Abstract
Available reports on brucellosis in Nigeria are largely confined to cattle while it is believed that other ruminants like sheep and goats are equally exposed to the disease. To have an insight into the role of goats in the epidemiology of brucellosis in Nigeria, we conducted a cross-sectional study between June 2011 and May 2013 to determine the seroprevalence of brucellosis in goats in some selected states in Nigeria. Serum samples were collected from goats at different locations and tested for antibodies to Brucella spp using the Rose Bengal Test (RBT), samples positive by RBT were further subjected to Competitive Enzyme Linked Immunosorbent Assay (cELISA). Data collected to determine risk factors were also analysed using chi-square and logistics regression statistics.
Out of a total of 2827 samples tested from the different states (Benue = 331; Borno =195; Oyo = 2155; Sokoto = 146), we recorded an overall seroprevalence of 2.83% (Benue = 17.30%; Borno = 2.05%; Oyo = 0.60% and Sokoto = 0.00%) by RBT. The cELISA further supported 9.45% (7/74) of the total RBT positive samples. Logistic regression analysis showed that the location (p = 0.004) and source (p < 0.0001); are probable risk factors to be considered in the epidemiology of brucellosis with sex (p = 0.179); age (p = 0.791) and breed (p = 0.369) not playing any major role.
Our findings reveal a relatively low seroprevalence of brucellosis among goats screened except for Benue State. Since most of the goats sampled in the present study were from the abattoirs, further farm level investigations are required to determine the role of goats in the epidemiology of brucellosis in Nigeria since they share common environment with sheep and cattle that are natural hosts of Brucella species which are of major public health threat.
INTRODUCTION
Brucellosis is caused by the bacteria of the genus Brucella, a Gram-negative intracellular coccobacilli that occurs in a wide variety of animals including cattle, sheep, goats, pigs and other livestock as well as humans. It is a contagious systemic disease characterized by the inflammation of the genital organs and foetal membranes, abortion, sterility and formation of localized lesions in the lymphatic system and joints (CDC, 2005). Brucellosis is a zoonotic disease noted for its public health importance and threat to food security globally. Though controlled in many developed countries (Corbel, 1997), brucellosis is endemic in Africa (Cadmus et al., 2013), South America (Lucero, 2005) and many Asian countries (Sofian et al., 2008).
In Nigeria, several serological studies have shown that brucellosis is endemic in the livestock population (Ocholi, 1993; Cadmus et al., 2006, Ibironke et al., 2008, Mai et al., 2012). Recently, the prevalence of 8.6% was recorded in Lagos State (Cadmus et al., 2009), 37% in three Northern States of Nigeria (Kaduna, Kano and Adamawa) (Mai et al., 2012) as well as 16.1% in Plateau State (Bertu et al., 2010) in north-central Nigeria. Incidentally, most of these studies have been concentrated on cattle; while few documented evidence have shown that the disease also exists in goats in Nigeria; with prevalence of 0.86%, 14.00% and 25.80% reported in south-western, north-eastern and north-central Nigeria respectively (Cadmus et al 2006; Tijjani et al., 2009; Kaltungo et al., 2013).
In many rural and nomadic communities in Nigeria, goats are continuously in close contact with humans. Thus, there is an increased likelihood of zoonotic transmission of brucellosis to individuals in such settings. In addition, abattoir workers involved in the slaughter and processing of goats are at high risk of being infected, especially from infected uterine and udder contents (European Commission., 2001) since Brucella can also be excreted through these routes. Among the Brucella species, Brucella melitensis which is the major cause of brucellosis in caprine, is noted to be the most pathogenic in humans (OIE, 2009). Hence, serologic studies focusing on brucellosis in goats is not only needful but useful. This study therefore sets out to investigate the sero-prevalence of brucellosis in farms/households as well as goats slaughtered at major abattoirs in some selected states in Nigeria.
