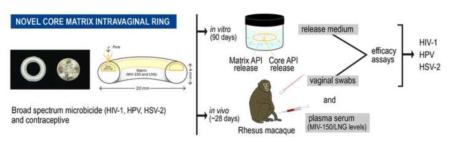
Abstract
Women urgently need a self-initiated, multipurpose prevention technology (MPT) that simultaneously reduces their risk of acquiring HIV-1, HSV-2, and HPV (latter two associated with increased risk of HIV-1 acquisition) and prevents unintended pregnancy. Here, we describe a novel core-matrix intravaginal ring (IVR), the MZCL IVR, which effectively delivered the MZC combination microbicide and a contraceptive. The MZCL IVR contains four active pharmaceutical ingredients (APIs): MIV-150 (targets HIV), zinc acetate (ZA; targets HIV and HSV-2), carrageenan (CG; targets HPV and HSV-2), and levonorgestrel (LNG; targets unintended pregnancy). The elastomeric IVR body (matrix) was produced by hot melt extrusion of the non-water swellable elastomer, ethylene vinyl acetate (EVA-28), containing the hydrophobic small molecules, MIV-150 and LNG. The solid hydrophilic core, embedded within the IVR by compression, contained the small molecule ZA and the macromolecule CG. Hydrated ZA/CG from the core was released by diffusion via a pore on the IVR while the MIV-150/LNG diffused from the matrix continuously for 94 days (d) in vitro and up to 28d (study period) in macaques. The APIs released in vitro and in vivo were active against HIV-1ADA-M, HSV-2, and HPV16 PsV in cell-based assays. Serum LNG was at levels associated with local contraceptive effects. The results demonstrate proof-of-concept of a novel core-matrix IVR for sustained and simultaneous delivery of diverse molecules for the prevention of HIV, HSV-2 and HPV acquisition, as well as unintended pregnancy.
Keywords: core-matrix design, intravaginal ring, multipurpose prevention technology, microbicides, contraception, efficacy
1. Introduction
Topical microbicides are usually self-administered pharmaceutical preparations for both women and men, containing antiretroviral (ARV) and or non-ARV active pharmaceutical ingredients (APIs) targeted to prevent sexually transmitted infections (STIs) [1]. Examples include formulations like gels, foams, intravaginal rings (IVRs), electrospun nanofibers, pessaries, douches, diaphragms, suppositories and tablets. The status of microbicide formulation development has been reported prior in comprehensive reviews [2, 3]. Focusing on vulnerable populations, microbicide developers seek to improve women’s sexual and reproductive health (SRH), by targeting primarily HIV-1 prevention [4, 5] and, in some instances, unwanted pregnancy [6-8]. This approach does not protect women from other prevalent non-curable viral STIs such as herpes simplex virus-2 (HSV-2) and human papilloma virus (HPV). While some APIs have anti-HIV/HSV-2 [9, 10] activity, there is no broad spectrum multipurpose prevention technology (MPT) that can simultaneously prevent these three major viral STIs and unintended pregnancy. HPV or HSV-2 can pose collateral risks to women’s SRH. There is an increased susceptibility to HIV-1 acquisition [11-14], HPV causes cervical cancer [15], and neonatal HSV-2 infections render high mortality rate in both, mothers and infants. Daily there are nearly 5,700 new HIV-1 infections [16], 4000 AIDS-related deaths [16], 35,000 new HSV-2 infections in women [17], 700 HPV-related deaths [18], 200,000 unplanned pregnancies [19], 125,000 abortions [19] and 800 pregnancy-related deaths worldwide [20]. Further, high-risk, low income populations of Sub-Saharan Africa and South/West Asia are vulnerable to SRH challenges and would benefit greatly from women-initiated products that simultaneously prevent STIs and unintended pregnancy [21].
While vaccine(s) will likely have substantial benefits, HIV-1 and HSV-2 [22] vaccines are still being developed or tested and the marketed HPV vaccines [23-25] are effective against (up to) nine HPV subtypes but not 31 other HPVs associated with anogenital infections [24, 26, 27]. Besides, vaccines may require cold storage which will limit accessibility in resource-poor regions and have low uptake mainly due to parental concerns and high cost. Thus, there is an urgent need to develop women initiated, low cost MPTs that are amenable to diverse populations [21].
The results of the Phase 2b CAPRISA-004 trial (39% reduction in HIV-1 and 50% reduction in HSV-2) of a coitally dependent 1% tenofovir (TFV) gel [28] has encouraged the field to develop topical intravaginal formulations. But the MTN-003 VOICE trial which assessed efficacy and safety of short term oral and vaginal formulations - tenofovir disoproxil fumarate (TDF) and tenofovir-emtricitabine (TDF-FTC) oral tablets, and 1% TFV vaginal gel to be used daily for 12-33 months [29], indicated that the effectiveness of the tested microbicides was not significantly different than the placebo group. However, a subset analysis of 1% TFV gel treated group confirmed that women with TFV detected in their plasma were at a significantly lower risk of acquiring HIV-1. On the heels of VOICE trial, recently published results from the FACTS 001 study [30] which was conducted to confirm the results of CAPRISA-004 trial but with a larger sample size (in order to warrant licensure to 1% TFV gel) also indicated that there was no significant protection between the groups using 1% TFV gel and placebo gel. However, moderate protection was observed in the group who used the product consistently [30]. Overall, the data from the clinical trials strongly suggest that the protection correlates with user adherence. For better adherence and longer term protection, locally acting, non-coitally dependent products such as sustained release intravaginal rings (IVRs) would be a better choice. IVR user adherence study in Sub-Saharan Africa has been reported earlier [31]. For the current development status of MPT IVRs, readers can refer to [32].
We designed an IVR to release an effective broad spectrum combination microbicide and a contraceptive for 90 days (d): MIV-150, ZA, CG, and LNG. MIV-150, a non-nucleoside reverse transcriptase inhibitor (NNRTI), significantly protected macaques from SHIV-RT challenge [33-38] and limited the emergence of NNRTI resistant mutations in SHIV-RT infected macaques carrying a high dose MIV-150 IVR [39]. Zinc salts have shown broad spectrum antiviral activity including HIV-1 [40] and HSV [41]. CG, a high molecular weight (MW) linear sulfated polysaccharide extracted from red seaweed showed potent in vitro and in vivo antiviral activity against HPV [24, 26, 42-44] with proven safety for topical use [45, 46]. Additionally, highly compliant Carraguard (3 wt. % carrageenan gel) users in the Phase 3 trial were associated with lower risk of HPV infection (vs. methyl cellulose placebo gel users) [47]. ZA (low MW metal salt)/CG, showed antiviral synergy against HSV-2 in vitro [48], significant protection in a high dose vaginal and rectal challenge in vivo in mice [48, 49] and reduced SHIV-RT infection in macaques [49]. LNG, a second generation progestin, is an FDA approved contraceptive with anti-ovulatory properties. It is supplied as different formulations and included in the WHO’s model list of essential medicines [50].
The MZC combination microbicide gel protected mice against SHIV-RT, HSV-2, and HPV infections - both vaginally and rectally [51], significantly protected macaques against SHIV-RT and partially against HSV-2 in a high dose as well as low dose repeat SHIV-RT/HSV-2 co-challenge [34, 52], and significantly reduced HSV-2 shedding in the latter [52, 53].
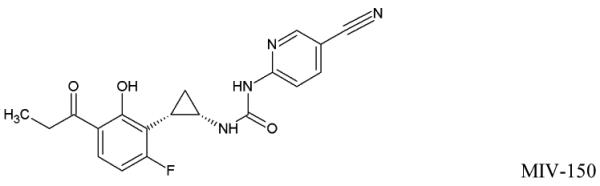
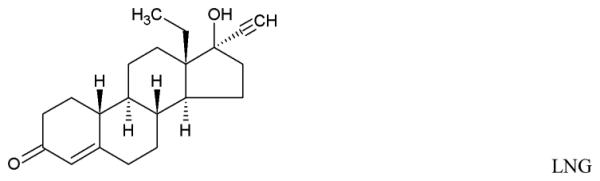
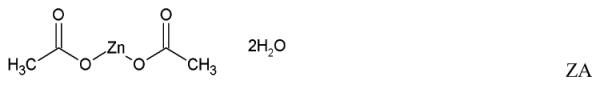
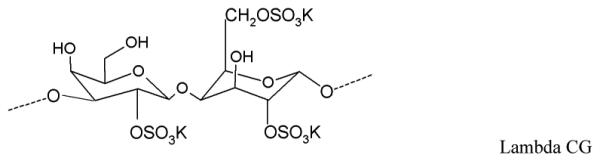
The promising results led to the development of an MZCL IVR. But, designing a long acting (90d) MPT IVR, to simultaneously release MZCL can be challenging for three main reasons: i) developing one-body IVR architecture to release two hydrophilic, one small and one large molecule (ZA and CG) and two hydrophobic small molecules (MIV-150 and LNG). The differences in size and solubilities of APIs in one polymer limits one-matrix system approach to deliver APIs (Table 1). ii) providing an extended release of the APIs for as long as 90d (vs. 1d to 1 week for short term formulations) and iii) keeping end user experience in mind, preserving the simple IVR form such that multiple APIs can be accommodated without manipulating the design (additional external attachments to hold more APIs).
Table 1.
