Abstract
Candida albicans is the most common fungal pathogen in humans, and most diseases produced by C. albicans are associated with biofilms. Previously, we developed nylon-3 polymers with potent activity against planktonic C. albicans and excellent C. albicans vs. mammalian cell selectivity. Here, we show that these nylon-3 polymers have strong and selective activity against drug-resistant C. albicans in biofilms, as manifested by inhibition of biofilm formation and by killing of C. albicans in mature biofilms. The best nylon-3 polymer (poly-βNM) is superior to the antifungal drug fluconazole for all three strains examined. This polymer is slightly less effective than amphotericin B (AmpB) for two strains, but the polymer is superior against an AmpB-resistant strain.
Fungal infections represent a major problem in human health care.1,2 C. albicans is the most common fungal pathogen, causing invasive infections that are associated with high mortality.1 Treatment of C. albicans infections is imperfect because current drugs have significant side effects, and resistance is developing for these drugs, including fluconazole and amphotericin B (AmpB).3,4 The majority of C. albicans infections are associated with biofilms.5 Development of strategies to attack biofilms formed by drug-resistant C. albicans, while limiting toxicity to the human host, is challenging because both organisms are eukaryotes.
Host-defense peptides have been widely explored for antimicrobial properties,6-8 as have synthetic peptide analogues and unnatural, sequence-specific oligomers intended to mimic host-defense peptides,9-18 but relatively few of these compounds have been evaluated for activity toward fungal biofilms.19 Histatin 5, a natural peptide from saliva, is active against planktonic C. albicans,20 i.e., free-floating cells, and this peptide can inhibit biofilm formation,21 but the effect of histatin 5 on mature biofilms has not be reported. ApoEdpLW, an 18-mer derived from human apolipoprotein E, inhibits Candida biofilm formation but has relatively little effect on mature biofilms.22 β-Amino acid oligomers developed by our group inhibit growth of planktonic C. albicans, and they inhibit biofilm formation by C. albicans; however, these β-peptides show little activity toward mature biofilms, and they are relatively toxic toward mammalian cells, as manifested by their hemolytic activity.12
The high cost of chemical synthesis for peptides or unnatural, sequence-specific oligomers such as β-peptides has inspired many groups to evaluate the antimicrobial actions of synthetic polymers. Antibacterial activity has been reported for a number of polymers,23-38 but antifungal activity has been documented in only a few cases. Peptidopolysaccharides active against planktonic forms of C. albicans have been described.32 Polyester-polycarbonate block copolymers that form hydrogels at high concentrations (20 mg/ml) are moderately active against fungal biofilms.39 Recently, we have described nylon-3 polymers that display potent and selective activity against planktonic forms of multiple fungal species.40,41 The best antifungal nylon-3 polymers contain the cationic subunit βNM and the hydrophobic subunit CH in varying proportions (Figure 1), and display minimum inhibitory concentration (MIC) = minimum fungicidal concentration (MFC) = 3 μg/mL for the planktonic form of the pathogenic K1 strain of C. albicans.40 Here we show that such polymers can inhibit C. albicans biofilm formation and kill C. albicans in mature biofilms.
Figure 1.
Structures of nylon-3 βNM:CH copolymers (left) and βNM homopolymers (right). R can be the sidechain of either subunit for βNM:CH copolymers. All of the polymers used in this work have an average ∼20-mer length, except for a long version of the βNM homopolymer (∼105-mer). All polymers are heterochiral.
Three strains of C. albicans were examined in this study: clinical isolate K1;42 clinical isolate Gu5, which is resistant to fluconazole;43 and lab strain E4, which is resistant to both fluconazole and AmpB.44 The activity of nylon-3 polymers was evaluated in terms of the minimum concentration necessary to inhibit 80% biofilm formation (“sessile minimum inhibitory concentration,” SMIC80).45 These values were determined based by measuring cell viability within biofilms via an XTT assay.46 The antifungal drugs fluconazole and AmpB were used as controls. Mammalian cell toxicity was assessed in terms of human red blood cell lysis (“hemolysis”), specifically, the minimum concentration necessary to cause 10% hemolysis (HC10).
