Abstract
Ephrin receptor A10 (EphA10), a transmembrane receptor that binds to ephrin, is a newly identified breast cancer marker protein that has also been detected in HER2-negative tissue. In this study, we report creation of a novel bispecific antibody (BsAb) binding both EphA10 and CD3, thereby forming a bridge between antigens expressed on both tumor and immune cells and promoting recognition of tumor cells by immune cells and redirection of cytotoxic T cells (CTL). This BsAb (EphA10/CD3) was expressed in supernatants of BsAb gene-transfected cells as monomeric and dimeric molecules. Redirected T-cell lysis was observed when monomeric and dimeric BsAb were added to EphA10-overexpressing tumor cells in vitro. Furthermore, dimeric BsAb (EphA10/CD3) was more cytotoxic than monomeric BsAb, with efficient tumor cell lysis elicited by lower concentrations (≤10−1 μg/mL) and a lower effector to target (E/T) cell ratio (E/T = 2.5). Dimeric BsAb (EphA10/CD3) also showed significant anti-tumor effects in human xenograft mouse models. Together, these results revealed opportunities to redirect the activity of CTL towards tumor cells that express EphA10 using the BsAb (EphA10/CD3), which could be tested in future clinical trials as a novel and potent therapeutic for breast cancer tumors.
Introduction
Monoclonal antibodies (mAbs) are important therapeutic molecules in the treatment of hematologic malignancies as well as solid tumors [1–4]. Trastuzumab, a humanized mAb directed against human HER2, was approved for clinical use for patients with HER2-overexpressing metastatic breast cancer [5]. The anti-proliferative and cytotoxic effects of trastuzumab likely result from a combination of antibody-dependent cellular cytotoxicity (ADCC), inhibition of extracellular domain cleavage, decreased intracellular signal transduction and anti-angiogenic effects [6]. However, many breast cancer patients with low-level or no HER2 expression do not respond to trastuzumab therapy. Furthermore, the majority of trastuzumab-treated patients will develop resistance to trastuzumab within one year of treatment initiation [7–11]. Moreover, the FcγRIII 158V/F polymorphism interferes with the ability to generate ADCC responses [12, 13]. Therefore, there is a significant need for novel biomarkers and anti-tumorigenic therapeutics for HER2-negative breast cancer patients.
Ephrin receptors, which are a subfamily of receptor tyrosine kinases (RTKs), play important roles in cell–cell communications regulating cell attachment, shape, and mobility in neuronal and endothelial cells [14–16]. Members of the Ephrin receptor family induce the formation of neural networks and promote angiogenesis, and they are overexpressed in various cancer cells [17–19]. Previously, we identified Ephrin receptor A10 (EphA10) as a new human breast cancer biomarker by proteomic analysis [20]. In the Immunohistological (IHC) studies, 49% of breast cancer tissues (93/189) and 44% of HER2 negative tissues (60/136) were EphA10-positive while normal breast tissues were negative. Furthermore, EphA10 was not expressed in 36 normal tissues, with the exception of testis [21]. We also confirmed the testis-specific expression profile of EphA10 at the mRNA level via real-time quantitative PCR methods. Almost all breast cancer patients are female, and therefore EphA10 targeted therapy is a promising therapeutic strategy for most HER2-negative breast cancer patients.
Recently, immune cell-mediated antitumor therapy has been achieved more effectively using bispecific antibodies (BsAb) that bind to surface target antigens on both cancer cells and immune cell such as T-cells [22, 23], NK cells [24, 25] and macrophages [26, 27]. Most BsAb have targeted the CD3 complex on T cells because cytotoxic T cells are some of the most potent killer cells of the immune system. However, monoclonal full IgG therapy is not effective in directly engaging T cells because they lack Fc-gamma receptors. In particular, a bispecific T-cell engager (BiTE), which has the potential to redirect tumor resident and cytotoxic T cells (CTL), showed significant cytotoxicity against tumor cells [22, 23, 28–30]. Actually, the trifunctional Ertumaxomab (HER2/CD3) showed high efficacy for breast cancer patients who express low levels of HER2 and therefore do not respond well to trastuzumab [31]. The development of novel therapeutics is essential because current therapies are inadequate to treat most HER2-negative breast cancer patients.
In this study, we report the construction of a novel BsAb that targets EphA10, thus resembling BiTE technology. We also demonstrate the specificity and potent efficacy of redirected target cell lysis and antitumor activity of BsAb (EphA10/CD3) using both in vitro and in vivo methods. Our findings demonstrate that BsAb (EphA10/CD3) could potentially be used to achieve potent antitumor T-cell responses in EphA10-positive breast cancer patients.
Materials and Methods
Cell lines and culture
Expi293F cells (Invitrogen; Life Technologies; Carlsbad, CA) were cultured in shaker incubators (37°C, 8% CO2) in Expi293 Expression Medium. Hybridoma OKT3 (CRL-8001), MDA-MB-435 (human cancer cell line; HTB-129) and Jurkat (human T lymphocyte; TIB-152) cells were obtained from American Type Culture Collection (ATCC, Rockville, MD) and cultured under the recommended conditions. Human cells that overexpressed EphA10, MDA-MB-435 (MDA-MB-435EphA10), were established in our laboratory. In brief, a lentiviral vector encoding human EphA10 was transfected into MDA-MB-435 cells and stably transfected cells were obtained by Blasticidin (Invitrogen) selection. A hybridoma producing anti-EphA10 IgG was established from splenocytes of a human EphA10-immunized mouse by fusion with a mouse myeloma line. No authentication was done by the authors.
Preparation of PBMC
PBMCs were prepared from the peripheral blood of healthy donors. All the healthy doners gave their written informed consent to participate in the study according to the Helsinki declaration. The study protocol was approved by the local ethics committee (Institutional Review Board of the National Institutes of Biomedical Innovation, Health and Nutrition registered under the number 78 detailed on its website. http://www.nibio.go.jp/part/strategy/ethics/pdf/rinrisinsa_31.pdf)
Cloning of variable (V) immunoglobulin domains
The genes of V light-chain (VL) and V heavy-chain (VH) domains from each hybridoma were subcloned using 5'-Full RACE kits (Takara Bio, Kyoto, Japan). The amplified DNA was directionally subcloned into a plasmid vector using the TOPO TA cloning kit (Invitrogen) and sequenced using a 3130xl Genetic Analyzer (Applied Biosystems, Carlsbad, CA).
Vector construction
The vectors to express the bispecific antibody or single chain Fv (scFv), respectively, were constructed as described previously [32]. The primer sequences are shown in Table 1.
