Abstract
The ubiquitin-proteasome pathway has gained attention as a potential chemotherapeutic target, owing to its importance in the maintenance of protein homeostasis and the observation that cancer cells are more dependent on this pathway than normal cells. Additionally, inhibition of histone deacetylases (HDACs) by their inhibitors like Vorinostat (SAHA) has also proven a useful strategy in cancer therapy and the concomitant use of proteasome and HDAC inhibitors has been shown to be superior to either treatment alone. It has also been reported that delta-aminolevulinic acid dehydratase (ALAD) is a proteasome-associated protein, and may function as an endogenous proteasome inhibitor. While the role of ALAD in the heme biosynthetic pathway is well characterized, little is known about its interaction with, and the mechanism by which it inhibits, the proteasome. In the present study, this ALAD-proteasome complex was further characterized in cultured prostate cancer cells and the effects of SAHA treatment on the regulation of ALAD were investigated. ALAD interacts with the 20S proteasomal core, but not the 19S regulatory cap. Some ubiquitinated species were detected in ALAD immunoprecipitates that have similar molecular weights to ubiquitinated proteasomal α2 subunits, suggesting preferred binding of ALAD to ubiquitinated α2. Additionally, SAHA treatment increases levels of ALAD protein and an acetylated protein with a molecular weight similar to the ubiquitinated α2 subunit. Thus, the results of this study suggest that ALAD may play a regulatory role in a previously unreported post-translational modification of proteasomal α subunits.
Keywords: ALAD, Proteasome, Ubiquitination, Acetylation, Prostate Cancer
The ubiquitin-proteasome pathway (UPP) is essential to cellular homeostasis as it is responsible for the majority of protein degradation within the cell. The UPP selectively degrades proteins involved in many biological processes, including development, differentiation, proliferation, signal transduction, and apoptosis [Nalepa et al., 2006]. The UPP degrades these proteins in a stepwise manner; first, an ubiquitin chain is added to the protein substrate and second, the ubiquitinated protein is degraded by the 26S proteasome. The ubiquitin chain is attached to the substrate through the actions of a series of enzymes: the E1 enzyme activates the ubiquitin, E2 enzyme conjugates the activated ubiquitin, and E3 enzyme ligates the ubiquitin chain to the specific substrate proteins. The 26S proteasome is comprised of two major components, the 20S catalytic core and one or two 19S regulatory caps. The 20S core consists of two identical alpha rings and two identical beta rings, each made up of seven subunits (α1–α7 and β1–β7, respectively). Three beta subunits, β1 (trypsin-like activity), β2 (caspase-like or PGPH-like activity), and β5 (chymotrypsin-like activity), carry out the proteolytic activity of the proteasome, while the alpha subunits’ major role is to serve as docking points for the 19S caps, as well as blocking unregulated entry of protein substrates into the core [Smith et al., 2007]. Thus, due to the importance of protein homeostasis to normal cellular function and the role that the ubiquitin-proteasome pathway plays in regulating protein turnover, the UPP has emerged as a possible target in cancer therapy. Indeed, studies have reported higher proteasome activity in cancer cells than in normal cells [Kumatori et al., 1990; Li and Dou, 2000; Loda et al., 1997] and that inhibition of the proteasome selectively leads to cell cycle arrest and apoptosis in cancer cells [An et al., 1998; Dou and Li, 1999]. In fact, the use of proteasome inhibition as an effective cancer therapy was validated by the 2003 USFDA approval of bortezomib for the treatment of relapsed and refractory multiple myeloma.
While bortezomib has shown success in the clinic, it has also been associated with toxicities and resistance, indicating that further exploration into new or improved proteasome inhibitors is warranted. In fact, an endogenous inhibitor of the proteasome, ALAD, was reported about twenty years ago [Guo et al., 1994]. ALAD, best known for its role in heme biosynthesis, is composed of eight identical subunits and catalyzes the condensation of two molecules of aminolevulinic acid (ALA) to form porphobilinogen (PBG), a heme precursor [Berlin and Schaller, 1974; Jaffe et al., 2001]. It has also been reported that ALAD is identical to, but not physically associated with, the inhibitory CF-2 subunit of the 19S regulatory cap [Guo et al., 1994]. ALAD and CF-2 were shown to have a common sequence of the first fourteen N-terminal amino acids, identical migration in gel electrophoresis, identical isoelectric points of pH 7.1, cross-reactivity of specific polyclonal antibodies, similar dehydratase and proteasome inhibitor specific activities in both proteins and the presence of both activities in recombinant ALAD [Guo et al., 1994]. Most recently, ALAD has been shown to physically interact with the 20S proteasomal core [Bardag-Gorce and French, 2011], although precisely how and where has yet to be elucidated.
