Summary
Adult somatic stem cells in various organs maintain homeostatic tissue regeneration and enhance plasticity. Since its initial discovery five decades ago, investigations of adult neurogenesis and neural stem cells have led to an established and expanding field that has significantly influenced many facets of neuroscience, developmental biology and regenerative medicine. Here we review recent progress and focus on questions related to adult mammalian neural stem cells that also apply to other somatic stem cells. We further discuss emerging topics that are guiding the field toward better understanding adult neural stem cells and ultimately applying these principles to improve human health.
Introduction
It is now established that neural progenitor/stem cells (NSCs) reside in the adult mammalian brain and contribute to brain plasticity throughout life (Kempermann and Gage, 1999). Joseph Altman first suggested that neurogenesis, or the generation of new neurons, occurs beyond development in the adult mammalian brain (Altman and Das, 1965). His findings sparked optimism that this endogenous process can be harnessed to repair the injured or diseased brain. Significant progress in the investigation of this phenomenon has since led to remarkable knowledge about adult NSCs and neurogenesis (Ming and Song, 2011). For example, pioneering in vitro analysis demonstrated self-renewal and multipotency of NSCs derived from the adult mammalian brain (Reynolds and Weiss, 1992). In vivo studies using nucleotide analog labeling, retroviral lineage-tracing and genetic fate-mapping later revealed NSC population dynamics, differentiation capacities, regulatory mechanisms and heterogeneity. Single-cell genetic lineage-tracing has illustrated the existence of endogenous adult mammalian NSCs with hallmark stem cell properties (Bonaguidi et al., 2011).
This review focuses on topics of adult NSCs that can be applied more broadly to somatic stem cells in many other tissues, such as bone marrow, blood, endothelium, skin, fat, gastrointestinal tract, liver, lung, endocrine organs, and skeletal muscle (Li and Clevers, 2010). Adult somatic stem cells share fundamental properties, including self-renewal, relative quiescence, differentiation capacity and residence within a specific environment or niche (Figure 1A). We focus on recent progress delineating the composition of specialized neurogenic niches, signaling mechanisms, and potential functions of NSCs in the adult mammalian brain. We also explore emerging topics in the adult somatic stem cell field, such as single-cell analysis, human stem cells, and reprogramming. We hope to provide an integrated view of adult NSCs and prompt new questions and theories about regulation and potential function of adult somatic stem cells.
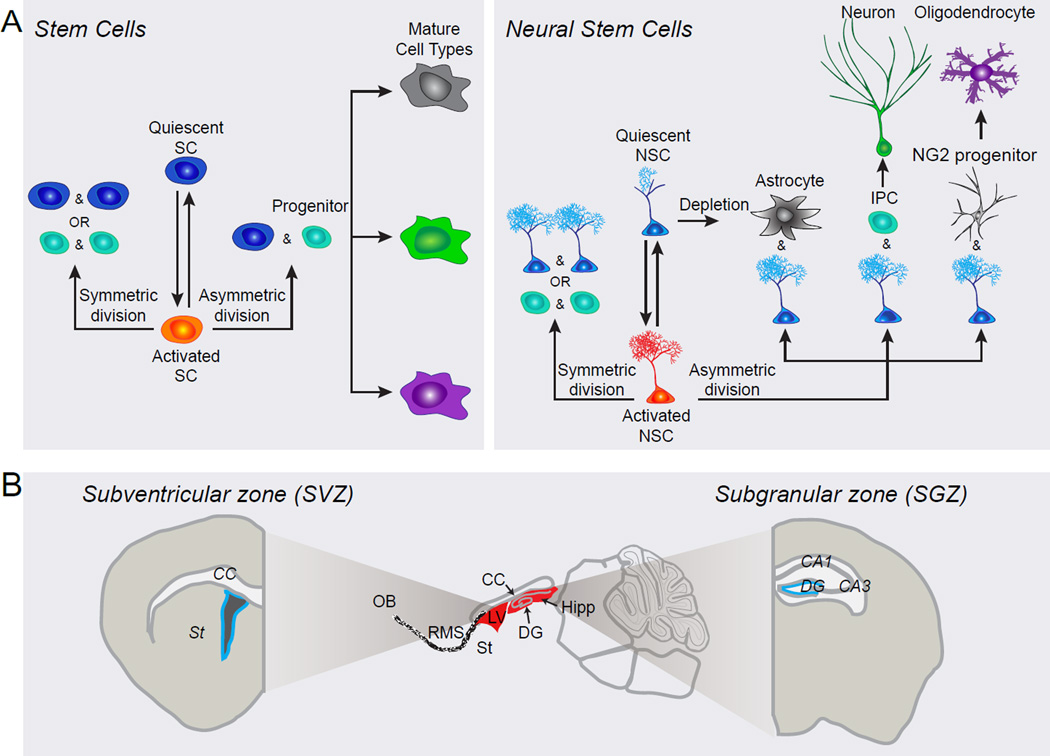
Figure 1. Behavior of neural stem cells within adult niches.
(A) A schematic diagram illustrating potential behavior of an adult stem cell and, more specifically, of an adult neural stem cell (NSC) over its life cycle. Adult NSCs can transition between quiescent and active states by exiting and entering cell cycle, respectively. Once activated, NSCs choose between different modes of division. Asymmetric division is self-renewing and yields an NSC and a progenitor, while symmetric division yields either two NSCs (self-renewing) or two progenitors (not self-renewing). Progenitors may be fate restricted, meaning that they can only differentiate into a particular cell type, or they may be multipotent and must make a fate choice before differentiation. It is also possible that NSCs may directly differentiate into mature glial cell types. Though this diagram depicts a wide range of NSC activities, specific NSC populations may exhibit a predisposition for certain activities, such as asymmetric division or quiescence.
(B) A sagittal view of the adult rodent brain, focusing on two major niches where adult NSCs reside: the subventricular zone (SVZ) and the subgranular zone (SGZ). The SVZ is located along the lateral ventricle in the forebrain, while the SGZ is located in the hippocampus along the dentate granule cell layer where it abuts the hilus. CC, corpus callosum; DG, dentate gyrus; Hipp, hippocampus; LV, lateral ventricle; NSC, neural stem cell; OB, olfactory bulb; RMS, rostral migratory stream; SC, stem cell; St, striatum.
Neural Stem Cells in the Adult Mammalian Brain
There are two major neurogenic niches in the adult mammalian brain where endogenous NSCs reside, the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) within the dentate gyrus of the hippocampus (Figure 1B). The SVZ is located along the ependymal cell layer, which separates the ventricular space from the SVZ (Figure 2A). Adult SVZ NSCs (also named Type B cells) extend a basal process to terminate on blood vessels and extend an apical process with a primary cilium that pokes through the ependymal cell layer to contact the cerebrospinal fluid (CSF) in the ventricle (Mirzadeh et al., 2008). Type B NSCs give rise to transient amplifying progenitors (C cells) (Doetsch et al., 1999), which divide a few times before becoming neuroblasts (A cells). Neuroblasts then form a chain and migrate into the olfactory bulb where they migrate radially and differentiate into different subtypes of interneurons. Radial glia-like NSCs (named RGLs or Type 1 cells) in the SGZ, at the border between the inner granule cell layer and hilus, give rise to intermediate progenitor cells (IPCs) (Seri et al., 2001), which exhibit limited rounds of proliferation before generating neuroblasts (Berg et al., 2015) (Figure 2B). Neuroblasts migrate tangentially along the SGZ and develop into immature neurons, which migrate radially into the granule cell layer to differentiate into dentate granule neurons (Sun et al., 2015).
