Abstract
AIM
Individuals with cerebral palsy (CP) have impaired movement due to a brain injury near birth. Understanding how neuromuscular control is altered in CP can provide insight into pathological movement. We sought to determine if individuals with CP demonstrate reduced complexity of neuromuscular control during gait compared with unimpaired individuals and if changes in control are related to functional ability.
METHOD
Muscle synergies during gait were retrospectively analyzed for 633 individuals (age range 3.9–70y): 549 with CP (hemiplegia, n=122; diplegia, n=266; triplegia, n=73; quadriplegia, n=88) and 84 unimpaired individuals. Synergies were calculated using non-negative matrix factorization from electromyography collected during previous clinical gait analyses. Synergy complexity during gait was compared with diagnosis subtype, functional ability, and clinical examination measures.
RESULT
Fewer synergies were required to describe muscle activity during gait in individuals with CP compared with unimpaired individuals. Changes in synergies were related to functional impairment and clinical examination measures including selective motor control, strength, and spasticity.
INTERPRETATION
Individuals with CP use a simplified control strategy during gait compared with unimpaired individuals. These results were similar to synergies during walking among adult stroke survivors, suggesting similar neuromuscular control strategies between these clinical populations.
Walking is an important activity of daily living that enhances independence, participation, and quality of life. However, for individuals with cerebral palsy (CP), walking can be a challenging and sometimes impossible activity. To improve mobility for individuals with CP and other neurological disorders, we need to understand how unimpaired individuals control walking and how control is altered after brain injury.
There are several theories for how humans control movement. Rhythmic activities such as walking are theorized to be partly controlled at the level of the spinal cord.1 Infants, spinalized animals, and individuals who have had a spinal cord injury can produce rhythmic stepping patterns.2–4 However, in addition to rhythmic stepping, walking requires dynamic balance and adaptability. Thus, muscle activity controlled via the spinal cord is theorized to be supplemented with cortically modulated muscle activity producing a versatile gait pattern.
Computational techniques, including matrix factorization algorithms, have been used to evaluate the complexity of different neuromuscular control strategies.5 Using experimentally measured muscle activity (electromyography [EMG]), matrix factorization algorithms identify low-dimensional spaces composed of weighted groups of muscles that can describe variation in muscle activity. These weighted groups of muscles, commonly referred to as synergies or modes, represent muscles that are consistently activated together and are theorized to represent a simplified control strategy compared with controlling each muscle individually. Evaluating the variance in muscle activity accounted for by a given number of synergies can provide a measure of the complexity of control used by an individual during a task. Previous studies have shown that muscle activity during a variety of tasks can be described by a small set of synergies.6,7 For example, less than six synergies have been shown to describe greater than 90% of the variance in muscle activity during unimpaired gait.8 The term synergy has been used clinically in many contexts. In this manuscript we use the term synergy to refer to weighted groups of muscles identified mathematically from EMG data.
Previous studies have also demonstrated that synergies identified from EMG data are altered after brain injury. After a stroke, fewer synergies are used during walking and upper-extremity tasks compared with unimpaired adults,9,10 possibly reflecting a simplified control strategy. However, the generalizability of these results to other clinical populations is unknown. While adult stroke survivors often have decades of walking experience before brain injury, individuals with CP learn to walk after brain injury, which may lead to different synergies. One preliminary study of eight children with CP suggested that synergies were altered compared with unimpaired adults,11 but the impact of age and relationship with functional ability remains unclear.
A variety of other strategies have previously been used to evaluate altered neuromuscular control among individuals with CP. Analyses of surface EMG and M-wave amplitude elicited via nerve stimulation during maximal contractions have demonstrated that individuals with CP have reduced voluntary neuromuscular activation.12 During tasks such as postural control and walking, analyses of EMG data have shown inappropriate timing and increased co-activation of antagonist muscles.13,14 The central mechanisms underlying these altered muscle activation patterns remain unclear and synergies provide one framework to evaluate neuromuscular control strategies.
The aims of this research were to (1) evaluate if and how synergies among individuals with CP differ from those exhibited by unimpaired individuals and (2) determine if synergies are related to functional ability, clinical examination measures, and diagnosis subtype. We hypothesized that, similar to adult stroke survivors, fewer synergies would be required to describe muscle activity during gait compared with unimpaired individuals, and that synergy complexity would be related to functional ability and selective motor control.
