Abstract
The genome of Tannerella forsythia, an etiologic factor of chronic periodontitis, contains several genes encoding putative proteases. Here, we characterized a subtilisin-like serine protease of T. forsythia referred to as mirolase. Recombinant full-length latent promirolase (85 kDa, without its signal peptide) processed itself through sequential autoproteolytic cleavages into a mature enzyme of 40 kDa. Mirolase latency was driven by the N-terminal prodomain (NTP). In stark contrast to almost all known subtilases, the cleaved NTP remained non-covalently associated with mirolase, inhibiting its proteolytic, but not amidolytic, activity. Full activity was observed only after the NTP was gradually, and fully, degraded. Both activity and processing was absolutely dependent on calcium ions, which were also essential for enzyme stability. As a consequence, both serine protease inhibitors and calcium ions chelators inhibited mirolase activity. Activity assays using an array of chromogenic substrates revealed that mirolase specificity is driven not only by the substrate-binding subsite S1, but also by other subsites. Taken together mirolase is a calcium-dependent serine protease of the S8 family with the unique mechanism of activation that may contribute to T. forsythia pathogenicity by degradation of fibrinogen, hemoglobin and the antimicrobial peptide LL-37.
Keywords: pathogenicity, periodontitis, protease specificity, protein expression, serine protease, virulence factor
Introduction
Peptidases (proteases) are enzymes that hydrolyze peptide bonds. Genes encoding peptidases constitute approximately 3% of all genes in prokaryotes. However, the number of peptidase genes in individual prokaryotic genomes differs greatly, ranging from very few in species of bacteria in the genus Mycoplasma to up to 179 genes encoding potential proteolytic enzymes in Bacillus cereus (Potempa and Pike, 2005). Due to the wide distribution and variety of enzymes, it is not surprising that bacterial peptidases play a key role in processes such as degradation of unwanted proteins, proteolytic modification of proteins and their precursors (e.g. removal of signal peptides), hydrolysis of extracellular polypeptides to generate nutrients, and regulation of gene expression through the limited proteolysis of regulatory proteins (Rao et al., 1998; Gupta et al., 2002; Koziel and Potempa, 2013).
Many bacterial proteases can be considered virulence factors because they can degrade the proteinaceous constituencies of infected tissues, facilitate host colonization and spreading of bacteria within organisms, or protect pathogens against the immune system response (O-Brien-Simpson et al., 2003; Ingmer et al., 2009; Koziel and Potempa, 2013; Jusko et al., 2014; Palm et al., 2014). Moreover, prokaryotic proteases are also required for the production of other virulence factors (e.g., peptidases are components of secretion systems) and thus indirectly contribute to pathogen virulence (Tuteja, 2005; Glew et al., 2012).
One example of a disease in which bacterial proteases play a crucial role is periodontitis, an infectious disease affecting the human oral cavity, leading to the destruction of supportive tissues of the teeth. It is estimated that up to 15% of adults suffer from severe forms of periodontitis (Fox, 1992; Hugoson et al., 2008). Progression of the disease is manifested by alveolar bone resorption and loss of attachment between teeth and gums, resulting in the formation of deep periodontal pockets. In severe cases, the disease can lead to loss of teeth (Armitage, 2004). In addition, due to its infectious and inflammatory character, periodontitis is considered a significant factor in the development and/or progression of more serious diseases, such as lung disease (Garcia et al., 2001), low-birth weight preterm births (Offenbacher et al., 1996), atherosclerosis (Behle and Papapanou, 2006), diabetes (Pihlstrom and Michalowicz, 2005) and rheumatoid arthritis (Maresz et al., 2013).
The etiology of periodontitis is multifactorial, and as a result, it is impossible to identify a single pathogen that is responsible for development of the disease (Hajishengallis and Lamont, 2012; Wright et al., 2013). The development of periodontitis can start when subgingival biofilm is colonized by three species of bacteria: Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia (previously Bacteroides forsythus and Tannerella forsythensis), referred to as the “red complex”. Due to the striking correlation between the presence of these bacteria and the severity of symptoms, members of the “red complex” are considered major periodontopathogens (Socransky et al., 1998). All three of these bacterial strains possess one important common feature, strong extracellular proteolytic activity, which is thought to be the crucial virulence factor of these pathogens (Holt and Ebersole, 2005). Hydrolysis of the synthetic trypsin substrate benzoyl-DL-arginine-naphthylamide (BANA) is used to detect “red complex” bacteria (Loesche et al., 1992).
The role of secretory proteases of P. gingivalis and T. denticola as virulence factors is well-described (Guo et al., 2010; Ishihara, 2010). The gingipains Rgps and Kgp, which are responsible for 85% of the extracellular proteolytic activity of P. gingivalis (Potempa et al., 1997), are involved in processes such as nutrient acquisition, corruption of the innate immune system and immunomodulation (Potempa et al., 2003; Potempa and Pike, 2009). In the case of T. denticola, dentilisin is the best-characterized secretory protease and has been shown to play a role in the etiology of periodontitis. This serine protease is associated with the surface of T. denticola and is responsible for coaggregation with P. gingivalis, activation of the complement system and hydrolysis of IL-6, IL-8, IgG, IgA, fibronectin, laminin and type IV collagen (Ishihara, 2010).
In stark contrast to the other two members of the “red complex”, our knowledge of the functions of T. forsythia proteases is still limited. Thus far, only two proteases in T. forsythia, PrtH (a peptidase with hemolytic activity) (Saito et al., 1997) and karilysin (Karim et al., 2010), have been identified and characterized. PrtH, a 41-kDa cysteine peptidase, was identified as a T. forsythia detachment factor and is proposed to be responsible for the disintegration of human epithelium (Tomi et al., 2008). Compared to PrtH, our knowledge of the metallopeptidase karilysin is more detailed. It is synthesized as a 472-amino acid, multidomain protein composed of a signal peptide (SP), a 14-amino acid propeptide, an 18-kDa catalytic domain (CD) similar to animal matrix metalloproteinases and a C-terminal extension (CTE) (Cerdà-Costa et al., 2011). Karilysin was shown to degrade LL-37 and inhibit activation of all pathways of the complement system (Koziel et al., 2010; Jusko et al., 2012). Thus, karilysin could be involved in the evasion of host innate immune system by T. forsythia.
Bacterial secretory proteases are likely to be promising targets for the treatment of periodontitis (Ingar and Potempa, 2014). However, the multietiological characteristics of the disease and redundant functions of the proteolytic enzymes produced by different periodontal pathogens suggest that the inhibition of proteases from only one periodontopathogen may be insufficient for effective treatment and/or prevention of periodontitis. Thus, it is crucial to characterize all of the proteases secreted by “red complex” bacteria. Here, we describe the characterization of a novel serine protease of T. forsythia, comprised of a CD flanked by N- and C-terminal domains, which may contribute to the pathogenesis of periodontitis.
Results
Loci bfor_c_1_10621 and 10622 constitute a single ORF encoding a serine protease
Recently, we detected several errors in the sequence of an ORF (bfor_c_1_10593) encoding a matrix metalloproteinase-like enzyme (karilysin) in the sequenced genome of T. forsythia (www.brop.org) (Karim et al., 2010). In addition to karilysin and several other presumed proteases, this genome contains two adjacent ORFs (bfor_c_1_10622 and bfor_c_1_10621) coding for putative serine proteases. Sequencing of these loci together with the surrounding regions revealed several errors, including insertion and deletion affecting the reading frame and substitutions that resulted in altered codon identity. When the sequence was corrected for insertion of 186 and deletion of 8 bp, it formed a single ORF encoding a novel subtilisin-like serine protease (Figure 1AB). We designated this enzyme mirolase.
Figure 1. Resequencing of the mirolase gene (loci bfor_c_1_10621 and 10622).
