Abstract
Asthma and chronic obstructive pulmonary disease co-exist in a significant proportion of patients. Whether asthma increases mortality risk among subjects with airflow limitation remains controversial.
We used data from 2121 adult participants in the population-based TESAOD cohort. At enrollment (1972–73), participants completed questionnaires and lung function tests. Participants were categorized into four groups based on the combination of airflow limitation (AL: FEV1/FVC<70%) and physician-confirmed asthma at baseline. Vital status as of January 2011 was assessed through the National Death Index. Cox proportional hazards models were used to test differences in mortality risk across the four AL/Asthma groups.
In multivariate Cox models, the AL+/Asthma+ group had a 114% increased mortality risk over the follow-up as compared with the AL-/Asthma- group (adjHR: 2.14, 1.64–2.79). The corresponding Hazard Ratios were 1.09 (0.89–1.34) and 1.34 (1.14–1.57) for the AL-/Asthma+ and AL+/Asthma- groups, respectively. Among subjects with AL, asthma was associated with increased mortality risk (1.58, 1.17–2.12). However, this increased risk was substantially reduced and no longer significant after further adjustment for baseline FEV1 levels. Similar results were obtained when AL was defined as FEV1/FVC<lower limit of normal.
In a population-based cohort subjects with concomitant AL and asthma had an increased risk of dying, which was mainly related to their baseline lung function deficits.
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are highly prevalent obstructive lung diseases that have partially distinct risk factors and clinical manifestations, although they sometimes co-exist in the same patients(1–3). Chronic airflow limitation is the hallmark of COPD(4) and at the population level asthma has been shown to be a major risk factor for persistent airflow limitation(5) and to be a co-existing condition in up to 55% of cases of non-fully reversible airflow limitation(6). In this framework, the asthma-COPD overlapping syndrome has been gaining increasing attention as a condition that may have unique characteristics and require targeted disease management(7, 8).
A growing body of evidence indicates that cases with co-existing asthma and COPD have higher health care costs(9–11) and higher degrees of disease severity(12, 13) than patients with either disease alone. In the COPDGene study(13), as compared with patients with COPD alone, those with both COPD and asthma were more likely to experience frequent disease exacerbations, which in turn are known to be related to worse quality of life and higher mortality risk(14, 15). In line with these observations, asthma phenotypes – such as asthma attacks with eosinophilia(16) and bronchial hyper-responsiveness(17) – have been associated with an increased risk of mortality from COPD and, conversely, the presence of airflow limitation or a concomitant diagnosis of COPD has been found to increase mortality risk among patients with asthma(18–21). In a population-based study(22), the combined presence of self-reported MD-diagnosed asthma and COPD was associated with mortality rates that were higher than those associated with either disease alone. However, in apparent contrast with the above studies, several reports that identified patients from health care databases through previous COPD-related hospitalizations or medication use found the presence of concomitant asthma to be associated with no significant effects on, or even with protection against, mortality risk(23–26).
The above discrepancies may be related to the use of population-based versus clinical cohorts, with the latter being more likely to include moderate to severe forms of disease and, in turn, less representative of the entire population of subjects with chronic airway obstruction. The goal of our study was to use the population-based Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD) to determine the combined effects of asthma and airflow limitation (defined as a low ratio between forced expiratory volume in one second – FEV1 - and forced vital capacity - FVC) on all-cause mortality risk over nearly 40 years of follow-up.
Methods
Study population and vital status
TESAOD is a population-based prospective cohort study on non-Hispanic white households initiated in Tucson, AZ in 1972. Details of the enrollment process have been previously reported(28). At enrollment, TESAOD participants completed both a standardized respiratory questionnaire and spirometric lung function tests according to methods previously described(29). Twelve additional follow-up surveys were completed approximately every two years up to 1996 and vital status of TESAOD participants was updated through contact with family and designated next-of-kin and collection of death certificates. In 2013, a review of vital status as of January 1st, 2011 for the TESAOD cohort was completed through linkage with the National Death Index (NDI)(30). Causes of death were determined based on death certificates for events that occurred up to 1978 and based on NDI records for events that occurred after 1978.
Baseline phenotype variables
Physician-confirmed asthma (hereafter referred to simply as asthma) was defined as a positive report in the enrollment survey that the participant was told by a physician that he or she had asthma. Years of formal education, smoking status, and number of pack-years were assessed at baseline based on questionnaire information.
Consistent with previous TESAOD studies, percent predicted values for spirometric indices were computed using reference equations generated in the same population by Knudson and colleagues(31) and lower limit of normal (LLN) equations were derived from Hankinson and colleagues(32). In this study, we used two definitions of airflow limitation at baseline based on a FEV1/FVC ratio either below 70% or below the sex- and age-specific LLN threshold. Low FEV1 was defined as FEV1 < 80% of the predicted value.