METHOD
Study population
Individuals with a diagnosis of CP were selected from a database of over 6600 individuals who had previously received gait analysis at Gillette Children's Specialty Healthcare, St. Paul, MN. Individuals were included if their gait analysis was between January 2004 and February 2013 and included EMG data (Table I). Individuals were not excluded because of previous surgeries to provide a representative sample of individuals with CP who had been referred for gait analysis. These criteria resulted in a total of 549 individuals with CP (hemiplegia, n=122; diplegia, n=266; triplegia, n=73; quadriplegia, n=88). We also included 84 unimpaired individuals who had undergone gait analysis with EMG data collected at Gillette as part of a previous study.15 The age range of participants was 3.9 to 70 years (Table I). Human participant approval was obtained from both the University of Washington and the University of Minnesota.
Table I.
Study population
| Diagnosis | n | Age (y) | Height (m) | Mass (kg) | Speed (m/s) | GMFCS, % of participants | FAQ, % of participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | NA | <6 | 7 | 8 | 9 | 10 | NA | ||||||
| Control | 84 | 10.3a | 1.43a | 37.6a | 1.14a | — | — | — | — | — | — | — | — | — | — | — |
| 7.6-13.0b | 1.29-1.57b | 26.4-50.9b | 1.01-1.23b | |||||||||||||
| 4.3-18.0c | 1.01-1.85c | 16.2-81.9c | 0.79-1.64c | |||||||||||||
| Hemiplegia | 122 | 10.7a | 1.41a | 36.5a | 1.05a | 52 | 13 | 1 | 0 | 34 | 3 | 3 | 14 | 41 | 35 | 3 |
| 8.1-14.2b | 1.26-1.59b | 24.8-51.4b | 0.91-1.18b | |||||||||||||
| 4.6-53.4c | 1.08-1.82c | 16.0-92.3c | 0.25-1.53c | |||||||||||||
| Diplegia | 266 | 9.1a | 1.29a | 26.2a | 0.89a | 29 | 27 | 11 | 0 | 33 | 11 | 10 | 24 | 39 | 14 | 1 |
| 6.8-12.4b | 1.17-1.48b | 21.0-43.4b | 0.75-1.06b | |||||||||||||
| 3.9-70.0c | 0.92-1.87c | 11.7-92.4c | 0.12-1.46c | |||||||||||||
| Triplegia | 73 | 9.4a | 1.27a | 26.7a | 0.82a | 12 | 30 | 19 | 5 | 33 | 14 | 19 | 22 | 34 | 8 | 3 |
| 7.1-12.7b | 1.16-1.43b | 20.4-42.6b | 0.53-0.98b | |||||||||||||
| 4.5-48.2c | 1.00-1.78c | 14.9-72.3c | 0.20-1.38c | |||||||||||||
| Quadriplegia | 88 | 9.8a | 1.29a | 26.9a | 0.54a | 0 | 12 | 38 | 10 | 40 | 55 | 11 | 18 | 11 | 2 | 2 |
| 7.4-13.7b | 1.15-1.47b | 20.6-39.5b | 0.34-0.71b | |||||||||||||
| 5.2-56.1c | 1.01-1.79c | 14.7-81.3c | 0.06-1.38c | |||||||||||||
Median.
25th-75th centiles.
Minimum-maximum. GMFCS, Gross Motor Functional Classification System; FAQ, Functional Activity Questionnaire; NA, measurement not available.
Total: 633 individuals (control n=84; hemiplegia n=122; diplegia n=266; triplegia n=73; quadriplegia n=88) aged 3.9 to 70 years.
Electromyography
Surface EMG data (Motion Laboratory Systems, Baton Rouge, LA, USA) from five muscles on each leg – the rectus femoris, medial hamstrings, lateral hamstrings, medial gastrocnemius, and anterior tibialis – is collected at a patient's initial visit to the gait analysis laboratory as part of normal clinical care. For this study, EMG data from one randomly selected barefoot gait cycle was analyzed. EMG data from the more affected side (right or left) was included for the individuals with hemiplegic or triplegic CP, whereas a random side was selected using a random number generator for the other individuals. EMG data was sampled at 1080Hz, bandpass filtered between 20 and 400Hz, rectified, and then low-pass filtered at 10Hz. To facilitate comparisons between participants, EMG data from each muscle was normalized to its peak value and resampled at each 1% of the gait cycle. Using one random gait cycle instead of EMG data averaged over multiple gait cycles incorporates variations in muscle activity that are often lost during averaging.