Table 1. Oligonucleotide sequences of PCR primers used for construction of BsAb vectors.
| Name of primer | Nucleotide sequence (5’-3’)* |
|---|---|
| 5’NcoI-VL (hEphA10) | NNNCCATGGCCAGTTTTGTGATGACCCAGACTCCC |
| 3’VL (hEphA10)-Linker | AGAGCCGCCGCCGCCGCTACCACCACCACCAGCCCGTTTGATTTCCAGCTTGG |
| 5’Linker-VH (hEphA10) | GGCGGCGGCGGCTCTGGTGGTGGTGGATCCCAGGTTCTGCTGCAGCAGTCT |
| 3’VH (hEphA10)-NotI | NNNGCGGCCGCTGAGGAGACGGTGACTGAGGTT |
| 5’NcoI-VL (hCD3) | NNNCCATGGCCCAAATTGTTCTCACCCAGTCTCCAG |
| 3’VL (hCD3)-Linker | AGAGCCGCCGCCGCCGCTACCACCACCACCTTTCAGCTCCAGCTTGGTCCC |
| 5’Linker-VH (hCD3)) | GGCGGCGGCGGCTCTGGTGGTGGTGGATCCCAGGTCCAGCTGCAGCAGT |
| 3’VH (hCD3)-NotI | NNNGCGGCCGCTGAGGAGACTGTGAGAGTGGTG |
| 5’HindIII-SP-VL(hCD3) | NNNAAGCTTGTCGACCATGGGATGGTCACTGATTCTGCTGTTTCTGGTCGCCGTCGCTACTGGTGTCCACTCAGATATCCAAATTGTTCTCACCCAGTCTC |
| 3’VH (hCD3)-Linker’ | ACTGCTACCACCACCACCTGAGGAGACTGTGAGAGTGGTG |
| 5’Linker’-VL(hEphA10) | TCAGGTGGTGGTGGTAGCAGTTTTGTGATGACCCAGACTCCC |
| 3’VH (hEphA10)-His tag-NotI | NNNGCGGCCGCTCAATGATGATGATGATGATGTGAGGAGACGGTGACTGAGGT |
| 5’Linker’-VL(His) | TCAGGTGGTGGTGGTAGCAGTTTTGTGATGACCCAGACTCCC |
| 3’VH (His)-His tag-NotI | NNNGCGGCCGCTCAATGATGATGATGATGATGTGAGGAGACGGTGACTGAGGT |
*The restriction enzyme site is underlined.
For subcloning of target genes, the E. coli TOP10 strain (Invitrogen) was used. To obtain an anti-EphA10 scFv and an anti-CD3 scFv fragment, the corresponding VL and VH regions were cloned into separate vectors as templates for VL- and VH-specific PCR using the primer pairs 5’ NcoI-VL (hEphA10 or hCD3) / 3’ VL (hEphA10 or hCD3)-Linker and 5’ Linker-VH (hEphA10 and hCD3) / 3’ VH (hEphA10 or hCD3)-NotI, respectively. Overlapping complementary sequences were introduced into the PCR products that combined to form the coding sequence of the 15-amino acid (G4S)3 Linker during the subsequent fusion PCR. This amplification step was performed with the primer pair 5’ NcoI-VL (hEphA10 or hCD3) / 3’ VH (hEphA10 or hCD3)-NotI, and the resulting fusion product was cleaved with the restriction enzymes NcoI and NotI, then cloned into the pET20b vector (Invitrogen). Next, to construct the bispecific antibody (EphA10/CD3) expression vector, two previously described scFv vectors were used as templates for scFv-specific PCR with the primer pairs 5’ HindIII-Signal Peptide (SP; MGWSLILLFLVAVATGVHS)-VL (hCD3) / 3’ VH (hCD3)-Linker’ and 5’ Linker’-VL (hEphA10) / 3’ VH (hEphA10)-His tag -NotI, respectively. Overlapping complementary sequences were introduced into the PCR products that combined to form the coding sequence of the 5-amino acid G4S Linker during the subsequent fusion PCR. This amplification step was performed with the primer pair 5’ HindIII-SP-VL (hCD3) / 3’ VH (hEphA10)-His tag-NotI, and the resulting fusion product was cleaved with the restriction enzymes HindIII and NotI, then cloned into the pcDNA3.1 (+) vector (Invitrogen). The amino acid sequence of BsAb (EphA10/CD3) is shown in S1 Fig. Furthermore, a vector for the control bispecific antibody (His/CD3) was prepared from an anti- hexahistidine tag (His tag) scFv and an anti-CD3 scFv fragment, following the same procedure.
Expression and purification of BsAb (EphA10/CD3)
BsAb (EphA10/CD3) and BsAb (His/CD3) were prepared using the Expi293 Expression System (Invitrogen). Plasmid DNA (300 μg) and ExpiFectamin 293 Reagent (800 μL) were mixed with Opti-MEM® I medium (15 mL final volume) and allowed to stand at room temperature for 25 min. The mixed solution was added to 7.5 x 108 Expi293 cells cultured in Expi293 Expression Medium and gently mixed in a shaker incubator at 37°C with a humidified atmosphere of 8% CO2 in air. At 18 hours post-transfection, 1.5 mL of ExpiFectamin 293 Transfection Enhancer 1 and 15 mL of ExpiFectamin 293 Transfection Enhancer 2 were added to each flask. The transfected cells were then incubated under the same conditions in a shaking incubator for one week.
Each BsAb was purified from the cell culture supernatant by immobilized metal affinity chromatography (IMAC) and gel filtration chromatography with a Superdex200 prep grade column (GE Healthcare, Little Chalfont Bucks, UK) equilibrated in phosphate-buffered saline (PBS). SDS-PAGE and western blot analysis were performed to detect and confirm the size and purity of each BsAb. Purified proteins were concentrated in PBS by ultrafiltration using a Centriprep® 30K or 50K device (Millipore, Billerica, MA, USA), and protein concentrations were estimated using a Coomassie Plus Protein Assay (Thermo Fisher Scientific, Rockford, IL).
Flow cytometric analysis
5 x 105 cells (MDA-MB-435, MDA-MB-435EphA10, Jurkat) were used for flow cytometry. Each cell was suspended in Suspension buffer (2% FBS containing PBS) and incubated with 6 μg monomeric or dimeric BsAb or control IgG (anti-EphA10, anti-CD3) for 1 hour on ice, respectively. After washing with Suspension buffer, the cells were incubated with Surelight P3 labeled antibodies against the His tag (Columbia Biosciences, Frederick, MD) and Surelight P3 labeled antibodies against the mouse IgG (Columbia Biosciences) for 1 hour on ice. The cells were washed again and resuspended in 500 μL Suspension buffer and flow cytometric analysis was performed (FACScanto; BD Biosciences, San Jose, CA). All tests were carried out in triplicate.
Cytotoxicity assays
Cytotoxicity assays were performed as described previously with slight modifications [32]. In brief, human PBMCs as effector cells were isolated from healthy donors. MDA-MB-435EphA10 cells and MDA-MB-435 parent cells were used as target cells. Target cells (103 cells/well) were added to 96-well plates (NUNC; Life Technologies, Gaithersburg, MD) with 10% FBS containing D-MEM (Wako, Osaka, Japan) at 37°C in a humidified atmosphere containing 5% CO2. After over-night culture, supernatants were removed and non-stimulated PBMC were added to an effector-to-target (E/T) ratio of 2.5 or 5 with each of the antibodies (10−2–10 μg/mL), respectively. In this case, Anti-EphA10 IgG and anti-CD3 IgG were prepared from Hybridomas. After 48 hours of incubation, lactate dehydrogenase (LDH) released into the supernatant was measured using a CytoTox 96® non-radioactive cytotoxicity assay (Promega, Madison, WI). Percentages of specific lysis were calculated according to the formula: % cytotoxicity = [(experimental release)—(effector spontaneous release)—(target spontaneous release)] / [(target maximum release)—(target spontaneous release)] x 100. All tests were carried out in triplicate.
Analysis of Th1 cytokine production
Two plates containing MDA-MB-435EphA10 cancer cells (103 cells/well) or no target cells, respectively, were cultured as described. After overnight culture, non-stimulated PBMC (5 x 103 cells/well) were added to each plate and incubated with various concentrations of each BsAb and control full IgG. Th1 Cytokine (IFN-γ, IL-2 and TNF-α) concentrations in the supernatants were analyzed using an Opti-ELISA kit (BD Biosciences) following the manufacturer’s instructions. All tests were carried out twice.