At the time of the spirometric test, study nurses measured participants’ weight and height. Body mass index (BMI) was computed and BMI categories were defined as underweight (<18.5), normal weight (≥18.5 and <25), overweight (≥25 and <30), and obese (≥30).
Skin prick tests for allergens common in the Tucson area (house dust; Bermuda grass; tree mix; weed mix; Dematiaceae mold mix) were completed at the baseline survey and positive skin prick tests were defined as a wheal at least three mm larger than the control wheal for at least one tested allergen.
Eosinophilia and serum IgE
Blood samples were collected at enrollment. Eosinophils were measured as percentages from stained slides and blood eosinophilia was defined as eosinophils > 4%. Measurements of serum total IgE were carried out in duplicate according to the paper radioimmunosorbent test (PRIST) (Pharmacia Diagnostics, Piscataway, NJ) method.
Statistical analyses
For main analyses, we categorized subjects into four mutually exclusive groups defined by the combination of airflow limitation and asthma status at baseline (airflow limitation/asthma: no/no; no/yes; yes/no; yes/yes). This process was repeated for each of the two definitions of airflow limitation. For secondary analyses, to evaluate the impact that the combination of low FEV1 and asthma had on mortality risk four mutually exclusive groups were also generated based on the combination of FEV1 % predicted below 80% and asthma status at baseline.
Analysis of variance (ANOVA) and χ2 tests were used to compare baseline characteristics across the four groups. IgE levels were log-transformed to achieve normality. Cox proportional hazards models were used to investigate the association between the airflow limitation / asthma groups and all-cause mortality. In these models, the starting date was the date of completion of the baseline survey and the end date was the date of death if the subject was deceased or January 1st, 2011 if the subject was still alive as of that date. Cause-specific mortality was analyzed in secondary analyses for the three most common causes of death: heart disease, COPD, and cancer. For these analyses, we used competing risk models(33) and results were also confirmed using Cox models with death events due to causes other than the specific cause of interest treated as censored observations. In all analyses, household clustered sandwich estimators of standard errors were used. Three subjects with missing smoking status and/or pack-year information were excluded from Cox models. Seventy-four subjects with missing BMI information were categorized into a BMI “missing” category and 392 subjects with missing eosinophilia information were categorized into an eosinophilia “missing” category so that they could be included in Cox models to maximize sample size in survival analyses.
Results
Baseline characteristics
At baseline, 2495 non-Hispanic white TESAOD participants were between 21 and 80 years of age. Of them, 2121 (85%) completed both questionnaire and lung function tests and were included in the present study. As compared with the 374 subjects with incomplete information, the 2121 subjects included in this study did not differ significantly in terms of sex, age, education, BMI, smoking, or mortality rates during the follow-up.
Tables 1a and 1b show the baseline characteristics of participants across the four groups defined by the combination of airflow limitation and asthma. When airflow limitation was defined as FEV1/FVC < 70% (Table 1a), 78% of participants had neither airflow limitation nor asthma, 8% asthma only, 11% airflow limitation only, and 3% both. Similar percentages were found when airflow limitation was defined as FEV1/FVC < LLN (table 1b). Thus, asthma occurred in 24% of subjects with airflow limitation and airflow limitation was present in 31% of cases of asthma at the population level.
Table 1a.