Physicochemical attributes and chemical structures of the APIs
| Compound | MW (g/mol) |
Water solubility | IVR compartment |
|---|---|---|---|
| Micronized MIV-150 (log P: 4.2 ± 0.2 at 25°C) |
368.36 | 0.8 ± 0.05 μg/ mL (at 37°C) |
Matrix |
| Micronized LNG (log P: 3.4 ± 0.3 at 25°C) |
312.45 | 1.19 ± 0.05 μg/ mL (at 37°C) |
Matrix |
| Zinc acetate dihydrate (lop P: not reported) |
219.51 | 0.43 g/mL (at 20°C) |
Core |
| Carrageenan (log P: not reported) |
1360000 | 1g in ≤ 30 mL (at 80°C) |
Core |
| Structures | |||
| |||
| |||
| |||
| |||
To address these challenges we developed a core-matrix IVR in which the hydrophilic and hydrophobic APIs were segregated. A core containing hydrophilic APIs was sandwiched between ethylene vinyl acetate copolymer, grade 2803G (EVA-28), matrix containing hydrophobic APIs. A pore was drilled on the IVR to create a pathway for the release of hydrated core components. Thus, hydrophilic APIs were released from EVA matrix (usually regarded as an unsuitable carrier for the hydrophilic API) in a simple IVR form. The in vitro target release profile was ≥4 μg/d MIV-150, ≥50 μg/d zinc acetate, ≥100 μg/d CG, and ≥2 μg/d LNG. Also it was unknown how in vivo factors like cervical mucus secretion, blood, shedding of epithelial cells, and limited vaginal fluid volume might affect elution of core APIs via pore(s). Here, we demonstrate a proof-of-concept working of this novel core-matrix IVR (macaque prototype) by presenting the in vitro release for 90d, in vivo release in macaques for nearly 28d, and in vitro efficacy in cell-based assays.
2. Materials and methods
2.1 Core-matrix IVR fabrication
CG (95:5, lambda: kappa) was supplied by Industrial Research Limited (Wellington, New Zealand). Crystalline ZA, USP grade, Spectrum Chemicals (New Brunswick, NJ), was jet milled to less than 5 μm at Particle Sciences Inc. (Bethlehem, PA) using a Sturtevant sanitary design micronizer. MIV-150 was manufactured by Uquifa (Barcelona, Spain) and micronized to about 5 μm by Particle Sciences Inc. LNG was purchased from Crystal Pharma (Valladolid, Spain). EVA (grade 2803G) was supplied by Celanese (Florence, KY) and cryoground by ICO polymer Inc. (Akron, OH). The pellets for IVR matrix were processed at Particle Sciences Inc. Cryoground EVA-28 was mixed with MIV-150 (0.5 wt. %) alone or with LNG (0.1 wt. %) in a GlenMills T2F Turbula mixer (Clifton, NJ) and extruded using a Leistritz hot melt extruder (Nürnberg, Germany). The extruded strands were pelletized with a Scheer Bay BT-25 pelletizer (Bay City, MI). Placebo pellets (no APIs) were produced similarly. Carbide drill bits, ~ 500 μm and ~ 800 μm (yielded pore size of 720 μm), used to fabricate pores on the IVRs were purchased from Drill Bit City (Chicago, IL). All solvents were HPLC grade.
Using customized molds, Garner Industries (Lincoln, Nebraska), EVA-28 pellets pre-compounded with MIV-150 (±LNG) were melt extruded (120 psi, 245°F) to produce half IVRs with a central cavity (concentric to the outer diameter) for the core (Suppl. Fig 1, method 1). ZA/CG (3/7) mixture was compressed within the core cavity to fill it. The same matrix was extruded on top to form a full IVR, MZC or MZCL IVR. Thus, one half of the IVR was a matrix containing the core (core side) while other half was pure matrix. The target loadings per IVR were 3 mg MIV-150, 30 mg ZA, 70 mg CG and or 0.6 mg LNG. The IVRs were subjected to an overnight leak test (Suppl. method 2). Core side pores (pores bored from the IVR surface into the core, 1 pore per IVR) of 500 μm and 800 μm and through pore (pore formed through the cross section of the IVR, 2 pores per IVR) of 500 μm were tested. The 800 μm pores (but not 500 μm due to small pore size) were plugged with the core material to provide instantaneous release and potentially limit pore clogging in vivo. Microscopy was performed on the pores (Suppl. method 3). Taken together, we tested 500 μm pored (core side and through) MZC/ MZCL, 800 μm (core side) MZC, and placebo (EVA-28 matrix) IVRs. Details of the different IVR configurations tested are listed in Table 2.
Table 2.
Different IVR formulations tested in this study
| S.No | IVR type | Pore size (μm) |
Pore location | No. of pores per IVR |
|---|---|---|---|---|
| 1. | MZC | 500 | Core side | 1 * |
| 2. | MZCL | 500 | Core side | |
| 3. | MZC | 800 | Core side | |
| 4. | MZC | 500 | Through | 2* |
| 5. | MZCL | 500 | Through | |
| 6. | Placebo | 500 | Core side | 1 |
| 7. | Placebo | 500 | Through | 2 |
Cross sections shown in Figures 1b and 1c
2.2 Quantification of APIs
A literature method for ZA [54] was optimized (Suppl. method 4), LLOQ: 0.1 μg/ 200 μl (volume plated in the well). CG was analyzed by colorimetic UV assay using methylene blue [55], LLOQ: 0.12 μg/ 200 μl. MIV-150, LLOQ: 0.096 μg/ mL at 10 μl injection volume, and LNG, LLOQ: 0.32 μg/ mL at 10 μl injection volume, were analyzed using the HPLC method described for MIV-150 in [37] except that the gradient progressed from 90% (mobile phase A-200 mM ammonium acetate buffer) to 10% (mobile phase B-acetonitrile) in 15 min. HPLC by Agilent systems (Santa Clara, CA), series 1100 was used. LNG was detected at 245 nm wavelength.
2.3 Determination of API content in the matrix
MIV-150 and LNG were extracted by dissolving ~ 50 mg of IVR matrix in 2 mL of chloroform with overnight shaking and then adding methanol [56]. The solution was filtered using a 0.2 μm PTFE filter, Pall (Port Washington, NY) and the MIV-150 and LNG content of the filtrate was analyzed using HPLC as per method in (2.2) by making 10 μL injections. Spike control recovery (known amount analyte added to a blank EVA-28 matrix) for MIV-150 and LNG were 99 ± 4%, and 95 ± 5% respectively.
2.4 In vitro release
The release testing was performed on a whole MZC/ MZCL IVR in 10 mL (sink condition for hydrophilic APIs and non-sink condition for hydrophobic APIs) of 25 mM acetate buffer, pH 4.2, at 37°C with shaking at 100 rpm for 94d with daily media replacement and periodic sampling. The release eluent was assayed for all APIs as described above.
The release of ZA and CG was modeled to a power function
| equation 1 |
Where, y = the average cumulative release, α = release coefficient, t = time, β = exponent that defines the type of release where β=1 denotes linear release and β ≠1 non-linear release
To utilize MIXED procedure model (Suppl., method 5) the power function was log-transformed so that a linear mixed-effect model that provides estimates of α and β on the log-scale while accounting for repeated measurements can be performed. This allows assessing differences in pore size and the effect of LNG (MZC vs. MZCL IVRs) on the release of core components.
2.5 Pharmacokinetic (PK) studies in macaque model
Ethics statement: Adult female rhesus macaques (Macaca mulatta) of 4-13 years and weighing 5-14 kgs were used. The macaques were housed and treated at the Tulane National Primate Research Center, TNPRC (Covington, LA) in accordance with the protocol approved by the Institutional Animal Care and Use Committee (OLAW Assurance A4499-01), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC000594). All procedures complied with the Animal Welfare Act [57], the Guide for the Care and Use of Laboratory Animals [58], and TNPRC standards for minimizing animal distress. Macaques were confirmed negative for SIV, simian type D retroviruses, simian T cell leukemia virus-1 and herpes B and were randomized to treatment groups.
Following IVR groups (Table 2): 500 μm core side - MZC (n=10) and MZCL (n=3), 500 μm through - MZC (n=11) and MZCL (n=3), 800 μm core side with a plug - MZC (n=4) and placebo IVRs (n=2), were tested in non-Depo-Provera (non-DP) treated macaques for 23 or 28d. The IVRs were inserted with the core side facing the cervix. Vaginal pH at the baseline and during the study was measured by pH paper, EMD Millipore Corp (Billerica, MA) which was inserted into the vaginal vault for about 5 minutes. Vaginal fluid (VF) was collected using Merocel® spears, Medtronic Xomed (Jacksonville, Florida) and suspended in 1 mL of saline, EDTA blood (< 10 mL/kg/month) was collected at indicated time points as described [37] and shipped overnight to the Population Council, NY for analysis. Plasma was isolated from EDTA blood [59]. CG in VF was quantified using ELISA (LLOQ: 40 ng/mL) [51] and MIV-150 by radio immunoassay (RIA), LLOQ: 1 ng/mL or 2.7 nM [35]. MIV-150 in plasma was analyzed by LCMS/MS (LLOQ: 20 pg/mL or 54 pM) [36]. LNG in serum was measured by RIA, Immunometrics Ltd (London, UK) at the Oregon National Primate Research Center (ONPRC), Endocrine Technology and Support Core Laboratory, ETSC, (Beaverton, OR). The range of detection of the assay was 23-375 fmol/sample with a sensitivity of 36-47 pg/ml. Post study, the IVRs were removed from the vaginal tract (without squeezing), rinsed in saline, dried, labeled as per the macaque ID and shipped overnight at room temperature (RT) to the Population Council, NY for residual API quantification. Post use, the IVRs were sectioned in half to elute the residual core components in 10 mL, pH 4.2, acetate buffer. APIs were analyzed as described above.
2.6 Assessment of progestational activity of LNG in presence of MZC combination
We separately tested the effect of MZC combination on the progestational activity of LNG as described [60], Suppl. method 6. Briefly, CG and MZC gels without LNG [44] and at two different doses of LNG (50 μg/mL and 100 μg/mL) were applied intravaginally for 6d. The endometrial glandular transformation was assessed histologically as per the McPhail index [61].