(A) The insertion and deletion of 186 bp and 8 bp, respectively, in the database sequence resulted in a putative protein with a truncated N-terminus (highlighted) and missing signal peptide sequence (underlined). (B) Alignment of the translated amino acid sequence of the mirolase gene directly from the database (original) and after corrections (corrected). The catalytic triad of mirolase, Asp, His and Ser, are boxed, and regions conserved in other proteases of T. forsythia are underlined and shown in red and blue font. (C) Predicted multidomain structure of mirolase.
The corrected mirolase sequence (Genbank Accession No. KM504523) was compared against protein sequences in the Uniprot database (NCBI blast program http://blast.ncbi.nlm.nih.gov/Blast.cgi). This analysis revealed a domain structure encompassing a SP, a unique N-terminal prodomain (NTP), a CD with significant similarity to peptidases from family S8 serine proteases, and a CTE ending with a C-terminal domain (CTD) bearing a high degree of sequence identity to the CTD of karilysin (Figure 1BC). Of note, the significant level of identity of the CD of mirolase to other known members of the S8 protease family was limited to sequential motifs around residues of the catalytic triad: Asp231, His283 and Ser477 (highest score, 147 bits, E = 2e-35, against a calcium-dependent protease from Anabaena variabilis ATCC 29413, sequence ID: sp|P23916.3|PRCA_ANAVT).
Mirolase is expressed as an 85-kDa zymogen that processes itself to an active protease
Conservation of the catalytic residues in mirolase strongly suggested that the protein would have proteolytic activity. To verify this and elucidate the role of the unique sequences flanking the CD, we cloned the coding sequence of mirolase (without a predicted signal peptide) into the pGex-6P-1 vector and expressed it as a fusion protein with an N-terminal GST tag in both its native form and as a catalytically inactive mutant in which the catalytic residue Ser477 was substituted by Ala. Purification of the recombinant proteins by affinity chromatography on glutathione-Sepharose, followed by several different chromatography steps, including gel filtration, failed to yield homogenous protein (Figure 2A). This was likely due to the propensity of the protein to form soluble aggregates with E. coli proteins and, in the case of the active form, to undergo autoproteolysis (see below). Furthermore, in contrast to the catalytic Ser477Ala mutant, very little native protein with the expected molecular mass of 85-kDa was obtained in the first step of purification (Figure 2A, lane 4). This band was confirmed by N-terminal sequencing analysis to be full-length mirolase with all identified domains (NTP, CD, preCTD and CTD). Despite the heterogeneous nature of the obtained samples, we were able to use them to confirm the proteolytic activity of mirolase. Mirolase was incubated for up to 168 h, and aliquots taken at different time points were subjected to SDS-PAGE (Figure 2B), zymography on casein (Figure 2C) and gelatin (Figure 2D) as substrates, active-site labeling and, finally, analysis of the activity released over time as quantified by hydrolysis of Suc-AAPF-pNA and azocoll (Figure 2F). As shown in Figure 2C, massive autodegradation/processing occurred during incubation, leading to the accumulation of three discrete proteolysis-resistant fragments of mirolase. At 48 h, 60- and 40-kDa bands were identified by Edman degradation as mirolase-derived peptides truncated at Gln183 in the N-terminal portion of the full-length protein, while a third band (20-kDa) constituted the preCTD domain (Figure 1C). Mass spectroscopy analysis confirmed that the CTD was not present in both 20-kDa (preCTD) and 60-kDa (Mirolase 60-kDa) bands (Figure 2H).
Figure 2. Expression, purification and proteolytic processing of mirolase.
Expression and purification of (A) full-length mirolase and (B) full-length mirolase S477A. E. coli extracts, the purified GST-fusion proteins and tag-free proteins after digestion with PreScission protease were resolved by SDS-PAGE. Lane 1, E. coli extracts before the addition of IPTG; lane 2, E. coli extracts at 6 h after stimulation of protein expression with IPTG; lane 3, GST-fusion proteins eluted from glutathione-Sepharose; and lane 4, tag-free proteins (84.6 kDa). (C–G) Proteolytic autoprocessing. Full-length mirolase was incubated at 37°C. At defined time points, the following took place: aliquots were withdrawn for (C) SDS-PAGE analysis, followed by zymography with (D) casein and (E) gelatin, (F) labeling with FP-biotin and (G) the measurement of residual activity using Suc-AAPF-pNA and azocoll as substrates. (H) MS analysis of two products: mirolase 60-kDa and preCTD obtained during mirolase processing. The N-terminal sequence of the analyzed protein bands is shown in brackets. Identified peptides are underlined.
The intensity of the 20-kDa band remained very strong until 24 h, when it suddenly decreased to the level observed in the sample incubated for 48 h, and stayed at this intensity. This suggests that in samples taken prior to 24 h, the 20-kDa band was comprised of two proteins, one of which was later degraded. Indeed, N-terminal sequence analysis revealed two amino acid residues in stoichiometric amounts in each cycle of Edman degradation aligning to the GSGSQQRYYW and AAVAPRLSGP sequences derived from the N-terminus of full-length mirolase and preCTD, respectively.
In stark contrast to the results of SDS-PAGE, the active band pattern obtained from zymography apparently reflecting activity of 60- and 40-kDa fragments of mirolase was similar across all time points, with one significant exception: no active bands were present at time zero (Figure 2DE). This corroborates with the lack of active-site labeling (Figure 2F) and hydrolysis of Suc-AAPF-pNA and azocoll (Figure 2G) observed at this time point. Activity appeared within 30 min of incubation and increased over time, reaching a maximum at 24 h (on Suc-AAPF-pNA) and 48 h (azocoll). Interestingly, active-site labeling of the 60-kDa band correlated far better with the hydrolysis of azocoll than the synthetic chromogenic substrate (Figure 2F and 2G) and is clearly related to the 40- and 60-kDa bands observed by SDS-PAGE. The lack of labeling of these bands and hydrolysis of azocoll in samples incubated for up to 24 h are assumed to be the result of the presence of the intact NTP, which functions as an inhibitor of mirolase. The active-site labeled 40-kDa band correlated well with SDS-PAGE bands that appeared after 24 h and bears 183-QTQTDPLYPQ as the N-terminal sequence. The identity of the N-terminal sequence of the 60-kDa and 40-kDa bands argues that the lower band was generated by proteolytic cleavage of the 20-kDa preCTD. Of note, no activity was detected in the catalytically inactive variant of mirolase, which correlated with the consistent SDS-PAGE band pattern observed throughout the incubation period (data not shown). Together, these findings indicate that mirolase is synthesized as a latent proenzyme, which then autoprocesses itself to the active form of the enzyme truncated at the N- and C-termini.