Baseline characteristics of participants across the four combination groups of airflow limitation (defined as FEV1/FVC < 70%) and asthma
| Total | NO air. limitation / NO asthma | NO air. limitation / YES asthma | YES air. limitation / NO asthma | YES air. limitation / YES asthma | p-value1 | |
|---|---|---|---|---|---|---|
| N (%) | 2121 (100) | 1645 (78) | 165 (8) | 237 (11) | 74 (3) | |
| Sex: N(%) males | 925 (44) | 692 (42) | 66 (40) | 124 (52) | 43 (58) | 0.001 |
| Age in yrs, mean±SD | 50±18 | 49±18 | 46±18 | 60±15 | 59±14 | <0.001 |
| BMI N(%) (N=2047) | 0.001 | |||||
| Normal (18.5–25) | 1140 (56) | 902 (57) | 76 (48) | 129 (57) | 33 (45) | |
| Underweight (<18.5) | 60 (3) | 42 (3) | 1 (1) | 14 (6) | 3 (4) | |
| Overweight (25–30) | 694 (34) | 532 (33) | 60 (38) | 69 (31) | 33 (45) | |
| Obese (≥30) | 153 (7) | 114 (7) | 21 (13) | 14 (6) | 4 (5) | |
| Education: N(%)with > 12 yrs | 913 (43) | 733 (45) | 78 (47) | 78 (33) | 24 (32) | 0.001 |
| Smoking status, N(%) (N=2120) | <0.001 | |||||
| Never smoker | 868 (41) | 737 (45) | 64 (39) | 45 (19) | 22 (30) | |
| Former smoker | 519 (24) | 357 (22) | 45 (27) | 85 (36) | 32 (43) | |
| Current smoker | 733 (35) | 550 (33) | 56 (34) | 107 (45) | 20 (27) | |
| Pack-years2, mean±SD | 26±24 | 23±21 | 21±22 | 40±28 | 39±29 | <0.001 |
| FEV1 % predicted, mean±SD | 93±20 | 98±17 | 89±17 | 73±22 | 59±24 | <0.001 |
| Skin prick tests: N(%) positive (N=2084) | 744 (36) | 552 (34) | 101 (64) | 61 (26) | 30 (43) | <0.001 |
| Eosinophilia: N(%)(N=1600) | 135 (8) | 86 (7) | 18 (14) | 17 (9) | 14 (23) | <0.001 |
| IgE, IU/mL (N=1903)3 | 28 (26, 30) | 24 (22, 26) | 86 (66, 111) | 27 (21, 33) | 82 (54, 125) | <0.001 |
| Self-reported COPD4: N(%) (N=2111) | 318 (15) | 143 (9) | 57 (35) | 69 (30) | 49 (66) | <0.001 |
| Deceased by Jan 1st, 2011: N(%) | 1367 (64) | 994 (60) | 97 (59) | 209 (88) | 67 (91) | <0.001 |
P values are either from oneway analysis of variance (ANOVA) for continuous variables, or Pearson χ2 test for categorical variables across the four groups.
Computed only among smokers (N=1250).
Reported as geometric means (95% CI). Statistical tests were done using log10 transformed values.
Defined as a self-report of having been seen by a doctor for chronic bronchitis and/or emphysema
Table 1b.
Baseline characteristics of participants across the four combination groups of airflow limitation (defined as FEV1/FVC < LLN) and asthma
| Total | NO air. limitation / NO asthma | NO air. limitation / YES asthma | YES air. limitation / NO asthma | YES air. limitation / YES asthma | p-value1 | |
|---|---|---|---|---|---|---|
| N (%) | 2121 (100) | 1667 (79) | 163 (8) | 215 (10) | 76 (4) | |
| Sex: N(%) males | 925 (44) | 717 (43) | 67 (41) | 99 (46) | 42 (55) | 0.146 |
| Age in yrs, mean±SD | 50±18 | 49±18 | 48±18 | 54±18 | 55±17 | 0.0002 |
| BMI N(%) (N=2047) | <0.001 | |||||
| Normal (18.5–25) | 1140 (56) | 905 (56) | 72 (46) | 126 (63) | 37 (49) | |
| Underweight (<18.5) | 60 (3) | 42 (3) | 1 (1) | 14 (7) | 3 (4) | |
| Overweight (25–30) | 694 (34) | 551 (34) | 66 (42) | 50 (25) | 27 (36) | |
| Obese (≥30) | 153 (7) | 117 (7) | 17 (11) | 11 (5) | 8 (11) | |
| Education: N(%)with > 12 yrs | 913 (43) | 738 (44) | 73 (45) | 73 (34) | 29 (38) | 0.027 |
| Smoking status, N(%)(N=2120) | <0.001 | |||||
| Never smoker | 868 (41) | 746 (45) | 65 (40) | 36 (17) | 21 (28) | |
| Former smoker | 519 (24) | 374 (22) | 46 (28) | 68 (32) | 31 (41) | |
| Current smoker | 733 (35) | 546 (33) | 52 (32) | 111 (52) | 24 (32) | |
| Pack-years2, mean±SD | 26±24 | 24±22 | 24±23 | 35±28 | 33±29 | <0.001 |
| FEV1 % predicted, mean±SD | 93±20 | 98±17 | 89±17 | 72±22 | 60±24 | <0.001 |
| Skin prick tests: N(%)positive (N=2084) | 744 (36) | 544 (33) | 99 (63) | 69 (32) | 32 (44) | <0.001 |
| Eosinophilia: N(%)(N=1600) | 135 (8) | 87 (7) | 17 (14) | 16 (10) | 15 (24) | <0.001 |
| IgE, IU/mL (N=1903)3 | 28 (26, 30) | 23 (21, 25) | 83 (64, 108) | 30 (24, 38) | 87 (58, 132) | <0.001 |
| Self-reported COPD4: N(%) (N=2111) | 318 (15) | 146 (9) | 55 (34) | 66 (31) | 51 (67) | <0.001 |
| Deceased by Jan 1st, 2011: N(%) | 1367 (64) | 1037 (62) | 103 (63) | 166 (77) | 61 (80) | <0.001 |
P values are either from oneway analysis of variance (ANOVA) for continuous variables, or Pearson χ2 test for categorical variables across the four groups.