Nylon-3 polymers were initially evaluated for inhibition of C. albicans biofilm formation (Table 1). In this study, C. albicans cells were combined with varying concentrations of polymer and then incubated 48 hours to allow biofilm formation. Each of the nylon-3 polymers could inhibit biofilm formation by all three strains of C. albicans, although efficacies varied, and SMIC80 values ranged from 9.4 to 75 μg/mL (Table 1). Polymers with a higher content of the cationic subunit (βNM) displayed stronger inhibitory activity (lower SMIC80 values). The longest homopolymer, (βNM)105, proved to be the most active, with SMIC values of 9.4, 9.4 and 12.5 μg/mL toward K1, Gu5 and E4 strains, respectively. For most of the polymers, no hemolysis could be detected at the maximum concentration evaluated (2000 μg/mL); the most hydrophobic polymer, 70:30 βNM:CH, displayed limited hemolysis (∼9%) at this concentration (Figure 2). Therefore, all of the nylon-3 polymers examined here demonstrate excellent selectivity in terms of their activity against the three C. albicans strains examined. The behavior of AmpB was inferior by some measures. This drug is very effective at inhibiting biofilm formation by the K1 and Gu5 strains (SMIC80 = 2.4 and 0.8 μg/mL respectively), but shows much lower activity for the E4 strain (SMIC80 > 100 μg/mL). In addition, AmpB is highly hemolytic, in sharp contrast to the nylon-3 polymers (Figure 2). All three strains of C. albicans are highly resistant to fluconazole, and the nylon-3 polymers were superior to this drug in each case.
Table 1.
The inhibitory effect of nylon-3 polymers and antifungal drugs against C. albicans biofilm formationa
| polymer | SMIC80, μg/ml | ||
|---|---|---|---|
| K1 | Gu5 | E4 | |
| 70:30 βNM:CH | 37.5 | 37.5 | 75 |
| 80:20 βNM:CH | 18.8 | 18.8 | 37.5 |
| 90:10 βNM:CH | 12.5 | 9.4 | 18.8 |
| βNM | 18.8 | 18.8 | 18.8 |
| (βNM)105 | 9.4 | 9.4 | 12.5 |
| AmpB | 2.4 | 0.8 | >100 |
| fluconazole | >500 | >500 | 375 |
SMIC80 is the concentration to inhibit 80% biofilm formation, as measured by assessing biofilm viability with an XTT assay. All nylon-3 polymers are of ∼20 mer length except (βNM)105, which is of ∼105 mer length.
Figure 2.

Dose-dependent hemolysis upon treatment with (a) nylon-3 polymers or (b) AmpB. Note the difference in horizontal scale between (a) and (b).
In the next set of experiments we asked whether the nylon-3 polymers could kill C. albicans cells in pre-established biofilms (Table 2). This type of activity is known to be more difficult to achieve than inhibiting growth of planktonic cells or inhibiting biofilm formation because an established biofilm presents physical barriers to permeation by antifungal agents, and the sessile cells within a biofilm are physiologically distinct from planktonic cells.5 Biofilms were allowed to form for 24 or 48 hours and then treated with polymers or antifungal drugs for another 48 hours, at which point the viability of C. albicans within the biofilm was assessed. In contrast to the biofilm formation assay results, where significant differences were observed among nylon-3 polymers, these polymers were similar to one another in terms of activity against 48-hour biofilms. For the most hydrophobic polymer, 70:30 βNM:CH, activity in this assay was comparable to the activity observed for inhibition of biofilm formation; however, for the polymers with greater cationic charge density, activity against 48-hour biofilm was somewhat lower (higher SMIC80 values) than inhibitory activity toward biofilm formation. Biofilms often contain polyanionic constituents, such as DNA,47 which may hinder entry by the polycationic nylon-3 chains. A polymer that is more hydrophobic than 70:30 βNM:CH (40% CH) displays similar or lower activity toward mature C. albicans biofilms and significantly lower activity in terms of inhibiting biofilm formation (Table S2), which is consistent with the trend in nylon-3 activities toward planktonic C. albicans.40
Table 2.