Synergy calculation
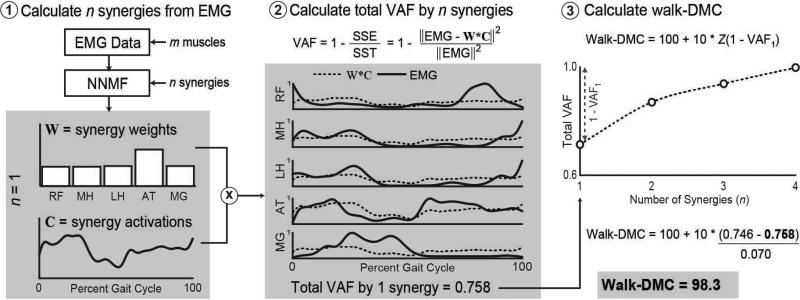
EMG data was used to calculate synergies using non-negative matrix factorization: a common matrix factorization algorithm used in previous studies of gait (see detailed descriptions and tutorials5,6). Briefly, this algorithm calculates synergies (W) and the relative activation of those synergies (C) such that muscle activations=W×C+error. W is an n×m matrix where n is the specified number of synergies (from one to four in this study) and m is the number of muscles (five in this study). C is an m×t matrix where t is the number of time points (101 across the normalized gait cycle in this analysis). Thus, each column of W represents the relative weighting of muscles in each synergy and each row of C represents the activation level of each synergy over the gait cycle. Non-negative matrix factorization was repeated within an iterative optimization that tested random initial estimates of W and C and selected the matrices that minimized the sum of squared error between the activations calculated by W×C and the EMG data. The total variance accounted for (VAF) by n synergies was calculated by comparing W*C and the EMG data (Fig. 1). We repeated this analysis with the number of synergies, n, varying from one to four synergies.
Figure 1.
Sample of process to calculate the dynamic motor control index during walking (walk-DMC) for one of the unimpaired individuals. (a) Non-negative matrix factorization is used to calculate n synergies, W, and the activation of those synergies, C, over a gait cycle. In this example we have calculated one synergy (n=1). The center figure shows a comparison of the experimental electromyography (EMG) data and reconstructed data from one synergy, W×C. (b) The variance in EMG data accounted for (VAF) by n synergies is calculated as one minus the ratio of the sum of squared errors (SSE) and the total sum of squares of the EMG data (SST) calculated across the 101 points of the gait cycle. In this example, one synergy accounted for about 75.8% of the variance in muscle activity for the five muscles with surface EMG. (c) This process can be repeated for n=2, 3, or 4 synergies to evaluate the change in total VAF for with increasing number of synergies. Walk-DMC is calculated from the total VAF of one synergy as a z score compared with the mean (0.746) and standard deviation (0.07) of the unimpaired individuals. Muscle abbreviations: RF, rectus femoris; MH, medial hamstring; LH, lateral hamstring; AT, anterior tibialis; MG, medial gastrocnemius.
The dynamic motor control index during walking (walk-DMC) was used as a summary measure of muscle activity complexity. For each participant, walk-DMC was calculated as a z-score:
where VAF1 is the variance accounted for when n=1 (one synergy) for a participant and (1–VAF1)AVE and (1–VAF1)SD are the average and standard deviation of the variance not accounted for by one synergy for the 84 unimpaired individuals. In other words, walk-DMC is a z-score for unaccounted variance, 1–VAF1.
We chose to examine VAF by one synergy to minimize the effects from choosing a given number of synergies. Since we used one random gait cycle for each participant in this analysis, we also evaluated the variability in VAF1 across gait cycles. The average standard deviation in VAF1 between gait cycles was 0.041 for the unimpaired individuals and 0.025 for the individuals with CP (coefficient of variation 5.5% and 3.0%).
We scaled walk-DMC to facilitate interpretation, such that 100 equals the average walk-DMC of unimpaired individuals and each 10-point increment represents one standard deviation. This normalization method has been used to evaluate gait in individuals with CP (e.g. Gait Deviation Index16) and will aid in comparing results of future studies, which may include different numbers of EMG channels or other factors that can impact VAF.17 A walk-DMC score less than 100 indicates a simplified muscle activation pattern during gait, where one synergy describes a greater variance in muscle activity compared with unimpaired individuals.