In vivo efficacy of BsAb (EphA10/CD3) against a xenograft model
In vivo efficacy of BsAb (EphA10/CD3) was evaluated using a xenograft model that consisted BALB/c nu/nu mice (Japan SLC, Inc., Shizuoka, Japan) that received a s.c. engraftment of 1 x 106 MDA-MB-435EphA10 cells with 1 x 106 non-stimulated PBMC. Six animals per group were treated intravenously with 1 or 10 μg dimeric BsAb (EphA10/CD3), 10 μg dimeric BsAb (His/CD3) and 10 μg control full IgG (anti-EphA10, anti-CD3) administered on study days 0, 1, 2 and 3. Tumor growth in two perpendicular directions was measured on the indicated days with calipers and tumor volumes (mm3) were calculated using the formula: V = (width2 x length) / 2. All experimental procedures were conducted in accordance with the Japanese regulations on animal experiments and approved by the Institutional Animal Care and Use Committee of National Institutes of Biomedical Innovation, Health and Nutrition, Osaka, Japan.
Results
Formulation of BsAb (EphA10/CD3)
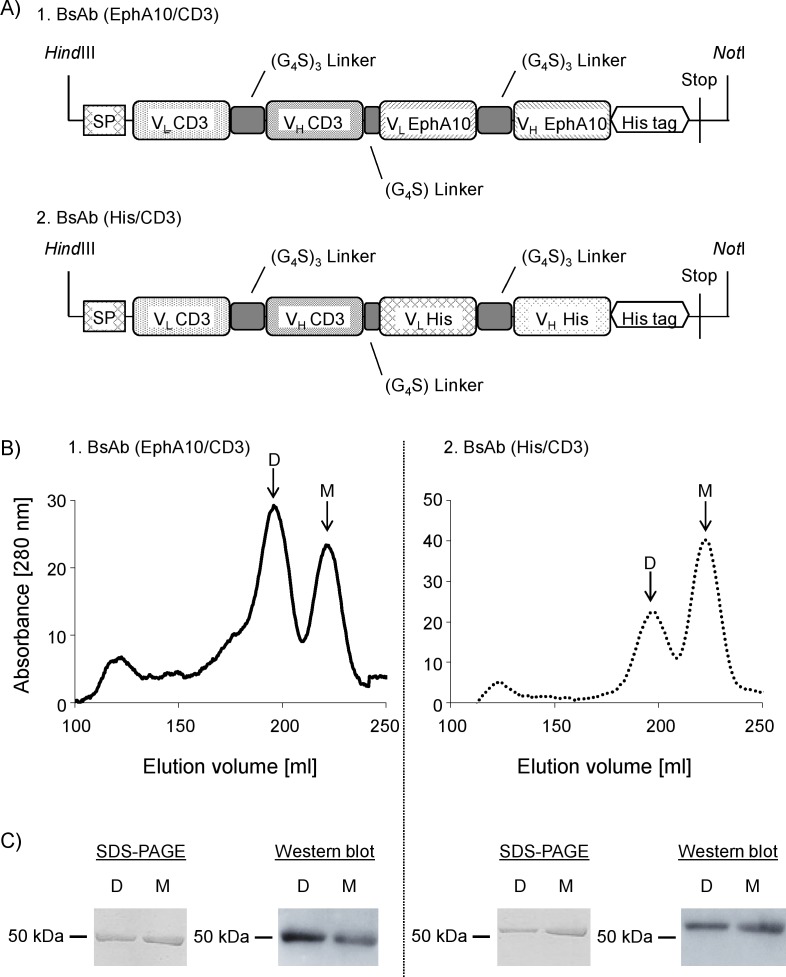
Each BsAb was constructed with the single-chain Fv fragment (VL-(G4S)3-VH) derived from the hybridomas (anti-EphA10 IgG, anti-CD3 IgG) or phage library (anti-His scFv) and then connected by a G4S linker (Fig 1A). The plasmid vector construct was designed by adding an N-terminal signal peptide to express BsAb in a soluble form and adding a C-terminal hexahistidine tag (His tag) to purify it using affinity chromatography on a Ni-Sepharose column. This plasmid vector was transfected into Expi293 cells. Western blot analysis of a small-scale culture (30 mL) revealed that each BsAb was expressed in culture supernatants (data not shown), thus large-scale culture (300 mL) was performed. Pooled supernatants were purified by IMAC, and eluted fractions containing each BsAb were further purified by gel-filtration chromatography, which produced two main peaks, respectively (Fig 1B). SDS-PAGE under reducing conditions followed by western blot analysis showed only a single band indicating a ~50 kDa protein (Fig 1C), consistent with the calculated molecular mass of approximately 53 kDa for each BsAb. Calculated molecular weights of prepared samples, determined using a calibration curve from samples with defined molecular weights run over a gel-filtration chromatography column, are shown in S1 Table. These results suggested that the BsAb (EphA10/CD3) and the BsAb (His/CD3) existed in both monomeric and dimeric forms in the cell supernatants. Other groups have also reported that BsAb of BiTE class could be produced as both monomers and dimers [33, 34]. We expected that the characteristics of the BsAb dimer would differ from those of the monomer. Therefore, the binding specificity and cytotoxic activity of each BsAb species was evaluated.
Fig 1. Characteristics of BsAb (EphA10/CD3) and BsAb (His/CD3).
(A) Gene construct of operons encoding each BsAb in plasmid pcDNA3.1. (B) Gel filtration chromatography profile of BsAb (EphA10/CD3) and BsAb (His/CD3), which were purified with IMAC. (C) SDS-PAGE and Western blot analysis of dimer (lane D) and monomer (lane M) fractions. The parallel line shows molecular size standards with their apparent molecular weights in kiloDalton (kDa). The down arrow shows elution peak of monomeric and dimeric BsAb. Abbreviations: D is dimer, M is monomer.
Binding specificity of BsAb (EphA10/CD3) for human EphA10 and CD3
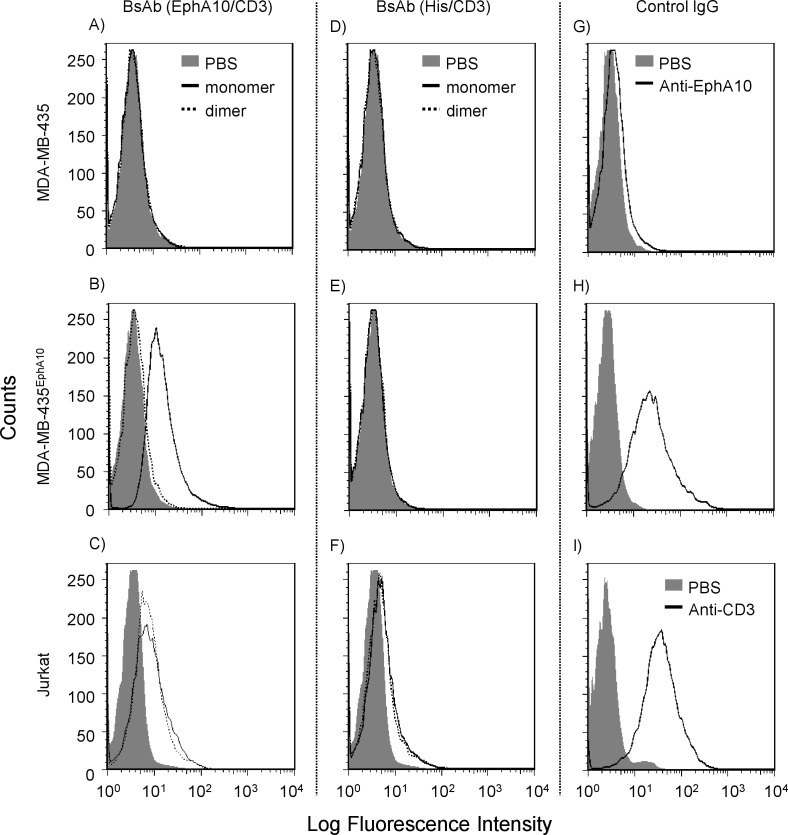
Binding activities of monomeric and dimeric BsAb (EphA10/CD3) were examined by flow cytometric analysis using the MDA-MB-435 parental cells, MDA-MB-435EphA10 cells and Jurkat cells. Specific binding of both EphA10 and CD3 antigens was observed for monomeric and dimeric BsAb (EphA10/CD3) (Fig 2). In this case, Dimeric BsAb (EphA10/CD3) could not be detected, however, we could confirm its binding activity via surface plasmon resonance (SPR) (S2 Fig). On the other hand, monomeric and dimeric BsAb (His/CD3) could bind CD3 not but EphA10. These results demonstrated that the binding activity of each domain was preserved after the bispecific format conversion. Furthermore, the structural differences between monomeric and dimeric forms did not significantly affect the binding activity.