To study the function of ALAD binding to the 20S proteasome, we performed a series of immunoprecipitation experiments in prostate cancer cells with or without ALAD overexpression. Additionally, we investigated whether HDAC inhibition by SAHA had any effects on ALAD or its interaction with the 20S proteasome. This study not only confirms a novel ALAD-20S proteasome complex structure, but also describes previously unreported post-translational modifications of 20S proteasome and preferred binding of ALAD to the acetylated, ubiquitinated proteasome subunits, indicating that ALAD may play a regulatory role in this process.
Materials & Methods
Reagents and Antibodies
Fetal bovine serum was purchased from Aleken Biologicals (Nash, TX). RPMI-1640 media, trypsin and penicillin/streptomycin were purchased from Life Technologies (Carlsbad, CA). Fugene-HD transfection reagent was from Promega (Madison, WI) and full-length C-terminal myc-DDK-tagged ALAD and PCMV6-Entry plasmids were from OriGene Technologies (Rockville, MD). Suberoylanilide hydroxamic acid (SAHA) and purified ATP and ubiquitin were from Sigma-Aldrich (St. Louis, MO). Purified human 20S proteasome was obtained from Boston Biochem (Cambridge, MA). Mouse monoclonal anti-ALAD, anti-α2, anti-ubiquitin and anti-myc, polyclonal rabbit anti-ALAD, anti-α2 and anti-ubiquitin and goat anti-actin and anti-α2 primary antibodies, as well as normal mouse and rabbit IgG and anti-goat secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal rabbit anti-Acetyl-Lysine antibody was from Cell Signaling Technology (Boston, MA), mouse monoclonal anti-H3 and anti-tubulin antibodies were from Abcam (Cambridge, MA), and polyclonal rabbit anti-S10a antibody was from BioMol (Farmingdale, NY). Mouse and rabbit secondary antibodies were purchased from Bio-Rad Laboratories (Hercules, CA). TRITC-conjugated anti-mouse secondary antibody and DAPI were from Sigma Aldrich (St. Louis, MO). Enhanced chemiluminescence reagent and autoradiography film were purchased from Denville Scientific (Metuchen, NJ). VectaSchield anti-fade solution was purchased from Vector Laboratories (Burlingame, CA).
Cell Culture and Whole-Cell Extract Preparation
Human prostate cancer DU145, LNCaP and PC-3 cell lines, purchased from American Type Culture Collection (Manassas, VA), were grown in RPMI 1640 supplemented with 10% FBS and 100 units/ml penicillin and 100 µg/ml streptomycin (Life Technologies; Carlsbad, CA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. Whole cell extracts were prepared as follows: cells were harvested, washed twice with phosphate-buffered saline and lysed in a whole cell lysis buffer (50 mM tris–HCl, pH 8.0, 150 mM NaCl, 0.5% NP40). Mixtures were vortexed for 20 min at 4°C, followed by centrifugation at 12,000 rpm for 12 minutes. Supernatants were collected as whole cell extracts and protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad; Hercules, CA).
Transfection
Full-length C-terminal myc-DDK-tagged human ALAD in a PCMV6-Entry vector (OriGene Technologies; Rockville, MD) was transformed into DH5α competent E. coli, followed by expansion and DNA purification using the QIAGEN (Venlo, Netherlands) EndoFree Plasmid Maxi Prep kit. After measurement of DNA purity and concentration, genes were transiently transfected into parental prostate cancer cells using Fugene-HD transfection reagent (Promega; Madison, WI) in Opti-MEM serum-free medium (Life Technologies; Carlsbad, CA). Empty PCMV6-Entry vector was used as a control.
Western Blot Analysis
Cell lysates (40 µg) were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE; 10–12%) and transferred to nitrocellulose membranes, followed by incubation with indicated primary and secondary antibodies and visualization using enhanced chemiluminescence reagent (Denville Scientific, Metuchen, NJ).