MATERIALS AND METHODS
Study site
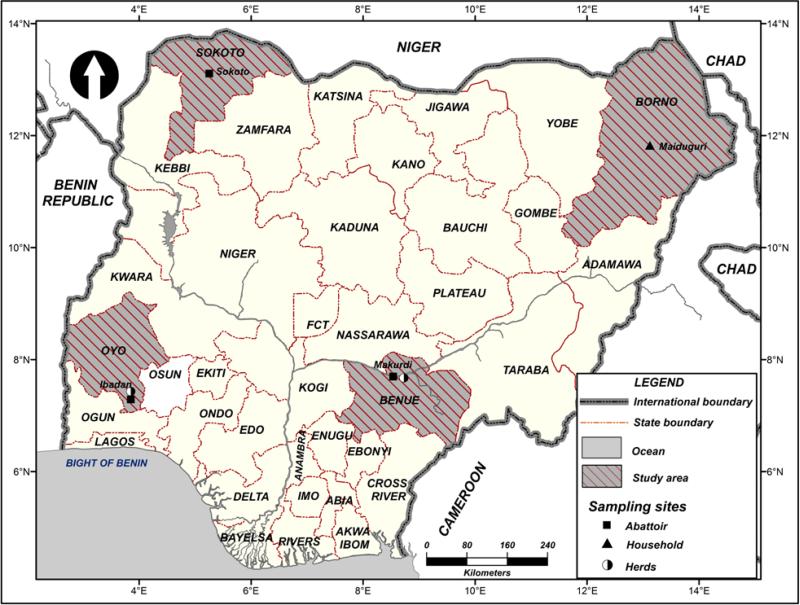
The study was conducted in selected states in Nigeria including Oyo, Benue, Borno and Sokoto States.
Oyo State
The state has a total land mass of 27,036km.2. The dry season lasts from November to March while the wet season starts from April and ends in October. Average daily temperature ranges between 25 °C (77.0 °F) and 35 °C (95.0 °F), almost throughout the year. Though Oyo State is situated in the forest belt in Nigeria, there are the derived savannah areas in the state that favour livestock rearing. Traditionally, the West African dwarf (WAD) goats are kept but the Sokoto Red goats and their crosses are common (Blench, 1999). However a good number of goats slaughtered in the state come from northern Nigeria. For this study, samples from goats were collected from the households and abattoirs.
Benue State
Benue has a land area of 30,755km2 and is situated in north-central Nigeria. Though the Sokoto Red goats are generally common in northern Nigeria, Benue State is one of the northern states of the country that still harbours a good population of the West African dwarf goats (Blench, 1999). In Benue State, blood samples were collected from goats in the market and goats slaughtered in the abattoirs.
Borno State
Borno State has a land mass of 72,767 km2. The state is located in the semi – arid zone of north-eastern Nigeria. It is noted for the presence of large numbers of the Sahel breed of goats (Blench, 1999). Blood samples were collected from goat herds from various farms.
Sokoto State
Sokoto State is in the north-western part of Nigeria and has a land area of 32,146 km2. Sokoto is the home of the Sokoto Red goats in Nigeria (Ngere et al., 1984) and samples were collected from the abattoirs.
Animal sampling, sample collection and handling
Goats from the selected states were sampled. Blood samples were collected from goats available at the various sites. However, due to varied level of support and cooperation received during the course of the study, sample collection did not follow a systematic approach. Thus, varying numbers of animals were screened across the four states. Animals’ parameters such as breed, sex and age, source and location were recorded. For each animal, about 10mls of blood was collected into 15ml sterile tubes. The blood samples were allowed to clot and centrifuged at 3000rpm for 15minutes. Serum samples were then decanted and stored at –20 °C until they were assayed. The serum samples were examined by Rose Bengal test (RBT) (Alton et al., 1988) and the positives were further examined with competitive enzyme-linked immunosorbent assay (cELISA) (MacMillan et al., 1990).