The structure of synergies (i.e. the relative weights of muscles in each synergy of W) was compared, between diagnosis subtypes and with the unimpaired individuals, using K-means cluster analysis. Non-negative matrix factorization estimates synergies that can describe variance in muscle activity for each individual, but we needed a robust method to compare the structure of synergies across individuals. To determine common synergies between individuals we used K-means cluster analysis, which identifies clusters of synergies that are similar across individuals, and returns the average weights of common synergies. For a given number of synergies, n, K-means cluster analysis identifies n clusters that minimize the sum over all clusters of the squared Euclidean distance from each individual's synergies to the center of each cluster. This process was repeated for n equals one to four synergies. The outputs of each analysis were the centers of each cluster, representing the average weights of common synergies across individuals. We used K-means cluster analysis to identify the average synergies for the unimpaired individuals and each CP diagnosis subtype (hemiplegic, diplegic, triplegic, and quadriplegic). For each group, the participants were divided based upon the number of synergies required to describe greater than 90% of the total variance in muscle activity.
Clinical assessments
Gait impairment was measured using two common scales: (1) the Gross Motor Functional Classification System (GMFCS18) which classifies individuals from level I (individuals who can walk without limitations), to level V (individuals who are transported in a manual wheelchair), and (2) the Gillette Functional Assessment Questionnaire (FAQ19) a 10-level parent-report walking scale from level 10 (individuals who walk, run, and climb without difficulty or assistance) to level 1 (individuals who cannot take any steps at all). GMFCS has only been collected as part of clinical care since 2006 (Table I).
Strength, spasticity, and selective motor control were assessed during a clinical examination. Strength at the hip, knee, and ankle was assessed using the Kendall scale.20 The Ashworth Score was used to assess spasticity of the hip flexors, hamstrings, rectus femoris, and plantarfexors.21 Selective motor control of the hip, knee, and ankle was assessed as follows: 0, patterned; 1, partly isolated; and 2, completely isolated. To combine measurements of strength, spasticity, or selective motor control across multiple joints or muscles into single summary values for each measure, we performed principal component analysis. Previous studies have shown that these clinical examination measures are correlated across joints and muscles and that the first principal component describes a large proportion of the variance.22 In our analysis, the first principal components explained 47% of the variance in strength, 46% of the variance in spasticity, and 54% of the variance in selective motor control.
Statistical comparisons
One-way analyses of variance were used to compare walk-DMC between the unimpaired individuals, the individuals with CP, diagnosis subtypes, GMFCS levels, and FAQ levels with post hoc comparison between groups, with a Tukey–Kramer correction for multiple comparisons. Regression analyses were used to evaluate the correlation of walk-DMC with the first principal component of clinical examination measures of strength, spasticity, and selective motor control. Individuals with CP tend to walk slower than unimpaired individuals, which may also impact walk-DMC. To evaluate the impact of walking speed, we analyzed walk-DMC for a subset of the unimpaired individuals (n=36) who walked at four walking speeds from very slow to fast. We used a linear mixed-effects analysis to evaluate walk-DMC versus walking speed with fixed effects for speed, and random effects for the intercept and slope of speed grouped by individuals. A likelihood ratio test was used to compare models with and without speed. Significance was set at p<0.05 for all tests.