Subcellular Fractionation
Cells were grown and harvested as described previously. Following collection of the cell pellet, 500 µL 1× hypotonic buffer (20mM Tris-HCl pH 7.4, 10mM NaCl, 3mM MgCl2) was added and the samples were mixed by pipetting. After 15 minute incubation on ice, 25 µL 10% NP-40 was added followed by vortexing for 15 seconds. The homogenate was then centrifuged for 10 minutes at 3000 rpm at 4°C. The supernatant was then transferred and saved as the cytosolic fraction. The pellet was resuspended in 50 µL complete cell extraction buffer (100mM Tris-HCl pH 7.4, 2mM Na3VO4, 100mM NaCl, 1% Triton X-100, 1mM EDTA, 10% glycerol, 1mM EGTA, 0.1% SDS, 1mM NAF, 0.5% deoxycholate, 20mM Na4P2O7, 1mM PMSF) and incubated on ice for 30 minutes with vortexing at 10 minute intervals, followed by centrifugation at 14,000 g for 30 minutes at 4°C. The supernatant was then transferred and saved as the nuclear fraction. Fractions were subjected to the protein concentration assay and Western blotting as previously described.
Immunoprecipitation
Cells were treated in the figure legends and lysed as described above. Immunoprecipitation was carried out using the Pierce Classic IP Kit (Thermo Fisher; Rockford, IL). Briefly, cell lysates (500–1500 µg) were incubated with 10µg primary antibody (anti-myc, anti-α2 or anti-S10a) or IgG (as control) with rocking overnight at 4°C. Protein A/G beads were then added and the mixtures were incubated in a spin column with a 10 µm pore size with rocking for 4–18 h at 4°C. Unbound proteins were separated by centrifugation at 3000 rpm for 1 min and saved as the supernatant fraction. Beads were then washed three times with 1× TBS, followed by elution of bound proteins with low pH elution buffer and centrifugation at 3000 rpm for 1 min. 4× SDS sample buffer was added to supernatant (20 µl) and eluate (pulldown) samples, which were subjected to analysis by Western blot.
Immunofluorescence
PC-3 cells were plated at a density of approximately 25,000 cells per well on 6 well chamber slides (Fisher Scientific; Pittsburgh, PA). Cells were transfected with either empty vector or full length ALAD after 24 h as described above. After 48 h, media was removed from the slide with a pipette and cells were washed with PBS. The plastic chamber was then removed and the slide was allowed to dry followed by three washes in ice cold 1:1 methanol:acetone to fix and rupture cell membranes. The slide was again allowed to dry before rehydration in a PBS wash followed by blocking in 5% BSA for 45 minutes at room temperature. The slides were then washed once with PBS and incubated with mouse anti-ALAD primary antibody in BSA overnight at 4°C. The slide was then washed with PBS three times followed by incubation in TRITC-conjugated anti-mouse secondary antibody (Sigma Aldrich; St. Louis, MO) for 2 h at room temperature. Following three PBS washes, DAPI (Sigma Aldrich; St. Louis, MO) was added to the slide for 5 minutes and the slide was then washed a final three times in PBS before the cover slip was applied with VectaShield anti-fade solution (Vector Laboratories; Burlingame, CA). Photomicrographs were taken with a Leica DM4000 fluorescence microscope (Leica Microsystems, Inc.; Buffalo Grove, IL) at 63× magnification and signals were documented with 5.0 Openlab Improvision software (Perkin Elmer; Waltham, MA).