The Rose Bengal test (RBT)
The RBT antigen consisting of standardized B. melitensis antigen was sourced from the Animal Health and Veterinary Laboratories Agency, Weybridge U.K. and used to carry out the test (Alton et al., 1988). Briefly, equal volumes (30μl) of antigen and test serum were mixed thoroughly on a plate using a stick applicator and the plate was rocked for 4 minutes. The appearance of agglutination within 2 minutes was scored 2+ (++), while the agglutination after 2 minutes was scored 1+ (+). The absence of agglutination after 4 minutes was scored negative (-). However, all RBT positive samples were further subjected to cELISA test.
Competitive Enzyme Linked Immnunosorbent Assay (cELISA)
The cELISA kit was sourced from the Animal Health and Veterinary Laboratories Agency, Weybridge U.K. The reagents in the kit were reconstituted as directed by the manufacturers. These included control sera, diluting buffer, conjugate, washing solution, chromogen and stopping solution. The test was performed according to the manufacturer's instructions. The optical density (OD) was measured at 450nm using a microplate ELISA reader (Intertek Multiscan M11®). A positive/negative cut off was calculated as 60% of the mean of the OD of the conjugate control wells. Samples in wells with OD equal to or less than the cut-off point were scored positive, while those above were scored negative.
Data analysis
All data were analysed using the STATA software version 12. Group differences were tested using chi-square statistics for categorical variables. A multi-variable adjusted logistic regression was carried out using all the variables that were statistically significant at the 10% level with the main outcome measure (RBT test) in bivariate analysis. All tests were two-tailed and statistical significance was set at p<0.05.
RESULT
The overall sero-prevalence of 2.83% (74/2827) was recorded in all the four States. The highest sero-prevalence of 17.30% was recorded in Benue State, followed by 2.05% in Borno, 0.60% in Oyo State, while a sero-prevalence of 0.00% was recorded in Sokoto using the RBT. Furthermore, the result of the breed prevalence showed the highest infection rate of 2.77% among the Red Sokoto, 2.73% among West African Dwarf and the least sero-prevalence of 1.37% was recorded among the other breeds (Sahel and Kaduna Red; Table 2).
Table 2.
Seroprevalence of brucellosis in goats according to sex, age, breed, source and location
| Brucella infection | |||
|---|---|---|---|
| Characteristic | Positive (n)% | Negative (n)% | p-value |
| Sex | |||
| Male | 16 | 1082 | 0.002 |
| Female | 58 | 1671 | |
| Age | |||
| Adult | 13 | 1991 | <0.0001 |
| Young adult | 61 | 762 | |
| Breed | |||
| Red Sokoto | 52 | 1824 | 0.369 |
| West African Dwarf | 18 | 641 | |
| **Others | 4 | 288 | |
| Source | |||
| Abattoir | 70 | 2335 | 0.020 |
| Herds | 4 | 418 | |
| Location | |||
| Oyo State | 13 | 2142 | <0.0001 |
| Benue State | 57 | 274 | |
| Borno State | 4 | 191 | |
| Sokoto State | 0 | 146 | |
| Total | 74 | 2753 | |
Sahel and Kaduna Red breeds were grouped into others for statistical analysis because they contained cells whose numbers were less than 5
In the same vein, the sex-specific sero-prevalence of 3.47% recorded in female animals was higher than the 1.46% recorded among the male animals. The age specific result showed a sero-prevalence of 7.42% in younger animals which was higher than the 0.65% recorded in older animals. On the basis of sources of samples, the sero-prevalence of 3.00% was recorded in animals sourced from the abattoir while the sero-prevalence of 1.00% was recorded in animals from the households/herds (Table 2).
Our findings as indicated by logistic regression showed that location (p = 0.004) and source (p < 0.0001) of animals were significantly associated with seropositivity of animals to Brucella antibodies. In addition to this, our results showed that animals in Benue State were more likely to be seropositive to Brucella infection than those in Oyo State (OR= 34.40; 95% CI: 18.59 – 63.66; Table 3). Also, goat samples sourced from the abattoir were more likely to be seropositive to Brucella spp antibodies when compared to those from the household/herds (OR= 1.61; 95% CI: 1.31 – 2.00). Furthermore, the logistic regression analysis revealed that there was no significant association between seropositivity and sex (OR= 1.19; 95% CI: 0.98 – 1.44) as well as age (OR= 1.56; 95% CI: 0.91 – 2.71; Table 3).