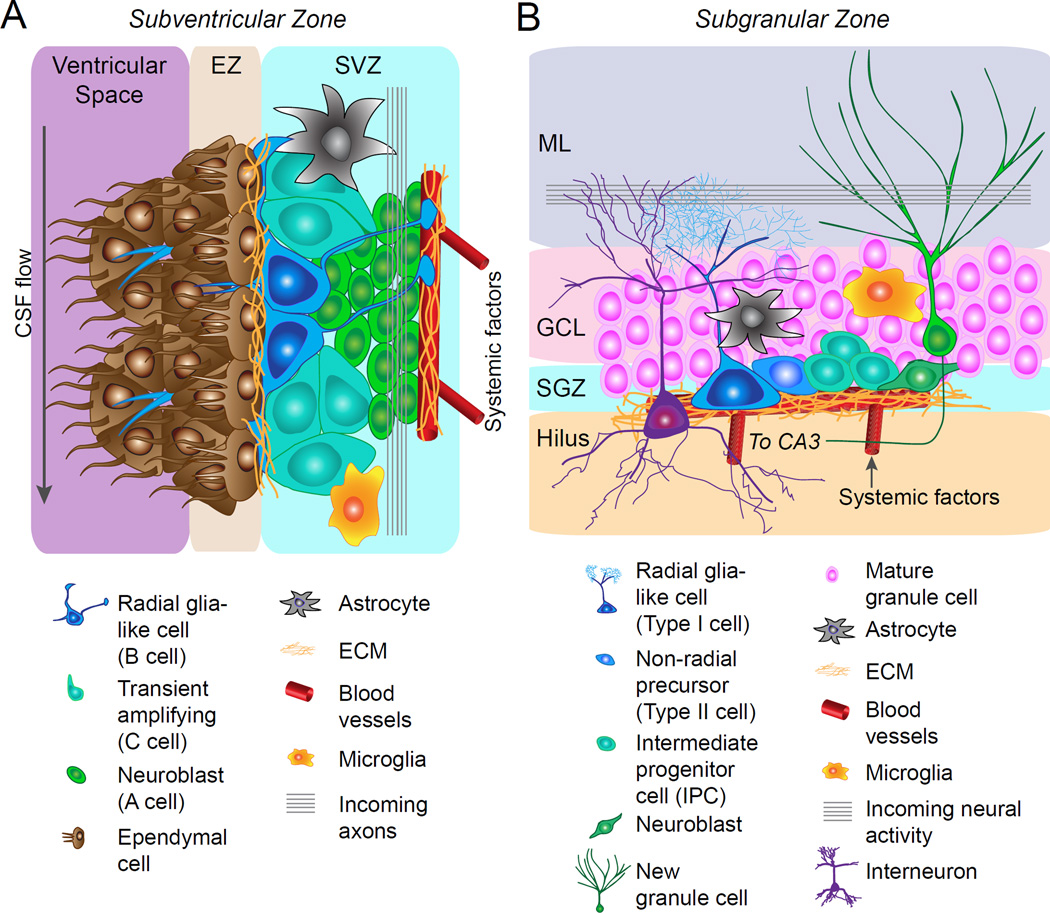
Figure 2. Adult neural stem cell niches.
(A) A schematic diagram depicting cellular and molecular components of the subventricular zone (SVZ) niche. Ependymal cells are organized into rosette shaped structures which line the lateral ventricle and border the SVZ. Radial glia-like neural stem cells (B cells) reside along the ependymal zone in the SVZ and extend a radial process to contact blood vessels. They also extend a single cilium through the ependymal rosettes to contact the cerebrospinal fluid in the ventricular space. Radial glia-like neural stem cells (NSCs) generate transit amplifying cells (C cells), which generate neuroblasts (C cells). Neuroblasts migrate down the rostral migratory stream to the olfactory bulb where they differentiate into olfactory bulb neurons. In addition to the aforementioned cell types, astrocytes and microglia contribute to the cellular architecture of the niche. Molecular niche signals contribute to both adult NSC niches, including morphogens (e.g. BMPs, SHH, Wnts, Notch), growth factors (e.g. VEGF, IGF, EGF), neurotrophins (e.g. BDNF, NT-3, NGF), cytokines (e.g. interleukins), neurotransmitters (GABA, 5-HT, Ach, dopamine), extracellular matrix (e.g. laminins, proteoglycans), cell-cell signaling molecules (e.g. Ephrins, Connexins) and systemic factors (e.g. CCL11, GDF11).
(B) A schematic diagram depicting cellular and molecular components of the subgranular zone (SGZ) niche. Radial glia-like NSCs (Type I cells) reside in the SGZ and extend a radial process through the granule cell layer of the dentate gyrus into the molecular layer. Radial glia-like NSCs generate intermediate progenitor cells (IPCs), which generate neuroblasts and these progenitor cells are closely associated with the vasculature. Neuroblasts differentiate into dentate granule cells which migrate into the granule cell layer of the dentate gyrus. In addition to the aforementioned cell types, astrocytes, microglia, and interneurons contribute to the cellular architecture of the niche. ECM, extracellular matrix; EZ, ependymal zone; GCL, granule cell layer; ML, molecular layer; SGZ, subgranular zone; SVZ, subventricular zone.
Two fundamental properties of stem cells are the ability to self-renew and to give rise to differentiated progeny (Figure 1A). It had long been postulated that adult neurogenesis originates from tri-potent NSCs with the capacity to generate neurons, astrocytes and oligodendrocytes. The existence of self-renewing, multipotent adult NSCs was originally suggested by long-term expansion and differentiation into three neural lineage by neurospheres - non-adherent, spherical cultures of clonally derived precursors, or monolayer cultures (Palmer et al., 1997; Reynolds and Weiss, 1992). However, recent genetic fate-mapping and clonal lineage-tracing of NSCs in the adult hippocampus in vivo have found generation of neurons and astrocytes, but not oligodendrocytes (Bonaguidi et al., 2011). In the adult SVZ, population fate-mapping studies have shown generation of both neurons and oligodendrocytes. However, recent in vivo clonal analysis has found only neuronal lineages from individual NSCs (Calzolari et al., 2015), whereas in vitro time-lapse analysis revealed generation of either neurons or oligodendrocytes from acutely isolated individual precursor cells, but never both (Ortega et al., 2013). Therefore, whether endogenous NSCs with an intrinsic tri-lineage potential exist in the adult mammalian brain remains a fundamental question. In one model, NSCs are gradually specified during development and become restricted in the lineage potential in adult (Kriegstein and Alvarez-Buylla, 2009); Alternatively, adult NSCs could be intrinsically tri-potent, but with some latent lineage potentials constitutively suppressed by the niche. Whether endogenous adult NSCs exhibit long-term self-renewal is also under debate. In the adult SGZ, one population level study suggested that NSC activation leads to multiple rounds of proliferation, which is coupled with depletion via differentiation into astrocytes without ever returning to quiescence (Encinas et al., 2011). Clonal analysis, on the other hand, showed cycles of activation, a return to quiescence, and re-activation of individual NSCs, with moderate depletion via astrocytic transformation (Bonaguidi et al., 2011). In the adult SVZ, clonal analysis revealed rapid progeny expansion and then depletion of NSCs (Calzolari et al., 2015). Different genetic labeling approaches could target different populations of NSCs and/or different states of the same NSC population, thereby producing seemingly disparate results. Given that the most primitive NSCs are rare, it will be critical to develop specific targeting approaches to examine their self-renewal capacity and lineage potential at the clonal level in the future.
Studies using an anti-mitotic drug to eliminate dividing precursors showed that adult NSCs are largely quiescent in vivo (Doetsch et al., 1999). Quiescence is thought to allow adult stem cells to withstand metabolic stress and to preserve genome integrity over a life time. The quiescent state has long been viewed as dormant and passive. Emerging evidence suggests just the opposite. Recent single-cell transcriptome analysis of quiescent adult SGZ NSCs showed active expression of various receptors for niche signals and downstream signaling components, the majority of which are down-regulated once NSCs become activated (Shin et al., 2015). Functionally, decreasing GABA (Song et al., 2012) or Wnt inhibitor expression (Jang et al., 2013) leads to quiescent NSC activation. The concept that quiescence is an actively maintained state is gaining broad empirical support in multiple somatic stem cell systems (Cheung and Rando, 2013).