Computed only among smokers (N=1250).
Reported as geometric means (95% CI). Statistical tests were done using log10 transformed values.
Defined as a self-report of having been seen by a doctor for chronic bronchitis and/or emphysema
When airflow limitation was defined as FEV1/FVC < 70% (Table 1a), male sex, older age, and lower education were associated with the presence of airflow limitation with or without asthma. In contrast, being overweight or obese was associated with asthma, independent of the concomitant presence of airflow limitation. The highest percentage of current smokers was found in the group with airflow limitation only and, among smokers, the two groups with airflow limitation had higher pack-years than did the two groups without airflow limitation. High percentages of positive skin prick tests and high serum IgE levels were found in the two groups with asthma. The group with both airflow limitation and asthma had the highest percentage of eosinophilia and, of note, the lowest FEV1 levels.
When airflow limitation was defined as FEV1/FVC < LLN (Table 1b), similar trends were found across the four groups, but sex distribution was not significantly different anymore and age differences were reduced in magnitude.
The relation of airflow limitation and asthma to all-cause mortality
As of January 2011, 1367 (64%) of the 2121 participants had died. Participants in the two groups with airflow limitation had the highest mortality rates (Tables 1a and 1b). After adjusting for age, sex, education, BMI categories, smoking status and pack-years, the two groups with airflow limitation still had a significantly higher mortality risk than subjects with no airflow limitation and no asthma (Table 2). This increased risk was greater in the group with both airflow limitation and asthma. When airflow limitation was defined as FEV1/FVC < 70% (Model 1), as compared with subjects with no airflow limitation and no asthma, the group with airflow limitation only had a 34% increase and the group with both airflow limitation and asthma a 114% increase in all-cause mortality risk. When the two groups were compared with each other, the risk associated with the presence of both airflow limitation and asthma was significantly higher than that associated with the presence of airflow limitation only (adjusted HR 1.60, 95% CI 1.19 to 2.14). In contrast, the presence of asthma only (i.e., without airflow limitation) was not associated with an increased mortality risk (adjusted HR 1.09, NS). Similar results were found when airflow limitation was defined as FEV1/FVC < LLN (Model 2). After full adjustment, significant increases by 37% and 135% in mortality risk were found for the group with airflow limitation only and for the group with both airflow limitation and asthma, respectively.
Table 2.
Cox PH models for all-cause mortality adjusted for sex, age, BMI categories, education, smoking status and pack years. In Model 1, the four combination groups were based on the definition of airflow limitation (AL) as FEV1/FVC < 70%. In Model 2, the four combination groups were based on the definition of airflow limitation as FEV1/FVC < LLN. N=2118.
| Model 1 | Model 2 | |
|---|---|---|
| Airflow limitation defined as | Airflow limitation defined as | |
| FEV1/FVC < 70% | FEV1/FVC < LLN | |
| HR (95% CI) | HR (95% CI) | |
| Sex | ||
| Male | 1 | 1 |
| Female | 0.73(0.65, 0.82)† | 0.73(0.65, 0.82)† |
| Age at baseline in years | 1.11(1.10, 1.11)† | 1.11(1.10, 1.12)† |
| BMI | ||
| Normal (18.5–25) | 1 | 1 |
| Underweight (<18.5) | 1.71(1.17, 2.48)* | 1.69(1.16, 2.47)* |
| Overweight (25–30) | 0.91(0.81, 1.02) | 0.91(0.81, 1.02) |
| Obese (≥30) | 1.32(1.09, 1.59)* | 1.31(1.08, 1.58)* |
| Education: > 12 yrs | 0.90(0.81, 1.00)* | 0.90(0.81, 1.00)* |
| Smoking status | ||
| Never | 1 | 1 |
| Former | 0.89(0.76, 1.05) | 0.89(0.76, 1.04) |
| Current | 1.42(1.21, 1.67)† | 1.41(1.20, 1.66)† |
| Pack-years | 1.01(1.01, 1.01)† | 1.01(1.01, 1.01)† |
| AL / Asthma groups | ||
| AL − / Asthma − | 1 | 1 |
| AL − / Asthma + | 1.09(0.89, 1.34) | 1.08(0.89, 1.31) |
| AL + / Asthma − | 1.34(1.14, 1.57)† | 1.37(1.14, 1.63)* |
| AL + / Asthma + | 2.14(1.64, 2.79)† | 2.35(1.77, 3.11)† |
p<0.05.
p<0.001.