Table 3.
Results of logistic regression analysis of variables significant at 10% level with the main outcome measure (RBT) in bivariate analysis
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Sex | |||
| Male | 1.0 (referent group) | 0.197 | |
| Female | 1.47 | 0.82 – 2.65 | |
| Age | |||
| Adult | 1.0 (referent group) | 0.791 | |
| Young adult | 1.56 | 0.91 – 2.72 | |
| Source | |||
| Abattoir | 1.0 (referent group) | <0.0001 | |
| Herds | 0.017 | 0.004 – 0.059 | |
| Location | |||
| Oyo State | 1.0 (referent group) | ||
| Benue State | 34.40 | 18.59 – 63.66 | <0.0001 |
| Borno State | 3.45 | 1.11 – 10.68 | 0.032 |
| Sokoto State | 0.016 | 0.003 – 0.015 | <0.0001 |
DISCUSSION
Our findings reveal an overall seroprevalence of 2.83% (with a range of 0.00% to 17.30%) of brucellosis among goats screened across the four states in Nigeria. This result must be put in the context of the fact that goats are generally not vaccinated against brucellosis, neither are there control programmes for the disease in the country; hence, the low grade persistence of the disease in the absence of any outbreak. Furthermore in Nigeria, cattle and goats are generally reared together on free range in rural communities with the possibility of cross infection from cattle. In this present study however, we did not confirm nor identify the Brucella species responsible for infection in the goats in the four states studied. Despite this, we cannot rule out infection of these goats by Brucella species like Brucella abortus as earlier reported in small ruminants in Nigeria (Ocholi et al., 2005). Furthermore, despite the paucity of data on brucellosis in goats in Nigeria, our current findings reveal that the disease is prevalent among goats and this has significant economic and public health implications. Judging from the different results obtained from earlier studies, the overall seroprevalence recorded in this study is comparable to the prevalence of 2.80% obtained in northern Nigeria (Brisibe et al., 1993) and 2.80% in Somalia (Falade and Hussein, 1997); but higher than1.9% in pastoral goats in eastern Ethiopia (Teshale et al., 2007) and 2.00% reported in Uganda (Kabagambe et al., 2001). It is however lower than 16.10% in northern Nigeria (Bale and Nuru.,1982) and 45.75% recorded in an outbreak of brucellosis in a goat flock in Abeokuta, south-western Nigeria (Ojo et al., 2007) as well as 13.60% in goat herds in north-eastern Ethiopia (Adugna et al., 2013). Despite these various findings, we believe that the varying prevalence could be due to geographical differences, sources of animals, sampling techniques, individual differences in interpretation of tests and the number of animals sampled.
Furthermore, our findings recorded a significant association (p <0.0001) between location and seropositivity, with goats sampled in the north having significantly higher sero-prevalence than those sampled in the south of Nigeria. However, it can be observed that the high seroprevalence recorded in the north was mainly due to results from Benue State (17.3%), which is relatively higher when compared to those from other states in this study. The reason for the high prevalence recorded in Benue State may not be unrelated to the fact that animals from this state are sourced from different areas of Nigeria some of which have reported high prevalence of brucellosis in goats (Bertu et al., 2010). For instance, Plateau State, an adjoining state where some of the animals are sourced from, reported a seroprevalence of 16.10% from goats (Bertu et al., 2010). Again, there are similar reports of 29.2%, 23.3% and 26 .7% prevalence reported in Adamawa, Kaduna and Kano (other adjoining states where goats are sourced into Benue State) respectively in cattle (Mai et al., 2012). The relevance of the rates of Brucella infections recorded in cattle in these states becomes useful because it is usual practice for cattle and small ruminants to share common grazing and watering points in the north. Again, Brucella abortus has been isolated from small ruminants kept together with cattle in Bauchi, northern Nigeria (Ocholi et al., 2005).
