccine development, although we are encouraged by the success of the HPV vaccine. Most efforts to develop a vaccine against gonorrhea have focused on conventional parenteral immunization, which generates circulating, predominantly IgG antibodies, but is generally ineffective at inducing secretory (S) IgA at mucosal surfaces. However, the genital tract secretions of both males and females contain more IgG derived largely from the circulation than SIgA produced locally and transported through epithelial cells [57]. Intranasal immunization has been found to be the most effective route for inducing antibodies in the genital tracts of female mice and monkeys [76–78], and possibly also in women [79]. Local, intravaginal immunization has been accomplished [79], but as the genital tract lacks organized immune inductive tissue equivalent to intestinal Peyer’s patches, responses are not disseminated through the “common” mucosal immune system. The generation and recall of memory responses in the mucosal immune system depends on the nature of the inducing antigen, being most effective with potent adjuvants such as CT. Persistence of SIgA responses after their generation, however, appears to depend on continued stimulation and is counteracted by competing antigenic stimuli [80]. The ideal route for vaccination against gonorrhea will depend upon whether induction of local SIgA antibodies is needed in addition to IgG; this in turn will require understanding the effector mechanisms of antibody-mediated defense against Gc in the genital tract.
4.5 Adjuvants
Few vaccine adjuvants have been specifically evaluated for generating responses against Gc, although many have been tested for their ability to enhance circulating and mucosal antibody or cellular responses against experimental HIV vaccines. CT, the related E. coli heat-labile enterotoxins (LT types I, IIa, IIb, and IIc), and their non-toxic derivatives (mutants or isolated B subunits) are among the most potent mucosal adjuvants and have been extensively studied in animals when administered by oral, nasal, or even vaginal routes [81–83]. Intranasal immunization with antigens administered with or coupled to the nontoxic B subunit of CT induces vaginal antibody responses in mice and monkeys [77, 84], but the use of such adjuvants in humans is precluded by the finding that these toxins can traffic from the nasal epithelium to the brain via the olfactory nerve [85]. While some mutants and derivatives of LT appear to retain adjuvant activity in the absence of toxicity, and lack the capacity for retrograde neural transmission, their applicability to gonorrhea vaccines will need careful evaluation.
Recent studies using microencapsulated IL-12 given intravaginally in mice infected with Gc showed enhanced Gc-specific vaginal and serum antibodies (Liu et al., J Infect Dis, in press), suggesting that IL-12 can serve as a potent intravaginal adjuvant. IL-12 administered intranasally is known to have an adjuvant effect with respiratory vaccines [86]. Other cytokines, including a combination of IL-1α, IL-12, and IL-18, are effective adjuvants for HIV peptide vaccines given intranasally [87]. Oligodeoxynucleotides containing the CpG motif also serve as adjuvants that engate TLR9 and induce genital tract responses [88]. Research on adjuvants will be an important aspect of gonorrhea vaccine development, especially when candidate antigens and the desired types of protective immune responses have been identified.
5.0 Future Objectives
A comprehensive effort is needed on many fronts to make a gonorrhea vaccine a reality, Inter-institutional collaborations with goal-oriented direction and novel, focused, and sustained funding mechanisms are essential. If a stable, long-term institutional commitment can be made, the following activities could lead to development of an effective vaccine:
- Continued research to understand basic aspects of pathology and host responses
-
◦Test in humans the hypotheses generated in animal and in-vitro models of infection, to determine the impact of Gc on human genital immune responsiveness.
-
◦Determine the mechanisms of immune defense against Gc infection in human female and male genital tracts.
-
◦Determine reproductive cycle effects on susceptibility to Gc infection in humans.
-
◦
- Focused efforts on vaccine development
-
◦identify conserved Gc antigens or multivalent vaccine constructs;
-
◦identify routes and schedules of immunization to generate required responses;
-
◦identify appropriate vaccine adjuvants and/or delivery systems;
-
◦define surrogate measures for assessing immunity.