Additional inclusion of total serum IgE and eosinophilia as covariates in the models did not modify the increased risk of mortality associated with the group with combined airflow limitation and asthma (Table E1). However, when combination groups were based on FEV1 % predicted and asthma (Tables E2 and E3), the groups with FEV1 % predicted < 80% showed similar mortality risks independent of whether they had or not asthma. These results suggest that the increased mortality risk seen among subjects with airflow limitation and asthma may be related to the lower FEV1 levels shown by this group.
Effects of asthma on mortality risk among subjects with airflow limitation
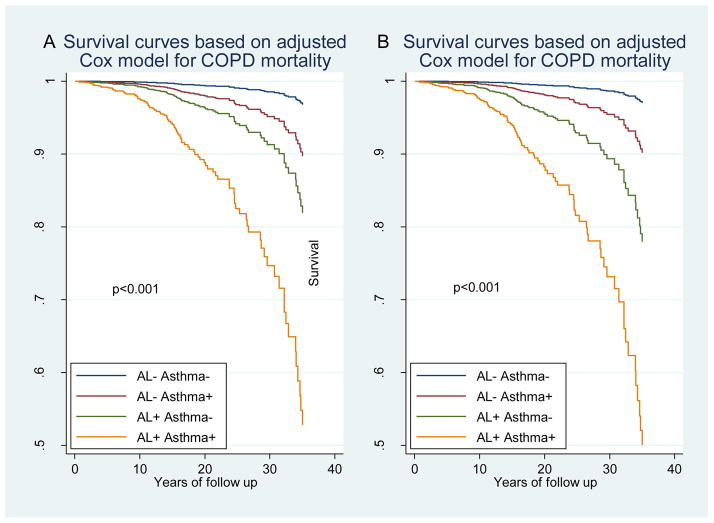
To test the above hypothesis and better characterize the effects of asthma on mortality risk among subjects with airflow limitation, we restricted Cox PH models to the 310 participants with FEV1/FVC < 70% (Table 3a) and to the 291 participants with FEV1/FVC < LLN (Table 3b) at baseline and tested the effects of asthma with and without concomitant adjustment for baseline FEV1 levels. After adjusting for sex, age, BMI, education, smoking status, and pack-years, asthma was significantly associated with a 58% (adjusted HR 1.58, 95% CI 1.17 to 2.12) and a 64% (adjusted HR 1.64, 95% CI 1.18 to 2.29) increased mortality risk among subjects with FEV1/FVC < 70% and among subjects with FEV1/FVC < LLN, respectively (Model 1 in Tables 3a and 3b). However when Cox PH models were further adjusted for baseline levels of % predicted FEV1, the association of asthma with mortality was reduced by more than 50% and was no longer significant (Model 2 in Tables 3a and 3b). These results suggest that lung function deficits explained a large proportion of the increased mortality risk associated with asthma. In line with this scenario, among subjects with baseline FEV1 % predicted < 80% no asthma effects were found on mortality risk (Table E3), and among subjects with airflow limitation at baseline COPD was the only leading cause of death that was increased by the presence of a concomitant asthma diagnosis at baseline (Table E4 and Figure 1).
Table 3a.
Cox PH models for all-cause mortality among the 310 subjects with airflow limitation (AL) at baseline (defined as FEV1/FVC < 70%). Model 1 included sex, age, BMI categories, education, smoking status, pack years, and asthma; and Model 2 was further adjusted for % predicted FEV1.
| Model 1 | Model 2 | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Sex | ||
| Male | 1 | 1 |
| Female | 0.66(0.50, 0.87)* | 0.72(0.55, 0.95)* |
| Age at baseline in years | 1.10(1.08, 1.12)† | 1.10(1.09, 1.12)† |
| BMI | ||
| Normal (18.5–25) | 1 | 1 |
| Underweight (<18.5) | 2.29(1.29, 4.06)* | 2.27(1.27, 4.07)* |
| Overweight (25–30) | 0.71(0.54, 0.93)* | 0.79(0.61, 1.03) |
| Obese (≥30) | 1.09(0.61, 1.96) | 1.04(0.56, 1.93) |
| Education: >12 yrs | 0.76(0.59, 0.98)* | 0.80(0.62, 1.03) |
| Smoking status | ||
| Never | 1 | 1 |
| Former | 0.99(0.66, 1.48) | 1.01(0.67, 1.52) |
| Current | 1.27(0.83, 1.92) | 1.29(0.86, 1.94) |
| Pack-years | 1.01(1.00, 1.02)† | 1.01(1.00, 1.01)* |
| AL / Asthma groups | ||
| AL + / Asthma − | 1 | 1 |
| AL + / Asthma + | 1.58(1.17, 2.12)† | 1.27(0.94, 1.73) |
| FEV1 % predicted at baseline | 0.98(0.98, 0.99)† | |
p<0.05.
p<0.001.