Another emerging theme in stem cell biology is heterogeneity. In most adult somatic stem cell systems, quiescent and active stem cell populations co-exist (Li and Clevers, 2010). The most obvious example of heterogeneity among adult NSCs is the difference in their progeny between two niches: SVZ NSCs generate olfactory bulb interneurons and corpus callosum oligodendrocytes, whereas SGZ NSCs generate dentate granule neurons and astrocytes. Notably, NSCs derived from both niches generate all three neural lineages once propagated in culture with high concentrations of growth factors (Palmer et al., 1997; Reynolds and Weiss, 1992), therefore the in vivo niche may limit adult NSC potential. Within the adult SVZ, progeny subtypes are further specified according to where the mother NSC is located along the ventricle. For example, ventral NSCs produce deep granule cells and calbindin-expressing periglomerular cells, whereas dorsal NSCs produce superficial granule cells and tyrosine hydroxylase-expressing periglomerular cells (Merkle et al., 2007). This form of positional heterogeneity could be due to regional differences in the niche and/or intrinsic differences in NSCs. In the adult dentate gyrus, multiple inducible reporter lines, such as GLAST-CreERT2 and Nestin-CreERT2, label adult NSC populations that react to environmental stimuli differently (DeCarolis et al., 2013). It should be noted that phenotypic heterogeneity could reflect distinct populations or different states of the same population of stem cells, which cannot be resolved via snap-shot or short-term lineage-tracing analyses. This represents a technical challenge for the whole somatic stem cell field, which may require a combination of single-cell long-term lineage-tracing, single-cell transcriptome analysis and modeling to resolve.
A related question is how is adult NSC heterogeneity established during development or more generally, what are the embryonic origin(s) of adult NSCs? Adult SGZ NSCs appear to originate from the ventral hippocampus during late gestation and then re-locate to the dorsal hippocampus (Li et al., 2013). Two recent studies suggested that adult SVZ NSCs are regionally specified at an early embryonic stage and then remain largely quiescent until re-activation postnatally (Fuentealba et al., 2015; Furutachi et al., 2015). In contrast to substantial knowledge about origins of other somatic stem cells (Barker et al., 2010), very little is known about the identity, location and properties of precursors that give rise to adult NSCs in the SGZ and SVZ.
Adult Neural Stem Cell Niche
Somatic stem cells reside in niches with specific molecular and cellular characteristics. Adult NSC niches include ependymal cells, vascular cells, and immature and mature lineages of NSCs (Ma et al., 2005). Unique to the adult SVZ, ependymal cells line the ventricular surface, and their apical surface is covered in cilia which beat the CSF, circulating it through the ventricle (Figure 2A). Ependymal cells are organized into pinwheel formations, and at the center of each pinwheel, NSCs extend a single apical cilium to directly contact the CSF (Mirzadeh et al., 2008) (Figure 2A). Ependymal cells secrete signaling factors, such as noggin, an inhibitor of BMP signaling, which allows for activation of NSCs. Compared to non-neurogenic regions, the vasculature is highly organized with unique architecture throughout both adult SGZ (Sun et al., 2015) and SVZ (Shen et al., 2008) and Adult NSCs extend processes that are closely associated with blood vessels (Figure 2). Both endothelial cells themselves, as well as factors transported through the blood, provide signals that impact NSCs. For example, endothelial cells secrete vascular endothelial growth factor (VEGF) which promotes NSC self-renewal, and neurotrophin-3 (NT-3), which promotes quiescence and long-term maintenance (Delgado et al., 2014). In addition, the blood-brain barrier is leaky near clusters of proliferating NSCs, which would allow these cells to more easily access factors in the blood (Tavazoie et al., 2008). Under homeostatic conditions, microglia phagocytose IPCs that have undergone apoptosis (Sierra et al., 2010), while activated microglia reduce neurogenesis (Ekdahl et al., 2003). It has become clear for multiple somatic stem cell systems that stem cells themselves, and their immature and mature progeny, are an integrated part of the niche. Adult NSCs release factors that contribute to autocrine and paracrine signaling (Alfonso et al., 2012). Immediate progeny of NSCs provide diffusible or contact-mediated signals, such as the neurotransmitter GABA (Liu et al., 2005) and Notch ligand Delta-like 1 (Dll1) (Kawaguchi et al., 2013), to regulate NSC quiescence as a part of the feedback mechanism for homeostasis. Astrocytes secrete cytokines, such as IL-1β and IL-6, which promote neuronal differentiation of NSCs (Barkho et al., 2006). Finally, adult NSCs extend long radial processes that potentially integrate signals along their length. In the SGZ, radial processes extend through the entire granule cell layer up into the molecular layer of the dentate gyrus where they branch out extensively, forming a tuft of processes. This structure may allow NSCs to sample and integrate neuronal circuitry activity. Indeed, adult NSCs are regulated by neurotransmitters released by mature neurons (Berg et al., 2013). Together, these results demonstrate that multiple cell types contribute to the signaling milieu of the niche, which represents a general theme for various adult somatic stem cells.
The extracellular matrix (ECM) is a network of molecules that provides a structural framework for surrounding cells and is involved in signaling. In adult neurogenic niches, the ECM contains molecules such as laminins, proteoglycans, and tenascin C (Kazanis and ffrench-Constant, 2011). Fractones, the first ECM structures described in the adult niches, contact precursors and trap growth factors (Kerever et al., 2007). NSCs are embedded in the ECM and express cell adhesion molecules, which allow them to interact with the ECM. For example, vascular cell adhesion molecule-1 (VCAM-1) is highly expressed at the apical endfeet of SVZ NSCs and is necessary for the structural organization of the ependymal cell pinwheel architecture (Kokovay et al., 2012). In addition to its role in maintaining niche structures, the ECM can activate signaling cascades. Integrin-linked kinase (ILK) interacts with multiple proteins, including integrins; loss of ILK in adult NSCs results in increased cell proliferation (Porcheri et al., 2014).
Morphogens, such as BMPs, Notch, Wnts, and sonic hedgehog (SHH), are critical for tissue patterning and specification during embryonic development, and continue to regulate adult NSCs (Faigle and Song, 2013). For example, BMP signaling negatively regulates neurogenesis by promoting SVZ NSC astroglial fate commitment and SGZ NSC quiescence (Lim et al., 2000; Mira et al., 2010). Notch signaling induces proliferation and maintenance of NSCs in both adult niches, whereas loss of signaling forces NSCs to exit cell cycle and transition to a progenitor stage (Breunig et al., 2007). Wnt signaling is important for NSC maintenance, and Wnt inhibitors Dickkopf-1 (Dkk1) and secreted frizzled-related protein 3 (sFRP3) promote NSC quiescence (Jang et al., 2013; Seib et al., 2013). SHH signaling is required for SVZ NSC maintenance, but over-activation promotes symmetric division, which initially leads to NSC expansion but ultimately depletion (Ferent et al., 2014). Growth factors and neurotrophins also play an important role in regulating late progenitors, but their impact on NSCs is less well described (Faigle and Song, 2013). Together, these studies highlight the importance of tight regulation of morphogens and growth factors within the niche; a disturbance in either direction can negatively impact the NSC population.
Though apparently lacking synapses, NSCs express receptors and respond to a variety of neurotransmitters (Berg et al., 2013). GABA, the major inhibitory neurotransmitter of the brain, promotes NSC quiescence by blocking cell cycle progression (Fernando et al., 2011). In the SVZ neuroblast-released GABA acts as a negative feedback mechanism to limit NSC division (Liu et al., 2005). SGZ NSCs can sense parvalbumin interneuron-released GABA; reducing GABA signaling results in NSC activation and symmetrical division (Song et al., 2012). The dopamine receptor antagonist haloperidol increases SVZ NSC division, suggesting that dopamine also promotes quiescence (Kippin et al., 2005). In contrast, serotonin and acetylcholine activate SVZ NSCs (Paez-Gonzalez et al., 2014; Tong et al., 2014).