RESULTS
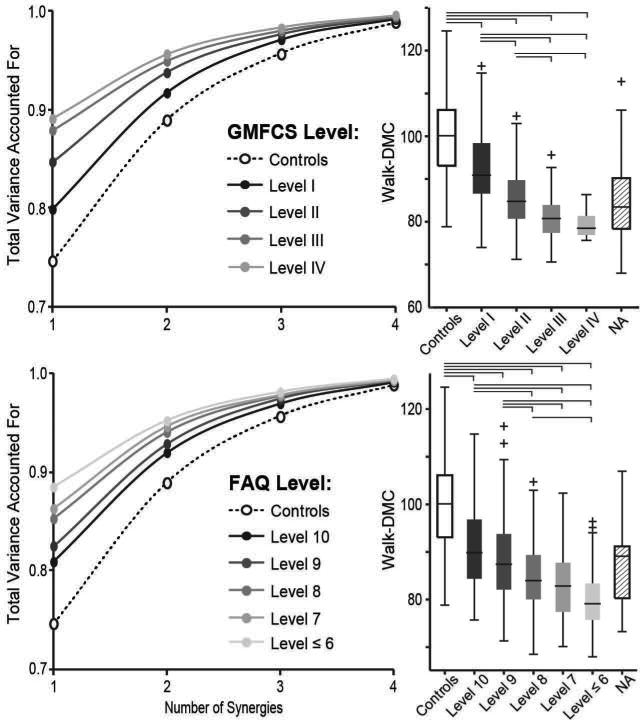
Fewer synergies were required to describe variations in muscle activity during gait among individuals with CP compared with unimpaired individuals (Fig. 2). While three synergies were required to describe greater than 90% of the total variance in muscle activity during gait for over 60% of the unimpaired individuals, only one or two synergies were required to describe greater than 90% of the variance in muscle activity for over 80% of the individuals with CP. One synergy accounted for 74.6% (95% confidence interval [CI] 73.1–76.1%) of the variance in muscle activity during gait at free speed for the unimpaired individuals compared with 84.2% (83.7–84.7%) among the individuals with CP. Thus, walk-DMC was significantly lower, 86.2 (85.5–86.9), among individuals with CP than unimpaired individuals, 100 (97.9–102.1). Walk-DMC decreased significantly with progressively more severe diagnosis subtypes. Individuals with hemiplegia, diplegia, triplegia, and quadriplegia had walk-DMC values of 89.2 (87.8–90.6), 86.9 (85.9–87.9), 84.4 (82.5–86.3), and 81.4 (80.0–82.8), respectively. All diagnosis subtypes were significantly different from the unimpaired individuals with average estimated differences from unimpaired of 10.8 (7.6–14.1), 13.1 (10.2–15.9), 15.6 (11.9–19.2), and 18.6 (15.1–22.1) for hemiplegia, diplegia, triplegia, and quadriplegia, respectively.
Figure 2.
Control complexity during gait, as measured by synergies, was significantly reduced among individuals with cerebral palsy (CP) compared with unimpaired individuals. Left: the mean total variance account for (tVAF) by one to four synergies increased with functional impairment level as measured by Gross Motor Functional Classification System (GMFCS) level (top) and Gillette Functional Assessment Questionnaire (FAQ) score (bottom). A GMFCS level of I and FAQ score of 10 represent the least impairment. Right: walk-DMC decreased with severity of impairment measured by both GMFCS level and FAQ score. The final boxplots (NA group) show results for participants missing GMFCS or FAQ data (see Table I). Brackets represent significant differences between groups (p<0.05).
Walk-DMC also decreased with functional impairment as measured by GMFCS and FAQ (Fig. 2). For high-functioning individuals, in GMFCS level I or with an FAQ of 10, the walk-DMC was still significantly less than unimpaired individuals at 92.4 (91.1–93.7) for GMFCS level I and 90.9 (89.2–92.6) for an FAQ of 10. The estimated differences between unimpaired individuals and GMFCS level I and FAQ 10 groups were 7.6 (4.8–10.4) and 9.1 (5.6–12.6), respectively. Among the most severely impaired individuals, who could still walk for analysis, the walk-DMC was greatly reduced: 79.2 (77.5–80.9) for GMFCS level IV and 80.0 (78.7–81.3) for an FAQ less than 7. The estimated differences between unimpaired individuals and GMFCS level IV and FAQ <7 groups were 20.8 (14.6–27.0) and 20.0 (16.5–23.5), respectively. The decrease in walk-DMC with GMFCS level and FAQ was consistent across diagnosis subtypes (Fig. S1, online supporting information).
The structure of the synergies (W) for the unimpaired individuals was similar to previously reported results of walking in adults.8,9 Three synergies described over 90% of the total VAF for unimpaired individuals and included synergies dominated by (1) the medial and lateral hamstrings, (2) the medial gastrocnemius, and (3) the rectus femoris and anterior tibialis. For the minority of individuals with CP who required three or more synergies to describe greater than 90% of total VAF, synergy structure was similar to the unimpaired individuals (Fig. 3a). The activations (C) of the synergies were similar, except for increased activation of the synergy dominated by the gastrocnemius in early stance and decreased activation of the synergy dominated by the rectus femoris and anterior tibialis in late swing. The majority of the individuals with CP required only two synergies to describe greater than 90% of the total VAF (Fig 3b). For these individuals, the hamstrings and gastrocnemius were activated more during late swing and early stance and the rectus femoris and anterior tibialis were activated more in late stance and early swing than the unimpaired individuals.