Intriguing however, is the fact that the 2.05% prevalence recorded in Borno State in this study was lower than the 6.00% earlier reported in Borno and Yobe States (Brisibe et al., 1996) and 4.00% in Yobe State (Tijjani et al., 2009). The difference recorded may be due to the fact that goats screened from Borno State in this study were sourced from herds/household animals as against the other studies where slaughter animals were used. It is important to note, that quite a large number of animals in slaughter houses are culled by farmers for poor performance (Mangen et al., 2002); which could be an indicator of brucellosis. Furthermore, the prevalence of 0.58% recorded in Oyo State is lower than the 4.75% (Falade, 1981) and 9.0% (Ogundipe et al., 1993) previously reported, but similar to 0.80% recorded in a relatively recent study in the same state (Cadmus et al., 2006).
Again, the result of our findings shows that there is no significant association (p = 0.197) between seropositivity to Brucella antibodies and sex of the animal though with a higher rate of infection in the female than male. This is consistent with the studies of Adugba et al. (2013), Teshale et al. (2006) and Ashenafi et al. (2007) who reported no significant association between sex and seropositivity, but in contrast to other studies (Junaidu et al., 2010 and Tijani et al., 2009). Although bucks and does are known to be equally susceptible to Brucella infection (E.C., 2001), however male animals are usually sold off at younger age than the females (Kebede et al., 2008). The higher infection rate of brucellosis in does than bucks reported by some workers may therefore be due to the fact that bucks are generally more aggressive and with few needed for breeding purposes; hence goat farmers rear fewer males. Generally therefore, female animals may have a possible longer time of exposure to Brucella infection than males.
Furthermore, our findings show significantly (p < 0.0001) higher seropositive cases among trade animals when compared to goat flocks kept in the farms and households. This is similar to earlier study carried out by Bale and Nuru (1982) in northern Nigeria. The plausible reason for the higher sero-prevalence recorded in slaughter animals could be attributed to the fact that most of the animals slaughtered in the abattoirs are purchased from livestock markets. Farmers are known to sell animals that are mostly underperforming reproductively or sickly (Mangen et al., 2002). This partly explains why majority (94.59%) of the seropositive goats in this study were from slaughter houses/markets.
In the same vein, although most goats sampled were adults (70.89%), our study recorded higher sero-positivity in young animals (61%) than adults (13%), however there was no significant association (p = 0.719) between Brucella infection and age of animals. While young animals may be infected, they generally do not show any clinical sign but only weak and transient serological response. Conversely, susceptibility to brucellosis increases after sexual maturity and especially with pregnancy (E. C., 2001) but most of the positive young animals in this study were in early puberty; hence the possibility of being infected. Although some of them may have been infected as kids through suckling infected dams, however many may have been exposed through mating with infected bucks. Goats are known to reach puberty between 4 to 8 months of age (Delgadillo et al., 2007) and because mating in free range is not controlled, they become sexually active during early puberty and are therefore exposed to Brucella infection. In addition, these young goats may as well have been exposed to Brucella infection at grazing or watering points through contact with contaminated pasture and water. However, in older animals the disease could become chronic resulting in the antibody titre falling to undetectable levels giving rise to false negative results in serological diagnosis of brucellosis (Godfroid et al., 2002; Tessaro et al., 2004).