2.7 Activity of eluted MZCL in cell based assays Cells and viruses
Human PBMCs were isolated from leukopacks, NY Blood Center (New York, NY) and were activated and grown as previously described [51]. TZM-bl (NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH), HeLa and Vero cells (ATCC, Rockville, MD) were cultured as previously described [48, 51]. HIV-1ADA-M and HIV-1BaL were kindly provided by Dr. Jeffrey Lifson at Leidos Biomedical Research, Inc. Frederick National Laboratory (Frederick, Maryland) and titered using the TZM-bl assay [62] or the Reed and Muench formula. HSV-2 G strain (ATCC) and HPV-16 PsV were propagated and titered as previously described [51].
Impact of LNG on antiviral properties of MZC
A concentration range (six-nine dilutions per API) of MIV-150 (11-0.04 nM), ZA (228-0.9 μM, anti-HIV assay) and (300-2.7 μg/mL, anti-HSV-2 assay), CG (300-2.7 ng/mL, anti-HSV-2 assay) and (2-0.007 μg/mL, anti-HPV assay) with or without LNG (500 nM) were prepared in cell culture media. For anti-HIV assay, MIV-150, ZA or their combination (± LNG) were added to activated PBMCs before HIV-1BaL challenge as per [44] except that for ZA treatments, same ZA concentrations were replaced at every media change. Anti-HSV-2 activity was measured using the HSV-2 plaque assay as previously described [48]; the anti-HPV assay previously described [26, 44] was used to test CG antiviral activity against HPV16-PsV. All analysis was performed in triplicate.
Efficacy of in vitro and in vivo released samples in cell based assays
Based on the HPLC assay results for CG and MIV-150, the in vitro release eluent and VF samples were diluted to generate dose-response curves from which CC50 and IC50 with 95% confidence intervals (CI) were computed. We tested the antiviral activity using the TZM-bl assay for anti-HIV-1ADA-M activity [62] and anti-HPV16 PsV activity using the luciferase assay (HeLa cells) [26, 44]. The dye (PrestoBlue) uptake assay was used to test the in vitro susceptibility of HSV-2 [63]. Briefly, Vero cells were seeded (104 cells/well) in 100 μl of medium and incubated overnight at 37°C, 5% CO2, and 98% humidity. Diluted release samples were added to cells immediately before adding 85 pfu/well of HSV-2 G. Cell controls and cytotoxicity samples received medium only (no virus). The plates were incubated for 6d at 37°C, 5% CO2, and 98% humidity. Cell monolayers were washed with DMEM without phenol red then 100 μl of 1× Presto Blue reagent was added. Fluorescence (emission 600 nm and excitation 560 nm) was read on Gemini EM microplate reader, Molecular Devices (Sunnyvale, CA). All samples and controls were tested in triplicates.
2.8 Stability of the IVRs
Freshly prepared MZCL IVRs (n=3) were cut into four sections and each section was sealed in an aluminum pouch, Sorbent systems (Los Angeles, CA), using Uline heat sealer (Pleasant Prairie, WI). One section per IVR was stored at −80°C (control) and the remaining at 40°C/75% RH for three months in a Caron incubator. All APIs were assayed as described earlier.
2.9 Statistics
Statistical analysis of the in vitro release of APIs was performed using a Power model in SAS (version 9.4). This model was fit using the MIXED procedure. One-way Analysis of variance (ANOVA) was performed by using JMP®, version 11.1.1, SAS Institute Inc. (Cary, NC). In vitro IC50 values with the 95% CI were calculated using a dose-response-inhibition analysis on GraphPad Prism v5.0c software for Windows (San Diego, CA). For the correlation between residual APIs (core and matrix), Spearman analysis in GraphPad Prism was used. For all tests, statistical significance was achieved at p ≤ 0.05.
3.0 Results
3.1 The novel core-matrix IVR contains hydrophilic and hydrophobic APIs of different MWs
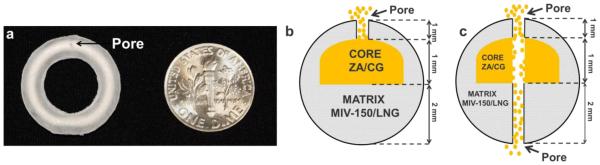
Figure 1a (left) shows a prototype macaque MZCL IVR containing a ZA/CG core in one half of the IVR matrix (EVA-28 loaded with MIV-150 and LNG); the other half comprised matrix only (no core). Each IVR contained ZA (34 ± 4 mg, n=15) and CG (81 ± 10 mg, n=15). The IVR matrix contained MIV-150 (3 ± 0.15 mg, n=15) and in MZCL IVRs, LNG (0.6 ± 0.04 mg, n=6). Figure 1b demonstrates placement of the core and the matrix APIs within the IVR.
Figure 1. Core-matrix IVR.
(a) MZCL IVR (20 mm X 4mm), macaque prototype, containing ZA/CG core (off-white ring) as seen through the translucent EVA-28 matrix containing MIV-150 and LNG and a pore to elute hydrated ZA/CG gel (scale: US dime = 17.91 mm diameter). (b and c) Cross sections depicting core and matrix compartments of the same IVR with a core side pore (b) and a drilled through pore (c) eluting ZA/CG gel.
3.2 The core – matrix IVR releases all four API for 94d in vitro
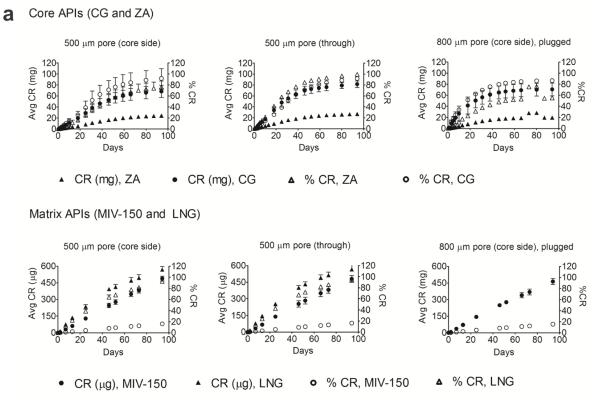
The cumulative and percent cumulative release (CR) of the hydrophilic ZA and CG (upper row) and hydrophobic MIV-150 and LNG (lower row) from IVRs with 500 μm pores (core side and through) and 800 μm pore (core side) is shown in Figure 2a. The daily release for the same IVRs is illustrated in Figure 2b. The release conditions were sink for ZA and CG, and non-sink for MIV-150 and LNG throughout the study. The buffer uptake (via the pore) and subsequent progression of the waterfront was observed in all IVRs (Figure 2c).
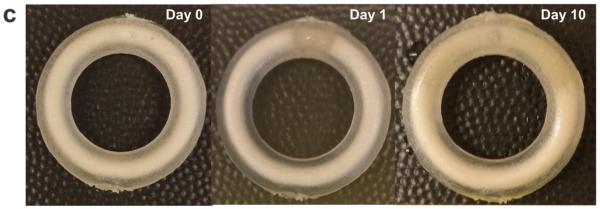
Figure 2. In vitro release of APIs from novel core-matrix IVRs.
(a) Cumulative release (mean ± SD) as a function of time of hydrophilic (core) APIs - CG and ZA (upper row) under sink conditions and hydrophobic (matrix) APIs - MIV-150 and LNG under non-sink conditions (bottom row) from the same rings. Release of APIs as seen from – 500 μm core side pore, n=5, (data pooled for MZC and MZCL IVRs), 500 μm through pore, n=5, (data pooled for MZC and MZCL IVRs) and 800 μm core side pore, n=3, (MZC IVRs). (b) Daily release as a function of time of the same rings as (a). Dotted lines represent target daily release. The data is pooled as the IVR type, MZC vs. MZCL, did not affect API release in vitro over time . (c) Unhydrated IVR core (d0) and progressive hydration of the core during 10d in vitro release. ZA/CG gel eluted from the pore by d1. The IVR dimensions are same as that in Figure 1.
CG release
CG release from 500μm pored core side IVRs was significantly lower than the release from the 500 μm through pored IVRs (p = 0.0028, adjusted p-value by Tukey-Kramer test method for multiple comparisons= 0.0078) and also from 800 μm pored core side IVRs (p = 0.0056, adjusted p-value by Tukey-Kramer method for multiple comparisons = 0.0154; Suppl. Table 1), Figures 2a and 2b. The differences in release rates can be distinguished from their daily release profiles (Figure 2b), where the 500 μm pored (core side) IVRs released CG in a sustained manner above or at the target release until the end of the study. At d50, the levels were above target release profile. In contrast, for the 500 μm pored (through) IVRs and 800 μm pored core side IVRs, the levels were at the target release by d50 but fell below the target release rate by d94. There was no significant difference between the release from 500 μm through pore and 800 μm pore (p = 0.6713, adjusted p-value by Tukey-Kramer method for multiple comparisons = 0.9053) or from MZC and MZCL IVRs with the same pore sizes (p=0.575), suggesting LNG did not affect CG release from the core. Therefore, CG release for the same pore configuration (Figure 2) is represented as pooled from MZC and MZCL IVRs. The 500 μm pored core side IVR released CG at a slower rate compared to 500 μm through and 800 μm configurations. Through d31, the average CR (mg/d) was 38 ± 9 (52 ± 17% CR), 56 ± 8 (68 ± 8% CR) and 56 ± 21 (69 ± 31% CR) from 500 μm (core side), 500 μm (through) and 800 μm core side pores, respectively. Through d31, the average daily release (mg/d) was 1.3 ± 0.4, 1.1 ± 0.2 and 0.9 ± 0.3 from 500 μm (core side), 500 μm (through) and 800 μm core side pores, respectively. Post 94d, the % CG left in 500 μm core, 500 μm through and 800 μm core IVRs was 10 ± 3, 7 ± 6 and 5 ± 5 respectively.