Fig 2. Binding activity of monomeric and dimeric BsAbs.
The left panels (A, B, C) are monomeric and dimeric BsAbs (EphA10/CD3) and the central panels (D, E, F) are monomeric and dimeric BsAbs (His/CD3) and the right panels (G, H, I) are control full IgG (anti-EphA10, anti-CD3). MDA-MB-435 parental cells, human EphA10 transfected cells (MDA-MB-435EphA10) and Jurkat cells (CD3 positive) were used for flow cytometric analysis. Binding activities of each antibody sample were measured at 6 μg. Cell-binding proteins were detected via SureLight P3 conjugated anti-His tag or anti-mouse IgG mAb. Gray filled-in area is vehicle control (PBS). Line indicates each antibody sample (dotted lines are dimeric BsAb).
Redirected target cell lysis of BsAb (EphA10/CD3) with PBMC
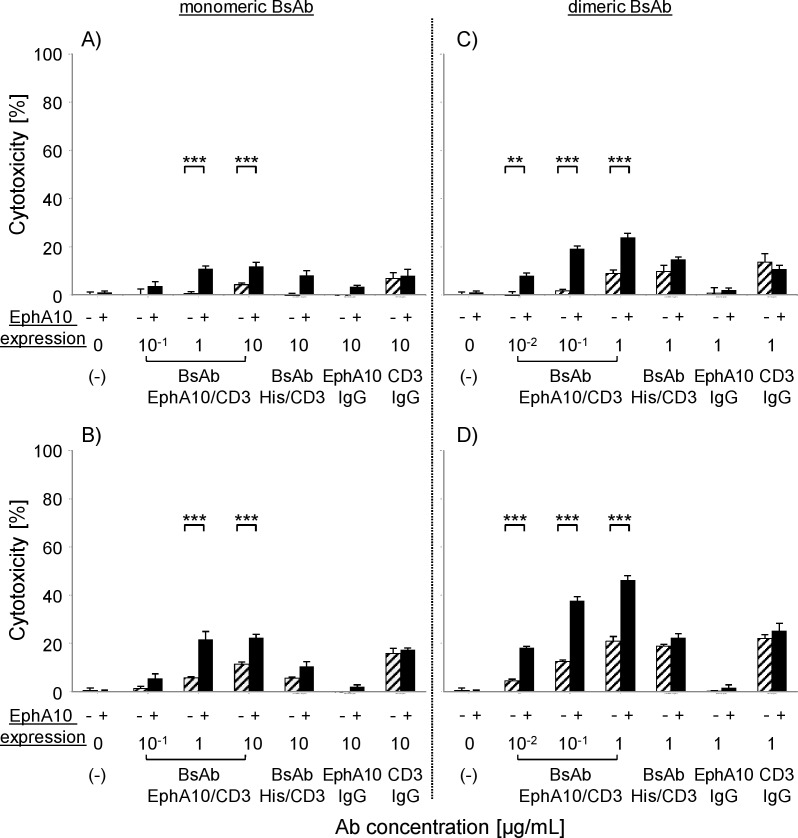
The efficacy of T-cell mediated redirected lysis of MDA-MB-435EphA10 cells and the parental cells following administration of BsAb (EphA10/CD3) was examined using an LDH cytotoxicity assay. Non-stimulated PBMC were used as effector cells at E/T ratios of 2.5 and 5, respectively. As shown in Fig 3, monomeric and dimeric BsAb (EphA10/CD3) showed potent, significant target-specific cytotoxicity against MDA-MB-435EphA10 cells compared with the full IgG constructs (anti-EphA10 IgG, anti-CD3 IgG) and BsAb (His/CD3) in a dose-dependent manner. Furthermore, the cytotoxic efficacy of dimeric BsAb (EphA10/CD3) was higher than that of the monomeric form at low antibody concentrations and low E:T ratios, indicating that dimerization would improve the avidity of binding to each antigen. We also confirmed the target-specific cytotoxicity of BsAb (EphA10/CD3) by testing other EphA10-positive cancer cell lines (S3 and S4 Figs). Although anti-CD3 IgG and dimeric BsAb (His/CD3) showed similar cytotoxicity against both types of cells, this might have been caused by non-specific activation of the PBMC. In this study, we tried to determine the EC50 values of all antibody samples, but we could not get credible data because of the small concentration range (only three different points could be tested). However, dimeric BsAb (EphA10/CD3) showed a significant cytotoxic effect against MDA-MB435EphA10 compared with control samples.
Fig 3. In vitro cytotoxicity of BsAb (EphA10/CD3) against MDA-MB-435EphA10 and parental cells.
The left panels are monomeric BsAb (A, B) and the right panels are dimeric BsAb (C, D). MDA-MB-435 parental cells (slashed column) and MDA-MB-435EphA10 (black column) cells were co-cultured with human PBMC at E/T ratios of 2.5 (A, C) and 5 (B, D). Each point represents the mean of triplicate determinations; Error bars represent the standard deviations of triplicate determinations. Asterisks label readings that were statistically significant (unpaired Student’s T-test) from MDA-MB-435 and MDA-MB-435EphA10 (***: P<0.001, **: P<0.01).