-
◦
- Expanded testing and modeling of vaccine candidates in human subjects
-
◦Consider early Phase I Clinical Trials of rational vaccine candidates to determine safety and nature of responses generated, regardless of evidence of efficacy in animal models.
-
◦Modeling studies to assess potential benefit of vaccines affording protection against only specific aspects of Gc disease, or targeting certain populations.
-
◦Identify potential clinical efficacy end-points or biomarkers to assess vaccine effects in the absence of complete protection that could be of public health importance
-
◦
Highlights.
Multiple drug resistance in Neisseria gonorrhoeae impels renewed vaccine efforts
Poor natural immunity, antigen variation, and gender factors pose challenges
Gonococcal subversion of immune responses requires new vaccine approaches
Improved animal models available for vaccine evaluation
Concerted goal-oriented research efforts and sustained support required
Acknowledgements
Funding for this work was provided to A.E.J. by grants RO1-AI 42053 and U19 AI31496 and to M.W.R. by grant R21 AI074791 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. M.W.R. was also supported by the John R. Oishei Foundation, Buffalo, New York. We thank John Nyquist, M.S., C.M.I, F.A.M.I. AND MARCIA HOBBS, PH.D. for preparation of the figures and Freyja LYNN and Amanda DeRocco for helpful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westrom LV. Sexually transmitted diseases and infertility. Sex Transm Dis. 1994;21:S32–S37. [PubMed] [Google Scholar]
- 2.Campbell MF. The surgical pathology of epididymitis. Ann Surg. 1928;88:98–111. doi: 10.1097/00000658-192807000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods CR. Gonococcal infections in neonates and young children. Semin Pediatr Infect Dis. 2005;16:258–270. doi: 10.1053/j.spid.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Jarvis GA, Chang TL. Modulation of HIV transmission by Neisseria gonorrhoeae: molecular and immunological aspects. Curr HIV Res. 2012;10:211–217. doi: 10.2174/157016212800618138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Geneva: World Health Organization; 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. [Google Scholar]
- 6.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012;7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahim SH, McKenna MT, Marks JS. Sexual behaviour: related adverse health burden in the United States. Sex Transm Infect. 2005;81:38–40. doi: 10.1136/sti.2003.008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndowa F, Lusti-Narasimhan M. The threat of untreatable gonorrhoea: implications and consequences for reproductive and sexual morbidity. Reprod Health Matters. 2012;20:76–82. doi: 10.1016/S0968-8080(12)40653-X. [DOI] [PubMed] [Google Scholar]
- 10.Tapsall JW. Geneva: World Health Organization; 2001. Antimicrobial resistance in Neisseria gonorrhoeae. [Google Scholar]
- 11.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 12.Madore DV. Impact of immunization on Haemophilus influenzae type b disease. Infect Agents Dis. 1996;5:8–20. [PubMed] [Google Scholar]
- 13.Chan CH, McCabe CJ, Fisman DN. Core groups, antimicrobial resistance and rebound in gonorrhoea in North America. Sex Transm Infect. 2012;88:200–204. doi: 10.1136/sextrans-2011-050049. [DOI] [PubMed] [Google Scholar]
- 14.Cahoon LA, Seifert HS. Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol Microbiol. 2011;81:1136–1143. doi: 10.1111/j.1365-2958.2011.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol Rev. 2011;35:498–514. doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 17.Rechner C, Kuhlewein C, Muller A, Schild H, Rudel T. Host glycoprotein Gp96 and scavenger receptor SREC interact with PorB of disseminating Neisseria gonorrhoeae in an epithelial invasion pathway. Cell Host Microbe. 2007;2:393–403. doi: 10.1016/j.chom.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Schryvers AB, Stojiljkovic I. Iron acquisition systems in the pathogenic Neisseria. Mol Microbiol. 1999;32:1117–1123. doi: 10.1046/j.1365-2958.1999.01411.x. [DOI] [PubMed] [Google Scholar]