ZA release
Similar to CG release, the ZA release is pooled from MZC and MZCL for the same pore configuration (Figures 2a and 2b). Also, in agreement with CG release, ZA release from 500 μm pored core side IVRs was significantly lower than the release from 500 μm through pored IVRs (p < 0.0001, adjusted p-value by Tukey-Kramer test method for multiple comparisons < 0.0001) and 800 μm pored core side IVRs (p= 0.0015, adjusted p-value by Tukey-Kramer method for multiple comparisons = 0.0042) while there was no significant difference between the release from 500 μm through and 800 μm core side IVRs (p=0.8393, adjusted p-value by Tukey-Kramer method for multiple comparisons = 0.9775). Similar to the daily release profile of CG, the larger pored (800 μm) IVR approached target release rate at d50 and the levels fell below the target value by d94. In contrast the 500 μm pored core side IVRs showed a sustained release maintaining the levels above or at the target values throughout the study. Also, release from MZC and MZCL IVRs over time was not statistically significant (p=0.2126). The release of ZA scaled proportionally to CG loading (ZA: CG, 3:7). Through d31, the average CR (mg/d) was 11 ± 2 (35 ± 9% CR), 16 ± 2 (45 ± 7 % CR) and 13 ± 3 (38 ± 7% CR) from 500 μm core side, 500 μm through and 800 μm core side IVRs, respectively. Through d31, the average daily release (mg/d) was 0.35 ± 0.1, 0.34 ± 0.1 and 0.23 ± 0.23 from 500 μm (core side), 500 μm (through) and 800 μm core side pores, respectively. Post 94 d release, the % ZA left in 500 μm core, 500 μm through and 800 μm core IVRs was 8 ± 5, 4 ± 3 and 2 ± 2 respectively.
MIV-150 release
The release medium was non-sink, yielding a linear CR (and % CR) vs. t plot, Figures 2a. The daily release rate was constant (Figure 2b). MIV-150 under the non-sink conditions crystallized on the surface of the IVRs (confirmed by HPLC analysis). Post 94d release, the % MIV-150 left in 500 μm core, 500 μm through, and 800 μm core IVRs was 35 ± 4, 31 ± 9 and 37 ± 2 respectively. A stability indicating HPLC method indicated that MIV-150 was stable during the study period. Under sink conditions in vitro (MIV-150 demonstrates a first order release as expected from matrix systems (unpublished).
LNG release
The release medium was non-sink for LNG (Figures 2a and b). Post 94d release, the % LNG left in 500 μm core and 500 μm through IVRs was 9 ± 1% and 9 ± 2%, respectively. There was no LNG crystallization observed during in vitro release studies as confirmed by HPLC analysis, and LNG was stable throughout the 94d release study.
3.3 Core – matrix IVRs released APIs in vivo for up to 28d
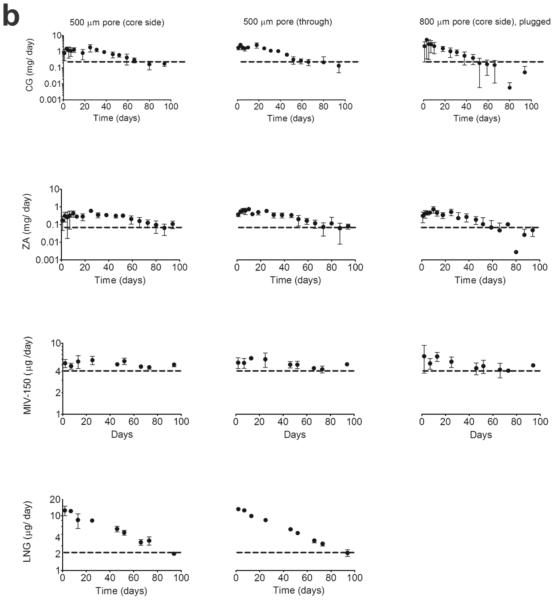
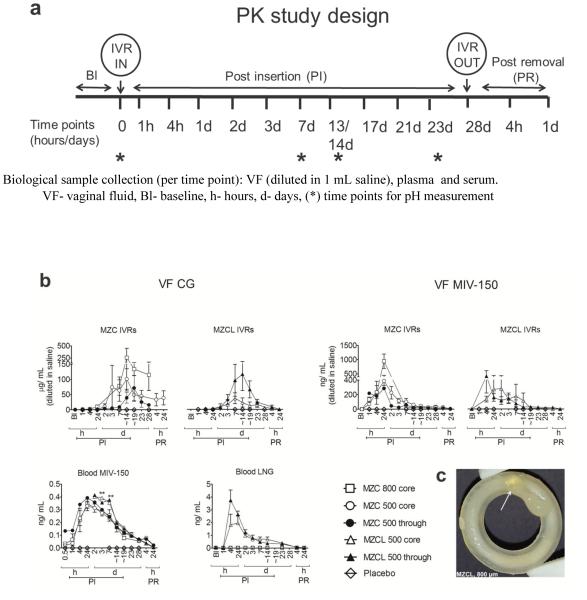
Although the IVRs released considerable levels of APIs in vitro, we staged a 28d PK study (Figure 3a) to test their performance in vivo in the presence of viscous cervical fluid, blood, epithelial shedding, bacterial growth (if any) in the core or pore, all of which could hinder the release of core APIs. There was no change in vaginal pH over this period: baseline pH was 7.0 ± 0.6 (n=15) vs. 7.3 ± 0.9 (mean ± SD) during and post study (n=53, pooled time points for MZC/MZC IVRs). It should be noted that the PK profiles generated in Figure 3b resulted from analyzing the concentrations of the swabs that were diluted in 1 mL of saline, the net swab mass being unknown. This is a limitation of this study. Therefore, the PK profiles are a conservative estimate of the VF concentration and that the actual VF concentrations will be higher than represented in Figure 3b.
Figure 3. PK profiles of APIs from MZC/ MZCL IVRs with varying pore configurations in non-DP treated macaques.
(a) Schematic representation of PK study timeline for IVR insertion and removal, pH measurement and sample collection at indicated timepoints (b) CG and MIV-150 levels (upper row) in VF from MZC IVRs of 500 μm pore (core side, n=10), 500 μm pore (through, n=11), 800 μm pore (core side, n= 4) and MZCL IVRs of 500 μm pore (core side, n=3) and 500 μm pore (through, n=3). MIV-150 and LNG levels (bottom left) in blood from the same rings. Data represents mean ± SEM. ** p < 0.01 (MZC vs. MZCL) (c) Image of an MZCL IVR with 800 μm pore IVR removed post 21 days in vivo insertion (as collected from the lumen with no further post-treatment). The ZA/CG from the core released as a gel (arrow) demonstrating proof-of-concept. The IVR dimensions are same as that in Figure 1A.
3.3.1 CG release into vaginal fluid (VF)
CG release from MZC/MZCL IVRs (Figure 3b, top left) showed a lag of about 24 h, not observed in vitro. But once CG release began, the levels increased steadily until d14 and thereafter persisted at ~50 μg/mL (levels that protect mice from HSV-2 and HPV [44]) until the IVRs were removed. There was a 2d lag in the 500 μm through MZC IVRs, but thereafter the CG levels built up steadily until d19. For both MZC/MZCL groups, the core side configurations (open symbols) showed a steady release; the through IVRs (filled symbols) a more rapid decline. The 800 μm core IVRs tended to release more CG relative to 500 μm core IVRs, as observed in vitro and in [53]. As expected, there was no CG detected in macaques carrying placebo IVRs. Although we were unable to achieve statistically significant comparison due to high variability seen in vivo and limited number of animals tested per group, when a larger sample size was tested this result was significant [53]. Taken together, the data strongly suggests that after the initial lag, IVRs (irrespective of pore configuration) continuously released CG until IVR removal and without any indication of pore blockage. A representative MZCL IVR eluting ZC gel from the core is shown in Figure 3c. Peak CG release from 800 μm and 500 μm (core and through) IVRs was 27,000 and 7000 x IC50 of CG control (0.031 μg/mL) in the same assay.
3.3.2 VF MIV-150
MIV-150 was detected (Figure 3b, top right) within 1-4h PI (unlike CG) and rapidly increased until 24h (burst) and declined gradually thereafter, which is characteristic of matrix based diffusion. The 800 μm IVRs demonstrated a greater release at 24h and the levels for all MZC IVR configurations decreased by 7d, but the MZCL IVRs showed sustained levels for 14-19d. The variability observed within the groups can be attributed to the same reasons as API variability in VF as discussed earlier. But overall, all IVRs showed a first order matrix based diffusion release. There was no MIV-150 detected in VF sampled from macaques carrying placebo IVRs. From this data, for MZC IVRs at 24 h, MIV-150 levels from 500 (core), 500 (through) and 800 (core) IVRs, were 242-1448, 88-1075 and 907-2851 x IC50 of MIV-150 control (0.584 ng/mL) in the same assay. Similarly, in MZCL 500 (core) and 500 (through) IVRs at 24h, the levels were 181-455 and 414-987 x IC50 of MIV-150 control.
3.3.3 Blood MIV-150
MIV-150 was detected within 30 min PI (Figure 3b, bottom left). The levels attained in the blood were more consistent than in VF. There were a few notable observations-the 500 through IVRs showed a higher release at the onset in the MZC group (no corresponding data available for that time point in MZCL group). Significantly greater amounts of MIV-150 were released from MZCL IVRs on days 3 (p= 0.0087) and 7 (p=0.0046), one-way ANOVA, relative to the MZC IVRs but thereafter the kinetics for both groups overlapped. The release was first order (burst and decline), comparable to the kinetics of MIV-150 in VF. There was no MIV-150 detected in plasma collected from macaques carrying placebo IVRs. The crystallization of MIV-150 seen in vitro was not observed post on IVRs examined post in vivo studies, which suggests that MIV-150 released under sink conditions in vivo. Based on this PK profile we computed other PK parameters as follows: AUC48h - ∞: 1.2 ng.d. mL−1 (MZC IVR) and 1.5 ng.d. mL−1 (MZCL IVR). For MZCL IVR, Cmax (0-∞) was 0.36 ng/mL at 24h (Tmax). For MZCL IVR the first time point was measured at 48h so Cmax (48h-∞) was 0.4 ng/mL at 48h (Tmax). Overall, only sub-nanogram levels of MIV-150 were detected in blood during IVR use.