Adult NSCs also communicate with other cells via gap junctions and direct cell-cell interactions. The majority of NSCs are coupled to each other by gap junctions formed by connexin 43 (Cx43) (Kunze et al., 2009). Loss of Cx30 and Cx43 in SGZ NSCs severely diminishes NSC numbers. NSCs also communicate via direct cell-cell interactions with endothelial cells of the vasculature and with astrocytes. In the SGZ, astrocytic expression of ephrinB2 promotes neuronal differentiation of NSCs via EphB4 receptor-mediated pro-neuronal transcription factor upregulation (Ashton et al., 2012). In the SVZ, endothelial cell expression of ephrinB2 and Jagged1 promote NSC quiescence by suppressing cell cycle entry and inhibiting differentiation, and loss of either gene results in NSC activation and depletion (Ottone et al., 2014). These studies illustrate that the same signal could have different effects in the two niches.
Beyond the local niche, adult NSCs can also sense systemic changes via circulating factors. SVZ NSCs have direct access to CSF, which represents a complex reservoir of diverse signals. While its role remains to be characterized for adult NSCs, CSF in the embryonic brain promotes cortical neural progenitor proliferation via IGF-2 (Lehtinen et al., 2011). Heterochronic parabiont studies have shown that elevated circulating factors in the blood of aged mice, such as cytokines CCL11 (Villeda et al., 2011) and β2-microglobulin (Smith et al., 2015), inhibit SGZ cell proliferation and neurogenesis, whereas factors in blood of young animals, such as GDF11, induce vascular remodeling and lead to increased SVZ NSC number and neurogenesis (Katsimpardi et al., 2014).
In summary, NSCs are dynamically regulated by a large number of synergistic and antagonistic factors in each neurogenic niche and beyond. Several key questions remain to be addressed. How does each individual NSC compute a constant barrage of niche signals and make the ultimate decision to stay in quiescence, or enter cell cycle and choose a particular fate? How does an animal’s experience translate into changes in niche signaling that impact NSC behavior? Answers to these questions may reveal general principles for various adult somatic stem cells.
Mechanisms Regulating Adult Neural Stem Cells
Transduction of extracellular niche signals trigger a signaling cascade that activates intracellular regulatory mechanisms, including transcription factors, epigenetics, and metabolism (Figure 3A). Although some of these regulators affect gene expression or cell function directly, there is a lot of crosstalk among regulators to form a complex web of interactions.
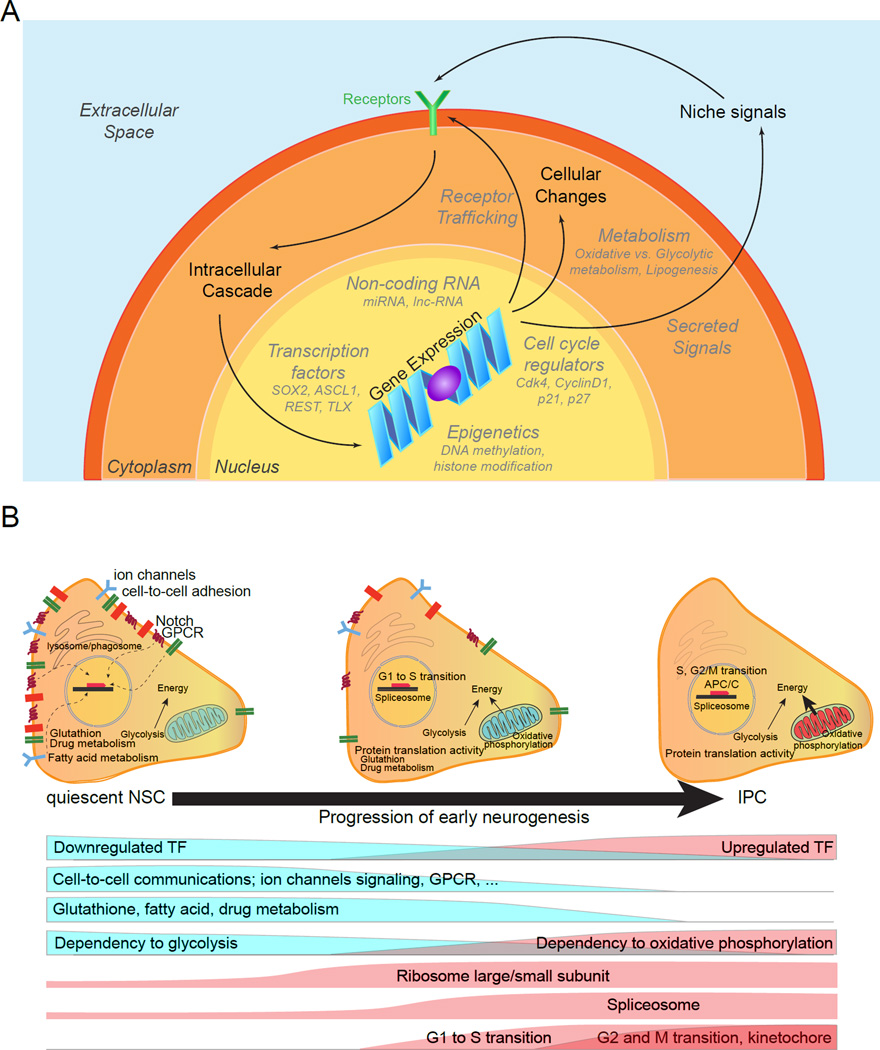
Figure 3. Adult neural stem cell regulation.
(A) A schematic diagram depicting extrinsic and intrinsic mechanisms that regulate adult NSCs. Extrinsic niche signals activate receptors, which trigger intracellular cascades that induce changes in gene expression. In addition, intrinsic transcriptional programs can direct gene expression in NSCs. Regardless of how it occurs, modulation of gene expression results in cellular changes, which affect NSC behavior. Not surprisingly, intrinsic and extrinsic mechanisms that regulate NSCs are interconnected and feedback on one another.
(B) Schematic summary of molecular signatures of quiescent NSCs and molecular cascades underlying their activation and neurogenesis in the adult NSCs revealed by single-cell RNA-seq and Waterfall analyses [adapted from (Shin et al., 2015)]. Shown on the top is an illustration of molecular signatures of adult qNSCs and their immediate progeny. Shown at the bottom are functional categories of genes that show a clear shift during adult qNSC activation and generation of IPCs. qNSCs exhibit intra- and inter-cellular signaling to actively sense the local niche, rely mostly on glycolysis for energy, and have highly active fatty acid, glutathione, and drug metabolism. Upon activation, NSCs increase translational capacity, followed by cell cycle entry with G1 to S transition. Oxidative phosphorylation starts to be active and stem cell specific properties are down-regulated. IPCs maintain active cell cycle genes, ribosomal activity and fully active oxidative phosphorylation for energy generation.
Regulation of gene expression by transcription factors represents one fundamental mechanism that regulates adult NSCs (Hsieh, 2012). For example, the transcription factor SRY (sex determining region Y)-box 2 (Sox2) is highly expressed by adult NSCs in both niches, and its deletion results in NSC depletion. Sox2 expression requires active Notch signaling, and SOX2 has many targets, including Shh and Tlx. Another important transcription factor, achaete-scute homolog 1 (Ascl1) is expressed in a small subset of adult NSCs at a given time and activates NSCs by targeting cell cycle regulators (Andersen et al., 2014). The nuclear receptor TLX is critical for NSC proliferation and maintenance, while repressor element 1-silencing transcription/neuron-restrictive silencer factor (REST/NRSF) blocks pro-neuronal genes to prevent precocious neuronal differentiation while maintaining the NSC pool in the SGZ (Hsieh, 2012). In the SVZ, there are a host of transcription factors that specify the diverse populations of olfactory bulb neurons (Sequerra, 2014). Classically, individual transcription factors have been investigated for their role in regulating adult NSCs, but the recent availability of single-cell RNA-seq datasets for adult NSCs in both SVZ (Llorens-Bobadilla et al., 2015) and SGZ (Shin et al., 2015) allows, for the first time, a means to understand the logic of combinatorial transcription factor regulation of NSC behavior at the whole-genome level.