Table 3b.
Cox PH models for all-cause mortality among the 291 subjects with airflow limitation (AL) at baseline (defined as FEV1/FVC < LLN). Model 1 included sex, age, BMI categories, education, smoking status, pack years, and asthma; and Model 2 was further adjusted for % predicted FEV1.
| Model 1 | Model 2 | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Sex | ||
| Male | 1 | 1 |
| Female | 0.73(0.54, 0.99)* | 0.81(0.60, 1.10) |
| Age at baseline in years | 1.10(1.08, 1.11)† | 1.10(1.08, 1.11)† |
| BMI | ||
| Normal (18.5–25) | 1 | 1 |
| Underweight (<18.5) | 2.48(1.29, 4.74)* | 2.46(1.30, 4.65)* |
| Overweight (25–30) | 0.73(0.53, 1.00)* | 0.80(0.60, 1.09) |
| Obese (≥30) | 0.96(0.50, 1.83) | 0.93(0.47, 1.84) |
| Education: > 12 yrs | 0.70(0.53, 0.92)* | 0.71(0.53, 0.94)* |
| Smoking status | ||
| Never | 1 | 1 |
| Former | 1.10(0.70, 1.71) | 1.15(0.73, 1.83) |
| Current | 1.22(0.77, 1.94) | 1.36(0.85, 2.20) |
| Pack-years | 1.01(1.00, 1.02)* | 1.01(1.00, 1.02)* |
| AL / Asthma groups | ||
| AL + / Asthma − | 1 | 1 |
| AL + / Asthma + | 1.64(1.18, 2.29)* | 1.30(0.91, 1.84) |
| FEV1 % predicted at baseline | 0.98(0.97, 0.99)† | |
p<0.05.
p<0.001.
Figure 1.
Survival curves for mortality by COPD across the four AL/asthma groups based on Cox PH models adjusted for sex, age, BMI categories, education, smoking status and pack-years. (A) Survival curves for the four groups based on airflow limitation (AL) defined as FEV1/FVC<70%. (B) Survival curves for the four groups based on airflow limitation defined as FEV1/FVC<LLN.
Discussion
In this study, we found that in a cohort representative of the general adult population the coexistence of airflow limitation and asthma doubled the risk of dying over the follow-up, but these effects were mainly related to the baseline lung function deficits of this group.
Asthmatics who develop airflow limitation and/or chronic lung function deficits have long been known to be at increased risk of dying(18–21). In a Danish cohort of more than 1,000 outpatients with asthma, having FEV1 % predicted levels below 70% increased the risk of dying during the study follow-up by several times(20). Similarly, a 10% increase in baseline FEV1 % predicted was associated with a >20% reduction in mortality risk in a group of 89 patients with chronic asthma followed for 17 years(34). Therefore, it is not surprising that in our study we found a mortality risk twice as high in subjects with asthma and airflow limitation than in subjects with asthma alone.
Nonetheless, whether the presence of asthma increases the risk of dying among subjects with COPD remains controversial. Using NHANES III data, Diaz-Guzman and colleagues found that, as compared with participants who did not report either asthma or COPD, those with COPD alone had a 44% increased risk but those with both COPD and asthma an 83% increased risk of dying(22), suggesting stronger effects on mortality for the latter group. In contrast, several studies that selected patients with COPD based on health care databases and records of hospitalizations and/or treatment did not find increased mortality effects(23), or even reported protective effects(24–26), of a concomitant asthma diagnosis. A possible explanation for these apparently conflicting findings is that co-existing asthma represents a marker of poor prognosis among subjects with airflow limitation in the general population but not necessarily in selected clinical cohorts of COPD patients, which are likely to represent the group of patients with the most severe forms of airflow limitation. At least three observations are consistent with this scenario. First, previous reports from the TESAOD study found decreased rather than increased mortality risk associated with asthma when analyses were restricted to subjects with moderate to severe COPD at baseline(35). Second, in our study only one third of the subjects with airflow limitation in the general population had been seen by a doctor for COPD and this group had baseline FEV1 levels that were >30% lower than those of subjects with airflow limitation but no COPD diagnosis (data not shown). Finally, we did not find different mortality risks associated with asthma when analyses were restricted to subjects with low lung function levels (i.e., FEV1 % predicted < 80%) at baseline.