Results
ALAD binds the 20S proteasome in place of the 19S proteasomal cap and associates with a modified form of α2 subunit protein
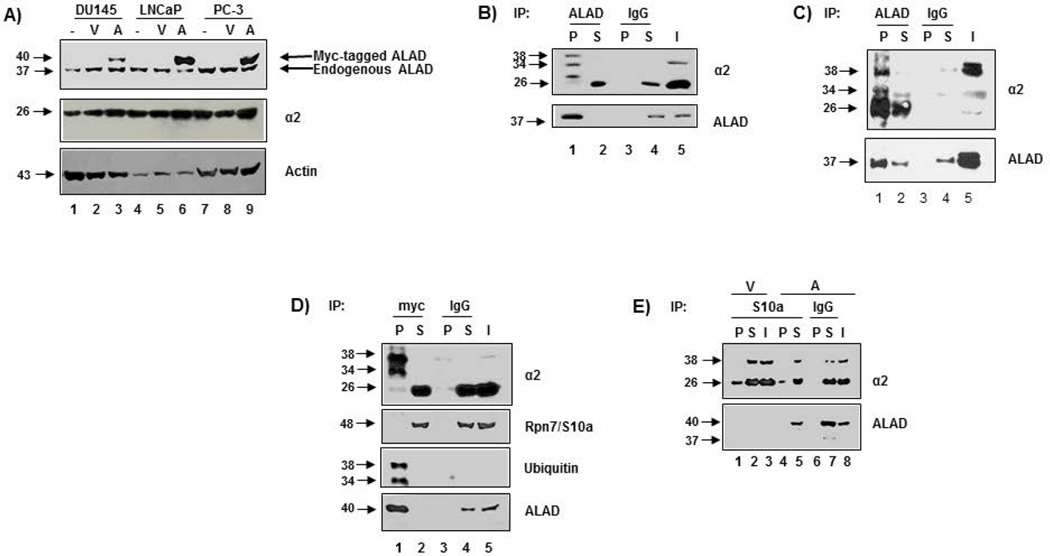
We first determined the levels of endogenous ALAD in a panel of prostate cancer cell lines by Western blot analysis. The chosen prostate cancer cell lines express varying levels of endogenous ALAD (Figure 1A). Next, to ascertain how ALAD interacts with the proteasome in cells, PC-3 cell and human erythrocyte lysates were immunoprecipitated with anti-ALAD. Immunoblotting with anti-ALAD antibody confirms the successful immunoprecipitation of ALAD with anti-ALAD but not the control IgG antibody (Figures 1B, 1C). The proteasomal α2 subunit (26 kD band), along with two bands with apparent molecular masses of 34 and 38–40 kD, respectively, were recognized by anti-α2 antibody in the anti-ALAD (but not anti-IgG) pulldown (Figure 1B, 1C). These 34 and 38 kD bands may be ubiquitinated forms of proteasomal α2 subunits (see below). Therefore, endogenous ALAD binds the 20S proteasome and is associated with potentially modified forms of proteasomal α2 subunit.
Figure 1.
ALAD binds the 20S proteaosme in place of the 19S regulatory cap and is associated with ubiquitinated forms of α2. A) Protein levels of endogenous and tagged ALAD protein in prostate cancer cells. Several prostate cancer cell lines were transfected with empty vector or myc-tagged full length ALAD for 48 h followed by Western blot analysis. B) Endogenous ALAD interacts with a modified form of α2. Parental PC-3 cells (1500 µg) were immunoprecipitated with anti-ALAD followed by Western blot analysis with indicated antibodies (anti-α2 = mouse). C) Endogenous ALAD interacts with unmodified and modified α2 in human erythrocytes. Human erythrocyte preparations (500 µg) were immunoprecipitated with anti-ALAD followed by Western blot analysis with indicated antibodies (anti-α2 = mouse). D) A ubiquitinated form of proteasomal α2, but not the 19S cap, is pulled down with myc-tagged ALAD in transfected PC-3 cells. Myc-tagged full length ALAD-transfected PC-3 cells (500 µg) were immunoprecipitated with anti-myc followed by Western blot analysis with the indicated antibodies (anti-α2 = rabbit). E) ALAD is not pulled down with the 19S proteasome. Empty vector or myc-tagged full length ALAD-transfected PC-3 (500 µg) cells were immunoprecipitated with anti-Rpn10 (a 19S subunit), followed by Western blot analysis. Matched IgG immunoprecipitation was performed as a control. Input was loaded at 5% of total protein used for immunoprecipitation. − = No transfection; V = Vector-transfected; A = ALAD-transfected; P = Pulldown; S = Supernatant; I = Input.
Because endogenous ALAD protein levels are relatively low (compared with human erythrocytes, Figure 1B vs. C, lane 5), these prostate cancer cells were then transfected with either myc-tagged full length ALAD or empty vector (as a control) for 48 h and subjected to analysis by Western blot. The transfection efficiency varies among these cell lines, with more successful transfection in LNCaP and PC-3 cells than DU145 cells (Figure 1A). Due to the high transfection efficiency in PC-3 cells, ALAD-transfected PC-3 cells were used for the remainder of experiments.