Th1 cytokine production from PBMC by BsAb (EphA10/CD3) stimulation
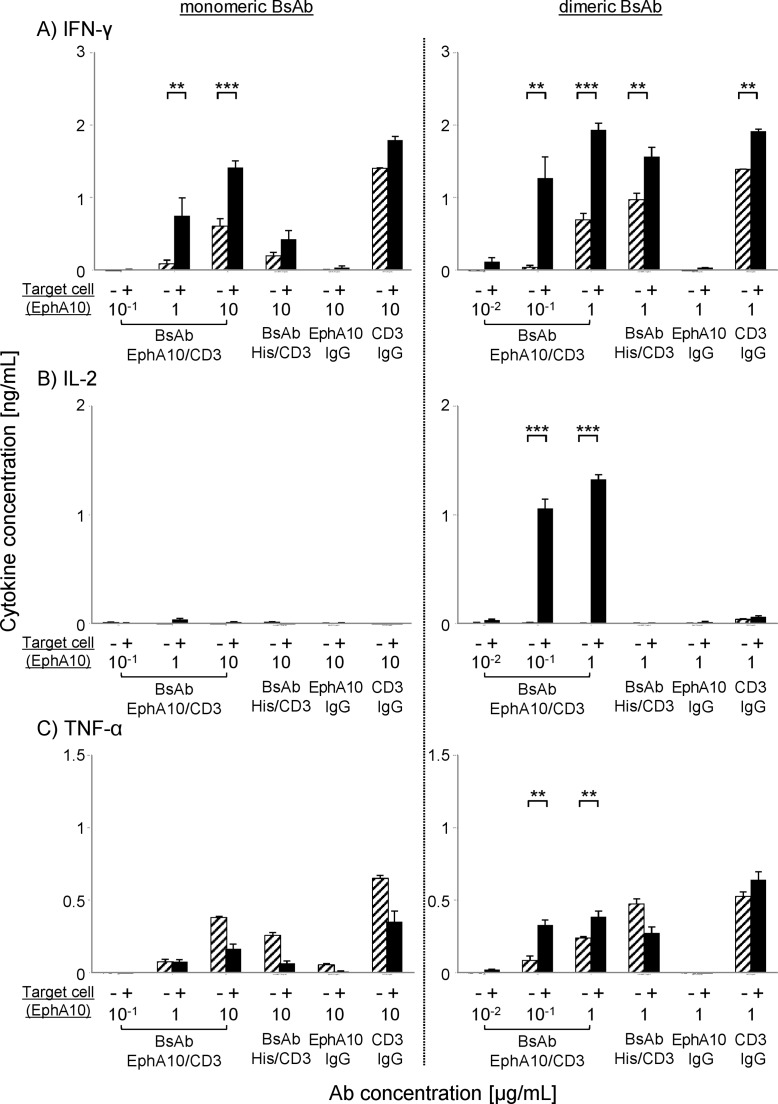
Other groups have reported that Th1 cytokines were secreted from PBMCs in the presence of target cells together with BsAb [33], thus Th1 cytokines were measured to verify the event of T-cell redirected tumor lysis. The immune stimulation of effector cells against target cells by BsAb (EphA10/CD3) was evaluated by measurement of IFN-γ, IL-2 and TNF-α production from activated PBMCs. The results showed that BsAb (EphA10/CD3) induced the production of IFN-γ, IL-2 and TNF-α from activated PBMC (Fig 4). Furthermore, the production of cytokines from PBMC was enhanced in the presence of target cells but not without these cells. This result was consistent with cytotoxicity assay results (Fig 3), and it suggested that the activation of T cells is effectively enhanced only when BsAb (EphA10/CD3) interact with cells expressing both of the target antigens. The production of IL-2 was accelerated in the presence of target cells and effector cells only with the dimeric form of the BsAb (EphA10/CD3), although anti-CD3 IgG and control BsAb (His/CD3) showed similar activation of PBMC not dependent on target cells. These results support our conclusion that the dimeric BsAb (EphA10/CD3) was more cytotoxic than the monomeric BsAb (EphA10/CD3) as a control Ab at low concentrations and low E/T ratios. Because IL-2 has the ability to potently stimulate CTL and NK cells, the dimeric BsAb is an attractive candidate for cancer immunotherapy [35].
Fig 4. Th1 cytokine production from human PBMCs after BsAb (EphA10/CD3) reaction.
Non-stimulated PBMC (E/T ratio of 5) were incubated in the presence of MDA-MB-435EphA10 breast cancer cells (black column) or no target cells (slashed column) with various concentrations of each BsAb (EphA10/CD3) and control sample (BsAb (His/CD3), full IgG). The production of (A) IFN-γ, (B) IL-2 and (C) TNF-α in the presence of monomer (left panel; 1–10 μg/mL) or dimer (right panel; 0.1–1 μg/mL) was determined from the cell culture supernatants. Control samples were incubated with BsAb (His/CD3) (1 or 10 μg/mL) or anti-EphA10 IgG (1 or 10 μg/mL) or anti-CD3 IgG (1 or 10 μg/mL). Each point represents the mean of triplicate determinations; Error bars represent the standard deviations of triplicate determinations. Asterisks label readings that were statistically significant (unpaired Student's T-test) from PBMC with or without MDA-MB-435EphA10 (***: P<0.001, **: P<0.01).
In vivo efficacy of BsAb (EphA10/CD3) in xenograft model
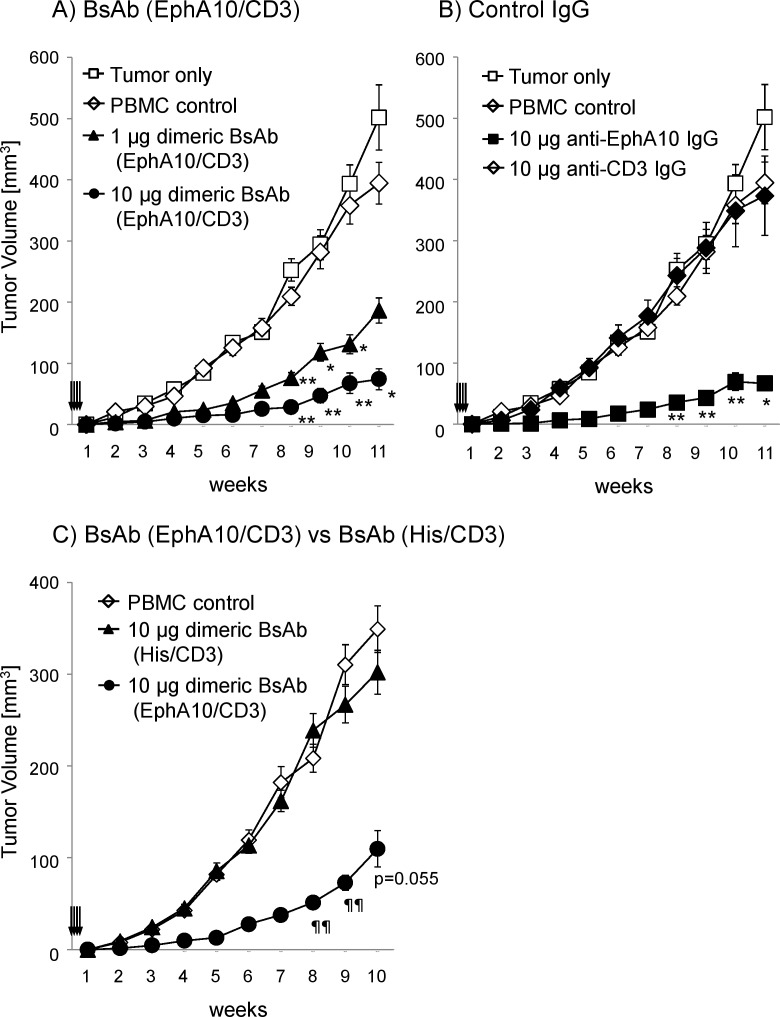
Finally, the antitumor activity of dimeric BsAb (EphA10/CD3) was evaluated in vivo. The xenograft model mice inoculated with MDA-MB-435EphA10 cells were tested. In this experiment, 1 x 106 non-stimulated human PBMC were injected with 1 x 106 tumor cells into BALB/c nu/nu mice. At the beginning of the experiment, mice were then given daily i.v. doses of dimeric BsAb (EphA10/CD3) (1 or 10 μg/mouse) or dimeric BsAb (EphA10/CD3) (10 μg/mouse) or control full IgG (anti-EphA10, anti-CD3; 10 μg/mouse). The in vivo growth of MDA-MB-435EphA10 cells was the same as that of the parental cells in the presence or absence of PBMC. On the other hand, the groups treated with dimeric BsAb (EphA10/CD3) showed tumor growth that was significantly inhibited in a dose-dependent manner (Fig 5A). The antibody concentration range was almost the same as that reported for other BsAbs of the BiTE class [22, 29]. Furthermore, injection with 10 μg dimeric BsAb (EphA10/CD3) produced tumor inhibition just as potent as that of anti-EphA10 IgG (Fig 5B) and dimeric BsAb (His/CD3) had no effect (Fig 5C). S5 Fig shows our evaluation of the anti-tumor effect of another dimeric BsAb (EphA10/CD3’) that was constructed with anti-CD3 IgM. Dimeric BsAb (EphA10/CD3’) did not show an anti-tumor effect because its affinity for CD3 was very low (The dissociation constant, KD≒10−7). Thus, the dimeric BsAb (EphA10/CD3) showed effectiveness against EphA10-positive cancer tumors in vivo only when bound to both EphA10 and CD3.