3.3.4 Blood LNG
LNG levels spiked rapidly in the first 24h (similar to MIV-150) and declined gradually, consistent with matrix based diffusion release. Also like MIV-150, the 500 μm through IVRs tended to release more LNG at the initial time points (4-24h) but not thereafter. No LNG was detected in the MZC IVR group, as expected. The average LNG levels from the MZCL IVRs on the last day (d23) of use were 355 ± 88 pg/mL, level associated with contraceptive activity [64, 65]. Based on the PK profile, other PK parameters were computed as follows: AUC48h - ∞ was 8.07 ng.d. mL−1 (MZCL, 500 core) and 10.2 ng.d. mL−1 (MZCL, 500 through) at Cmax (0-∞) of 24h. Average LNG AUC0 - ∞for MZCL IVRs was 9.14 ng.d. mL−1.
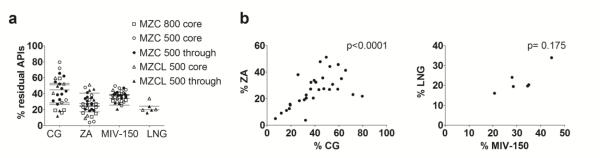
The residual APIs in the IVRs after the PK study is seen in Figure 4. Overall, the % of APIs remaining in the different IVRs were not different (Figure 4a), (p=0.55, one-way ANOVA). The residual ZA and CG levels (Figure 4b) correlated significantly (Spearman analysis, p<0.0001, two-tailed), but the correlation was not significant for residual MIV-150 and LNG probably because of the small number of IVRs available for analysis (Figure 4b).
Figure 4. Residual APIs in the IVRs post-PK study.
(a) IVRs used in the PK study were analyzed for residual API content. The plot shows % APIs (core and matrix APIs) remaining in the IVRs. (b) Plot indicating correlation between residual core APIs, CG and ZA, using Spearman analysis. The correlation was significant indicated by p<0.0001. (c) The correlation was not significant for MIV-150 and LNG at the ‘n’s tested.
3.3.5 Vaginal fluid ZA release
Zinc levels in VF were below the LLOQ of our current assay (15 μg/mL in VF). But the correlation between residual % CG and %ZA (Figures 4b) was significant, implying that CG and ZA were released concomitantly.
3.4 APIs in MZCL IVR are compatible
McPhail index scores (Table 3) and histological evaluations (data not shown) confirmed that the progestational activity observed in response to LNG was not significantly different when LNG was co-administered with MIV-150 and ZA. LNG did not interfere with the potency of individual or combined APIs (Table 4). This LNG concentration represents a much higher ratio ([LNG]/ [antiviral APIs]) than the amounts released from the IVRs, suggesting no interference with the activities of other APIs. There was no toxicity at all concentrations tested in the antiviral and cytotoxicity assays (data not shown).
Table 3.
MZC combination does not interfere with progestational activity of LNG
| Test gel | McPhail Index* (Mean)** | Histology |
|---|---|---|
| CG/LNG (50 μg LNG/ml) | 1.25 | Low glandular transformation |
| CG/LNG (150 μg LNG/ml) | 3.40 | High glandular transformation |
| MZC (0 μg LNG/ml) | 0 | No Glandular transformation |
| MZCL (50 μg LNG/ml) | 1.13 | Low glandular transformation |
| MZCL (150 μg LNG/ml) | 3.25 | High glandular transformation |
McPhail index scored by histological evaluation of endometrial glandular development and transformation.
Average index from 4 female rabbits per group
Table 4.
LNG does not impede antiviral activity of APIs included in MZC combination
| IC50 (95% confidence interval) | |||
|---|---|---|---|
| Compound* | Anti-HIV (nM for MIV-150, μm for ZA) |
Anti-HSV-2 (μg/mL) |
Anti-HPV (ng/mL) |
| MIV-150 | 1.03 (0.79-1.34) | n/a | n/a |
| MIV-150 + LNG | 0.60 (0.32-1.13) | n/a | n/a |
| CG | n/a | 0.010 (0.007-0.014)¶ | 22.8 (18.2-22.5) |
| CG+LNG | n/a | 0.008 (0.004-0.010)¶ | 11.5 (10.3-12.8) |
| ZA | 57.6 (29.9-110.8) | 18.5 (14.3 to 23.9) | n/a |
| ZA+LNG | 71.9 (43.5-119) | 13.6 (10.6-17.5) | n/a |
| ZA+MIV-150 | 20.7 (10.9-39.5) | n/a | n/a |
| ZA+MIV-150+LNG | 30.1 (23.7-38.2) | n/a | n/a |
| CG+ZA | n/d | 0.006 (0.006-0.007)¶ | n/d |
| CG+ZA+LNG | n/d | 0.005 (0.005-0.006)¶ | n/d |
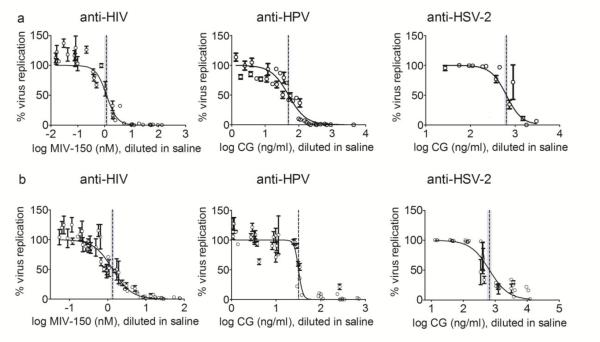
3.5 In vitro and in vivo released APIs are active
After confirming that LNG and MZC did not interfere with their respective activities, we verified the activities of in vitro and in vivo released APIs using established in vitro cell-based assays. APIs released in vitro and in vivo were active against HIV-1 ADA-M, HSV-2 and HPV 16- PsV (Figure 5, Table 5). The IC50 values of the test samples were comparable to the respective controls (within 95% CI), native API components, which were tested concurrently in the same assay. No CC50 values are shown since cell viability was nearly 100% at all dilutions tested, in all antiviral assays. In order to assess ZA contribution, the assay requires a pre-incubation step of 6h, which skews the signal-to-noise ratio. Hence only CG’s efficacy (but not zinc’s) against HSV-2 could be tested, yielding higher IC50 (otherwise much lower as seen in Table 4).
Figure 5. APIs release from MZC and MZCL IVRs were active in vitro and in vivo.
(a) Eluent from the in vitro release study (days 1, 25, 46 and 94, pooled). The figure represents the mean ± SD of samples from 12 replicate MZCL IVRs with each dilution run in triplicate. (b) Vaginal fluid was collected in swabs from macaques that received MZC or MZCL IVRs (n=8) and placed in 1 mL of saline. Data is for 48h for HIV-1, 7 days for HPV and day17 HSV-2 (with exception of 1 sample which was 48h) swab sample activity. The vertical lines are the IC50 values with 95% confidence intervals. All dilutions from each vaginal swab sample were run in triplicate, and the graph shows mean ± SD.
Table 5.
In vitro and in vivo released MZCL combination is active
| IC50 (95% confidence interval) | |||
|---|---|---|---|
| Test sample | Anti-HIV (nM) | Anti-HSV-2 (ng/mL) | Anti-HPV (ng/mL) |
| In vitro release eluent (control)* |
1.17 (0.98-1.4) 1.27 (0.7-2.3) |
637 (575-706) 496 (222-1108) |
49.56 (44.8-54.9) 20.3 (19-52.7) |
| Vaginal fluid (control)* |
0.5 (0.44-.56) 1.26 (0.62-2.57) |
659 (545-798) 438 (207-923) |
31.9 (30.6-33) 32 (25.9-40.5) |
Controls are pure API diluted in cell propagation media
3.6 Stability of APIs in the MZCL IVRs
The percent recoveries of all four APIs in MZCL IVRs (n=3, mean ± SD) at three months, 40°C/75% RH were 91 ± 11 % MIV-150 (p= 0.19), 97 ± 5 % LNG (p= 0.69), 106 ± 3.8 % CG (p= 0.08) and 96 ± 6 % ZA (p=0.33). The stability indicating HPLC method showed no additional API peaks, thereby confirming stability of MIV-150 and LNG in the IVR matrix. ZA/CG are stable at pH 4.2 for 3 months at 40°C/75% RH (unpublished).
4.0 Discussion
Common viral STIs like HSV-2 and HPV increase susceptibility to HIV-1 infection and cause other serious health conditions in women [11-15]. An MPT-IVR that can simultaneously prevent these STIs and unintended pregnancy for 90d could be an affordable and acceptable [31] solution particularly for women in resource-poor regions. Recognizing the limitations of the conventional matrix type IVRs, several groups have developed new IVR technologies for controlled and sustained delivery of hydrophilic APIs for up to 30 d. Release of biological macromolecules from an insert IVR [66], and a flux control pump technology [67] has been reported. Baum et al., [68] demonstrated release of two hydrophilic small molecules from a pod-IVR (can accommodate up to 10 pods). Essentially, the APIs were formulated in elastomeric or highly compressible hydrophilic matrices which were inserted in the main body of the IVR. The resulting reservoirs were discontinuous along the IVR length whereas the IVR design proposed here has a continuous core. The MPT IVR we developed contained a combination microbicide composed of three APIs (active against HIV-1, HSV-2 and HPV) and a contraceptive which we tested in vitro for 94d and in vivo in macaques release for up to 28d. A notable feature of the IVR is its ability to simultaneously deliver hydrophilic and hydrophobic APIs of different MWs from a simple polymeric toroid.