In line with these observations is also our finding that the increased mortality rates observed in asthmatic subjects with airflow limitation were largely mediated by their decreased lung function because adjustment for baseline FEV1 levels reduced by >50% the effects of asthma on mortality risk among subjects with airflow limitation. In addition, the excess mortality risk associated with asthma was mainly accounted for by death events that listed COPD as the underlying cause of death, even though these cause-specific analyses should be interpreted with caution because of the relatively small sample size.
We have previously reported(5) that, in the TESAOD cohort, subjects who developed persistent airflow limitation in association with asthma and those who developed airflow limitation without asthma showed different profiles of risk factors, with the main risk factor being eosinophilia for the former and smoking for the latter. They also had different trajectories of lung function, with lung function impairment largely related to early adulthood deficits in the group with asthma and to accelerated decline of lung function throughout adult life in the group without asthma. Whether and how these differences are related to the different mortality risks of these two groups remains to be determined. Also, our study did not address specifically clinical differences at baseline between asthmatics with and without airflow limitation that may, in turn, be related to their different mortality risks. It is conceivable that the former are more likely to have more severe and persistent forms of asthma, but this hypothesis should be addressed in prospective studies starting ideally from childhood or young adult age. Finally, in TESAOD no bronchodilator test was completed at baseline and therefore we do not know whether our findings would have been any different if the four groups were defined using post-bronchodilator FEV1/FVC values as recommended by the GOLD guidelines(4). Among the strengths of our study are the population-based nature of the TESAOD cohort, the availability of objectively assessed airflow limitation both based on fixed and LLN cut-offs of FEV1/FVC, and the nearly 40-years long mortality follow-up.
In conclusion, in a sample of the general adult population we found subjects with the concomitant presence of airflow limitation and a diagnosis of asthma to be at increased risk of dying over the follow-up and these effects to be mainly related to their baseline lung function deficits.
Supplementary Material
Acknowledgments
Sources of support This study was supported by awards HL107188 and HL095021 from the National Heart, Lung, and Blood Institute
References
- 1.Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional venn diagram of obstructive lung disease: Two approximations from the united states and the united kingdom. Chest. 2003;124:474–481. doi: 10.1378/chest.124.2.474. [DOI] [PubMed] [Google Scholar]
- 2.Viegi G, Matteelli G, Angino A, Scognamiglio A, Baldacci S, Soriano JB, Carrozzi L. The proportional venn diagram of obstructive lung disease in the italian general population. Chest. 2004;126:1093–1101. doi: 10.1378/chest.126.4.1093. [DOI] [PubMed] [Google Scholar]
- 3.Guerra S, Martinez FD. Epidemiology of the origins of airflow limitation in asthma. Proc Am Thorac Soc. 2009;6:707–711. doi: 10.1513/pats.200908-085DP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 5.Guerra S, Sherrill DL, Kurzius-Spencer M, Venker C, Halonen M, Quan SF, Martinez FD. The course of persistent airflow limitation in subjects with and without asthma. Respir Med. 2008;102:1473–1482. doi: 10.1016/j.rmed.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, Hansell AL, Nowitz MR, McNaughton AA, Soriano JB, Beasley RW. Proportional classifications of copd phenotypes. Thorax. 2008;63:761–767. doi: 10.1136/thx.2007.089193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piras B, Miravitlles M. The overlap phenotype: The (missing) link between asthma and copd. Multidiscip Respir Med. 2012;7:8. doi: 10.1186/2049-6958-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson PG, Simpson JL. The overlap syndrome of asthma and copd: What are its features and how important is it? Thorax. 2009;64:728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 9.Shaya FT, Dongyi D, Akazawa MO, Blanchette CM, Wang J, Mapel DW, Dalal A, Scharf SM. Burden of concomitant asthma and copd in a medicaid population. Chest. 2008;134:14–19. doi: 10.1378/chest.07-2317. [DOI] [PubMed] [Google Scholar]
- 10.Blanchette CM, Gutierrez B, Ory C, Chang E, Akazawa M. Economic burden in direct costs of concomitant chronic obstructive pulmonary disease and asthma in a medicare advantage population. J Manag Care Pharm. 2008;14:176–185. doi: 10.18553/jmcp.2008.14.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanchette CM, Broder M, Ory C, Chang E, Akazawa M, Dalal AA. Cost and utilization of copd and asthma among insured adults in the us. Curr Med Res Opin. 2009;25:1385–1392. doi: 10.1185/03007990902875927. [DOI] [PubMed] [Google Scholar]