Figure 3.
Mean synergy weights (W, left) and synergy activations (C, right) calculated from non-negative matrix factorization among unimpaired individuals and individuals with cerebral palsy (CP) who required (a) three synergies or (b) two synergies to describe greater than 90% of total variance in muscle activity. RF, rectus femoris; MH, medial hamstrings; LH, lateral hamstrings; AT, anterior tibialis; MG, medial gastrocnemius.
Clinical examination measures used to evaluate control among individuals with CP, including strength, spasticity, and selective motor control, were all correlated with walk-DMC (Fig. S2, online supporting information). Strength had the greatest correlation with walk-DMC (r=0.49, slope=0.11) followed by selective motor control (r=0.44, slope=0.07) and spasticity (r=−0.34, slope=−0.06). Individuals with greater strength, less spasticity, and more selective motor control had a walk-DMC more similar to unimpaired individuals.
The individuals included in this study covered a wide age range; however, the relationship between age and walk-DMC was weak both among the unimpaired individuals and among individuals with CP (r2=0.01 and slope=−0.13 for individuals with CP; r2=0.001 and slope=−0.14 for unimpaired individuals). In our analysis of the relationship between walking speed and walk-DMC for 36 unimpaired individuals who walked at four speeds, we did find a moderate relationship between walking speed and walk-DMC (slope=7.2, SE=2.3; p<0.01; [Fig. S3, online supporting information]). Among the unimpaired individuals, walk-DMC increased with increasing walking speed. However, even at the very slow walking speed, walk-DMC was 93.0 (89.8–96.2), greater than the individuals with CP.
DISCUSSION
Complexity of control, as measured by synergies, was reduced during gait in individuals with CP compared with unimpaired individuals, and was related to functional ability and clinical examination measures. The large, heterogeneous population in this study included diverse brain injuries, gait patterns, and functional ability levels, which was reflected in the variability in walk-DMC between individuals.
Changes in synergies were also similar to adult stroke survivors. Previous studies have determined that the number of synergies required to describe muscle activity is reduced after stroke.9,10 During walking, the muscle activities of individuals with stroke and CP are largely described by a few synergies characterized by a bimodal activation pattern similar to synergies during rhythmic stepping in infants23 (see Fig. 3b). Thus, altered control strategies appear to be independent of when the injury occurs (e.g. early in life or after decades of walking) and reflect a control strategy present early in development.
The variability and spread in walk-DMC in this study reflects the heterogeneity of this population, but also suggests potential limitations in using walk-DMC clinically. Although we found walk-DMC was related to GMFCS, FAQ, and clinical examination measures, there was a high degree of overlap between functional levels and significant spread in relation to clinical examination measures. There was also a dependence of walk-DMC on walking speed, and thus comparisons need to account for this possible confounding effect. Future studies will determine if an individual's walk-DMC is a clinically useful measure for evaluation or treatment planning. A recent study of adult stroke survivors found that individuals with synergies more similar to unimpaired individuals had greater improvements in walking after a treadmill training program,24 suggesting synergy analysis may be useful for treatment planning.
In this study we analyzed a large population who had previously received clinical motion analysis. This provided a powerful group for evaluating synergies, but also introduced limitations. We were limited to the EMG data and clinical examination measures that are included as the standard of care. In particular, our measures of strength, spasticity, and selective motor control are all ordinal scales with poor sensitivity; other evaluations such as torque measurements for strength or the Tardieu Scale for spasticity could be superior. EMG data was only available from five muscles per leg. Synergies calculated with non-negative matrix factorization are sensitive to the number of muscles in the analysis, and using fewer muscles can over-estimate total VAF.17 These limitations motivated using the normalized walk-DMC as a summary measure of synergy complexity. Despite the limited number of EMG channels, the synergy weights, W, of the unimpaired individuals were similar to previous studies of unimpaired adults.9
Clinical motion-analysis laboratories evaluate gait in individuals with CP, to inform surgical and rehabilitation planning. The results of this study demonstrated that synergies are altered among individuals with CP, and walk-DMC can provide a measure of altered neuromuscular control from data collected as part of clinical care. Future studies will determine if synergies change after treatment or predict clinical outcomes. The similarity of synergies in CP, stroke, and infant rhythmic-stepping indicates that there are common changes in control after brain injury that may reflect control in early development. Quantifying these changes and evaluating the plasticity of synergies may provide pathways to new treatments for individuals with CP and other neurological disorders.