The dose ranging studies of ZA in CG gels at a high HSV-2 challenge in mice showed that ZA content had to be at least 1/10 of CG loading [48] to show significant protection. The IVR core loading of 3/7 (ZA/CG) maintains this criterion and yields a compact core upon compression. We selected MIV-150 loading based on dose ranging studies where 3 mg MIV-150/ IVR significantly protected macaques from SHIV-RT infection in a high dose viral challenge [52]. The LNG dose for a human IVR was scaled down for a macaque IVR [69]. The target release values for the microbicides are at least 1000 times their reported EC50 values and for LNG, 10,000 times above its reported minimum effective level, 200 pg/mL [65].
In vitro, ZA/CG release from MZCL IVRs was sustained for 94d; controlled (zero order) for first 18-31d, but first order thereafter (rate of release declined steadily). Upon contact with the aqueous release medium, there was influx of buffer into the ZA/CG reservoir through the pore. Within the core, the compressed ZA/CG pellet started transitioning into a supersaturated gelled mass. The solvent front was seen progressing gradually in the dry core for the first few days until the core became fully hydrated. As the hydration continued, volume expansion led to the expulsion of the ZA/CG gel which subsequently dissolved in the release medium. ZA/CG gel eluted by d1. Initially, under sink conditions, the concentration of the APIs in the core (C0) is much greater than the concentration in the release medium (Cm); C0 >>> Cm. The core APIs released at a steady state (SS) until (constant) concentration gradient was maintained between the core and the outside medium. But as the core continued to deplete, the constant concentration gradient between C0 and Cm that drives the zero order, or SS, release was not maintained and the release rate declined steadily and transitioned to a first order release. Nevertheless, the levels of released CG were greater than 100 μg/mL throughout; CG at ~50 μg/mL protected mice from HPV and HSV-2 infections [26]. ZA release kinetics mimicked that of CG except that release rate scaled proportionally to the ZA/CG loading indicating that CG acts as a carrier for ZA.
The pore configuration influenced the API release rates. The small pored (core side) IVRs (500 μm) maintained a longer linear release profile (up to 31d) relative to the 500 μm through pored IVRs, (two pores) or larger pored (800 μm) IVRs (up to 18d). For both CG and ZA, the overall release rate from the 500 μm pored IVRs was significantly lower than 500 μm pored through and 800 μm pored core side IVRs. Gunawardana [70] also reported different release rates of human monoclonal antibodies from an IVR as a function of pore size. Tobias et al., [71] showed that SS steady can be extended by increasing the payload in an osmotic device made from biodegradable elastomer but keeping the pore size constant. Thus, by scaling the current macaque prototype to a human IVR where the core capacity will increase by 2.5-3 folds, we can expect that the SS can be extended. Release rate is an inverse function of the membrane thickness and along with pore size can be optimized to yield the desired release rate. The membrane thickness in all the IVRs we tested was 1mm. The core API release was not affected by the addition of LNG to MZC combination. In vivo, LNG and MZC combination was compatible; neither LNG nor MZC affected each other’s bioactivity. The release conditions were non-sink for matrix APIs, MIV-150 and LNG, and the SS observed was a result of the saturation of the media. The primary goal of the release studies was to characterize CG and ZA elution from the IVRs via a pore continuously for 90d. Therefore, we did not adjust the release conditions by incorporating solubilizers or by increasing the volume (compromised detection of ZA) to provide sink conditions for MIV-150 and LNG. The non-sink conditions used in in vitro studies demonstrates that hydrophobic APIs were released from the prototype IVRs but does not characterize actual device behavior which is typically tested under sink conditions. Under sink conditions, release of hydrophobic APIs from matrix EVA systems is first order release [37, 72] and this was also supported by release of MIV-150 and LNG in vivo in blood in the current study.
We staged in vivo studies to test the performance of the IVRs (particularly release of core APIs from a pore). The in vivo lag and variability in CG release can be attributed to the physiological release conditions (not encountered in vitro) and inter-macaque related differences-volume of fluids (vaginal, cervical and blood) that vary during cyclical phases and which are generally more viscous (contributing to lag) than the in vitro release medium, varying distribution of CG as a function of different vaginal vault sizes, and possibility of microbial growth within the core or pore channel (no visual evidence of bacterial growth). In addition, the amount of the VF diluted in 1 mL of saline was not known, contributing to overall variability. But all the IVRs released CG, following the lag, and the release persisted until the IVRs were removed. Similar to in vitro release, the 800 μm pored IVR tended to show greater CG release than 500 μm pored IVRs and this result was significant [53]. These data demonstrate the feasibility of releasing a hydrophilic high MW API in a sustained manner from a non-water swellable EVA matrix, otherwise suitable to elute hydrophobic small molecules. The ZA and CG correlation upon use was significant, supporting the hypothesis that ZA released with CG. MIV-150 levels in vaginal fluid showed a first order release. Macaques using MZCL IVRs had significantly higher levels of MIV-150 in blood at early time points (d3 and d7) compared to macaques using MZC IVRs. A similar trend was observed in a macaque efficacy study [53]. At the levels of LNG achieved in serum, contraceptive efficacy has been reported [65] in women. However it should be noted that Dusterberg et al., [73] reported inter-species related variations in (%) bioavailabilities and plasma half-lives of three different progestogens including LNG. As per their findings, % bioavailability and plasma half-life of LNG in rhesus macaques was nearly 10 and 6 times (respectively) less than that reported for women. Therefore, by accounting for the inter species related variation, the results presented here inform that the MZCL IVR is a suitable system to deliver LNG at effective levels, however, serum LNG levels as seen in macaques may in fact translate to much higher levels in humans. The IVR formulations had not altered the vaginal pH during the study period. Although the total loading of LNG per IVR was nearly 5 times less than that of MIV-150, the levels of LNG detected in serum were nearly 10 times greater than that of MIV-150 in plasma, suggesting a more rapid systemic clearance of MIV-150. The effect of LNG in vivo is being further investigated. In both in vitro and in vivo studies, MIV-150 and LNG being more hydrophobic will preferentially partition from the surface of the IVR in to the surrounding (in vitro release medium or tissues) by the Fick’s law of diffusion. However, the inside of the matrix is directly in contact with the hydrated core which has a volume of ~100 μl. So, there will also likely be inward flux of the hydrophobic APIs in the core. But MIV-150 and LNG, being hydrophobic, have limited solubility in the aqueous phase and therefore the amount of hydrophobic APIs dissolved in the core will be considerably less than the amount released in the external medium. The dissolved hydrophobic APIs in the core will be transported (along with CG and ZA) to the outside medium from the pore. Therefore, the levels detected in the surrounding medium will be due to the diffusion of APIs from the IVR surface and a fraction resulting from the core. The eluent from in vitro released studies and VF containing MIV-150, CG and ZA (and LNG) retained their activity against HIV-1, HSV-2 and HPV in cell-based assays. Also, in the presence of semen, efficacy of the APIs in VF was maintained [53, 74].
After seeing promising PK and efficacy results, we evaluated MZCL IVRs for efficacy in macaques under repeat SHIV-RT and HSV-2 co-challenge [53]. There was significant protection from SHIV-RT infection and reduced HSV-2 shedding frequency.
The results from the proof-of-concept PK studies presented here and the efficacy studies reported elsewhere [53] are encouraging. The next steps for the human IVR development will involve evaluation of EVA-28 and other suitable non-water swellable clinical grade polymer (thermoplastic or silicone) that are approved for a long term use (90 d). Evaluation criteria include release characteristics of the APIs, ease of scale-up, mechanical properties, stability and cost. Semi-solid core formulations will also be explored as it may help to overcome the lag in vivo. The wall thickness, pore size, number of pores, core composition, loading and mechanical strength will be optimized. Based on the IVR dimensions, we expect to see a 3-4 fold increase in the core API payload in the human IVR (relative to macaque IVR) which will likely yield an extended SS for up to 90d.
4.1 Clinical significance of this IVR
Millions of women worldwide can benefit from an MPT like the MZCL IVR as it simultaneously protects against HIV-1, HSV-2, HPV and unintended pregnancy. Its extended duration of action (one IVR per 90d) will also help alleviate the cost, making it affordable for low income populations. As the IVR can deliver APIs of diverse physicochemical properties, it can deliver APIs to prevent and/or treat other STIs like bacterial vaginosis, gonorrhea, and syphilis.
Supplementary Material
Acknowledgments
This work was supported by the United States Agency for International Development (USAID) under the terms of Cooperative Agreement GPO-A-00-04-00019-00, NIH Grant R21AI098645 and the Danish International Development and Agency (DANIDA). The contents of this paper are the sole responsibility of the Population Council and do not necessarily reflect the views of the funding agencies. We thank Dr. Kyle Kleinbeck for providing LNG loaded gels and the veterinary staff at TNPRC for their continued support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Turpin JA. Topical microbicides to prevent the transmission of HIV: formulation gaps and challenges. Drug delivery and translational research. 2011;1:194–200. doi: 10.1007/s13346-011-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Friend DR, editor. Recent Trends in Microbicide Formulations. Antivir Res. 2010;88:S1–S66. doi: 10.1016/j.antiviral.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [3].Fernandez-Romero JA, Teleshova N, Zydowsky TM, Robbiani M. Preclinical assessments of vaginal microbicide candidate safety and efficacy. Advanced drug delivery reviews. 2014 doi: 10.1016/j.addr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- [4].Devlin B, Nuttall J, Wilder S, Woodsong C, Rosenberg Z. Development of dapivirine vaginal ring for HIV prevention. Antiviral Res. 2013;100:S3–8. doi: 10.1016/j.antiviral.2013.09.025. [DOI] [PubMed] [Google Scholar]
- [5].Smith JM, Rastogi R, Teller RS, Srinivasan P, Mesquita PM, Nagaraja U, McNicholl JM, Hendry RM, Dinh CT, Martin A, Herold BC, Kiser PF. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16145–16150. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Clark JT, Clark MR, Shelke NB, Johnson TJ, Smith EM, Andreasen AK, Nebeker JS, Fabian J, Friend DR, Kiser PF. Engineering a segmented dual-reservoir polyurethane intravaginal ring for simultaneous prevention of HIV transmission and unwanted pregnancy. Plos One. 2014;9:e88509. doi: 10.1371/journal.pone.0088509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Friend DR. Drug delivery in multiple indication (multipurpose) prevention technologies: systems to prevent HIV-1 transmission and unintended pregnancies or HSV-2 transmission. Expert Opin Drug Del. 2012;9:417–427. doi: 10.1517/17425247.2012.668183. [DOI] [PubMed] [Google Scholar]
- [8].CAMI health MPT Product Development Database. 2012 http://mpts101.org/mpt-database. Access date: January 20, 2015, in, CAMI/Public Health Institute.