Epigenetic modifications, which are potentially heritable changes in gene expression that are not encoded in the DNA sequence, play important roles in adult neurogenesis (Ma et al., 2010). A common form of chromatin modification is DNA methylation, which normally suppresses gene expression. The DNA methyl transferase DNMT3a methylates intergenic regions of some neurogenic genes and, unlike most DNMTs, promotes their expression in adult SVZ NSCs (Wu et al., 2010). Active DNA demethylation, mediated by the TET-GADD45 pathway (Guo et al., 2011), regulates SGZ NSC proliferation both cell autonomously (Zhang et al., 2013) and non-cell autonomously via modulation of growth factor expression in mature granule neurons (Ma et al., 2009). Methyl-CpG binding proteins bind to methylated DNA to regulate gene expression and neurogenesis (Ma et al., 2010). For example, loss of Methyl-CpG binding protein 1 increases NSC proliferation (Liu et al., 2010). Methyl-CpG binding protein 2 balances NSC proliferation and differentiation by regulating specific miRNAs, including repression of miR-137 (Szulwach et al., 2010). Histone modifications also contribute to the epigenetic regulation of adult neurogenesis. For example, Bmi-1, a member of the polycomb group of chromatin remodeling factors, is critical for SVZ NSC self-renewal (Molofsky et al., 2003). Enhancer of zeste homolog2 (Ezh2) is a subunit of the polycomb repressive complex 2 and the methyltransferase of histone H3K27. Ezh2 is expressed in actively dividing NSCs in the SGZ, and is thought to promote NSC proliferation by suppressing Pten expression and activating Akt-mTOR (Zhang et al., 2014). Histone deacetylase 3 regulates NSC proliferation via stabilization of cyclin-dependent kinase 1 (Jiang and Hsieh, 2014).
Epigenetic mechanisms often target other intracellular regulators, such as non-coding RNAs, creating a network of crosstalk interactions. Non-coding RNAs, including miRNAs and long non-coding RNAs (lnc-RNAs), play a regulatory role in adult neurogenesis (Li and Jin, 2010). miRNAs are small non-coding RNAs that regulate gene expression by binding to untranslated regions of target messenger RNAs, thus inhibiting protein synthesis. miR-124 promotes lineage progression in the SVZ by repressing SOX9 (Cheng et al., 2009), whereas miR-137 inhibits differentiation and promotes NSC proliferation through modulation of Ezh2 (Szulwach et al., 2010). Lnc-RNAs are non-coding RNAs greater than 200 nucleotides that can regulate gene transcription, post-transcriptional mRNA processing, and epigenetic modifications. Two lnc-RNAs, Six3os and Dlx1as, promote neuronal differentiation and repress astrocytic differentiation of NSCs, whereas another lnc-RNA, Pnky, inhibits NSC differentiation (Ramos et al., 2015; Ramos et al., 2013).
Recent studies have revealed changes in energetic demands during different stem cell behaviors (Jessberger, 2015). SVZ NSCs are more resistant to hypoxia and have a lower requirement for oxidative metabolism, but are more dependent on glycolytic metabolism than neurons (Candelario et al., 2013). Spot14, a gene previously implicated in lipid metabolism, reduces lipogenesis and negatively regulates SGZ NSC proliferation (Knobloch et al., 2013). Recent single-cell RNA-seq analysis of adult NSC dynamics showed that activation of quiescent NSCs is accompanied by downregulation of glycolytic metabolism and upregulation of mitochondrial oxidation in both SGZ and SVZ (Figure 3B) (Llorens-Bobadilla et al., 2015; Shin et al., 2015). Emerging evidence supports a model that stem cells predominantly utilize glycolytic metabolism, which is thought to be important for maintaining self-renewal and lineage potency; upon lineage differentiation, cells switch to oxidative metabolism, which is needed to support the growing energetic demands of specialized progeny.
One hallmark of adult neurogenesis is its dynamic regulation by various physiological, pathological and pharmacological stimuli, such as exercise, antidepressants, aging, epilepsy, and stroke (Ming and Song, 2011). The specific target(s) and underlying signaling mechanisms for many stimuli remain to be identified. It is also not always clear whether a particular signaling mechanism directly affects quiescent NSCs or their immediate progeny or whether subtypes of NSCs are differentially regulated. New tools that precisely target specific NSC sub-populations or different states of the same NSCs are needed to address these questions.
Potential Functions of Adult Neural Stem Cells
Adult somatic stem cells normally play a homeostatic role in maintaining tissue organization. When adult NSCs were initially discovered, it was assumed that their function was to provide a regenerative source for new neurons, and there were hopes that these NSCs might be involved in functional brain repair after injury. Instead, cumulative evidence suggests that the primary function of endogenous adult NSCs is to confer an additional layer of plasticity to the brain via both direct and indirect mechanisms (Christian et al., 2014).
In both neurogenic niches, adult NSCs primarily generate new neurons, which are hyper-excitable and exhibit a lower threshold for long-term potentiation induction during a critical period when compared to mature neurons (Ge et al., 2008). These properties of developing neurons could allow them to differentially contribute to information coding compared to mature neurons in the same circuit (Figure 4). In addition, adult-born neurons promote plasticity of the existing circuitry by making new synaptic contacts with mature neurons (Ming and Song, 2011). Behavioral analyses suggest that adult neurogenesis is important for certain types of hippocampus- or olfactory bulb-dependent learning and memory (Christian et al., 2014; Deng et al., 2010; Sahay et al., 2011). Specifically, new dentate granule cells have been implicated in long-term spatial memory and pattern separation, a function that permits discrimination between two similar inputs, whereas new olfactory bulb interneurons are important for short-term olfactory memory and flexible olfactory associative learning. Excitements in this field continues with suggestions of additional functions of adult neurogenesis, such as buffering stress response (Snyder et al., 2011) and regulating “forgetting” or memory consolidation (Akers et al., 2014).
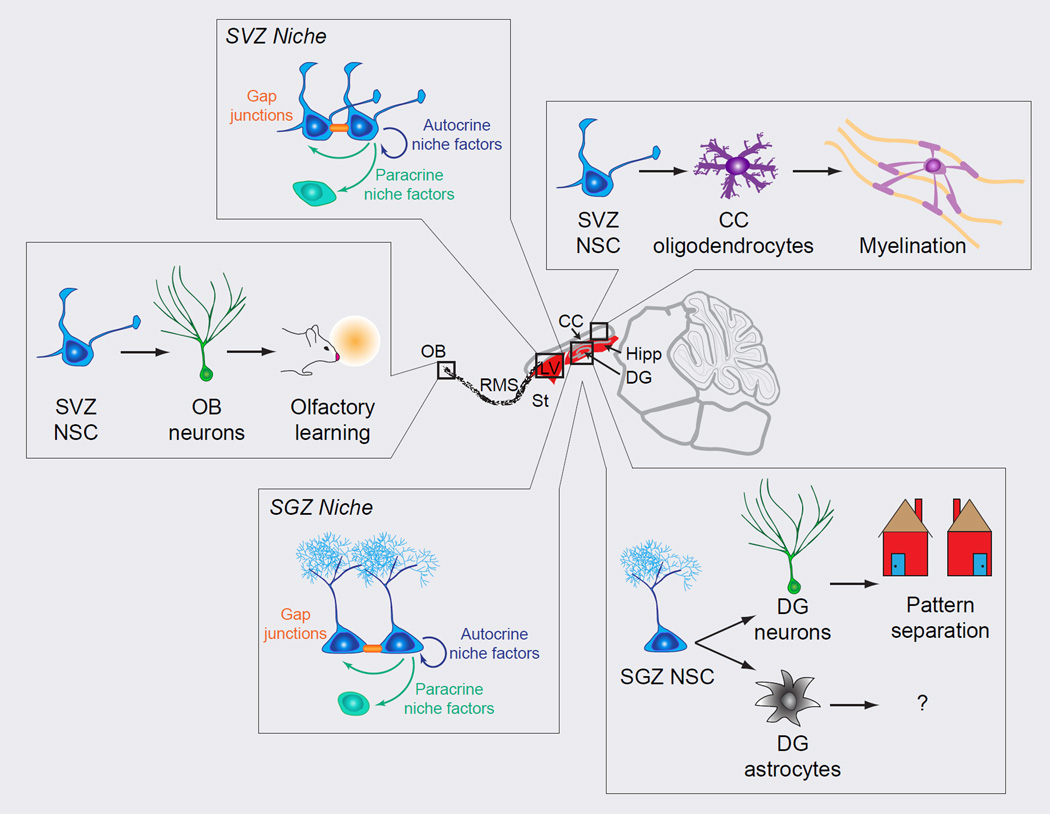
Figure 4. Impact of neural stem cells in the adult mammalian brain.