Mirolase forms an inhibitory complex with the NTP
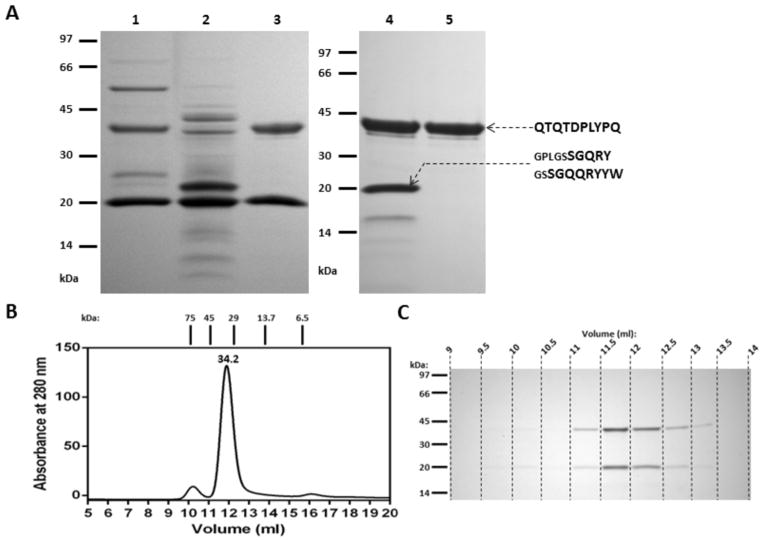
Analysis of the processing of full-length mirolase revealed that the boundaries of the fully processed CD of the 40-kDa protein encompass residues Gln193 to His529 (Figure 1). Based on this finding, we expressed the protein without the CTE (the preCTD and CTD) but with the intact NTP (Figure 3) to confirm the role of the NTP in mirolase latency. The 60-kDa expressed promirolase promptly cleaved itself to the lower molecular mass forms, but the NTP was not degraded. This corroborates our analysis of the autoprocessing of the full-length mirolase. To fully convert promirolase to the mature protease, a sample obtained after affinity chromatography on glutathione-Sepharose was subjected to autoproteolysis. This treatment yielded only two bands of 40- and 20-kDa in the soluble fraction (Figure 3A, lane 3). Edman degradation identified these bands as the CD and the NTP, respectively. Size exclusion chromatography of this fraction yielded a single symmetrical peak that eluted at the volume corresponding to 34.2 kDa. SDS-PAGE analysis of fractions taken from across the peak clearly revealed the stoichiometric presence of both components in each fraction, arguing that the NTP and the CD were resolved as a complex. A discrepancy between the molecular mass of the complex (34.2 kDa) inferred from gel filtration and the calculated mass (60 kDa) can be explained by the putative, non-globular shape of the complex. Although the complex had almost no proteolytic activity on azocoll, the activity was released during incubation at 37°C (not shown), apparently due to proteolytic degradation of the NTP, as clearly shown by the presence of a single 40-kDa band of the CD in the incubated sample (Figure 3A, lane 5). Of note, this procedure enabled the purification of approximately 0.25 mg of highly purified recombinant CD of mirolase from 1 liter culture of E. coli.
Figure 3. Purification of the final form of mirolase.
C-terminally truncated mirolase encompassing residues Ser19 to Pro535 (kDa) was expressed in E. coli as a fusion protein with a GST tag and purified on glutathione-Sepharose with in-column cleavage (PreScission protease) to remove GST. (A) The tag-free CD (lane 1) was supplemented with CaCl2 (final concentration: 30 mM), incubated overnight at 37°C and centrifuged. The pellet (lane 2) was discarded and supernatant (lane 3) was concentrated, passed through HiLoad 16/600 Superdex 75 pg (lane 4) and allowed to undergo autoproteolysis (overnight incubation at 37°C), which resulted in 40-kDa mature mirolase (lane 5). All protein samples were analyzed by SDS-PAGE, and the N-terminal sequence of some of the bands was determined. (B) Mirolase (100 μl) obtained after initial autoproteolysis (lane 3) was subjected to size exclusion chromatography on a molecular mass standardized Superdex 75 10/300 GL column. Collected fractions were resolved by SDS-PAGE (C).
Together, these data confirm that the NTP maintains mirolase latency by forming a stable, non-covalent complex in trans with the CD. Inhibition of mirolase activity is relieved by proteolysis of the NTP, and this result corroborates with our analysis of the autoprocessing of full-length mirolase, thus explaining the sudden burst of proteolytic activity on azocoll and active-site labeling after 24 h incubation (Figure 2FG).
Biochemical characterization of mirolase
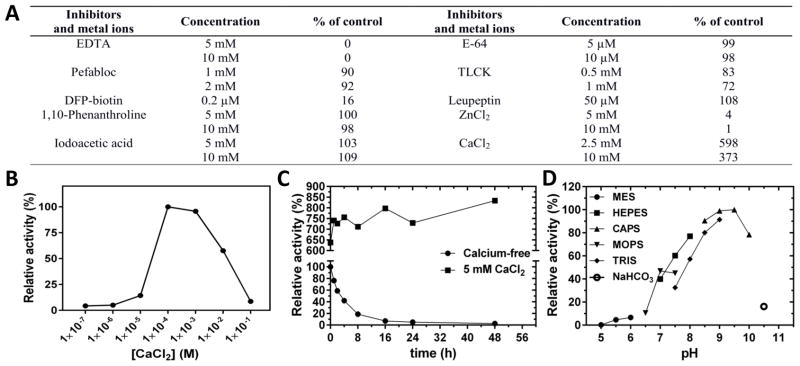
The availability of the active forms of mirolase allowed us to perform biochemical characterization of the protease. First, we investigated the effect of different inhibitors and divalent cations on mirolase activity (Figure 4A). The enzyme was inhibited by Zn2+, EDTA and, as expected, by DFP (FP-biotin), the diagnostic inhibitor of serine proteases, but surprisingly not by the serine proteinase inhibitor pefabloc. Compounds targeting serine- and/or cysteine proteases, including leupeptin, iodoacetic acid, and E-64, as well as the metalloproteinase inhibitor o-phenanthroline, had no effect, while TLCK inhibited mirolase only slightly. Inhibition by EDTA correlates with the dependence of mirolase on the presence of Ca2+, which substantially increases its enzyme activity. To study the calcium-dependence of mirolase activity in more detail, we preincubated full-length mirolase with EDTA, then removed EDTA by dialysis (at 4 °C) and assayed enzyme activity in buffers with increasing Ca2+ concentrations. At up to 10 μM calcium, enzyme activity was negligible. Activity reached a maximum in the 100 μM to 1 mM range, and then decreased at 10 mM and dropped to baseline at 100 mM (Figure 4B). Interestingly, the effect of incubation of 40-kDa mirolase with EDTA was irreversible; enzyme activity could not be restored even after only a short preincubation at 20 °C (not shown). By contrast, incubation for 16 h at 37 °C was required for EDTA to irreversibly inhibit full-length mirolase (Figure 4C).
Figure 4. Effect of inhibitors and divalent cations, optimum pH and the effect of calcium on mirolase activity and stability.
(A) Mirolase activity determined at 37°C in 50 mM Tris-HCl, pH 8.0, using N-Suc-Ala-Ala-Pro-Phe-pNA as a substrate, was taken as 100%. (B) The activity of mirolase was determined in buffers with different pH values. The activity in 0.1 M CAPS pH 9.5 was arbitrarily set at 100%. (C) Calcium-free full-length mirolase (1 μg) was added to 250 μl of 50 mM Tris (pH 8.0) containing increasing concentrations of CaCl2 (0 to 100 nM). After preincubation, the substrate Suc-AAPF-pNA was added at a final concentration of 250 μM, and residual activity was measured. (D) Calcium-free full-length mirolase (1 μg per 250 μl) in 50 mM Tris (pH 8.0), alone and in buffer supplemented with 5 mM CaCl2, was incubated at 37°C. At defined time points (t = 0, 1, 2, 4, 8, 16, 24 and 48 h), aliquots (250 μl) were withdrawn, supplemented with CaCl2, and residual activity was determined using Suc-AAPF-pNA as the substrate.
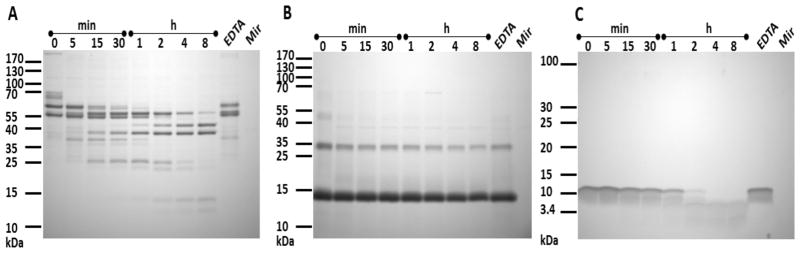
Mirolase is active at alkaline pH from 7 to 10, with optimum activity observed at 9.5 when using Suc-AAPF-pNA as a substrate (Figure 4D). Despite using buffers with identical molarity (100 mM), the mirolase activity at the same pH varied. This could be explained by difference in ionic strength of the buffers as calculated using a software developed by Rob Beynon (http://www.liv.ac.uk/buffers/buffercalc.html). For example, ionic strength of buffers at pH 7.5 is 0.041 (HEPES), 0.062 (MOPS) and 0.086 M (Tris), which corresponds to observed differences in activity at this pH. In addition to Suc-AAPF-pNA, mirolase unexpectedly hydrolyzed low numbers of the structurally related chromogenic substrates Suc-AAPL-pNA and Suc-AAPA-pNA (Table 1). Surprisingly, substitution of the P1 residue with chemically and structurally related amino acids, such as Val and Ile, resulted in the lack of recognition and thus cleavage of such substrates. In addition, changes in the P2 and P3 positions from that in the cleaved substrates also had a detrimental effect on their recognition and hydrolysis. This argues that mirolase is a specific protease that cleaves peptide bonds in the context of sequential motifs. Finally, to determine whether mirolase is a potential virulence factor, we assessed its ability to degrade the human proteins hemoglobin, fibrinogen, fibronectin, collagen type I, and LL-37, an antimicrobial peptide from the cathelicidin family. Mirolase efficiently degraded both fibrinogen and LL-37, and it is of note that the α-chain of fibrinogen was completely degraded within 5 min. By contrast, hemoglobin was cleaved with much lower efficacy (Figure 5), while fibronectin and collagen type I were resistant to cleavage by mirolase (data not shown).