- [9].Andrei G, Lisco A, Vanpouille C, Introini A, Balestra E, van den Oord J, Cihlar T, Perno CF, Snoeck R, Margolis L, Balzarini J. Topical Tenofovir, a Microbicide Effective against HIV, Inhibits Herpes Simplex Virus-2 Replication. Cell Host Microbe. 2011;10:379–389. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nixon B, Jandl T, Teller RS, Taneva E, Wang Y, Nagaraja U, Kiser PF, Herold BC. Vaginally delivered tenofovir disoproxil fumarate provides greater protection than tenofovir against genital herpes in a murine model of efficacy and safety. Antimicrobial agents and chemotherapy. 2014;58:1153–1160. doi: 10.1128/AAC.01818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brown JM, Wald A, Hubbard A, Rungruengthanakit K, Chipato T, Rugpao S, Mmiro F, Celentano DD, Salata RS, Morrison CS, Richardson BA, Padian NS. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. Aids. 2007;21:1515–1523. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- [12].Cohen MS. Classical Sexually Transmitted Diseases Drive the Spread of HIV-1: Back to the Future. J Infect Dis. 2012;206:1–2. doi: 10.1093/infdis/jis303. [DOI] [PubMed] [Google Scholar]
- [13].Houlihan CF, Larke NL, Watson-Jones D, Smith-McCune KK, Shiboski S, Gravitt PE, Smith JS, Kuhn L, Wang C, Hayes R. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. Aids. 2012;26:2211–2222. doi: 10.1097/QAD.0b013e328358d908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corey L. Herpes simplex virus type 2 and HIV-1: The dialogue between the 2 organisms continues. J Infect Dis. 2007;195:1242–1244. doi: 10.1086/513570. [DOI] [PubMed] [Google Scholar]
- [15].Burchell AN, Winer RL, de Sanjose S, Franco EL. Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24:52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- [16].amfAR AIDS epidemic Statistics. 2012 amfAR. [Google Scholar]
- [17].Looker KJ, Gamett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. B World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].WHO Human papillomavirus (HPV) and cervical cancer. Fact sheet N°380. Updated November 2014. http://www.who.int/mediacentre/factsheets/fs380/en/ (date accessed: January 20, 2015)
- [19].The Alan Guttmacher Institute UNPLANNED PREGNANCY COMMON WORLDWIDE. 2015 http://www.guttmacher.org/media/nr/abortww_nr.html/. Date accessed: January 20.
- [20].UNICEF data: Monitoring the situation of children and women Maternal mortality: Current status and progress. 2014 http://data.unicef.org/maternal-health/maternal-mortality. Date accessed: January 20, 2015.
- [21].Brady M, Manning J. Lessons from reproductive health to inform multipurpose prevention technologies: Don't reinvent the wheel. Antivir Res. 2013;100:S25–S31. doi: 10.1016/j.antiviral.2013.09.019. [DOI] [PubMed] [Google Scholar]
- [22].Zhu XP, Muhammad ZS, Wang JG, Lin W, Guo SK, Zhang W. HSV-2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses. 2014;6:371–390. doi: 10.3390/v6020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cervarix--a second HPV vaccine. The Medical letter on drugs and therapeutics. 2010;52:37–38. [PubMed] [Google Scholar]
- [24].Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer prevention research. 2012;5:18–23. doi: 10.1158/1940-6207.CAPR-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm. Date accessed: January 22, 2015.
- [26].Rodriguez A, Kleinbeck K, Mizenina O, Kizima L, Levendosky K, Jean-Pierre N, Villegas G, Ford BE, Cooney ML, Teleshova N, Robbiani M, Herold BC, Zydowsky T, Fernandez Romero JA. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antiviral Res. 2014;108:88–93. doi: 10.1016/j.antiviral.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].S.J. Howley PM, Lowy DR. Papillomaviruses. In: PM KDH, editor. Fields Virology. Lippincott Williams and Wilkins; Philadelphia: 2013. pp. 1662–1703. [Google Scholar]
- [28].Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, Group CT. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, Hendrix CW, Dai JY, Ganesh S, Mkhize B, Taljaard M, Parikh UM, Piper J, Masse B, Grossman C, Rooney J, Schwartz JL, Watts H, Marzinke MA, Hillier SL, McGowan IM, Chirenje ZM, Team VS. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2015;372:509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rees H, Delany-Moretlwe S, Baron D, Lombard C, Gray G, Myer L, Panchia R, Schwartz J, Doncel G. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. CROI; Seattle, Washington: 2015. [Google Scholar]
- [31].Montgomery ET, van der Straten A, Cheng H, Wegner L, Masenga G, von Mollendorf C, Bekker L, Ganesh S, Young K, Romano J, Nel A, Woodsong C. Vaginal ring adherence in sub-Saharan Africa: expulsion, removal, and perfect use. AIDS and behavior. 2012;16:1787–1798. doi: 10.1007/s10461-012-0248-4. [DOI] [PubMed] [Google Scholar]
- [32].CAMI health MPT Product Development Database. 2012 http://mpts101.org/mpt-database/mpts-topical-rings. Access date: February 17, 2015, in, CAMI/Public Health Institute.
- [33].Fernandez-Romero JA, Thorn M, Turville SG, Titchen K, Sudol K, Li J, Miller T, Robbiani M, Maguire RA, Buckheit RW, Jr., Hartman TL, Phillips DM. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sexually transmitted diseases. 2007;34:9–14. doi: 10.1097/01.olq.0000223287.46097.4b. [DOI] [PubMed] [Google Scholar]
- [34].Hsu M, Aravantinou M, Menon R, Seidor S, Goldman D, Kenney J, Derby N, Gettie A, Blanchard J, Piatak M, Jr., Lifson JD, Fernandez-Romero JA, Zydowsky TM, Robbiani M. A combination microbicide gel protects macaques against vaginal simian human immunodeficiency virus-reverse transcriptase infection, but only partially reduces herpes simplex virus-2 infection after a single high-dose cochallenge. AIDS Res Hum Retroviruses. 2014;30:174–183. doi: 10.1089/aid.2013.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kenney J, Aravantinou M, Singer R, Hsu M, Rodriguez A, Kizima L, Abraham CJ, Menon R, Seidor S, Chudolij A, Gettie A, Blanchard J, Lifson JD, Piatak M, Jr., Fernandez-Romero JA, Zydowsky TM, Robbiani M. An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. Plos One. 2011;6:e15835. doi: 10.1371/journal.pone.0015835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kenney J, Singer R, Derby N, Aravantinou M, Abraham CJ, Menon R, Seidor S, Zhang S, Gettie A, Blanchard J, Piatak M, Jr., Lifson JD, Fernandez-Romero JA, Zydowsky TM, Robbiani M. A single dose of a MIV-150/Zinc acetate gel provides 24 h of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8-24 h after gel use. AIDS Res Hum Retroviruses. 2012;28:1476–1484. doi: 10.1089/aid.2012.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Singer R, Mawson P, Derby N, Rodriguez A, Kizima L, Menon R, Goldman D, Kenney J, Aravantinou M, Seidor S, Gettie A, Blanchard J, Piatak M, Lifson JD, Fernandez-Romero JA, Robbiani M, Zydowsky TM. An Intravaginal Ring That Releases the NNRTI MIV-150 Reduces SHIV Transmission in Macaques. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ouattara LA, Barnable P, Mawson P, Seidor S, Zydowsky TM, Kizima L, Rodriguez A, Fernandez-Romero JA, Cooney ML, Roberts KD, Gettie A, Blanchard J, Robbiani M, Teleshova N. MIV-150-containing intravaginal rings protect macaque vaginal explants against SHIV-RT infection. Antimicrobial agents and chemotherapy. 2014;58:2841–2848. doi: 10.1128/AAC.01529-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hsu M, Keele BF, Aravantinou M, Krawczyk N, Seidor S, Abraham CJ, Zhang S, Rodriguez A, Kizima L, Derby N, Jean-Pierre N, Mizenina O, Gettie A, Grasperge B, Blanchard J, Piatak MJ, Jr., Lifson JD, Fernandez-Romero JA, Zydowsky TM, Robbiani M. Exposure to MIV-150 from a high-dose intravaginal ring results in limited emergence of drug resistance mutations in SHIV-RT infected rhesus macaques. Plos One. 2014;9:e89300. doi: 10.1371/journal.pone.0089300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antivir Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- [41].Arens M, Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. Journal of clinical microbiology. 2000;38:1758–1762. doi: 10.1128/jcm.38.5.1758-1762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. Plos Pathog. 2006;2:671–680. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- [44].Kizima L, Rodriguez A, Kenney J, Derby N, Mizenina O, Menon R, Seidor S, Zhang S, Levendosky K, Jean-Pierre N, Pugach P, Villegas G, Ford BE, Gettie A, Blanchard J, Piatak M, Jr., Lifson JD, Paglini G, Teleshova N, Zydowsky TM, Robbiani M, Fernandez-Romero JA. A Potent Combination Microbicide that Targets SHIV-RT, HSV-2 and HPV. Plos One. 2014;9:e94547. doi: 10.1371/journal.pone.0094547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kilmarx PH, Blanchard K, Chaikummao S, Friedland BA, Srivirojana N, Connolly C, Witwatwongwana P, Supawitkul S, Mock PA, Chaowanachan T, Tappero J. A randomized, placebo-controlled trial to assess the safety and acceptability of use of carraguard vaginal gel by heterosexual couples in Thailand. Sexually transmitted diseases. 2008;35:226–232. doi: 10.1097/OLQ.0b013e31815d6e0d. [DOI] [PubMed] [Google Scholar]