The inhibitory effect of nylon-3 polymers and antifungal drugs against 48 hr mature C. albicans biofilmsa
| polymer | SMIC80 | ||
|---|---|---|---|
| K1 | Gu5 | E4 | |
| 70:30 βNM:CH | 37.5 | 50 | 75 |
| 80:20 βNM:CH | 37.5 | 75 | 50 |
| 90:10 βNM:CH | 37.5 | 75 | 37.5 |
| βNM | 37.5 | 75 | 37.5 |
| (βNM)105 | 50 | 75 | 37.5 |
| AmpB | 6.3 | 6.3 | >200 |
| fluconazole | >1000 | >1000 | >1000 |
SMIC80 is the concentration to inhibit 80% biofilm growth, as measured by biofilm viability using an XTT assay. All nylon-3 polymers are of ∼20 mer length except (βNM)105 which is of ∼105 mer length. See Table S1 for results with 24 hr fungal biofilms.
AmpB is more active than any polymer against 48-hour biofilms formed by susceptible K1 or Gu5 strains; however, the polymers have a clear advantage against biofilms formed by the AmpB-resistant E4 strain. Biofilms from all three strains are unaffected by fluconazole. Comparison of SMIC80 values for polymers and AmpB against susceptible strains measured for 24-hour (see Figure S1) vs. 48-hour biofilms reveals a modest but interesting trend: the polymers seem to be slightly more active at the later time point. In contrast, AmpB seems to be slightly less active at the later time. The origin of this behavior is unclear.
We conducted live-dead staining followed by fluorescence microscopy to assess the impact of selected agents on K1 C. albicans cells within pre-formed biofilms (Figure 3). Mature C. albicans biofilms (48-hour) were treated with (βNM)105, AmpB or fluconazole; for the polymer and AmpB, the concentrations used for these studies correspond to the SMIC80 (50 and 6.3 μg/ml respectively), while for fluconazole the concentration was 1000 μg/ml). After treatment of the biofilm with each agent for 48 hours, the biofilms were incubated with SYTO 9 and propidium iodide to stain live cells (green fluorescence) and dead cells (red fluorescence), respectively. Biofilms treated with (βNM)105 or AmpB showed a high density of dead cells, but fluconazole-treated biofilm was similar to the control (no antifungal agent), with many cells evident, very few of which were dead. Biofilms treated with other nylon-3 polymers described here gave results qualitatively similar to those for (βNM)105 (data not shown). These imaging results are consistent with biofilm viability assays (Table 2) in indicating that fungicidal nylon-3 polymers can enter mature C. albicans biofilms and kill cells residing within.
Figure 3.
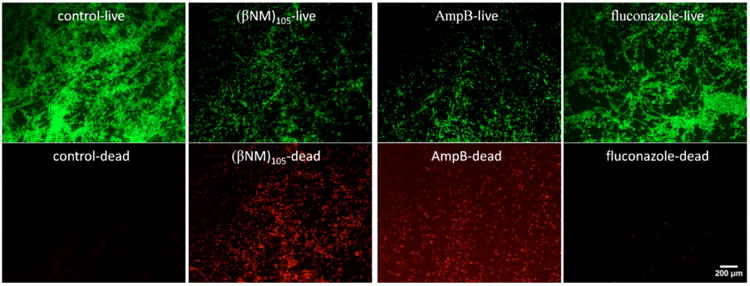
Fluorescence micrographs mature K1 C. albicans biofilms (48 hr) treated with antifungal agents and then subjected to Live-Dead staining. Biofilms were treated with antifungal agents at a concentration corresponding to the SMIC80 for (βNM)105 and AmpB (50 and 6.3 μg/ml respectively), and at 1000 μg/ml for fluconazole. Untreated biofilms were used as a control. Scale bar is 200 μm.
We have shown that nylon-3 polymers containing the βNM subunit can block biofilm formation by C. albicans and target cells within a mature biofilm. This activity is manifested even against strains that are resistant to the antifungal drugs fluconazole and/or AmpB. The activity observed against C. albicans resident in existing biofilms is noteworthy because such cells typically display features that dampen drug efficacy, such as upregulation of efflux pumps in the outer membrane.48 These observations suggest that the nylon-3 polymers discussed here may be useful for blocking fungal biofilm formation on biomedical device surfaces or for disinfecting those surfaces after infection has become established. The large-scale preparative accessibility of the nylon-3 polymers make them attractive for surface-modification applications.