- 19.Shafer WM, Veal WL, Lee EH, Zarantonelli L, Balthazar JT, Rouquette C. Genetic organization and regulation of antimicrobial efflux systems possessed by Neisseria gonorrhoeae and Neisseria meningitidis. J Mol Microbiol Biotechnol. 2001;3:219–224. [PubMed] [Google Scholar]
- 20.Gill MJ, McQuillen DP, van Putten JP, et al. Functional characterization of a sialyltransferase-deficient mutant of Neisseria gonorrhoeae. Infect Immun. 1996;64:3374–3378. doi: 10.1128/iai.64.8.3374-3378.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massari P, Ram S, Macleod H, Wetzler LM. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 2003;11:87–93. doi: 10.1016/s0966-842x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 22.Shafer WM, Datta A, Kolli VS, et al. Phase variable c hanges in genes lgtA and lgtC within the lgtABCDE operon of Neisseria gonorrhoeae can modulate gonococcal susceptibility to normal human serum. J Endotoxin Res. 2002;8:47–58. [PubMed] [Google Scholar]
- 23.Criss AK, Seifert HS. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Microbiol. 2012;10:178–190. doi: 10.1038/nrmicro2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Packiam M, Veit SJ, erson DJ, Ingalls RR, Jerse AE. Mouse strain-dependent differences in susceptibility to Neisseria gonorrhoeae infection and induction of innate immune responses. Infect Immun. 2010;78:433–440. doi: 10.1128/IAI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramsey KH, Schneider H, Kuschner RA, Trofa AF, Cross AS, Deal CD. Inflammatory cytokine response to experimental human infection with Neisseria gonorrhoeae. Ann N Y Acad Sci. 1994;730:322–325. doi: 10.1111/j.1749-6632.1994.tb44280.x. [DOI] [PubMed] [Google Scholar]
- 26.Hedges SR, Sibley DA, Mayo MS, Hook EW, 3rd, Russell MW. Cytokine and antibody responses in women infected with Neisseria gonorrhoeae: effects of concomitant infections. J Infect Dis. 1998;178:742–751. doi: 10.1086/515372. [DOI] [PubMed] [Google Scholar]
- 27.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packiam M, Wu H, Veit SJ, Mavrogiorgos N, Jerse AE, Ingalls RR. Protective role of Toll-like receptor 4 in experimental gonococcal infection of female mice. Mucosal Immunol. 2012;5:19–29. doi: 10.1038/mi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vliet SJ, Garcia-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008;86:580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- 30.Fisette PL, Ram S, ersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 31.Singleton TE, Massari P, Wetzler LM. Neisserial porin-induced dendritic cell activation is MyD88 and TLR2 dependent. J Immunol. 2005;174:3545–3550. doi: 10.4049/jimmunol.174.6.3545. [DOI] [PubMed] [Google Scholar]
- 32.Zhu W, Ventevogel MS, Knilans KJ, et al. Neisseria gonorrhoeae suppresses dendritic cell-induced, antigen-dependent CD4 T cell proliferation. PLoS One. 2012;7:e41260. doi: 10.1371/journal.pone.0041260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duncan JA, Gao X, Huang MT, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedges SR, Mayo MS, Mestecky J, Hook EW, 3rd, Russell MW. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W, Chen CJ, Thomas CE, erson JE, Jerse AE, Sparling PF. Vaccines for gonorrhea: can we rise to the challenge? Front Microbiol. 2011;2:124. doi: 10.3389/fmicb.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imarai M, Candia E, Rodriguez-Tirado C, et al. Regulatory T cells are locally induced during intravaginal infection of mice with Neisseria gonorrhoeae. Infect Immun. 2008;76:5456–5465. doi: 10.1128/IAI.00552-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Islam EA, Jarvis GA, Gray-Owen SD, Russell MW. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-beta-dependent mechanisms. Mucosal Immunol. 2012;5:320–331. doi: 10.1038/mi.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardi MC, Starnino S, Teloni R, et al. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS Immunol Med Microbiol. 2011;61:129–132. doi: 10.1111/j.1574-695X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 39.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 40.Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, Chen T. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun. 2005;73:4171–4179. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youssef AR, van der Flier M, Estevao S, Hartwig NG, van der Ley P, Virji M. Opa+ and Opa− isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infect Immun. 2009;77:5170–5180. doi: 10.1128/IAI.00355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.So NS, Ostrowski MA, Gray-Owen SD. Vigorous response of human innate functioning IgM memory B cells upon infection by Neisseria gonorrhoeae. J Immunol. 2012;188:4008–4022. doi: 10.4049/jimmunol.1100718. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MS, Sparling PF. Mucosal infection with Neisseria gonorrhoeae. Bacterial adaptation and mucosal defenses. J Clin Invest. 1992;89:1699–1705. doi: 10.1172/JCI115770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Britigan BE, Cohen MS, Sparling PF. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985;312:1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- 45.Plummer FA, Simonsen JN, Chubb H, et al. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989;83:1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, Cohen MS. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 47.Rice PA, Vayo HE, Tam MR, Blake MS. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164:1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arko RJ. Animal models for pathogenic Neisseria species. Clin Microbiol Rev. 1989;2(Suppl):S56–S59. doi: 10.1128/cmr.2.suppl.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen MS, Cannon JG. Human experimentation with Neisseria gonorrhoeae: progress and goals. J Infect Dis. 1999;179(Suppl 2):S375–S379. doi: 10.1086/513847. [DOI] [PubMed] [Google Scholar]
- 50.Hobbs MM, Sparling PF, Cohen MS, Shafer WM, Deal CD, Jerse AE. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae Strains FA1090 and MS11mkC. Front Microbiol. 2011;2:123. doi: 10.3389/fmicb.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt KA, Schneider H, Lindstrom JA, et al. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis. 2001;28:555–564. doi: 10.1097/00007435-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Johnson AP, Tuffrey M, Taylor-Robinson D. Resistance of mice to genital infection with Neisseria gonorrhoeae. J Med Microbiol. 1989;30:33–36. doi: 10.1099/00222615-30-1-33. [DOI] [PubMed] [Google Scholar]
- 53.Jerse AE, Wu H, Packiam M, Vonck RA, Begum AA, Garvin LE. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Front Microbiol. 2011;2:107. doi: 10.3389/fmicb.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plante M, Jerse A, Hamel J, et al. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J Infect Dis. 2000;182:848–855. doi: 10.1086/315801. [DOI] [PubMed] [Google Scholar]
- 55.Blake M, Holmes KK, Swanson J. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhea. J Infect Dis. 1979;139:89–92. doi: 10.1093/infdis/139.1.89. [DOI] [PubMed] [Google Scholar]
- 56.Hedges SR, Mayo MS, Kallman L, Mestecky J, Hook EW, 3rd, Russell MW. Evaluation of immunoglobulin A1 (IgA1) protease and IgA1 protease-inhibitory activity in human female genital infection with Neisseria gonorrhoeae. Infect Immun. 1998;66:5826–5832. doi: 10.1128/iai.66.12.5826-5832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell MW, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–677. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 58.Ngampasutadol J, Tran C, Gulati S, et al. Species-specificity of Neisseria gonorrhoeae infection: do human complement regulators contribute? Vaccine. 2008;26(Suppl 8):I62–I66. doi: 10.1016/j.vaccine.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 59.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–769. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price GA, Masri HP, Hollander AM, Russell MW, Cornelissen CN. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine. 2007;25:7247–7260. doi: 10.1016/j.vaccine.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price GA, Russell MW, Cornelissen CN. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect Immun. 2005;73:3945–3953. doi: 10.1128/IAI.73.7.3945-3953.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boslego JW, Tramont EC, Chung RC, et al. Efficacy trial of a parenteral gonococcal pilus vaccine in men. Vaccine. 1991;9:154–162. doi: 10.1016/0264-410x(91)90147-x. [DOI] [PubMed] [Google Scholar]