- [46].Kilmarx PH, van de Wijgert JH, Chaikummao S, Jones HE, Limpakarnjanarat K, Friedland BA, Karon JM, Manopaiboon C, Srivirojana N, Yanpaisarn S, Supawitkul S, Young NL, Mock PA, Blanchard K, Mastro TD. Safety and acceptability of the candidate microbicide Carraguard in Thai Women: findings from a Phase II Clinical Trial. Journal of acquired immune deficiency syndromes. 2006;43:327–334. doi: 10.1097/01.qai.0000243056.59860.c1. [DOI] [PubMed] [Google Scholar]
- [47].Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, Gopolang F, Hoffman M, Ramjee G, Williamson AL. The effectiveness of Carraguard, a vaginal microbicide, in protecting women against high-risk human papillomavirus infection. Antivir Ther. 2011;16:1219–1226. doi: 10.3851/IMP1890. [DOI] [PubMed] [Google Scholar]
- [48].Fernandez-Romero JA, Abraham CJ, Rodriguez A, Kizima L, Jean-Pierre N, Menon R, Begay O, Seidor S, Ford BE, Gil PI, Peters J, Katz D, Robbiani M, Zydowsky TM. Zinc acetate/carrageenan gels exhibit potent activity in vivo against high-dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrobial agents and chemotherapy. 2012;56:358–368. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kenney J, Rodriguez A, Kizima L, Seidor S, Menon R, Jean-Pierre N, Pugach P, Levendosky K, Derby N, Gettie A, Blanchard J, Piatak M, Jr., Lifson JD, Paglini G, Zydowsky TM, Robbiani M, Fernandez Romero JA. A modified zinc acetate gel, a potential nonantiretroviral microbicide, is safe and effective against simian-human immunodeficiency virus and herpes simplex virus 2 infection in vivo. Antimicrobial agents and chemotherapy. 2013;57:4001–4009. doi: 10.1128/AAC.00796-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].WHO Model List of Essential Medicines 2013 http://www.who.int/medicines/publications/essentialmedicines/18th_EML_Final_web_8Jul13.pdf (date accessed: January 20,2015)
- [51].Kizima L, Rodriguez A, Kenney J, Derby N, Mizenina O, Menon R, Seidor S, Zhang SM, Levendosky K, Jean-Pierre N, Pugach P, Villegas G, Ford BE, Gettie A, Blanchard J, Piatak M, Lifson JD, Paglini G, Teleshova N, Zydowsky TM, Robbiani M, Fernandez-Romero JA. A Potent Combination Microbicide that Targets SHIV-RT, HSV-2 and HPV. Plos One. 2014;9 doi: 10.1371/journal.pone.0094547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kenney J, Derby N, Aravantinou M, Kleinbeck K, Frank I, Gettie A, Grasperge B, Blanchard J, Piatak M, Jr., Lifson JD, Zydowsky TM, Robbiani M. Short communication: a repeated simian human immunodeficiency virus reverse transcriptase/herpes simplex virus type 2 cochallenge macaque model for the evaluation of microbicides. AIDS Res Hum Retroviruses. 2014;30:1117–1124. doi: 10.1089/aid.2014.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kenney J, Aravantinou M, Ugaonkar S, et al. A novel microbicide/contraceptive intravaginal ring protects macaques against SHIV-RT infection and reduces HSV-2 shedding after repeated co-challenge. Submitted. [Google Scholar]
- [54].Thanasarakhan W, Liawruangrath S, Wangkarn S, Liawruangrath B. Sequential injection spectrophotometric determination of zinc(II) in pharmaceuticals based on zinc(II)-PAN in non-ionic surfactant medium. Talanta. 2007;71:1849–1855. doi: 10.1016/j.talanta.2006.08.034. [DOI] [PubMed] [Google Scholar]
- [55].Soedjak HS. Colorimetric Determination of Carrageenans and Other Anionic Hydrocolloids with Methylene-Blue. Anal Chem. 1994;66:4514–4518. [Google Scholar]
- [56].Bart JCJ. Additives in Polymers: Industrial Analysis and Applications. John Wiley and Sons; 2005. pp. 146–152. [Google Scholar]
- [57].Animals and animal products . Animal Welfare Act and Regulation of 2001. Department of Agriculture; Belstsville, MD: 2001. Code of Federal Regulations. [Google Scholar]
- [58].Guide for the Care and Use of Laboratory Animals. The National Academies Press; 2011. [PubMed] [Google Scholar]
- [59].Crostarosa F, Aravantinou M, Akpogheneta OJ, Jasny E, Shaw A, Kenney J, Piatak M, Lifson JD, Teitelbaum A, Hu LY, Chudolij A, Zydowsky TM, Blanchard J, Gettie A, Robbiani M. A Macaque Model to Study Vaginal HSV-2/Immunodeficiency Virus Co-Infection and the Impact of HSV-2 on Microbicide Efficacy. Plos One. 2009;4 doi: 10.1371/journal.pone.0008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65:629–636. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]
- [61].McPhail MK. The assay of progestin. J Physiol-London. 1934;83:145–156. doi: 10.1113/jphysiol.1934.sp003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Begay O, Jean-Pierre N, Abraham CJ, Chudolij A, Seidor S, Rodriguez A, Ford BE, Henderson M, Katz D, Zydowsky T, Robbiani M, Fernandez-Romero JA. Identification of Personal Lubricants That Can Cause Rectal Epithelial Cell Damage and Enhance HIV Type 1 Replication in Vitro. Aids Res Hum Retrov. 2011;27:1019–1024. doi: 10.1089/aid.2010.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ashley R. Herpes simplex virus. In: Lennette E, editor. Diagnostic Procedures for Viral, Rickettsial, and Chlamydial Infections. American Public Health Association; Washington D.C.: 1995. pp. 375–395. [Google Scholar]
- [64].Nilsson CG, Lahteenmaki PL, Luukkainen T, Robertson DN. Sustained intrauterine release of levonorgestrel over five years. Fertility and sterility. 1986;45:805–807. doi: 10.1016/s0015-0282(16)49397-0. [DOI] [PubMed] [Google Scholar]
- [65].Gunardi E, Affandi B. Serum levonorgestrel concentration and cervical mucus viscosity after six months of monoplant® implantation. Med J Indones. 2014;23:25–29. [Google Scholar]
- [66].Morrow RJ, Woolfson AD, Donnelly L, Curran R, Andrews G, Katinger D, Malcolm RK. Sustained release of proteins from a modified vaginal ring device. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2011;77:3–10. doi: 10.1016/j.ejpb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Teller RS, Rastogi R, Johnson TJ, Blair MJ, Hitchcock RW, Kiser PF. Intravaginal flux controlled pump for sustained release of macromolecules. Pharmaceutical research. 2014;31:2344–2353. doi: 10.1007/s11095-014-1331-5. [DOI] [PubMed] [Google Scholar]
- [68].Baum MM, Butkyavichene I, Gilman J, Kennedy S, Kopin E, Malone AM, Nguyen C, Smith TJ, Friend DR, Clark MR, Moss JA. An intravaginal ring for the simultaneous delivery of multiple drugs. J Pharm Sci. 2012;101:2833–2843. doi: 10.1002/jps.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rowe PJ. Microdose Intravaginal Levonorgestrel Contraception - a Multicenter Clinical-Trial .1. Contraceptive Efficacy and Side-Effects. Contraception. 1990;41:105–124. doi: 10.1016/0010-7824(90)90141-h. [DOI] [PubMed] [Google Scholar]
- [70].Gunawardana M, Baum MM, Smith TJ, Moss JA. An Intravaginal Ring for the Sustained Delivery of Antibodies. J. Pharm. Sci. 2014;103:3611–3620. doi: 10.1002/jps.24154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Tobias IS, Lee H, Engelmayr GC, Macaya D, Bettinger CJ, Cima MJ. Zero-order controlled release of ciprofloxacin-HCl from a reservoir-based, bioresorbable and elastomeric device. Journal of Controlled Release. 2010;146:356–362. doi: 10.1016/j.jconrel.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Loxley A, Mitchnick M, Okoh O, McConnell J, Goldman L, Morgan C, Clark M, Friend DR. Ethylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonorgestrel. Drug Delivery Transl. Res. 2011;1:247–255. doi: 10.1007/s13346-011-0031-5. [DOI] [PubMed] [Google Scholar]
- [73].Dusterberg B, Humpel M, Speck U. Terminal half-lives in plasma and bioavailability of norethisterone, levonorgestrel, cyproterone acetate and gestodene in rats, beagles and rhesus monkeys. Contraception. 1981;24:673–683. doi: 10.1016/0010-7824(81)90018-4. [DOI] [PubMed] [Google Scholar]
- [74].Rodriguez A, Kleinbeck K, Mizenina O, Kizima L, Levendosky K, Jean-Pierre N, Villegas G, Ford BE, Cooney ML, Teleshova N, Robbiani M, Herold BC, Zydowsky T, Romero JAF. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir Res. 2014;108:88–93. doi: 10.1016/j.antiviral.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.