Supplementary Material
Acknowledgments
This research was supported by the NIH (R01GM093265).
Footnotes
Associated Content: Supporting Information: Bioassay protocols and results. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J. Value in Health. 2002;5:26. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 3.Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, Schumacher U, Einsele H. Febs Letters. 1997;400:80. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 4.Khan MS, Malik A, Ahmad I. Med Mycol. 2012;50:33. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
- 5.Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Giannini MJSM. J Med Microbiol. 2013;62:10. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff M. Nature. 2002;415:389. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 7.Yeaman MR, Yount NY. Pharmacological Reviews. 2003;55:27. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 8.Hancock REW, Sahl HG. Nature Biotechnology. 2006;24:1551. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 9.Hamuro Y, Schneider JP, DeGrado WF. J Am Chem Soc. 1999;121:12200. [Google Scholar]
- 10.Porter EA, Wang XF, Lee HS, Weisblum B, Gellman SH. Nature. 2000;404:565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]
- 11.Niu YH, Padhee S, Wu HF, Bai G, Harrington L, Burda WN, Shaw LN, Cao CH, Cai JF. Chem Commun. 2011;47:12197. doi: 10.1039/c1cc14476f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson AJ, Pomerantz WC, Neilsen KJ, Gellman SH, Palecek SP. Acs Chem Biol. 2009;4:567. doi: 10.1021/cb900093r. [DOI] [PubMed] [Google Scholar]
- 13.Kuriakose J, Hernandez-Gordillo V, Nepal M, Brezden A, Pozzi V, Seleem MN, Chmielewski J. Angew Chem Int Edit. 2013;52:9664. doi: 10.1002/anie.201302693. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson AJ, Pomerantz WC, Weisblum B, Gellman SH, Palecek SP. J Am Chem Soc. 2006;128:12630. doi: 10.1021/ja064630y. [DOI] [PubMed] [Google Scholar]
- 15.Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:4761. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papo N, Shai Y. Biochemistry. 2004;43:6393. doi: 10.1021/bi049944h. [DOI] [PubMed] [Google Scholar]
- 17.Patch JA, Barron AE. J Am Chem Soc. 2003;125:12092. doi: 10.1021/ja037320d. [DOI] [PubMed] [Google Scholar]
- 18.Olsen CA, Bonke G, Vedel L, Adsersen A, Witt M, Franzyk H, Jaroszewski JW. Organic Letters. 2007;9:1549. doi: 10.1021/ol070316c. [DOI] [PubMed] [Google Scholar]
- 19.van der Weerden NL, Bleackley MR, Anderson MA. Cell Mol Life Sci. 2013;70:3545. doi: 10.1007/s00018-013-1260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oppenheim FG, Xu T, Mcmillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. J Biol Chem. 1988;263:7472. [PubMed] [Google Scholar]
- 21.Pusateri CR, Monaco EA, Edgerton M. Arch Oral Biol. 2009;54:588. doi: 10.1016/j.archoralbio.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol T, Kelly B, Dobson C, d'Enfert C. Antimicrob Agents Chemother. 2011;55:4670. doi: 10.1128/AAC.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelman MA, Weisblum B, Lynn DM, Gellman SH. Organic Letters. 2004;6:557. doi: 10.1021/ol036341+. [DOI] [PubMed] [Google Scholar]
- 24.Lienkamp K, Madkour AE, Musante A, Nelson CF, Nusslein K, Tew GN. J Am Chem Soc. 2008;130:9836. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang YJ, Yang X, Zhu R, Hu K, Lan WW, Wu F, Yang LH. Macromolecules. 2013;46:3959. [Google Scholar]
- 26.Kuroda K, DeGrado WF. J Am Chem Soc. 2005;127:4128. doi: 10.1021/ja044205+. [DOI] [PubMed] [Google Scholar]