Table 1.
Mirolase cleavage specificity.
| P4 | P3 | P2 | P1 | KM[μM] | kcat[s−1] | kcat/KM [M−1s−1] | ||
|---|---|---|---|---|---|---|---|---|
| Suc | Ala | Ala | Pro | Glu | pNA | n.h. | ||
| Suc | Ala | Ala | Pro | Phe | pNA | 62±19 | 2.27±0.16 | (3.7±1.4)×104 |
| Suc | Ala | Ala | Pro | Val | pNA | n.h. | ||
| Suc | Ala | Ala | Pro | Leu | pNA | 625±42 | 5.8±0.21 | (9.3±0.9)×103 |
| Suc | Ala | Ala | Pro | Ile | pNA | n.h. | ||
| Suc | Ala | Ala | Pro | Asp | pNA | n.h. | ||
| Suc | Ala | Ala | Pro | Arg | pNA | n.h. | ||
| Suc | Ala | Ala | Pro | Ala | pNA | 713±259 | 1.24±0.25 | (1.7±1)×103 |
| MSuc | Ala | Ala | Phe | Ala | pNA | n.h. | ||
| Suc | Phe | Pro | Phe | pNA | n.h. | |||
| Suc | Ala | Ala | Ala | pNA | n.h. | |||
| Suc | Ala | Ala | Val | pNA | n.h. | |||
| Z | Gly | Gly | Leu | pNA | n.h. | |||
| ptos | Gly | Pro | Lys | pNA | n.h. | |||
| Suc | Val | Pro | Phe | pNA | n.h. | |||
| Suc | Phe | Pro | Phe | pNA | n.h. | |||
| Ala | Ala | Phe | pNA | n.h. | ||||
| Val | Leu | Arg | pNA | n.h. | ||||
| Suc | Gly | Arg | pNA | n.h. | ||||
| Z | Gly | Pro | pNA | n.h. | ||||
| Ala | Phe | pNA | n.h. | |||||
| benzo | Arg | pNA | n.h. | |||||
| Phe | pNA | n.h. | ||||||
| Leu | pNA | n.h. | ||||||
| Gly | pNA | n.h. |
The activity assay was performed in 50 mM Tris-HCl, pH 8.0. with the appropriate substrate at final concentration 250 μM. Substrates hydrolysed by mirolase are in bold. (n.h. – no hydrolysis).
Figure 5. Degradation of (A) fibrinogen, (B) hemoglobin and (C) LL-37 by mature mirolase (Mir).
Fibrinogen (110 μg), hemoglobin (110 μg) and LL-37 (13.75 μg) were preincubated with mature (40-kDa) mirolase in 110 μl of 100 mM Tris-HCl, 150 mM NaCl, 5 mM CaCl2 and 0.02% NaN3 (pH 8.0) at a substrate/enzyme weight ratio of 100:1 (fibrinogen and hemoglobin) and 1000:1 (LL-37). At the indicated time points, aliquots (10 μl) were withdrawn from the reaction mixture, mixed with 10 μl of hot reducing SDS-PAGE sample buffer and denatured at 95°C for 5 min to stop the reaction. The samples were then resolved by SDS-PAGE. Fibrinogen, hemoglobin and LL-37 incubated with mirolase in the presence of 50 mM EDTA (EDTA) served as a non-digested protein/peptide control.
Discussion
Proteases play multiple, often essential roles in eukaryotic and prokaryotic organisms. Since proteolysis is irreversible, unregulated proteolysis can cause significant damage; therefore, it is tightly controlled in a spatial and temporal manner in all organisms. The most common mechanism for regulating proteolysis is the synthesis of proteases as catalytically inactive zymogens (Khan and James, 1998). This strategy is especially common among subtilisin-like serine proteases (subtilases), which are often secreted as latent proenzymes consisting of a profragment, a CD and in some cases a CTE (Siezen and Leunissen, 1997). In most subtilases, the prodomain acts as an intermolecular chaperone that catalyses proper folding of an enzymatic domain and then acts as a temporal inhibitor, keeping the proenzyme latent until it is autoproteolytically removed. This type of domain structure is present in mirolase.
The primary structure of the nascent translational product inferred from the corrected mirolase gene sequence is a multidomain preproprotein encompassing an 18-residue SP, a 164-residue NTP, a 348-residue subtilisin-like CD and a 261-residue CTE. The amino acid sequence of this CTE is unique to T. forsythia proteases, including karilysin. It can be subdivided into two parts (Figure 1C): a variable region beginning with a conserved stretch of 30 residues, and the highly conserved C-terminal domain (CTD) (Karim et al., 2010). The CTD shares some similarity to the analogical region of P. gingivalis proteins secreted via the newly discovered type 9 secretion system (T9SS) operating in many bacterial species belonging to the phylum Bacteroidetes, including T. forsythia (Tomek et al., 2014). Indeed, T9SS-dependent secretion of several CTD-bearing proteases by T. forsythia has been recently shown (Narita et al., 2014).
In agreement with the zymogenic nature of pro-subtilases, full-length mirolase (proMir) showed no proteolytic activity. Nevertheless, the protein was very prone to autoprocessing, making purification of the recombinant proMir impossible. Maturation occurred through sequential proteolytic events (Figure 6). First, the cleavage at the N-terminus yielded the non-covalent complex of the NTP with the 70-kDa form of the enzyme. Subsequent cleavage occurred at an unidentified position, releasing the CTD and generating the stable 60-kDa form of mirolase (Mir60) still tightly associated with the NTP. The complex exhibited amidolytic (Suc-AAPF-pNA), but not proteolytic activity. Apparently the NTP acts as an inhibitor of proteolytic activity through steric hindrance. The lack of active-site labeling with FP-biotin further supports the contention that the NTP renders the catalytic cleft inaccessible to proteinaceous substrates. The gradual degradation of the NTP during prolonged incubation was accompanied by the incremental increase of amidolytic and proteolytic activity. A final cleavage at His530-Ala released the 20-kDa preCTD, which was resistant to proteolytic degradation but did not affect the inhibition of proteolytic activity by the NTP. Proteolytic activity on azocoll reached maximum only after the NTP was completely degraded.
Figure 6. Schematic drawing of sequential autoprocessing of full-length mirolase.
The cleavage sites were determined by N-terminal sequencing. Solid short arrows indicate major primary cleavage sites, and dotted arrows indicate secondary minor cleavage sites. Amino acids derived from the expression vector are indicated by lower case letters.
Despite a unique CTE, autoprocessing of mirolase is typical for subtilisin-like enzymes, with one important exception. In contrast to the absolute majority of previously characterized prokaryotic subtilases, which immediately degrade their prodomain after autoprocessing (Shinde et al., 2011), the cleaved NTP stays bound to mirolase in the inhibitory complex and full proteolytic activity is released after the NTP is totally degraded. In this respect, mirolase resembles the CspB protease of Clostridium difficile, the first, and thus far, only bacterial subtilase, which forms a stable inhibitory complex with its profragment after autoprocessing. In this complex, the C-terminus of the CspB prodomain extends deep into the catalytic cleft and acts as a gatekeeper, preventing access of a small reactive site-based probe (FP-Rh) to the catalytic serine residue (Adams et al., 2013). The same mechanism is likely to operate in the NTP-mirolase complex, since access of the related probe (FP-biotin) to the catalytic site is blocked.