It must be noted that although there was no evidence of vaccination of the goats sampled, however the subsequent screening of RBT positive samples with cELISA showed a significantly fewer number of positives (p<0.001). This is comparable to findings in Ethiopia (Teshale et al., 2006). Specificity has been one of the limitations of the RBT test as it provides more likely false positive results than false negative (Omer et al., 2001). This sometimes may be as a result of an overestimation of agglutination reaction by the individual investigator. Again, cross reaction between smooth Brucella antigens and other bacteria species especially Yersinia enterocolitica 0:9, which have been recorded in goats (E. C., 2001), may be the likely cause of divergent in result. This can be related to the fact that antibodies to smooth lipopolysaccharide (SLPs) of Brucella spp are mainly responsible for hummoral immune responses to Brucellae (Cherwonogrodzky et al, 1990). However, the immunodorminant O-side chain of Yersinia enterocolitica 0:9 and Brucella spp are identical (Caroff et al., 1984), resulting in cross reaction between both organisms. On the other hand, while the use of ELISA for the diagnosis of brucellosis in bovines have been well developed, its use in small ruminants still requires more extensive field validation (EC, 2009). Also, while ELISA protocols could be useful in the differentiation of vaccinated and unvaccinated small ruminants (Debbarh et al., 1996), their sensitivity is not very reliable (EC, 2001).
From our findings, the breed specific result revealed no significant association (p = 0.369) between seropositivity and breed of animals. Though the highest seroprevalence was recorded among the Red Sokoto breed, this is not significant when compared to that of WAD and others (Sahel and Kaduna Red). This is comparable to reports of Junaidu et al. (2010) and Tijjani et al, (2009) which also reported highest prevalence in Red Sokoto and Borno white respectively. Junaidu et al, (2010) also reported that there was no significant association between seropositivity and breed of animals.
Despite the results of the study, some limitations were observed. Firstly, more than three-quarters of the total animals screened were from Oyo State as more samples could not be collected from the northern parts of Nigeria due to security concerns; this could have introduced bias into the results of the study. Second, majority of the breed of animals screened were of the Red Sokoto breed, thus making the data to be skewed towards this particular breed. Third, cultures which would have helped to confirm the infection status of the serologically positive animals were not carried out.
Conclusion
Despite some of the limitations in the present study, our findings reveal that brucellosis is endemic in the population of goat screened with a significantly higher prevalence in Benue State compared to other states. The source and location of animals are implicated as potential risk factors in the epidemiology of brucellosis in goats. This can therefore constitute potential risks of infection to the human population given the close interactions between goats, livestock farmers and rural dwellers in such settings in Nigeria and other endemic areas of the world. Again, given close human and animal co-habitations, occupational exposure coupled with consumption of unpasteurised milk and other products from goats, zoonotic transmission of the disease could ensue. Finally, since majority of the animals screened in the present study were from markets/ slaughter houses, further studies should be focused on goats under farm settings living alone or co-habiting or sharing common pastures and water points with sheep and goats to shed more light on the role of goats in transmitting of B. melitensis and B. abortus which are of important zoonotic importance to humans.
Figure 1.
Map of Nigeria showing the study areas