- 64.Cornelissen CN, Kelley M, Hobbs MM, et al. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 65.Gulati S, McQuillen DP, Mandrell RE, Jani DB, Rice PA. Immunogenicity of Neisseria gonorrhoeae lipooligosaccharide epitope 2C7, widely expressed in vivo with no immunochemical similarity to human glycosphingolipids. J Infect Dis. 1996;174:1223–1237. doi: 10.1093/infdis/174.6.1223. [DOI] [PubMed] [Google Scholar]
- 66.Ngampasutadol J, Rice PA, Walsh MT, Gulati S. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine. 2006;24:157–170. doi: 10.1016/j.vaccine.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 67.Banerjee A, Wang R, Uljon SN, Rice PA, Gotschlich EC, Stein DC. Identification of the gene (lgtG) encoding the lipooligosaccharide beta chain synthesizing glucosyl transferase from Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1998;95:10872–10877. doi: 10.1073/pnas.95.18.10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulanger MJ, Murphy ME. Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: a new class of copper-containing nitrite reductases. J Mol Biol. 2002;315:1111–1127. doi: 10.1006/jmbi.2001.5251. [DOI] [PubMed] [Google Scholar]
- 69.Shewell LK, Ku SC, Schulz BL, et al. Recombinant truncated AniA of pathogenic Neisseria elicits a non-native immune response and functional blocking antibodies. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2012.12.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark VL, Knapp JS, Thompson S, Klimpel KW. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb Pathog. 1988;5:381–390. doi: 10.1016/0882-4010(88)90038-1. [DOI] [PubMed] [Google Scholar]
- 71.Falsetta ML, Steichen CT, McEwan AG, et al. The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front Microbiol. 2011;2:75. doi: 10.3389/fmicb.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keiser PB, Biggs-Cicatelli S, Moran EE, et al. A phase 1 study of a meningococcal native outer membrane vesicle vaccine made from a group B strain with deleted lpxL1 and synX, over-expressed factor H binding protein, two PorAs and stabilized OpcA expression. Vaccine. 2011;29:1413–1420. doi: 10.1016/j.vaccine.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 73.Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zeal, and Neisseria meningitidis serogroup B disease epidemic strain. Vaccine. 2005;23:2191–2196. doi: 10.1016/j.vaccine.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 74.Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2):B87–B97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hadad R, Jacobsson S, Pizza M, et al. Novel meningococcal 4CMenB vaccine antigens - prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. APMIS. 2012;120:750–760. doi: 10.1111/j.1600-0463.2012.02903.x. [DOI] [PubMed] [Google Scholar]
- 76.Gallichan WS, Rosenthal KL. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 77.Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu HY, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunization. Infect Immun. 2000;68:5539–5545. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001;69:7481–7486. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Hajishengallis G, Connell TD. Type II heat-labile enterotoxins: Structure, function, and immunomodulatory properties. Vet Immunol Immunopathol. 2012 doi: 10.1016/j.vetimm.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hajishengallis G, Connell TD. Type II heat-labile enterotoxins: structure, function, and immunomodulatory properties. Vet Immunol Immunopathol. 2013;152:68–77. doi: 10.1016/j.vetimm.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu HY, Russell MW. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 86.Metzger DW. IL-12 as an adjuvant for the enhancement of protective humoral immunity. Expert Rev Vaccines. 2009;8:515–518. doi: 10.1586/erv.09.13. [DOI] [PubMed] [Google Scholar]