As is true for the majority of subtilases, mirolase stability and activity is calcium-dependent. This provides T. forsythia with an additional mechanism by which to control the maturation and activity of the enzyme. Recent studies have shown that calcium concentrations in prokaryotes are regulated as tightly as in eukaryotic cells. In E. coli and several other tested bacteria, the cytoplasmic concentration of Ca2+ is stable at approximately 1 μM (Jones et al., 2002), and while the periplasmic concentration may change depending on the extracellular calcium concentration, it is maintained in the range of 60–300 μM (Jones et al., 2002; Dominguez, 2004). Although no data is available for T. forsythia, it is expected that similar calcium concentrations exist in the subcellular compartments of this bacterium. This means that mirolase, which folds prematurely in the cytoplasm, will remain inactive in the full-length form. It corroborates with our finding that promirolase does not processed itself in the absence of calcium (data not shown). As other proteins secreted via T9SS, mirolase is first exported into the periplasm where it undergoes instant autoprocessing but without the release of proteolytic activity due to inhibition by the NTP (Figure 2,3). In this way the transient latency of the complex allows secretion of still inactive mirolase into the extracellular environment were the NTP is degraded and the fully active protease is generated. This double-control mechanism likely evolved as a means to efficiently prevent damage to cellular compartments. In keeping, all proteases secreted via T9SS, including the archetypal gingipains of P. gingivalis (Veillard et al., 2013) and karilysin (Karim et al., 2010), are maintained in a zymogenic form until secretion, although none appear to be controlled as tightly as mirolase.
Limited specificity screening indicates that mirolase is a specific enzyme with an expanded substrate-binding cleft, but more precise mapping of substrate specificity will require the use of high-throughput methods. Mirolase cleaves several human substrates, the degradation of which may impact the development of periodontitis. One of these, cathelicidin LL-37, is a bactericidal peptide with strong immunomodulatory properties that seems to play a crucial role in the maintenance of homeostasis in the periodontium (Hosokawa et al., 2006, Eick et al., 2014). Levels of LL-37 are increased in patients with periodontitis compared to healthy controls (Türkoğlu et al., 2009), and the genetic lack of LL-37 is associated with severe periodontal disease (Pütsep et al., 2002). Thus, hydrolysis of LL-37 by mirolase may not only contribute to the survival of T. forsythia, which is resistant to LL-37, but also disturb the regulation of inflammation during periodontitis. Taking into account that proteases from different periodontopathogens synergistically inactivate the complement system (Jusko et al., 2012) and supply nutrients (Hajishengallis, 2014), it is tempting to speculate that mirolase allies with karilysin and gingipains, also known to degrade LL-37 (Gutner et al., 2009; Koziel et al., 2010), to synergistically eliminate the peptide from infected sites. A similar concerted action is likely to occur in the case of hemoglobin degradation, as has been described for proteases produced by two other major periodontal pathogens, P. gingivalis (gingipains) and Prevotella intermedia (interpain) (Byrne et al., 2013). Finally, rapid and efficient degradation of the α-chain of fibrinogen by mirolase may contribute to clotting deficiency in the periodontal pocket. However, because the mirolase transcript was found in gingival crevicular fluid from patients suffering from periodontitis and positive for T. forsythia (unpublished data courtesy of Sigrun Eick), all of the potential mechanisms by which mirolase may contribute to the pathogenicity of T. forsythia need to be verified experimentally.
Materials and methods
Reagents
Restriction endonucleases BamHI and XhoI, T4 DNA ligase, dNTP, GeneJET™ Gel Extraction Kit, GeneJET™ PCR Purification Kit, and GeneJET™ Plasmid Miniprep Kit were purchased from Thermo Scientific Fermentas (Vilnius, Lithuania). Phusion DNA Polymerase was obtained from Thermo Scientific Finnzyme (Woburn, MA, USA). QuikChange Lightning Site-Directed Mutagenesis Kit was from Stratagene (La Jolla, CA, USA). All primers used in the study were synthesized by Genomed (Warsaw, Poland). The expression vector pGEX-6P-1, glutathione-Sepharose 4 Fast Flow, 3C protease (PreScission), gel filtration columns HiLoad 16/600 Superdex 75 pg and Superdex 75 10/300 GL, and Gel Filtration LMW Calibration Kit were purchased from GE Healthcare Life Sciences (Little Chalfont, UK). Polyvinylidene difluoride (PVDF) membranes were from Millipore (Billerica, MA, USA). Protein Concentrators, 9K MWCO, 7mL and Pierce ECL Western Blotting Substrate were from Thermo Scientific Pierce (Rockford, Il, USA). Dialysis membranes Spectra/Por 4 12,000 to 14,000 Dalton MWCO were obtained from Spectrum Laboratories (Rancho Dominguez, CA, USA). FP-Biotin was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Human fibrinogen, human haemoglobin, Streptavidin-Peroxidase Polymer, all chromogenic substrates (p-nitroalanine (pNA)-derivatives), protease inhibitors (Table 1) and human proteins were purchased from Sigma (St. Louis, MO, USA). Synthetic LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was purchased from GenScript (Piscataway, NJ, USA). All other chemical reagents were obtained from BioShop Canada (Burlington, ON, Canada).
Resequencing of mirolase locus
The region encompassing the bfor_c_1_10622 and the bfor_c_1_10621 ORF (The Bioinformatics Resource for Oral Pathogens, www.brop.org) were amplified by PCR (primers 347PCRF 5′-AGGTATAGGACTTGAAGGGAAAGGC-3′ and 347PCRR 5′-CTATAGGGCTGTCATTGTCGTTCG-3′) using Phusion DNA Polymerase. The obtained PCR product was separated in 1% agarose gel, cut out from the gel and purified using gel extraction kit. The purified PCR product was sent for DNA sequencing using primers NG1s1F (5′-TCATCCGTATCGTCGCATCAG-3′) and NG1s1R (5′-CGTGTAAAGAAAGCGCTTCGG-3′).
Cloning and mutant construction
Genomic DNA from T. forsythia was extracted from strain ATCC 43037. The coding sequence of the full length mirolase gene, proMir (Ser19-Lys791, bfor_c_1_10621/10622; http://brop.org/) and mirolase without C-terminal extension, Mircat (Ser19-Pro535), both without the nucleotide sequence that encodes a signal peptide) were amplified by PCR, the amplicon purified and cloned into the pGEX-6P-1 expression vector using BamHI/XhoI sites and the following PCR primers (restriction sites are underlined):
proMir_F: 5′-GCAGGATCCTCCGGACAGCAGCGCTATTA -3′
proMir_R: 5′-CCGCTCGAGTTATTTTTTGATCAATTTCTG -3′
Mircat_F: 5′-ATAGGATCCCAGCCGGCAGAGCGCGGT-3′
Mircat_R: 5′-CCGCTCGAGTTAAGGGGCTACGG -3′
The resulting recombinant product included an N-terminal glutathione-S-transferase (GST) tag, a PreScission protease cleavage site followed by the cloned protein. This genetic manipulation inserted five residues (Gly-Pro-Leu-Gly-Ser) before the N-terminal serine residue of mirolase after the GST moiety was removed by cleavage with PreScission. The correctness of recombinant plasmids (pGEX-6P-1_proMir, pGEX-6P1_Mircat) were confirmed by DNA sequencing and then they were transformed into Escherichia coli strain BL21 (DE3) (New England Biolabs, Ipswich, MA, USA) under the control of the T7 promoter. The “wild-type” constructs were also used to produce Ser477Ala (S477A) mutation using following primers (S477A-F: 5′-CACTTTTAATGGTACTGCCGCCGCCTGTCCTC-3′; S477A-R: 5′-GAGGACAGGCGGCGGCAGTACCATTAAAAGTG-3′) and the QuikChange Lightning Site-Directed Mutagenesis Kit according to the manufacturer’s instructions. The mutated constructs were verified (pGEX-6P-1_MirS477A, pGEX-6P1_MircatS477A) by DNA sequencing.