Table 1.
Distribution of goats screened according to sex, age, breed, source and location
| Characteristic | Frequency | Percent |
|---|---|---|
| Sex | ||
| Male | 1098 | 38.84 |
| Female | 1729 | 61.16 |
| Age | ||
| Adult | 2004 | 70.89 |
| Young adult | 823 | 29.11 |
| Breed | ||
| Red Sokoto | 1876 | 66.36 |
| West African Dwarf | 659 | 23.31 |
| **Others | 292 | 10.33 |
| Source | ||
| Abattoir | 2405 | 85.07 |
| Household/Herds | 422 | 14.93 |
| Location | ||
| Oyo State | 2155 | 76.23 |
| Benue State | 331 | 11.71 |
| Borno State | 195 | 6.90 |
| Sokoto State | 146 | 5.16 |
| Total | 2827 | 100.00 |
Sahel and Kaduna Red
Acknowledgement
Data analysis and writing of this paper was supported by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by Fogarty International Centre, the Office of AIDS Research, and the National Human Genome Research Institute of the National Institute of Health, the Health Resources and Services Administration (HRSA) and the Office of the U.S. Global AIDS Coordinator under Award Number R24TW008878. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
References
- Adugna W, Tessema TS, Keskes S. Sero-prevalence of small ruminants’ brucellosis in four districts of Afar National Regional State. Northeast Ethiopia. 2013;5(12):358–364. [Google Scholar]
- Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the Brucellosis Laboratory. National Institute for Agricultual research, rue de I'Universitie, 7500 Paris. 1988 [Google Scholar]
- Ashenafi F, Teshale S, Ejeta G, Fikru R, Laikemariam Y. Distribution of Brucellosis among small ruminants in the pastoral region of Afar. Eastern Ethiopia. Rev. Sci. Tech. Int. 2007;26:731–739. doi: 10.20506/rst.26.3.1781. [DOI] [PubMed] [Google Scholar]
- Bale J, Nuru S. Serological Study of Sheep and goats Brucellosis in Northern Nigeria. Bull. Anim. Hlth. Prod. Afr. 1982;30:73–79. [PubMed] [Google Scholar]
- Brisibe F, Nawathe DR, Bot CJ. Serological prevalence of brucellosis in sheep, goats and human beings in Maiduguri Metropolis. Tropical Veterinarian. 1993;11:27–33. [Google Scholar]
- Blench R. Overseas Development Institute Portland House Stag Place London. SW1E; 1999. Traditional Livestock Breeds: Geographical Distribution and Dynamics in Relation to the Ecology of West Africa. Working Paper 122. p. 5DP. [Google Scholar]
- Brisibe F, Nawathe DR, Bot CJ. Sheep and goat brucellosis in Borno and Yobe States of arids northern Nigeria. J. Small Rum. Res. 1996;20:83–85. [Google Scholar]
- Cadmus SIB, Ijagbone IF, Oputa HE, Adesokan HK, Stack JA. Serological Survey of Brucellosis in Livestock Animals and Workers in Ibadan, Nigeria. African Journal of Biomedical Research. 2006;9:163–168. [Google Scholar]
- Cadmus SIB, Alabi PI, Adesokan HK, Dale EJ, Stack JA. Serological investigation of bovine brucellosis in three cattle production systems in Yewa Division, south-western Nigeria. Journal of South African veterinary Association. 2013;84(1) doi: 10.4102/jsava.v84i1.217. Art. #217, 6 pages. http://dx.doi.org/10.4102/jsava.v84i1.217. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention Brucellosis. 2005 http://www.cdc.gov/ncidod/dbmd/diseaseinfo/brucellosis_t.htm.
- Chenais E, Bagge E, Lambertz ST, Artursson K. Yersinia enterocolitica serotype O:9 cultured from Swedish sheep showing serologically false-positive reactions for Brucella melitensis. Infection Ecology and Epidemiology. 2012;2:19027. doi: 10.3402/iee.v2i0.19027. http://dx.doi.org/10.3402/iee.v2i0.19027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwonogrodzky JW, Dubray G, Moreno F, Mayer H. Antigens of Brucella. In: Nielsen K, Ducan R, editors. Animal Brucellosis. CRC Press; Orlando: 1990. pp. 19–64. [Google Scholar]
- Delgadillo JA, De Santiago-Miramontes MA, Carrillo E. Season of birth modifies puberty in female and male goats raised under subtropical conditions. Animal. 2007;1(6):858–864. doi: 10.1017/S1751731107000080. doi: 10.1017/S1751731107000080. [DOI] [PubMed] [Google Scholar]