Adult neural stem cells (NSCs) directly impact the surrounding niche and indirectly impact neural circuitry through their progeny. NSCs in the SVZ and SGZ release autocrine and paracrine niche factors that contribute to the signaling milieu. In addition, NSCs form gap junctions to directly communicate with other NSCs. SVZ NSCs generate OB neurons and CC oligodendrocytes. OB neurons contribute to olfactory learning, while CC oligodendrocytes myelinate CC axons. SGZ NSCs generate DG neurons and astrocytes. DG neurons are important for pattern separation functions, while the functions of astrocytes remain to be explored. Thus adult NSCs impact not only the surrounding niche, but also the circuitry of multiple brain regions and ultimately behavior. CC, corpus callosum; DG, dentate gyrus; Hipp, hippocampus; LV, lateral ventricle; NSC, neural stem cell; OB, olfactory bulb; ; RMS, rostral migratory stream; SGZ, subgranular zone; St, striatum; SVZ, subventricular zone.
Though adult NSCs primarily generate new neurons, they also produce a small but significant proportion of glia. SGZ NSCs generate astrocytes, but the function of newborn glia cells remains largely unknown (Figure 4). Astrocytes are connected by gap junctions and form networks that can modulate the surrounding neural circuitry through multiple avenues. For example, astrocytes release factors, such as glutamate, adenosine triphosphate (ATP), and D-serine, collectively termed gliotransmitters, to modulate neuronal excitability, synaptic activity, and plasticity (Araque et al., 2014). Gliogenesis also occurs in the SVZ with predominantly oligodendrocytes. Oligodendrocyte precursors migrate to the corpus callosum to myelinate axons (Figure 4) (Xing et al., 2014). After demyelinating injuries, significantly more oligodendrocytes contribute to remyelination in the corpus callosum, suggesting that these precursors could potentially be a target for enhancing myelin repair. Though the proportion of gliogenic progeny may be modest, this is an understudied area and further studies are needed to determine the contribution of adult gliogenesis to brain function in order to fully understand functions of adult NSCs.
Adult NSCs themselves may also contribute to the niche through autocrine and paracrine signaling (Figure 4). Because it is challenging to determine the origin of niche signals, only a few studies have been able to present direct evidence that adult NSCs release factors to impact the niche. For example, upon increases in intracellular calcium, NSCs release vasodilating factors that activate pericytes on the vasculature to increase blood flow (Lacar et al., 2012). Adult SVZ NSCs and their immediate progeny secrete diazepam binding inhibitor, which antagonizes GABA signaling and promotes proliferation of neuroblasts (Alfonso et al., 2012). In addition to regulating the vasculature and progenitors, NSCs might also directly influence each other. For example, NSCs in the adult SGZ express both the VEGF receptor 3 and its ligand VEGF-C; VEGF receptor stimulation promotes NSC activation (Han et al., 2015). In addition, NSCs are connected to each other by gap junctions, potentially coordinating their actions (Kunze et al., 2009).
The role of adult NSCs in brain function is now expanding beyond being a mere source of neuronal progeny. Future studies will be needed to explore whether signaling or transcriptional modulation of adult NSCs in vivo can further expand their lineage potential and function to include therapeutic neural repair.
Emerging Topics in the Adult Stem Cell Field
Adult somatic stem cells are difficult to study because they are rare, heterogeneous, and exhibit dynamic states. There is a need for technologies with precision to better probe adult stem cells so that we can take advantage of their endogenous regenerative capacity for the benefit of human health. Classic studies of adult NSCs mostly relied on population level analyses, which may mask properties of distinct groups of stem cells. Recent studies have begun using single-cell analysis to distinguish among different stem cell populations, including NSCs. For example, clonal genetic lineage-tracing has started to reveal properties of individual NSCs and their heterogeneity (Bonaguidi et al., 2011; Calzolari et al., 2015). It is expected that clonal analysis with genetic manipulations using different drivers to target subpopulations will lead to novel insights into adult NSC biology. Given recent advances in miniaturized microscope technology, it will also become possible to directly image the behavior of individual adult NSCs in vivo to explore how experience impacts NSC activation and division directly, as was recently achieved in adult fish (Barbosa et al., 2015) as well as for other adult somatic stem cells (Ritsma et al., 2014; Rompolas et al., 2012). Another major technical advance is single-cell omics analyses (Shin et al., 2014), including transcriptomes via RNA-seq, epigenomes via methyl-seq, HiC and ACTA-seq, and proteomics via mass cytometry. For example, single-cell RNA-seq analyses of adult NSCs in both SVZ (Llorens-Bobadilla et al., 2015; Luo et al., 2015) and SGZ (Shin et al., 2015) have started to provide a holistic picture of molecular signatures of quiescent adult NSCs and molecular dynamics of NSC activation and neurogenesis, including a switch in transcription factor expression and energy sources, changes in niche signaling capacity and priming of protein translation machinery (Figure 3B). Instead of snapshot analyses of discrete time points, future studies will be greatly facilitated by novel bioinformatic tools, such as Monocle (Trapnell et al., 2014), SCUBA (Marco et al., 2014) and Waterfall (Shin et al., 2015), and novel sequencing technologies, such as FISSEQ (Lee et al., 2014), to reconstruct temporal, spatial and lineage information to get a complete picture of the continuous and dynamic stem cell behavior.
Adult neurogenesis is conserved in mammals. Previous studies of postmortem tissues pulsed with BrdU have demonstrated the presence of adult neurogenesis in humans (Eriksson et al., 1998) and an innovative approach using 14C from nuclear bomb testing has started to reveal quantitatively the extent of human neurogenesis over a lifetime. Understanding human adult NSCs and neurogenesis is not only important for therapeutic applications, but also for basic knowledge of NSC biology given differences observed between humans and rodents. For example, the 14C-based birth-dating strategy suggested that the age-dependent decline in hippocampal neurogenesis is much less dramatic in humans as compared to rodents (Spalding et al., 2013). In addition, NSCs residing in the adult human SVZ may generate neurons that migrate to the striatum, rather than into the olfactory bulb as in rodents (Bergmann et al., 2012; Ernst et al., 2014; Sanai et al., 2011; Wang et al., 2011). The major barrier to studying adult human stem cells is the inaccessibility of living tissue. Most studies have relied upon histological analysis using postmortem samples, but we are limited to a few markers, such as NESTIN and DCX (Knoth et al., 2010). Recent single-cell RNA-seq analysis may identify new markers for adult NSCs to facilitate the effort. In addition, spontaneous somatic nuclear and mitochondrial mutations have been used as clonal marks to track cell lineage in human brains (Evrony et al., 2015) and intestinal stem cells (Baker et al., 2014). After further development, these approaches may enable clonal analysis of endogenous adult NSCs in humans via massive single-cell sequencing. Non-invasive imaging methods, such as magnetic resonance imaging and positron emission tomography, also have the potential to revolutionize adult NSC studies in humans, but remain to be validated.