Expression and purification of recombinant proteins
Transformed E. coli host were grown in LB Lennox media at 37°C to OD600 ranging from 0.75 to 1.0, cooled for 30 min at 4°C and expression of recombinant proteins were induced by the addition of 0.25 mM isopropyl-1-thio-β-Dgalactopyranoside (IPTG). After 6 h cultivation at 20°C, cells were harvested by centrifugation (6000 g, 15 min, 4°C) and resuspended in PBS (15 ml per pellet from 1 l of culture) and subsequently lysed by sonication (cycle of 30 × 0.5 s pulses at amplitude of 70% per pellet from 1 l of culture) using Branson Sonifier Digital 450 (Branson Ultrasonics, Danbury, CT, USA). The cell lysates were clarified by centrifugation (40 000 g, 40 min, 4°C), filtered through a 0.45-μm syringe filter and applied at 4°C onto a glutathione-Sepharose 4 Fast Flow column (bed volume 5 ml) equilibrated with PBS. Recombinant proteins were eluted using 50 mM Tris-HCl, pH 8.0, supplemented with fresh 10 mM reduced glutathione. Alternatively, 10 ml of PBS containing 100 μl of PreScission protease stock solution (1 U/ml) was applied onto the column and incubated for 40 h at 4°C. Protein concentration was determined by measurement of absorbance at 280 nm using Nanodrop (NanoDrop products, Wilmington, DE, USA) and BCA assay (Thermo Scientific Pierce). Purity of obtained protein was verified by SDS-PAGE electrophoresis.
Gel electrophoresis and zymography
Mirolase purification, autocatalytic processing and protein substrate degradation were monitored by SDS-PAGE using 10% and 18% (T:C ratio, 33:1) gels and the Tris-HCl/Tricine buffer system (Schägger and von Jagow, 1987). The 18% gels were used only for monitoring of degradation of LL-37. Gels were stained with 0.1% Coomassie Brilliant Blue R-250 in 10% acetic acid and destained in 30% methanol, 10% acetic acid and 1% acetic acid.
Zymographic analysis was performed on samples mixed 1:1 with non-reducing SDS sample buffer (0.125 M Tris-HCl, pH 7.8, 20% glycerol, 4% SDS, 0.1% Bromophenol Blue) for 15 min at 20°C and electrophoresed on 12% SDS-PAGE gels (T:C, 27.5:1) containing gelatin or casein at final concentration of 0.1 mg/ml. Gels were incubated 2x in 2.5% Triton X-100 for 30 min, followed by incubation in developing buffer (0.2 M Tris-HCl, pH 7.8, 5 mM CaCl2, 1 mM DTT) for 3h at 37°C. Finally, the gel was incubated in destaining/fixing solution (methanol:acetic acid: water (30:10:60)), then stained with 0.1% amido black in 10% acetic acid for 1 h and destained successively in destaining/fixing solution, 10% acetic acid, 1% acetic acid, which revealed clear zones of substrate hydrolysis on a blue background.
Proteolytic processing and activation of mirolase
Full-length mirolase was incubated in 50 mM Tris, 1 mM CaCl2, 0.01% SDS, 0.02% NaN3, pH 8.0 at 37°C for up to 168 h. At specific time points, aliquots were withdrawn for proteolytic activity measurement using Azocoll and Suc-AAPF-pNA as substrates, labelling with FP-biotin, zymography and SDS-PAGE analysis. MirolaseS477A served as a control.
Labelling with FP-biotin
Protein sample were incubated with 50 μM FP-biotin in 50 mM Tris, 150 mM NaCl, 5 mM CaCl2, pH 8.0, for 30 min at 20°C. Mixtures were resolved on a 10% SDS-PAGE gel. After electrophoresis proteins were electrotransferred in 25 mM Tris, 192 mM glycine and 20% methanol in a Semi-Dry Transfer Cell (Bio-Rad Life Science Research, Hercules, CA, USA) onto a PVDF membrane. The membrane was blocked overnight at 4°C in blocking buffer: 5% bovine serum albumin (BSA) in TTBS (20 mM Tris, 0.5 M NaCl, 0.1% Tween-20, pH 7.5). Next membrane was incubated with Streptavidin-HRP (1:10000) (Sigma) in blocking buffer for 1 h at RT followed by 4 washes with TTBS. The membrane was developed using ECL blotting substrate and Kodak Biofilm plate (Eastman Kodak, Rochester, NY, USA).
Purification of mature mirolase
To mirolase without C-terminal extension (Ser19-Pro535) expressed in E. coli and purified on glutathione-Sepharose as described above was supplemented with CaCl2 to the final concentration of 30 mM. After overnight incubation at 37°C, the mixture was centrifuged (16 100 g, 10 min, 4°C) to remove a precipitated protein. Obtained supernatant was concentrated and resolved by size exclusion chromatography on HiLoad 16/600 Superdex 75 pg using an AKTA purifier 900 FPLC system (GE Healthcare) at a flow rate of 1 ml/min in 20 mM Tris, 50 mM NaCl, 1 mM CaCl2, 0.02% NaN3, pH 8.0. Elution profile was followed at 280 nm and 1 ml fractions were collected. Samples containing mirolase were pooled, incubated overnight at 37°C then dialysed at 4°C against 20 mM Tris, 50 mM NaCl, 1 mM CaCl2, pH 8.0.
To determine the molecular mass and confirm heterodimeric composition of mirolase obtained after first digestion, the digest clarified of preciptate was resolved on a Superdex 75 10/300 GL column calibrated with Calibration Kits LMW and HMW (GE Healthcare) following the manufacturer’s instructions (Protein standards used: aprotinin, 6.5 kDA; ribonuclease A, 13.7 kDa; carbonic anhydrase, 29 kDa; ovalbumin, 44 kDa; conalbumin, 75 kDa). Elution profile was followed at 280 nm and 0.5 ml fractions were collected and analysed (30 μl samples) by SDS-PAGE.
N-Terminal sequence analysis
Samples were resolved on 10% SDS-PAGE gels were electrotransferred in 10 mM CAPS, 10% methanol, pH 11 in a Semi-Dry Transfer Cell onto a PVDF membrane. Protein bands were visualized by Coomassie brilliant blue G-250 staining, excised and analysed by automated Edman degradation using a Procise 494HT amino acid sequencer (Applied Biosystems, Carlsbad, CA, USA).
Mass spectroscopy analysis
Protein samples were separated on 10% SDS-PAGE gels and stained as describe earlier. After 15 min incubation in ultra-pure water chosen protein bands were excised and subjected to mass spectroscopy analysis. First, they were washed in water and submerge in 50% H2O, 50% acetonitrile (ACN) solution for 15 min. After the addition of 50 μL ACN the sample were left until gel pieces shrunk. Then, they were submerged in 50 μl of 0.1 M NH4HCO3 containing trypsin and protein was digested overnight at 37 °C. The peptides were extracted and analysed by NanoLC-MS/MS using an EASY-nLC II system (ThermoScientific) connected to a TripleTOF 5600 mass spectrometer (AB SCIEX; Framingham, MA, USA). Peptides were dissolved in 5% formic acid, injected, trapped and desalted on a Biosphere C18 column (5 μm, 2 cm × 100 μm I.D; Nano Separations). Next, peptides eluted from a trap column were separated on a 15 cm analytical column (75 μm i.d.) packed in-house in a fritted silica tip (New objectives; Woburn, MA, USA) with RP ReproSil-Pur C18-AQ 3 μm resin (Dr. Marisch GmbH, Ammerbuch-Entringen, Germany) connected in-line to the mass spectrometer. Peptides were eluted at a flow rate of 250 nl/min, using a 50 min gradient from 5% to 35% phase B (0.1% formic acid and 90% acetonitrile). The collected MS files were converted to Mascot generic format (MGF) using the AB SCIEX MS Data Converter beta 1.1 (AB SCIEX) and the “proteinpilot MGF” parameters. The generated peak lists were searched against the Swiss-Prot database using in-house Mascot search engine (matrix science). Search parameters used for the protein identification included trypsin, two missed cleavages, propionamide (Cys) as fixed modification and oxidation (Met) as variable modification. Peptide tolerances were set to 10 ppm and MS/MS tolerance to 0.6 Da.