Fig 5. Dose-dependent effect of dimeric BsAb (EphA10/CD3) in BALB/c nu/nu mice.
Each mouse (n = 6) was inoculated subcutaneously with a mixture of 106 MDA-MB-435EphA10 cells and 106 human PBMC at an E/T ratio of 1 and the indicated doses of dimeric BsAb (EphA10/CD3) were administered intravenously on study days 0 to 3 (arrows). A) Mean values of tumor growth curves are shown for mice that were untreated (⬜) or only PBMC-treated (◇), or treated with PBMC and 1 μg dimeric BsAb (EphA10/CD3) (▲) or 10 μg dimeric BsAb (EphA10/CD3) (●). B) Mean values of tumor growth curves are shown for mice that were treated with PBMC and 10 μg anti-EphA10 IgG (■) or 10 μg anti-CD3 IgG (◆). C) Mean values of tumor growth curves are shown for mice that were only PBMC-treated (◇), or treated with PBMC and 10 μg dimeric BsAb (EphA10/CD3) (●) or 10 μg dimeric BsAb (HIs/CD3) (▲). Values represent mean tumor sizes (in mm3) ± SEM (n = 6 per group). Asterisks label readings that were statistically significant (unpaired Student’s T-test) from the Ab treated group and the untreated group (**: P<0.01, *: P<0.05). Daggers indicate that differences were statistically significant from BsAb (EphA10/CD3) and BsAb (His/CD3) (¶¶: P<0.01).
Discussion
Breast cancer patients are divided into the following four therapeutically-relevant subtypes on the basis of HER2, estrogen receptor (ER) and progesterone receptor (PR) expression levels in tumor cells; luminal A (HER2-, ER+ and/or PR+), luminal B (HER2+, ER+ and/or PR+), HER2-enriched (HER2+, ER-, PR-) and TNBC (HER2-, ER-, PR-). Currently available anticancer mAbs (e.g. Trastuzumab) recognize extracellular or cell surface proteins such as HER2, however, these proteins comprise only a small fraction of cellular proteins and are not completely tumor-specific. EphA10, originally identified as a breast cancer biomarker, is a cell surface receptor and a specific antigen for cancer tissue, including HER2-negative cases [20]. Furthermore, IHC studies revealed that the above four tumors subtypes contained the following percentages of EphA10-positive cells: luminal A (54%), luminal B (68%), HER2-enrich (64%), TNBC (67%). [21]. TNBC is an aggressive disease that is associated with a high proliferation index, visceral and central nervous system metastases, and poor outcomes [36–38]. Therefore, the identification of novel biological markers and generation of more efficient therapeutics against TNBC are needed. In this study, we examined the antitumor effect of BsAb (EphA10/CD3) by cytotoxic assays using PBMC and by administration of this BsAb to xenograft model mice. The results demonstrated a proof of this concept and indicated that EphA10-targeted therapy could be a highly potent new therapy against not only HER2-negative breast cancer but also against TNBC.
Similar to BsAb of the BiTE class, BsAb (EphA10/CD3) induced efficient tumor cell lysis at much lower concentrations than those required for standard mAb therapies. For example, the FDA approved Blinatumomab (human CD19/human CD3), which is a BiTE molecule against acute lymphoblastic leukemia (ALL). Blinatumomab showed highly potent efficacy at a low dose, which was approximately 1000-fold lower than the existing mAb drug doses used for Ph-relapsed/refractory B-precursor patients [39, 40]. The results of an in vitro cytotoxicity assay also demonstrated that a dimeric BsAb (EphA10/CD3) could induce redirected tumor killing by CTL at a low concentration (10−1 μg/mL). Furthermore, the in vivo efficacy of dimeric BsAb (EphA10/CD3) in a xenograft model showed that it could inhibit tumor growth at low doses (1, 10 μg), similar to the effective concentration ranges for other BsAbs [22, 29]. These results indicate that the dimeric BsAb is a potent new BiTE format. Secreted IL-2 is known to be a key factor for T-cell activation and for the anti-tumor effect of BsAb therapy [35]. Dimeric BsAb showed a more highly cytotoxic effect than monomeric BsAb because the production of IL-2 was accelerated only following administration of the dimeric form, as shown in Fig 4B. Multimerization of antibody fragments could improve not only the pharmacokinetic properties but also the rate and/or extent of tumor accumulation [41, 42]. For example, a single-chain diabody format molecule, Tandab, is a dimeric molecule that is expected to show improved potency and efficacy for the reasons described above, and it is now being tested in clinical trials [41, 43]. Thus, dimeric BsAb molecules appear to have significant advantages over monomeric BsAbs.
Although the dimeric form of the BsAb against EphA10/CD3 shows promise as a new therapeutic molecule for the treatment of EphA10-positive cancer, there are still potential problems that could affect its translation to the clinic. First, the drug design of BsAb (EphA10/CD3) should be optimized to improve its therapeutic effects. In vivo studies have indicated the dimeric BsAb (EphA10/CD3) is effective against EphA10-positive cancer tumors, however, we did not demonstrate a significant difference compared with anti-EphA10 IgG. In a previous report, we showed that anti-EphA10 IgG could induce CDC activity [44]. The anti-tumor effect of anti-EphA10 IgG in vivo was caused primarily by CDC effects. The present model could not be simply compared to the individual effects of BsAb and CDC. Furthermore, there is a possibility that this drug could elicit Human Anti-Mouse Antibodies (HAMA) [45] because the product was derived from mouse antibodies. Most antibody drugs are derived from humanized or human antibodies, which have a lower immunogenic potential [46]. In the future, the efficacy of humanized dimeric BsAb (EphA10/CD3) and humanized anti-EphA10 IgG should be compared in human clinical trials. We are currently developing human antibodies against EphA10 and CD3 antigens by using a human antibody library generated in our laboratory. Thus, we are now constructing various BsAbs (EphA10/CD3) from other BsAb formats (include tandem scFv and single-chain Diabody) and different anti-CD3 Ab in order to improve its anti-tumor effect. Of course, we should compare their characteristics in vitro and in vivo, including comparisons with monomeric constructs, to optimize the potential therapeutic effects. Next, it will be necessary to demonstrate the effect of BsAb (EphA10/CD3) against HER2-negative breast cancer derived from patients. The present study demonstrates a high efficacy of BsAb (EphA10/CD3) only against a cell line that overexpresses EphA10 in an early-stage xenograft model mouse. However, it is unclear whether this BsAb (EphA10/CD3) would be effective in terminal cancer patients. Thus, evaluation of other mouse models, for example, tumor-established models and TNBC tissue engrafted xenograft models, should be carried out in the future. The method of creating cancer tissue originated spheroid cells (CTOS) is the most powerful technology to evaluate such a mouse model [47]. This method could be used to generate highly purified and viable primary cancer cells from patients, which could be effectively prepared and cultured in vitro. Furthermore, CTOS formed xenograft tumors in vivo that retained the features of the parental tumors. Thus, it should be possible to evaluate the effect of BsAb (EphA10/CD3) both in vitro and in vivo, and results of these studies could predict clinical responses of breast cancer patients who are presently considered incurable.