- 87.Thompson AL, Staats HF. Cytokines: the future of intranasal vaccine adjuvants. Clin Dev Immunol. 2011;2011:289597. doi: 10.1155/2011/289597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rahman M, Kallstrom H, Normark S, Jonsson AB. PilC of pathogenic Neisseria is associated with the bacterial cell surface. Mol Microbiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 90.Rudel T, Scheurerpflug I, Meyer TF. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature. 1995;373:357–359. doi: 10.1038/373357a0. [DOI] [PubMed] [Google Scholar]
- 91.Haghi F, Peerayeh SN, Siadat SD, Zeighami H. Recombinant outer membrane secretin PilQ(406–770) as a vaccine candidate for serogroup B Neisseria meningitidis. Vaccine. 2012;30:1710–1714. doi: 10.1016/j.vaccine.2011.12.076. [DOI] [PubMed] [Google Scholar]
- 92.Callaghan MJ, Lewis S, Sadarangani M, et al. Potential of recombinant Opa proteins as vaccine candidates against hyperinvasive meningococci. Infect Immun. 2011;79:2810–2818. doi: 10.1128/IAI.01338-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Jonge MI, Hamstra HJ, Jiskoot W, et al. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine. 2004;22:4021–4028. doi: 10.1016/j.vaccine.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 94.Cole JG, Jerse AE. Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops. PLoS One. 2009;4:e8108. doi: 10.1371/journal.pone.0008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cornelissen CN, Hollander A. TonB-dependent transporters expressed by Neisseria gonorrhoeae. Front Microbiol. 2011;2:117. doi: 10.3389/fmicb.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stork M, Bos MP, Jongerius I, et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 2010;6:e1000969. doi: 10.1371/journal.ppat.1000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shell DM, Chiles L, Judd RC, Seal S, Rest RF. The Neisseria lipooligosaccharide-specific alpha-2,3-sialyltransferase is a surface-exposed outer membrane protein. Infect Immun. 2002;70:3744–3751. doi: 10.1128/IAI.70.7.3744-3751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Serino L, Nesta B, Leuzzi R, et al. Identification of a new OmpA-like protein in Neisseria gonorrhoeae involved in the binding to human epithelial cells and in vivo colonization. Mol Microbiol. 2007;64:1391–1403. doi: 10.1111/j.1365-2958.2007.05745.x. [DOI] [PubMed] [Google Scholar]
- 99.Zhu P, Klutch MJ, Derrick JP, Prince SM, Tsang RS, Tsai CM. Identification of opcA gene in Neisseria polysaccharea: interspecies diversity of Opc protein family. Gene. 2003;307:31–40. doi: 10.1016/s0378-1119(02)01208-8. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y, Russell MW. Diversion of the immune response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by treatment with anti-transforming growth factor beta antibody generates immunological memory and protective immunity. MBio. 2011;2:e00095-11. doi: 10.1128/mBio.00095-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol. 2011;2:52. doi: 10.3389/fmicb.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koch ML. A study of cervical cultures taken in cases of acute gonorrhea with special reference to the phases of the menstrual cycle. Am. J. Obstet. Gyn. 1947;54:861–866. doi: 10.1016/s0002-9378(16)39663-6. [DOI] [PubMed] [Google Scholar]
- 103.McCormack WM, Reynolds GH. Effect of menstrual cycle and method of contraception on recovery of Neisseria gonorrhoeae. Jama. 1982;247:1292–1294. [PubMed] [Google Scholar]
- 104.Cole JG, Fulcher NB, Jerse AE. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect Immun. 78:1629–1641. doi: 10.1128/IAI.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Edwards JL. Neisseria gonorrhoeae survival during primary human cervical epithelial cell infection requires nitric oxide and is augmented by progesterone. Infect Immun. 2010;78:1202–1213. doi: 10.1128/IAI.01085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis. 2003;36:663–668. doi: 10.1086/367658. [DOI] [PubMed] [Google Scholar]
- 107.Muench DF, Kuch DJ, Wu H, et al. Hydrogen peroxide-producing lactobacilli inhibit gonococci in vitro but not during experimental genital tract infection. J Infect Dis. 2009;199:1369–1378. doi: 10.1086/597390. [DOI] [PMC free article] [PubMed] [Google Scholar]