Supplementary Material
What this paper adds
Synergies were simplified during walking for individuals with cerebral palsy compared with unimpaired individuals.
Synergy complexity was correlated with diagnosis, functional impairment, and selective motor control.
Changes in synergies were similar to previous reports from adult stroke survivors.
ACKNOWLEDGEMENTS
We thank the staff at the James R. Gage Center for Gait and Motion Analysis at Gillette Children's Specialty Healthcare for data collection and feedback. This work was funded by National Institutes of Health grant K12HD073945.
ABBREVIATIONS
- DMC
Dynamic motor control
- FAQ
Gillette Functional Assessment Questionnaire
- VAF
Variance accounted for
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
Developmental Medicine & Child Neurology 2015, 57: 000–000
Synergies during Gait in CP Katherine M Steele et al.
SUPPORTING INFORMATION
The following additional information may be found online:
Figure S1: Decrease in walk-DMC with GMFCS and FAQ levels across diagnosis subtypes.
Figure S2: Correlation of walk-DMC with clinical examination measures.
Figure S3: The relationship between walking speed and walk-DMC for unimpaired individuals.
REFERENCES
- 1.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 2.Forssberg H. Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res. 1985;57:480–93. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- 3.Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–61. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- 4.Calancie B, Needham-Shropshire B, Jacobs P, Willer K, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Evidence for a central rhythm generator for locomotion in man. Brain. 1994;117:1143–59. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- 5.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature. 1999;401:788–91. doi: 10.1038/44565. [DOI] [PubMed] [Google Scholar]
- 6.Ting LH, Chvatal SA. In: Motor Control: Theories, Experiments, and Applications. Danion F, Latash ML, editors. Oxford University Press; New York, NY: 2010. pp. 102–21. [Google Scholar]
- 7.Cheung VC, d'Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci. 2005;25:6419–34. doi: 10.1523/JNEUROSCI.4904-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JL, Neptune RR. Three-dimensional modular control of human walking. J Biomech. 2012;45:2157–63. doi: 10.1016/j.jbiomech.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophys. 2010;103:844–57. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung VCK, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A. 2012;109:14652–6. doi: 10.1073/pnas.1212056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Wang Q, Cao S, Wu D, Wang Q, Chen X, editors. Lower-limb muscle synergies in children with cerebral palsy.. 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER); San Diego, CA. 2013 6–8 November; IEEE; 2013. [Google Scholar]
- 12.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol. 2005;47:329–36. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 13.Nashner L, Shumway-Cook A, Marin O. Stance posture control in select groups of children with cerebral palsy: deficits in sensory organization and muscular coordination. Exp Brain Res. 1983;49:393–409. doi: 10.1007/BF00238781. [DOI] [PubMed] [Google Scholar]
- 14.Unnithan V, Dowling J, Frost G, Volpe AB, Bar-Or O. Cocontraction and phasic activity during GAIT in children with cerebral palsy. Electromyogr Clin Neurophysiol. 1996;36:487–94. [PubMed] [Google Scholar]
- 15.Schwartz MH, Rozumalski A, Trost JP. The effect of walking speed on the gait of typically developing children. J Biomech. 2008;41:1639–50. doi: 10.1016/j.jbiomech.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture. 2008;28:351–7. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci. 2013;7:105. doi: 10.3389/fncom.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 19.Novacheck TF, Stout JL, Tervo R. Reliability and validity of the Gillette Functional Assessment Questionnaire as an outcome measure in children with walking disabilities. J Pediatr Orthop. 2000;20:75–81. [PubMed] [Google Scholar]
- 20.Kendall H, Kendall F. Muscles: Testing and Function. Williams and Wilkins; Baltimore: 1949. [Google Scholar]
- 21.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 22.Rozumalski A, Schwartz MH. Crouch gait patterns defined using k-means cluster analysis are related to underlying clinical pathology. Gait Posture. 2009;30:155–60. doi: 10.1016/j.gaitpost.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Ivanenko YP, Dominici N, Cappellini G, et al. Changes in the spinal segmental motor output for stepping during development from infant to adult. J Neurosci. 2013;33:3025–36a. doi: 10.1523/JNEUROSCI.2722-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 2013;38:511–7. doi: 10.1016/j.gaitpost.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.