Enzymatic activity assays
Mirolase activity was routinely determined in 50 mM Tris, pH 8.0 using 250 μM Suc-AAPF-pNA. The assay for the activity of the full-length mirolase was performed in the eppendorf tubes. Briefly, the substrate (2.5 μl of 25 mM Suc-AAPF-pNA in DMSO) was added to 247.5 μl of reaction buffer containing 1 μg of the enzyme. After 2 h incubation at 37°C with vigorous shaking, 200 μl of obtained solution was transferred into wells of microplate and absorbance at 410 nm was measured using a SpectraMAX microplate reader (Molecular Devices, Sunnyvale, CA, USA). Mature mirolase was assayed directly on microplates (Nunc, Roskilde, Denmark). Briefly, to 100 μl of 20 mM enzyme the substrate (Suc-AAPF-pNA) was added to the final concentration of 250 μM. The enzymatic hydrolysis of the substrate was recorded as the increase of absorbance at 410 nm for 30 min at 37°C using the microplate reader. To determine activity against Azocoll, 125 μl of 15 mg/ml suspension of substrate in assay buffer was added to the same volume of the enzyme solution in eppendorf tubes and mixtures were incubate for 2 h at 37°C with shaking. Undigested Azocoll was removed by centrifugation (16 100 g, 5 min) and the absorbance at 520 nm of the supernatant was measured using a SpectraMAX microplate reader.
The pH optimum was determined using the following buffers at a concentration of 100 mM: MES (pH 5.5, 6.0, and 6.5), MOPS (pH 6.5, 7.0, and 7.5), HEPES (pH 7.0, 7.5, and 8.0), Tris (pH 7.5, 8.0, and 8.5), CAPS (pH 8.5, 9.0, 9.5 and 10.0), and NaHCO3 (pH 10.5). To test the effect of inhibitors and divalent metal ions on mirolase activity, the full length mirolase or the mature protease were preincubated with tested compound in 50 mM Tris, pH 8.0, for 15 min at room temperature and the residual activity was measured. In order to determine the optimal concentration of calcium for proteolytic activity, the full-length mirolase was incubated with 10 mM EDTA for 30 min at 4°C followed by dialysis against 20 mM Tris, 150 mM NaCl, pH 7.5. Such prepared full-length mirolase (1 μg) was added to 250 μl of 50 mM Tris, pH 8.0 containing CaCl2 at concentration ranging from 0 to 100 mM. After 15 min incubation at room temperature the residual activity was measured using Suc-AAPF-pNA as described above. A sample of full-length mirolase prepared in the same way was also used to check the effect of calcium on enzyme stability. The enzyme (1 μg/250 μl) was incubated in 50 mM Tris, pH 8.0, in the presence or absence of 5 mM CaCl2 for up to 48 h at 37°C. At specific time points 250 μl of mixture was withdrawn supplemented with CaCl2 (2.5 μl of 100 mM CaCl2) and residual activity was measured using Suc-AAPF-pNA.
Human plasma fibrinogen, human haemoglobin and antimicrobial peptide LL-37 were incubated with mature mirolase at the enzyme/substrate weight ratios 1:100 (fibrinogen and haemoglobin) and 1:1000 (LL-37) for 8 h at 37°C in 100 mM Tris, 150 mM NaCl, 5 mM CaCl2, 0.02% NaN3, pH 8.0. At specific time-points aliquots were withdrawn for SDS-PAGE analysis.
Mirolase substrate specificity and determination of kinetic parameters of pNA substrates hydrolysis
The specificity on pNA substrates was determined in 50 mM Tris, 150 mM NaCl, 5 mM CaCl2, pH 8.0, using both the full-length and mature mirolase and a panel of substrates listed in Table 2. The final concentration of each substrate was 250 μM.
In order to determine KM and kcat of enzymatic hydrolysis of substrates wells of a microplate were filled with 100 μl of substrate at concentration range 0.1–2 mM in assay buffer (50 mM Tris, 150 mM NaCl, 5 mM CaCl2, pH 8.0), then 100 μl of enzyme solution was added in assay buffer (20 nM final concentration of the enzyme) and the rate of substrate hydrolysis was recorded as the increase of absorbance at 410 nm was recorded for 60 min using a SpectraMAX microplate reader. The KM and kcat were determined using GpraphPad Software and Michelis-Menten model:
where: V is enzyme velocity, [E] active enzyme concentration, [S] substrate concentration, kcat the turnover number, and KM Michelis-Menten constant.
Acknowledgments
This study was supported by grants from: Faculty of Biochemistry, Biophysics and Biotechnology of Jagiellonian University (K/DSC/000361), National Science Center, Poland (UMO-2011/03/N/NZ1/00586 and 2012/04/A/NZ1/00051 to M.K. and J.P., respectively), US NIH (DE 09761 and DE 022597), the European Commission (FP7-PEOPLE-2011-ITN-290246 “RAPID” and FP7-HEALTH-F3-2012-306029 “TRIGGER”), and Polish Ministry of Science and Higher Education (UMO-2795/7.PR/13/2017/2). M.K. has obtained funding for the preparation of a doctoral dissertation from the National Science Center (Poland) as part of the funding of a doctoral scholarship on the basis of the decision’s number DEC-2013/08/T/NZ1/00315. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08).