In this study, we report the creation and initial testing of a novel BiTE class BsAb, (EphA10/CD3), which stimulates T cells to kill tumor cells that express EphA10 antigens. BsAb (EphA10/CD3) has two advantages as a potential novel breast cancer therapy. One is its specificity for a cancer-associated target molecule, and the other is the highly cytotoxic effect of the dimeric form of this BsAb against cancer cells. Our present findings suggest opportunities for using a BsAb (EphA10/CD3) to treat breast cancer patients whose tumors express EphA10. In the future, this type of BsAb could also be used as a novel drug to treat currently incurable cancers such as TNBC.
Supporting Information
(TIF)
Binding curves were determined by surface plasmon resonance (SPR) measurements with a BiacoreT200 (GE Healthcare). Anti-human antibodies (Human Antibody Capture Kit; GE Healthcare) were immobilized on CM5 chips (at ~10,000 RU) using standard amine-coupling chemistry. After hEphA10-Fc (5 μg/mL) was captured on anti-human antibodies, each BsAb sample (1–240 nM) was injected into the flow cell. Binding response was corrected by subtracting RU from a blank flow cell. A) monomeric BsAb (EphA10/CD3), B) monomeric BsAb (His/CD3), C) dimeric BsAb (EphA10/CD3), D) dimeric BsAb (His/CD3).
(TIF)
The method was describedd in the manuscript.
(TIF)
The left panels are monomeric BsAb (A, B) and the right panels are dimeric BsAb (C, D). Upper panels are MDA-MB-468 and lower panels are LN-Cap. Target cells were co-cultured with human PBMC at E/T ratios of 5. Each point represents the mean of triplicate determinations; Error bars represent the standard deviations of triplicate determinations. Asterisks label readings that were statistically significant (unpaired Student’s T-test) from BsAb (EphA10/CD3) and BsAb (His/CD3) (**: P<0.01, *: P<0.05).
(TIF)
We evaluated the anti-tumor effect of another dimeric BsAb (EphA10/CD3’) that was constructed with anti-CD3 IgM. Each mouse (n = 6) was inoculated subcutaneously with a mixture of 106 MDA-MB-435EphA10 cells and 106 human PBMC at an E/T ratio of 1 and the indicated doses of dimeric BsAb were administered intravenously on study days 0 to 3 (arrows). Mean values of tumor growth curves are shown for mice that were untreated (⬜) or only PBMC-treated (◇), or treated with PBMC and 10 μg dimeric BsAb (EphA10/CD3) (●),10 μg dimeric BsAb (EphA10/CD3’) (×). Values represent mean tumor sizes (in mm3) ± SEM (n = 6 per group). Section signs indicate statistically significant differences from BsAb (EphA10/CD3) and BsAb (EphA10/CD3’) (§§: P<0.01, §: P<0.05).
(TIF)
Calibration curve was obtained by Gel Filtration Calibration Kit LMW and HMW (GE Healthcare).
(TIF)
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Project for Development of Innovative Research on Cancer Therapeutics, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the JSPS. This study was also supported in part by Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan.
Abbreviations
- EphA10
Ephrin receptor A10
- BsAb
Bispecific antibody
- mAbs
Monoclonal antibodies
- ADCC
Antibody-dependent cellular cytotoxicity
- RTKs
Receptor tyrosine kinases
- BiTE
Bispecific T-cell engager
- CTL
Cytotoxic T cells
- ATCC
American Type Culture Collection
- scFv
Single chain Fv
- His
tag Hexahistidine tag
- IMAC
Immobilized metal affinity chromatography
- PBS
Phosphate-buffered saline
- LDH
Lactate dehydrogenase
- TNBC
Triple-Negative Breast Cancer
- ALL
Acute lymphoblastic leukemia
- HAMA
Human Anti-Mouse Antibodies
- CTOS
Cancer tissue originated spheroid cells
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by a Grants-in-Aid for Scientific Research and a Project for the Development of Innovative Research on Cancer Therapeutics from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Japan Society for the Promotion of Science (JSPS) (S. Taki, H. Kamada, S. Tsunoda). This study was also supported in part by Health Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan (H. Kamada, S. Tsunoda).
References
- 1. Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1(11):1311–8. . [PubMed] [Google Scholar]
- 2. Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188–95. . [PubMed] [Google Scholar]
- 3. Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64(6):2127–33. . [DOI] [PubMed] [Google Scholar]
- 4. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87. 10.1038/nrc3236 . [DOI] [PubMed] [Google Scholar]
- 5. Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, et al. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol. 1991;11(3):117–27. . [DOI] [PubMed] [Google Scholar]
- 6. Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2009;27(34):5838–47. 10.1200/JCO.2009.22.1507 . [DOI] [PubMed] [Google Scholar]
- 7. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26(10):1642–9. 10.1200/JCO.2007.11.6699 . [DOI] [PubMed] [Google Scholar]
- 8. Gogineni K, DeMichele A. Current approaches to the management of Her2-negative metastatic breast cancer. Breast Cancer Res. 2012;14(2):205 10.1186/bcr3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6(2):117–27. 10.1016/j.ccr.2004.06.022 . [DOI] [PubMed] [Google Scholar]
- 10. Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17(4):461–9. 10.1038/nm.2309 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reslan L, Dalle S, Dumontet C. Understanding and circumventing resistance to anticancer monoclonal antibodies. MAbs. 2009;1(3):222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koene HR, Kleijer M, Algra J, Roos D, von dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–14. . [PubMed] [Google Scholar]
- 13. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–6. 10.1038/74704 . [DOI] [PubMed] [Google Scholar]
- 14. Xu Q, Wilkinson DG. Eph-related receptors and their ligands: mediators of contact dependent cell interactions. J Mol Med (Berl). 1997;75(8):576–86. . [DOI] [PubMed] [Google Scholar]
- 15. Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3(11):1091–7. 10.1038/80606 . [DOI] [PubMed] [Google Scholar]
- 16. Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3(7):475–86. 10.1038/nrm856 . [DOI] [PubMed] [Google Scholar]
- 17. Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19(49):5614–9. 10.1038/sj.onc.1203856 . [DOI] [PubMed] [Google Scholar]
- 18. Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–80. 10.1038/nrc2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kandouz M. The Eph/Ephrin family in cancer metastasis: communication at the service of invasion. Cancer Metastasis Rev. 2012;31(1–2):353–73. 10.1007/s10555-012-9352-1 . [DOI] [PubMed] [Google Scholar]
- 20. Imai S, Nagano K, Yoshida Y, Okamura T, Yamashita T, Abe Y, et al. Development of an antibody proteomics system using a phage antibody library for efficient screening of biomarker proteins. Biomaterials. 2011;32(1):162–9. 10.1016/j.biomaterials.2010.09.030 . [DOI] [PubMed] [Google Scholar]
- 21. Nagano K, Maeda Y, Kanasaki S, Watanabe T, Yamashita T, Inoue M, et al. Ephrin receptor A10 is a promising drug target potentially useful for breast cancers including triple negative breast cancers. J Control Release. 2014;189:72–9. Epub 2014/06/20. 10.1016/j.jconrel.2014.06.010 S0168-3659(14)00397-6 [pii]. . [DOI] [PubMed] [Google Scholar]