- 27.Palermo EF, Sovadinova I, Kuroda K. Biomacromolecules. 2009;10:3098. doi: 10.1021/bm900784x. [DOI] [PubMed] [Google Scholar]
- 28.Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. J Am Chem Soc. 2007;129:15474. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]
- 29.Song AR, Walker SG, Parker KA, Sampson NS. Acs Chem Biol. 2011;6:590. doi: 10.1021/cb100413w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambhy V, Peterson BR, Sen A. Angew Chem Int Edit. 2008;47:1250. doi: 10.1002/anie.200702287. [DOI] [PubMed] [Google Scholar]
- 31.Sellenet PH, Allison B, Applegate BM, Youngblood JP. Biomacromolecules. 2007;8:19. doi: 10.1021/bm0605513. [DOI] [PubMed] [Google Scholar]
- 32.Li P, Zhou C, Rayatpisheh S, Ye K, Poon YF, Hammond PT, Duan HW, Chan-Park MB. Advanced Materials. 2012;24:4130. doi: 10.1002/adma.201104186. [DOI] [PubMed] [Google Scholar]
- 33.Nederberg F, Zhang Y, Tan JPK, Xu KJ, Wang HY, Yang C, Gao SJ, Guo XD, Fukushima K, Li LJ, Hedrick JL, Yang YY. Nature Chemistry. 2011;3:409. doi: 10.1038/nchem.1012. [DOI] [PubMed] [Google Scholar]
- 34.Costanza F, Padhee S, Wu HF, Wang Y, Revenis J, Cao CH, Li Q, Cai JF. Rsc Adv. 2014;4:2089. [Google Scholar]
- 35.Liu R, Chen X, Chakraborty S, Lemke JJ, Hayouka Z, Chow C, Welch RA, Weisblum B, Masters KS, Gellman SH. J Am Chem Soc. 2014;136:4410. doi: 10.1021/ja500367u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Suarez JM, Weisblum B, Gellman SH, McBride SM. J Am Chem Soc. 2014;136:14498. doi: 10.1021/ja506798e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakraborty S, Liu R, Hayouka Z, Chen X, Ehrhardt J, Lu Q, Burke E, Yang Y, Weisblum B, Wong GC, Masters KS, Gellman SH. J Am Chem Soc. 2014;136:14530. doi: 10.1021/ja507576a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dane EL, Ballok AE, O'Toole GA, Grinstaff MW. Chem Sci. 2014;5:551. doi: 10.1039/C3SC52777H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Fukushima K, Coady DJ, Engler AC, Liu SQ, Huang Y, Cho JS, Guo Y, Miller LS, Tan JPK, Ee PLR, Fan WM, Yang YY, Hedrick JL. Angew Chem Int Edit. 2013;52:674. doi: 10.1002/anie.201206053. [DOI] [PubMed] [Google Scholar]
- 40.Liu RH, Chen XY, Hayouka Z, Chakraborty S, Falk SP, Weisblum B, Masters KS, Gellman SH. J Am Chem Soc. 2013;135:5270. doi: 10.1021/ja4006404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu R, Chen X, Falk SP, Mowery BP, Karlsson AJ, Weisblum B, Palecek SP, Masters KS, Gellman SH. J Am Chem Soc. 2014;136:4333. doi: 10.1021/ja500036r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andes D, Lepak A, Nett J, Lincoln L, Marchillo K. Antimicrob Agents Chemother. 2006;50:2384. doi: 10.1128/AAC.01305-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franz R, Ruhnke M, Morschhauser J. Mycoses. 1999;42:453. doi: 10.1046/j.1439-0507.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 44.Pierce AM, Pierce HD, Unrau AM, Oehlschlager AC. Canadian Journal of Biochemistry. 1978;56:135. doi: 10.1139/o78-023. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar S, Uppuluri P, Pierce CG, Lopez-Ribot JL. Antimicrob Agents Chemother. 2014;58:1183. doi: 10.1128/AAC.01745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nett JE, Cain MT, Crawford K, Andes DR. J Clin Microbiol. 2011;49:1426. doi: 10.1128/JCM.02273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martins M, Uppuluri P, Thomas DP, Cleary IA, Henriques M, Lopez-Ribot JL, Oliveira R. Mycopathologia. 2010;169:323. doi: 10.1007/s11046-009-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee PK, Chandra J, Kuhn DA, Ghannoum MA. Infect Immun. 2003;71:4333. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