References
- Adams CM, Eckenroth BE, Putnam EE, Doublié S, Shen A. Structural and functional analysis of the CspB protease required for Clostridium spore germination. PLoS Pathog. 2013;9:e1003165. doi: 10.1371/journal.ppat.1003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004;34:9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- Behle JH, Papapanou PN. Periodontal infections and atherosclerotic vascular disease: an update. Int Dent J. 2006;56:256–262. doi: 10.1111/j.1875-595x.2006.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Byrne DP, Potempa J, Olczak T, Smalley JW. Evidence of mutualism between two periodontal pathogens: co-operative haem acquisition by the HmuY haemophore of Porphyromonas gingivalis and the cysteine protease interpain A (InpA) of Prevotella intermedia. Mol Oral Microbiol. 2013;28:219–229. doi: 10.1111/omi.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdà-Costa N, Guevara T, Karim AY, Ksiazek M, Nguyen KA, Arolas JL, Potempa J, Gomis-Rüth FX. The structure of the catalytic domain of Tannerella forsythia karilysin reveals it is a bacterial xenologue of animal matrix metalloproteinases. Mol Microbiol. 2011;79:119–132. doi: 10.1111/j.1365-2958.2010.07434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez DC. Calcium signalling in bacteria. Mol Microbiol. 2004;54:291–7. doi: 10.1111/j.1365-2958.2004.04276.x. [DOI] [PubMed] [Google Scholar]
- Fox CH. New considerations in the prevalence of periodontal disease. Curr Opin Dent. 1992;2:5–11. [PubMed] [Google Scholar]
- Eick S, Puklo M, Adamowicz K, Kantyka T, Hiemstra PS, Stennicke H, Guentsch A, Schacher B, Eickholz P, Potempa J. Lack of cathelicidin processing in Papillon-Lefèvre-Patients reveals essential role of LL–37 in periodontal homeostasis. Orphanet J Rare Diseases. 2014;9:148. doi: 10.1186/s13023-014-0148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RI, Nunn ME, Vokonas PS. Epidemiologic associations between periodontal disease and chronic obstructive pulmonary disease. Ann Periodontol. 2001;6:71–77. doi: 10.1902/annals.2001.6.1.71. [DOI] [PubMed] [Google Scholar]
- Glew MD, Veith PD, Peng B, Chen YY, Gorasia DG, Yang Q, Slakeski N, Chen D, Moore C, Crawford S, Reynolds EC. PG0026 is the C-terminal signal peptidase of a novel secretion system of Porphyromonas gingivalis. J Biol Chem. 2012;287:24605–24617. doi: 10.1074/jbc.M112.369223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Beg QK, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol. 2002;59:15–32. doi: 10.1007/s00253-002-0975-y. [DOI] [PubMed] [Google Scholar]
- Gutner M, Chaushu S, Balter D, Bachrach G. Saliva enables the antimicrobial activity of LL-37 in the presence of proteases of Porphyromonas gingivalis. Infect Immun. 2009;77:5558–5563. doi: 10.1128/IAI.00648-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014 doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–112. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M, Taubman MA, Kawai T. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugoson A, Sjödin B, Norderyd O. Trends over 30 years, 1973–2003, in the prevalence and severity of periodontal disease. J Clin Periodontol. 2008;35:405–414. doi: 10.1111/j.1600-051X.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Ingar O, Potempa J. Strategies for the inhibition of gingipains for the potential treatment of periodontitis and associated systemic diseases. J Oral Microbiol. 2014;6:24800. doi: 10.3402/jom.v6.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingmer H, Brøndsted L. Proteases in bacterial pathogenesis. Res Microbiol. 2009;160:704–710. doi: 10.1016/j.resmic.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Ishihara K. Virulence factors of Treponema denticola. Periodontol 2000. 2010;54:117–35. doi: 10.1111/j.1600-0757.2009.00345.x. [DOI] [PubMed] [Google Scholar]
- Jones HE, Holland IB, Campbell AK. Direct measurement of free Ca(2+) shows different regulation of Ca(2+) between the periplasm and the cytosol of Escherichia coli. Cell Calcium. 2002;32:183–192. doi: 10.1016/s0143416002001537. [DOI] [PubMed] [Google Scholar]
- Jusko M, Potempa J, Kantyka T, Bielecka E, Miller HK, Kalinska M, Dubin G, Garred P, Shaw LN, Blom AM. Staphylococcal proteases aid in evasion of the human complement system. J Innate Immun. 2014;6:31–46. doi: 10.1159/000351458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko M, Potempa J, Karim AY, Ksiazek M, Riesbeck K, Garred P, Eick S, Blom AM. A metalloproteinase karilysin present in the majority of Tannerella forsythia isolates inhibits all pathways of the complement system. J Immunol. 2012;188:2338–2349. doi: 10.4049/jimmunol.1101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim AY, Kulczycka M, Kantyka T, Dubin G, Jabaiah A, Daugherty PS, Thogersen IB, Enghild JJ, Nguyen KA, Potempa J. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol Chem. 2010;391:105–117. doi: 10.1515/BC.2010.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815–836. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel J, Potempa J. Protease-armed bacteria in the skin. Cell Tissue Res. 2013;351:325–337. doi: 10.1007/s00441-012-1355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel J, Karim AY, Przybyszewska K, Ksiazek M, Rapala-Kozik M, Nguyen KA, Potempa J. Proteolytic inactivation of LL-37 by karilysin, a novel virulence mechanism of Tannerella forsythia. J Innate Immun. 2010;2:288–293. doi: 10.1159/000281881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ, Lopatin DE, Giordano J, Alcoforado G, Hujoel P. Comparison of the benzoyl-DL-arginine-naphthylamide (BANA) test, DNA probes, and immunological reagents for ability to detect anaerobic periodontal infections due to Porphyromonas gingivalis, Treponema denticola, and Bacteroides forsythus. J Clin Microbiol. 1992;30:427–433. doi: 10.1128/jcm.30.2.427-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, Gawron K, Mizgalska D, Marcinska KA, Benedyk M, Pyrc K, Quirke AM, Jonsson R, Alzabin S, Venables PJ, Nguyen KA, Mydel P, Potempa J. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD. PLoS Pathog. 2013;9:e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Sato K, Yukitake H, Shoji M, Nakane D, Nagano K, Yoshimura F, Naito M, Nakayama K. Lack of a surface layer in Tannerella forsythia mutants deficient in the type IX secretion system. Microbiology. 2014 doi: 10.1099/mic.0.080192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien-Simpson O-NM, Veith PD, Dashper SG, Reynolds EC. Porphyromonas gingivalis gingipains: the molecular teeth of a microbial vampire. Curr Protein Pept Sci. 2003;4:409–426. doi: 10.2174/1389203033487009. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 67:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- Palm E, Khalaf H, Bengtsson T. Suppression of inflammatory responses of human gingival fibroblasts by gingipains from Porphyromonas gingivalis. Mol Oral Microbiol. 2014 doi: 10.1111/omi.12073. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike RN. Bacterial peptidases. Contrib Microbiol. 2005;12:132–180. doi: 10.1159/000081693. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa J, Pike R, Travis J. Titration and mapping of the active site of cysteine proteinases from Porphyromonas gingivalis (gingipains) using peptidyl chloromethanes. Biol Chem. 1997;378:223–230. doi: 10.1515/bchm.1997.378.3-4.223. [DOI] [PubMed] [Google Scholar]
- Potempa J, Sroka A, Imamura T, Travis J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr Protein Pept Sci. 2003;4:397–407. doi: 10.2174/1389203033487036. [DOI] [PubMed] [Google Scholar]
- Pütsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62:597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Ishihara K, Kato T, Okuda K. Cloning, expression, and sequencing of a protease gene from Bacteroides forsythus ATCC 43037 in Escherichia coli. Infect Immun. 1997;65:4888–4891. doi: 10.1128/iai.65.11.4888-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shinde U, Thomas G. Insights from bacterial subtilases into the mechanisms of intramolecular chaperone-mediated activation of furin. Methods Mol Biol. 2011;768:59–106. doi: 10.1007/978-1-61779-204-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen RJ, Leunissen JA. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Tomek MB, Neumann L, Nimeth I, Koerdt A, Andesner P, Messner P, Mach L, Potempa JS, Schäffer C. The S-layer proteins of Tannerella forsythia are secreted via a type IX secretion system that is decoupled from protein O-glycosylation. Mol Oral Microbiol. 2014 doi: 10.1111/omi.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomi N, Fukuyo Y, Arakawa S, Nakajima T. Pro-inflammatory cytokine production from normal human fibroblasts is induced by Tannerella forsythia detaching factor. J Periodontal Res. 2008;43:136–142. doi: 10.1111/j.1600-0765.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- Türkoğlu O, Emingil G, Kütükçüler N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 2009;80:969–976. doi: 10.1902/jop.2009.080532. [DOI] [PubMed] [Google Scholar]
- Tuteja R. Type I signal peptidase: an overview. Arch Biochem Biophys. 2005;441:107–11. doi: 10.1016/j.abb.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Veillard F, Sztukowska M, Mizgalska D, Ksiazek M, Houston J, Potempa B, Enghild JJ, Thogersen IB, Gomis-Rüth FX, Nguyen KA, Potempa J. Inhibition of gingipains by their profragments as the mechanism protecting Porphyromonas gingivalis against premature activation of secreted proteases. Biochim Biophys Acta. 2013;1830:4218–4228. doi: 10.1016/j.bbagen.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, Lamont RJ, Jenkinson HF. Microbial interactions in building of communities. Mol Oral Microbiol. 2013;28:83–101. doi: 10.1111/omi.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongqing T, Potempa J, Pike RN, Wijeyewickrema LC. The lysine-specific gingipain of Porphyromonas gingivalis: importance to pathogenicity and potential strategies for inhibition. Adv Exp Med Biol. 2011;712:15–29. doi: 10.1007/978-1-4419-8414-2_2. [DOI] [PubMed] [Google Scholar]