- 22. Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100(6):690–7. 10.1002/ijc.10557 . [DOI] [PubMed] [Google Scholar]
- 23. Flieger D, Kufer P, Beier I, Sauerbruch T, Schmidt-Wolf IG. A bispecific single-chain antibody directed against EpCAM/CD3 in combination with the cytokines interferon alpha and interleukin-2 efficiently retargets T and CD3+CD56+ natural-killer-like T lymphocytes to EpCAM-expressing tumor cells. Cancer Immunol Immunother. 2000;49(8):441–8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartmann F, Renner C, Jung W, Deisting C, Juwana M, Eichentopf B, et al. Treatment of refractory Hodgkin's disease with an anti-CD16/CD30 bispecific antibody. Blood. 1997;89(6):2042–7. . [PubMed] [Google Scholar]
- 25. Zhang T, Sentman CL. Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res. 2011;71(6):2066–76. 10.1158/0008-5472.CAN-10-3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. James ND, Atherton PJ, Jones J, Howie AJ, Tchekmedyian S, Curnow RT. A phase II study of the bispecific antibody MDX-H210 (anti-HER2 x CD64) with GM-CSF in HER2+ advanced prostate cancer. Br J Cancer. 2001;85(2):152–6. 10.1054/bjoc.2001.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fury MG, Lipton A, Smith KM, Winston CB, Pfister DG. A phase-I trial of the epidermal growth factor receptor directed bispecific antibody MDX-447 without and with recombinant human granulocyte-colony stimulating factor in patients with advanced solid tumors. Cancer Immunol Immunother. 2008;57(2):155–63. 10.1007/s00262-007-0357-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagorsen D, Bargou R, Ruttinger D, Kufer P, Baeuerle PA, Zugmaier G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk Lymphoma. 2009;50(6):886–91. 10.1080/10428190902943077 . [DOI] [PubMed] [Google Scholar]
- 29. Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67(8):3927–35. 10.1158/0008-5472.CAN-06-2760 . [DOI] [PubMed] [Google Scholar]
- 30. Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69(12):4941–4. 10.1158/0008-5472.CAN-09-0547 . [DOI] [PubMed] [Google Scholar]
- 31. Jager M, Schoberth A, Ruf P, Hess J, Lindhofer H. The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res. 2009;69(10):4270–6. 10.1158/0008-5472.CAN-08-2861 . [DOI] [PubMed] [Google Scholar]
- 32. Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95(6):2098–103. . [PubMed] [Google Scholar]
- 33. Brischwein K, Schlereth B, Guller B, Steiger C, Wolf A, Lutterbuese R, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43(8):1129–43. 10.1016/j.molimm.2005.07.034 . [DOI] [PubMed] [Google Scholar]
- 34. Asano R, Ikoma K, Shimomura I, Taki S, Nakanishi T, Umetsu M, et al. Cytotoxic enhancement of a bispecific diabody by format conversion to tandem single-chain variable fragment (taFv): the case of the hEx3 diabody. J Biol Chem. 2011;286(3):1812–8. 10.1074/jbc.M110.172957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–90. 10.1038/nri3156 . [DOI] [PubMed] [Google Scholar]
- 36. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. 10.1158/1078-0432.CCR-06-3045 . [DOI] [PubMed] [Google Scholar]
- 37. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. 10.1056/NEJMra1001389 . [DOI] [PubMed] [Google Scholar]
- 38. Crown J, O'Shaughnessy J, Gullo G. Emerging targeted therapies in triple-negative breast cancer. Ann Oncol. 2012;23 Suppl 6:vi56–65. 10.1093/annonc/mds196 . [DOI] [PubMed] [Google Scholar]
- 39. Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–8. 10.1200/JCO.2010.32.7270 . [DOI] [PubMed] [Google Scholar]
- 40. Hoffman LM, Gore L. Blinatumomab, a Bi-Specific Anti-CD19/CD3 BiTE((R)) Antibody for the Treatment of Acute Lymphoblastic Leukemia: Perspectives and Current Pediatric Applications. Front Oncol. 2014;4:63 Epub 2014/04/20. 10.3389/fonc.2014.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Von der Lieth CW, Matys ER, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293(1):41–56. 10.1006/jmbi.1999.3156 . [DOI] [PubMed] [Google Scholar]
- 42. Todorovska A, Roovers RC, Dolezal O, Kortt AA, Hoogenboom HR, Hudson PJ. Design and application of diabodies, triabodies and tetrabodies for cancer targeting. J Immunol Methods. 2001;248(1–2):47–66. . [DOI] [PubMed] [Google Scholar]
- 43. Reusch U, Burkhardt C, Fucek I, Le Gall F, Le Gall M, Hoffmann K, et al. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6(3):728–39. Epub 2014/03/29. 10.4161/mabs.28591 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nagano K, Yamashita T, Inoue M, Higashisaka K, Yoshioka Y, Abe Y, et al. Eph receptor A10 has a potential as a target for a prostate cancer therapy. Biochem Biophys Res Commun. 2014;450(1):545–9. Epub 2014/06/14. 10.1016/j.bbrc.2014.06.007 . [DOI] [PubMed] [Google Scholar]
- 45. Klee GG. Human anti-mouse antibodies. Arch Pathol Lab Med. 2000;124(6):921–3. . [DOI] [PubMed] [Google Scholar]
- 46. Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010;9(10):767–74. 10.1038/nrd3229 . [DOI] [PubMed] [Google Scholar]
- 47. Kondo J, Endo H, Okuyama H, Ishikawa O, Iishi H, Tsujii M, et al. Retaining cell-cell contact enables preparation and culture of spheroids composed of pure primary cancer cells from colorectal cancer. Proc Natl Acad Sci U S A. 2011;108(15):6235–40. 10.1073/pnas.1015938108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Binding curves were determined by surface plasmon resonance (SPR) measurements with a BiacoreT200 (GE Healthcare). Anti-human antibodies (Human Antibody Capture Kit; GE Healthcare) were immobilized on CM5 chips (at ~10,000 RU) using standard amine-coupling chemistry. After hEphA10-Fc (5 μg/mL) was captured on anti-human antibodies, each BsAb sample (1–240 nM) was injected into the flow cell. Binding response was corrected by subtracting RU from a blank flow cell. A) monomeric BsAb (EphA10/CD3), B) monomeric BsAb (His/CD3), C) dimeric BsAb (EphA10/CD3), D) dimeric BsAb (His/CD3).
(TIF)
The method was describedd in the manuscript.
(TIF)
The left panels are monomeric BsAb (A, B) and the right panels are dimeric BsAb (C, D). Upper panels are MDA-MB-468 and lower panels are LN-Cap. Target cells were co-cultured with human PBMC at E/T ratios of 5. Each point represents the mean of triplicate determinations; Error bars represent the standard deviations of triplicate determinations. Asterisks label readings that were statistically significant (unpaired Student’s T-test) from BsAb (EphA10/CD3) and BsAb (His/CD3) (**: P<0.01, *: P<0.05).
(TIF)
We evaluated the anti-tumor effect of another dimeric BsAb (EphA10/CD3’) that was constructed with anti-CD3 IgM. Each mouse (n = 6) was inoculated subcutaneously with a mixture of 106 MDA-MB-435EphA10 cells and 106 human PBMC at an E/T ratio of 1 and the indicated doses of dimeric BsAb were administered intravenously on study days 0 to 3 (arrows). Mean values of tumor growth curves are shown for mice that were untreated (⬜) or only PBMC-treated (◇), or treated with PBMC and 10 μg dimeric BsAb (EphA10/CD3) (●),10 μg dimeric BsAb (EphA10/CD3’) (×). Values represent mean tumor sizes (in mm3) ± SEM (n = 6 per group). Section signs indicate statistically significant differences from BsAb (EphA10/CD3) and BsAb (EphA10/CD3’) (§§: P<0.01, §: P<0.05).
(TIF)
Calibration curve was obtained by Gel Filtration Calibration Kit LMW and HMW (GE Healthcare).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.