- 12.Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, Aldington S, Beasley R. Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Respir J. 2009;34:812–818. doi: 10.1183/09031936.00174408. [DOI] [PubMed] [Google Scholar]
- 13.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, Crapo JD, Hersh CP. The clinical features of the overlap between copd and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 15.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hospers JJ, Schouten JP, Weiss ST, Rijcken B, Postma DS. Asthma attacks with eosinophilia predict mortality from chronic obstructive pulmonary disease in a general population sample. Am J Respir Crit Care Med. 1999;160:1869–1874. doi: 10.1164/ajrccm.160.6.9811041. [DOI] [PubMed] [Google Scholar]
- 17.Hospers JJ, Postma DS, Rijcken B, Weiss ST, Schouten JP. Histamine airway hyper-responsiveness and mortality from chronic obstructive pulmonary disease: A cohort study. Lancet. 2000;356:1313–1317. doi: 10.1016/S0140-6736(00)02815-4. [DOI] [PubMed] [Google Scholar]
- 18.Silverstein MD, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Yunginger JW. Long-term survival of a cohort of community residents with asthma. N Engl J Med. 1994;331:1537–1541. doi: 10.1056/NEJM199412083312301. [DOI] [PubMed] [Google Scholar]
- 19.Lange P, Ulrik CS, Vestbo J. Mortality in adults with self-reported asthma. Copenhagen city heart study group. Lancet. 1996;347:1285–1289. doi: 10.1016/s0140-6736(96)90937-x. [DOI] [PubMed] [Google Scholar]
- 20.Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995;108:10–15. doi: 10.1378/chest.108.1.10. [DOI] [PubMed] [Google Scholar]
- 21.Panizza JA, James AL, Ryan G, de Klerk N, Finucane KE. Mortality and airflow obstruction in asthma: A 17-year follow-up study. Intern Med J. 2006;36:773–780. doi: 10.1111/j.1445-5994.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Guzman E, Khosravi M, Mannino DM. Asthma, chronic obstructive pulmonary disease, and mortality in the u.S. Population. COPD. 2011;8:400–407. doi: 10.3109/15412555.2011.611200. [DOI] [PubMed] [Google Scholar]
- 23.Soriano JB, Vestbo J, Pride NB, Kiri V, Maden C, Maier WC. Survival in copd patients after regular use of fluticasone propionate and salmeterol in general practice. Eur Respir J. 2002;20:819–825. doi: 10.1183/09031936.02.00301302. [DOI] [PubMed] [Google Scholar]
- 24.Mapel DW, Nelson LS, Lydick E, Soriano J, Yood MU, Davis KJ. Survival among copd patients using fluticasone/salmeterol in combination versus other inhaled steroids and bronchodilators alone. COPD. 2007;4:127–134. doi: 10.1080/15412550701341111. [DOI] [PubMed] [Google Scholar]
- 25.Mapel DW, Hurley JS, Roblin D, Roberts M, Davis KJ, Schreiner R, Frost FJ. Survival of copd patients using inhaled corticosteroids and long-acting beta agonists. Respir Med. 2006;100:595–609. doi: 10.1016/j.rmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 26.McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of copd. Chest. 2007;132:1748–1755. doi: 10.1378/chest.06-3018. [DOI] [PubMed] [Google Scholar]
- 27.Bobadilla A, Guerra S, Sherrill D, Barbee R. How accurate is the self-reported diagnosis of chronic bronchitis? Chest. 2002;122:1234–1239. doi: 10.1378/chest.122.4.1234. [DOI] [PubMed] [Google Scholar]
- 28.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102:137–152. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 29.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. The American review of respiratory disease. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 30.Davis KB, Fisher L, Gillespie MJ, Pettinger M. A test of the national death index using the coronary artery surgery study (cass) Control Clin Trials. 1985;6:179–191. doi: 10.1016/0197-2456(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 31.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general u.S. Population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 33.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: Possibilities and pitfalls. International journal of epidemiology. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huovinen E, Kaprio J, Vesterinen E, Koskenvuo M. Mortality of adults with asthma: A prospective cohort study. Thorax. 1997;52:49–54. doi: 10.1136/thx.52.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrows B, Bloom JW, Traver GA, Cline MG. The course and prognosis of different forms of chronic airways obstruction in a sample from the general population. N Engl J Med. 1987;317:1309–1314. doi: 10.1056/NEJM198711193172103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.