A series of discoveries on cellular reprogramming by Gurdon and Jaenisch, culminated by Yamanaka’s demonstration of reprogramming somatic cells into pluripotent stem cells by defined transcription factors, has broken the conceptual barrier that development is a largely one-way process. Studies have since shown that mammalian cells can be reprogrammed in vivo, either by molecular or environmental factors, to exhibit expanded potentials that are not seen under physiological conditions (Arlotta and Berninger, 2014). For example, viral-mediated expression of exogenous Sox2 in resident astrocytes in the adult mouse reprograms them into proliferating neuroblasts in striatum and spinal cord, regions outside canonical neurogenic niches (Niu et al., 2013; Su et al., 2014). In the absence of exogenous reprogramming factors, striatal astrocytes turn on nestin expression and generate neurons in both an adult mouse stroke model (Magnusson et al., 2014) and in an excitotoxic model of Huntington's disease (Nato et al., 2015), via downregulation of Notch signaling. Neuroblasts in the adult SVZ are converted to oligodendrocytes in an injury model of corpus callosum demyelination via upregulation of chordin (Jablonska et al., 2010). Though trans-differentiation could be a powerful method to generate new cells in regions that lack a stem cell niche, many unknowns remain. The molecular mechanisms, especially epigenetic mechanisms, underlying induced potential remain to be determined. One interesting mechanistic question is whether such plasticity in vivo involves a return to NSC state, even transiently, followed by differentiation (Bar-Nur et al., 2015). Newly generated cells need to develop outside a niche in an environment that may lack appropriate signals to direct cellular identity and integration. Thus, the clinical relevance of in vivo reprogramming will hinge upon demonstration of proper integration, functional recovery and minimal side effects.
Concluding Remarks
Over the past five decades, adult neurogenesis and NSCs has evolved from a topic of interest to a few optimistic scientists to an established field that has made tremendous progress to significantly impact our perspective of brain plasticity. Initial work focused on characterizing the process of adult neurogenesis, followed by efforts to identify underlying cellular and molecular mechanisms, and its functional significance. Current work continues to examine and manipulate NSC regulatory mechanisms in an effort to take advantage of the regenerative capacity of adult NSCs. Though our understanding of adult NSCs has come a long way, there are still significant challenges for future research.
First, adult NSCs are a very heterogeneous population of cells, making it difficult to distinguish between different temporal states or distinct populations in vivo. Future studies will benefit from identification of subpopulation- or state-specific markers, as well as from single-cell analysis to dissect properties of various NSC sub-populations and dynamic states. Second, despite substantial advances in understanding numerous signaling pathways, little is known about how NSCs integrate many diverse signals to ultimately make choices, such as quiescence versus activation, symmetric versus asymmetric cell division, and maintenance versus fate commitment. Future research will need to focus on interactions between signaling pathways to identify signaling hubs and the hierarchy that coordinates the barrage of incoming signals. Third, human neurogenesis research has recently gained traction, and we have learned that despite sharing similar neurogenic niches, there are many differences between rodent and human adult neurogenesis. Significant advancements in the adult NSC field will require more human studies, as well as the development of new methods to probe NSCs in vivo. Non-invasive monitoring will be critical not only for human studies, but also for real time and longitudinal studies to understand the life-long impact of neurogenesis. Finally, while the prospect of using adult NSCs therapeutically as a regenerative source for neural repair is very exciting, a major issue that must be addressed is the functional consequence of new neuron generation, particularly outside of the traditional neurogenic niches via reprogramming. Vigorous examination of neuronal integration and the impact of new neurons on the surrounding neuronal circuitry and behavior will be imperative for this line of research to be clinically relevant.
Adult NSCs and neurogenesis confer a unique mode of plasticity to the mature mammalian brain. One overarching goal of adult neurogenesis research is to manipulate NSCs to improve brain health. The field has spent past five decades accumulating knowledge regarding neurogenesis and is starting to apply that knowledge in conjunction with new technologies to work toward this ultimate goal.
Acknowledgements
We apologize for not able to cite many original papers because constrains on reference numbers. We thank K. Christian and three anonymous reviewers for suggestions. The research in authors’ laboratories was supported by grants from NIH (NS047344, NS093772) and MSCRF to H.S., and from NIH (NS048271, MH105128, NS09538), and Dr. Miriam and Sheldon G. Adelson Medical Research Foundation to G-l.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A, Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, et al. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Alfonso J, Le Magueresse C, Zuccotti A, Khodosevich K, Monyer H. Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell stem cell. 2012;10:76–87. doi: 10.1016/j.stem.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. The Journal of comparative neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Andersen J, Urban N, Achimastou A, Ito A, Simic M, Ullom K, Martynoga B, Lebel M, Goritz C, Frisen J, et al. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron. 2014;83:1085–1097. doi: 10.1016/j.neuron.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Berninger B. Brains in metamorphosis: reprogramming cell identity within the central nervous system. Current opinion in neurobiology. 2014;27:208–214. doi: 10.1016/j.conb.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton RS, Conway A, Pangarkar C, Bergen J, Lim KI, Shah P, Bissell M, Schaffer DV. Astrocytes regulate adult hippocampal neurogenesis through ephrin-B signaling. Nature neuroscience. 2012;15:1399–1406. doi: 10.1038/nn.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AM, Cereser B, Melton S, Fletcher AG, Rodriguez-Justo M, Tadrous PJ, Humphries A, Elia G, McDonald SA, Wright NA, et al. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 2014;8:940–947. doi: 10.1016/j.celrep.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Nur O, Verheul C, Sommer AG, Brumbaugh J, Schwarz BA, Lipchina I, Huebner AJ, Mostoslavsky G, Hochedlinger K. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotechnol. 2015;33:761–768. doi: 10.1038/nbt.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa JS, Sanchez-Gonzalez R, Di Giaimo R, Baumgart EV, Theis FJ, Gotz M, Ninkovic J. Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science. 2015;348:789–793. doi: 10.1126/science.aaa2729. [DOI] [PubMed] [Google Scholar]
- Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, Gage FH, Zhao X. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DA, Belnoue L, Song H, Simon A. Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development. 2013;140:2548–2561. doi: 10.1242/dev.088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DK, Yoon KJ, Will B, Xiao AY, Kim NS, Christian KM, Song H, Ming G-l. Tbr2-expressing intermediate progenitor cells in the adult mouse hippocampus are unipotent neuronal precursors with limited amplification capacity under homeostasis. Front Biol (Beijing) 2015;10:262–271. [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari F, Michel J, Baumgart EV, Theis F, Gotz M, Ninkovic J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat Neurosci. 2015;18:490–492. doi: 10.1038/nn.3963. [DOI] [PubMed] [Google Scholar]