- European Commission Brucellosis in Sheep and Goats (B.mellitensis). Scientific committee on Animal Health and Animal Welfare. 2001 [Google Scholar]
- Falade S, Hussein AH. Brucella sero – activity in Somali goats. Trop Anim hlth Prod. 1997;17:93–99. doi: 10.1007/BF02237804. [DOI] [PubMed] [Google Scholar]
- Godfroid J, Saegerman C, Wellemans V, Walravens K, Letesson J-J, Tibor A, Mc MA, Spencer S, Sanna M, Bakker D, et al. How to substantiate eradication of bovine brucellosis when aspecific serological reactions occur in the course of brucellosis testing. Vet Microbiol. 2002;90:461–477. doi: 10.1016/s0378-1135(02)00230-4. [DOI] [PubMed] [Google Scholar]
- Junaidu AU, Daneji AI, Salihu MD, Magaji AA, Tambuwal FM, Abubakar MB, Nawawi H. Sero Prevalence of Brucellosis in Goat in Sokoto, Nigeria. Curr. Res. J. Biol. Sci. 2010;2(4):275–277. [Google Scholar]
- Kabagambe EK, Elzer PH, Geaghan JP, Scholl DT. Risk factors for Brucella seropositivity in goat herds in eastern and western Uganda. Prev. Vet. Med. 2001;52:91–108. doi: 10.1016/s0167-5877(01)00251-3. [DOI] [PubMed] [Google Scholar]
- Kaltungo BY, Saidu SNA, Sackey AKB, Kazeem HM. Serological Evidence of Brucellosis in Goats in Kaduna North Senatorial District of Kaduna State, Nigeria. ISRN Veterinary Science. 2013:1–6. doi: 10.1155/2013/963673. http://dx.doi.org/10.1155/2013/963673. [DOI] [PMC free article] [PubMed]
- Kebede T, Ejeta G, Ameni G. Seroprevalence of bovine brucellosis in smallholder farms in central Ethiopia (Wuchale-Jida district). Revue de Médecine Vétérinaire. 2008;159(1):3–9. [Google Scholar]
- Lucero NE, Ayala SM, Escobar GI, Jacob NR. Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiology of Infectious Diseases. 2008;136:496–503. doi: 10.1017/S0950268807008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai H, Irons P, Thompson P. A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Veterinary Research. 2012;8:144. doi: 10.1186/1746-6148-8-144. doi:10.1186/1746-6148-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manhica AP. The Prevalence of brucellosis in cattle, sheep and goat in Maputo Province, Mocambique. A thesis submitted for the Partial Fulfillment for the Award of Masters degree. Dept. of Veterinary tropical diseases, Faculty of Veterinary Science, University of Pretoria; 2010. [Google Scholar]
- Mohammed FU, Ibrahim S, Ajogi I, Bale JOO. Prevalence of bovine brucellosis and risk factors assessment in cattle herd in Jigawa State. ISRN Veterinary Science. 2011;2011 doi: 10.5402/2011/132897. Article ID 132897, 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocholi RA, Kwaga JKP, Ajogi I, Bale JOO. Abortion due to Brucella abortus in sheep in Nigeria. Rev. sci. tech. Off. int. Epiz. 2005;24(3):973–979. [PubMed] [Google Scholar]
- Ojo OE, Oyekunle MA, Omotainse SO, Ocholi RA, Ogunleye AO, Bertu WJ. Serological evidence of Brucellosis in a goat flock with recurrent abortion Abeokuta. Nigeria. Trop. Vet. 2007;25(1):26–33. [Google Scholar]
- Sofian M, Aghakhani A, Velayati AA, Banifazl M, Eslamifar A, Ramezani A. Risk factors for human brucellosis in Iran: a case—control study. International Journal Infectious Disease. 2008;12:157–161. doi: 10.1016/j.ijid.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Tessaro SV, Forbes LB. Experimental Brucella abortus infection in wolves. J Wildl Dis. 2004;40:60–65. doi: 10.7589/0090-3558-40.1.60. [DOI] [PubMed] [Google Scholar]
- Teshale S, Muhie Y, Dagne A, Kidanemariam A. Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of Eastern Ethiopia : the impact of husbandry practice. Revue Med. Vet. 2006;157(11):557–563. [Google Scholar]
- Tijjani AO, Musa HI, Ousoumanou O, Akintola OO. Prevalence of Brucellosis in Food Animals Slaughtered at Damaturu Abattoir, Yobe State. Sahel Journal of Veterinary Science. 2009;8(1):55–60. [Google Scholar]