- Candelario KM, Shuttleworth CW, Cunningham LA. Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. Journal of neurochemistry. 2013;125:420–429. doi: 10.1111/jnc.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature neuroscience. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nature reviews Molecular cell biology. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annual review of neuroscience. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Mechanic M, Petrik D, Carlton A, Ables JL, Malhotra S, Bachoo R, Gotz M, Lagace DC, Eisch AJ. In vivo contribution of nestin- and GLAST-lineage cells to adult hippocampal neurogenesis. Hippocampus. 2013;23:708–719. doi: 10.1002/hipo.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P, Farinas I. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Evrony GD, Lee E, Mehta BK, Benjamini Y, Johnson RM, Cai X, Yang L, Haseley P, Lehmann HS, Park PJ, et al. Cell lineage analysis in human brain using endogenous retroelements. Neuron. 2015;85:49–59. doi: 10.1016/j.neuron.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferent J, Cochard L, Faure H, Taddei M, Hahn H, Ruat M, Traiffort E. Genetic activation of Hedgehog signaling unbalances the rate of neural stem cell renewal by increasing symmetric divisions. Stem cell reports. 2014;3:312–323. doi: 10.1016/j.stemcr.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando RN, Eleuteri B, Abdelhady S, Nussenzweig A, Andang M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A. 2011;108:5837–5842. doi: 10.1073/pnas.1014993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A. Embryonic Origin of Postnatal Neural Stem Cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutachi S, Miya H, Watanabe T, Kawai H, Yamasaki N, Harada Y, Imayoshi I, Nelson M, Nakayama KI, Hirabayashi Y, et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat Neurosci. 2015;18:657–665. doi: 10.1038/nn.3989. [DOI] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. The Journal of physiology. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Su Y, Zhong C, Ming GL, Song H. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell cycle. 2011;10:2662–2668. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Calvo CF, Kang TH, Baker KL, Park JH, Parras C, Levittas M, Birba U, Pibouin-Fragner L, Fragner P, et al. Vascular Endothelial Growth Factor Receptor 3 Controls Neural Stem Cell Activation in Mice and Humans. Cell reports. 2015 doi: 10.1016/j.celrep.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012;26:1010–1021. doi: 10.1101/gad.187336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, et al. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell. 2013;12:215–223. doi: 10.1016/j.stem.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S. Special issue for stem cell metabolism: be quiet, grow, and differentiate. Front Biol (Beijing) 2015;10:99. [Google Scholar]
- Jiang Y, Hsieh J. HDAC3 controls gap 2/mitosis progression in adult neural stem/progenitor cells by regulating CDK1 levels. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:13541–13546. doi: 10.1073/pnas.1411939111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi D, Furutachi S, Kawai H, Hozumi K, Gotoh Y. Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nature communications. 2013;4:1880. doi: 10.1038/ncomms2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, ffrench-Constant C. Extracellular matrix and the neural stem cell niche. Developmental neurobiology. 2011;71:1006–1017. doi: 10.1002/dneu.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Wang Y, Kusek G, Wurster R, Lederman P, Lowry N, Shen Q, Temple S. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell stem cell. 2012;11:220–230. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Congreso MR, Hartmann C, Wallraff-Beck A, Huttmann K, Bedner P, Requardt R, Seifert G, Redecker C, Willecke K, et al. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11336–11341. doi: 10.1073/pnas.0813160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacar B, Herman P, Platel JC, Kubera C, Hyder F, Bordey A. Neural progenitor cells regulate capillary blood flow in the postnatal subventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:16435–16448. doi: 10.1523/JNEUROSCI.1457-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Daugharthy ER, Scheiman J, Kalhor R, Yang JL, Ferrante TC, Terry R, Jeanty SS, Li C, Amamoto R, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fang L, Fernandez G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jin P. Roles of small regulatory RNAs in determining neuronal identity. Nat Rev Neurosci. 2010;11:329–338. doi: 10.1038/nrn2739. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Bobadilla E, Zhao S, Baser A, Saiz-Castro G, Zwadlo K, Martin-Villalba A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Luo Y, Coskun V, Liang A, Yu J, Cheng L, Ge W, Shi Z, Zhang K, Li C, Cui Y, et al. Single-cell transcriptome analyses reveal signals to activate dormant neural stem cells. Cell. 2015;161:1175–1186. doi: 10.1016/j.cell.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Marchetto MC, Guo JU, Ming GL, Gage FH, Song H. Epigenetic choreographers of neurogenesis in the adult mammalian brain. Nat Neurosci. 2010;13:1338–1344. doi: 10.1038/nn.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- Marco E, Karp RL, Guo G, Robson P, Hart AH, Trippa L, Yuan GC. Bifurcation analysis of single-cell gene expression data reveals epigenetic landscape. Proc Natl Acad Sci U S A. 2014;111:E5643–E5650. doi: 10.1073/pnas.1408993111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, Hortiguela R, Marques-Torrejon MA, Nakashima K, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nato G, Caramello A, Trova S, Avataneo V, Rolando C, Taylor V, Buffo A, Peretto P, Luzzati F. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington's disease. Development. 2015;142:840–845. doi: 10.1242/dev.116657. [DOI] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature cell biology. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, Gascon S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nature cell biology. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH, Parrinello S. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nature cell biology. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Gonzalez P, Asrican B, Rodriguez E, Kuo CT. Identification of distinct ChAT(+) neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat Neurosci. 2014;17:934–942. doi: 10.1038/nn.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Porcheri C, Suter U, Jessberger S. Dissecting integrin-dependent regulation of neural stem cell proliferation in the adult brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5222–5232. doi: 10.1523/JNEUROSCI.4928-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AD, Andersen RE, Liu SJ, Nowakowski TJ, Hong SJ, Gertz CC, Salinas RD, Zarabi H, Kriegstein AR, Lim DA. The Long Noncoding RNA Pnky Regulates Neuronal Differentiation of Embryonic and Postnatal Neural Stem Cells. Cell stem cell. 2015 doi: 10.1016/j.stem.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AD, Diaz A, Nellore A, Delgado RN, Park KY, Gonzales-Roybal G, Oldham MC, Song JS, Lim DA. Integration of genome-wide approaches identifies lncRNAs of adult neural stem cells and their progeny in vivo. Cell stem cell. 2013;12:616–628. doi: 10.1016/j.stem.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ritsma L, Ellenbroek SI, Zomer A, Snippert HJ, de Sauvage FJ, Simons BD, Clevers H, van Rheenen J. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature. 2014;507:362–365. doi: 10.1038/nature12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Deschene ER, Zito G, Gonzalez DG, Saotome I, Haberman AM, Greco V. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature. 2012;487:496–499. doi: 10.1038/nature11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell stem cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Sequerra EB. Subventricular zone progenitors in time and space: generating neuronal diversity. Frontiers in cellular neuroscience. 2014;8:434. doi: 10.3389/fncel.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Berg DA, Zhu Y, Shin JY, Song J, Bonaguidi MA, Enikolopov G, Nauen DW, Christian KM, Ming GL, et al. Single-Cell RNA-Seq with Waterfall Reveals Molecular Cascades underlying Adult Neurogenesis. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Ming GL, Song H. Decoding neural transcriptomes and epigenomes via high-throughput sequencing. Nat Neurosci. 2014;17:1463–1475. doi: 10.1038/nn.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, et al. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–937. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhong C, Bonaguidi MA, Sun GJ, Hsu D, Gu Y, Meletis K, Huang ZJ, Ge S, Enikolopov G, et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489:150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nature communications. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GJ, Zhou Y, Stadel RP, Moss J, Yong JH, Ito S, Kawasaki NK, Phan AT, Oh JH, Modak N, et al. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1508545112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189:127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CK, Chen J, Cebrian-Silla A, Mirzadeh Z, Obernier K, Guinto CD, Tecott LH, Garcia-Verdugo JM, Kriegstein A, Alvarez-Buylla A. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14:500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol. 2014;32:381–386. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu YY, Zhao CH, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell research. 2011;21:1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE. Dnmt3a–dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science. 2010;329:444–448. doi: 10.1126/science.1190485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing YL, Roth PT, Stratton JA, Chuang BH, Danne J, Ellis SL, Ng SW, Kilpatrick TJ, Merson TD. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ji F, Liu Y, Lei X, Li H, Ji G, Yuan Z, Jiao J. Ezh2 regulates adult hippocampal neurogenesis and memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:5184–5199. doi: 10.1523/JNEUROSCI.4129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, et al. Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell. 2013;13:237–245. doi: 10.1016/j.stem.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]