We then confirmed the interaction of ALAD with the modified forms of proteasomal α2 (Figures 1B, 1C) in myc-tagged ALAD-transfected PC-3 cells (Figure 1D). Similar to results observed in parental PC-3 cells and human erythrocytes (Figures 1B, 1C), anti-myc antibody pulled down two similarly modified forms of proteasomal α2 subunit (MW 34 & 38–40 kD), but not the 19S regulatory cap Rpn7/S10a (Figure 1D), confirming that ALAD binds to a modified form of α2, in place of the 19S regulatory cap. Consistently, an antibody to Rpn7/S10a (a 19S proteasomal subunit) pulled down unmodified proteasomal α2, but not ALAD or the modified form(s) of α2 (Figure 1E). Importantly, the ALAD-associated, modified form of α2 appears to be ubiquitinated, as an anti-ubiquitin antibody recognizes two bands at the same molecular weights (34 & 38–40 kD) in the anti-myc pull down fraction (Figure 1D). Because both 34 and 38–40 kDa bands are recognized by anti-α2 and anti-ubiquitin antibodies, it is unlikely that the 34 kD band is a degraded form of the 38–40 kD band. Thus, these results indicate that ALAD binds to the 20S proteasomal core in the position of the 19S regulatory cap, associated with ubiquitination of α2 subunits.
ALAD inhibits proteasomal chymotrypsin-like activity and is a proteasomal target protein
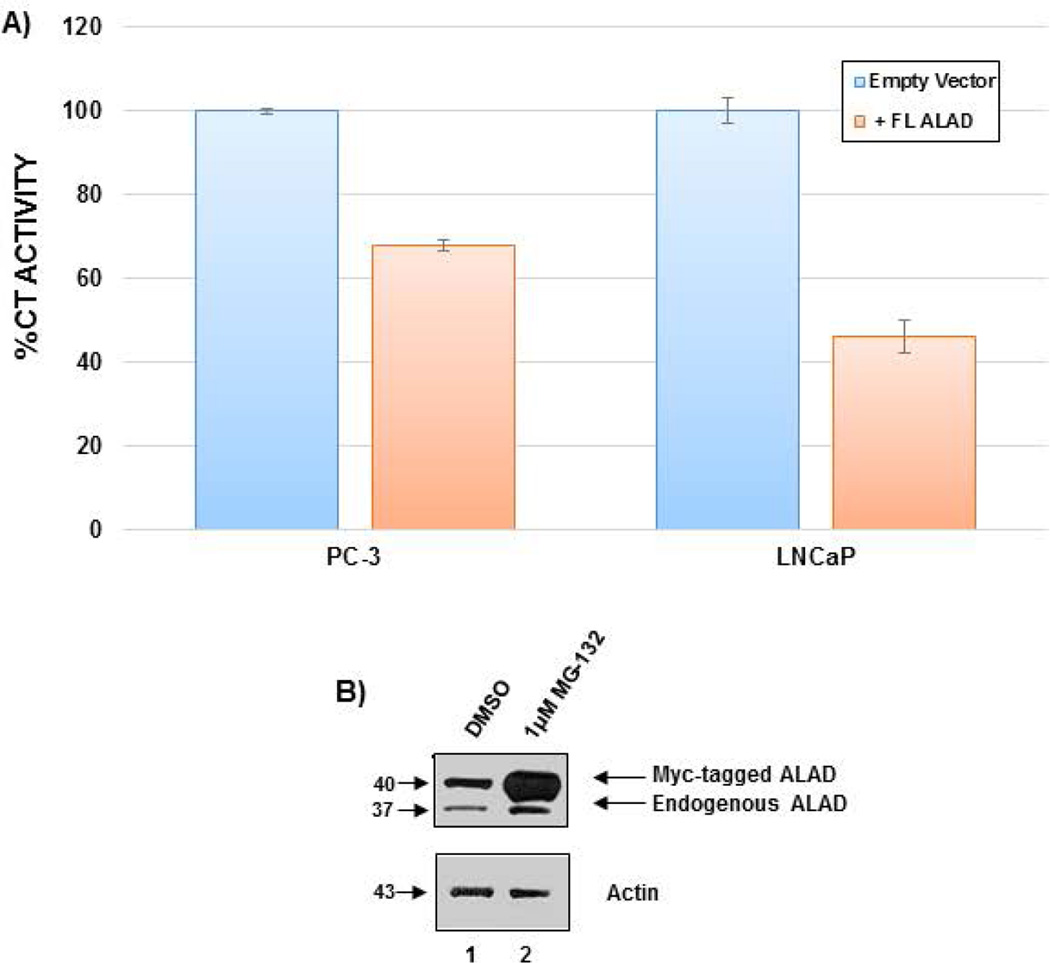
To verify the proteasome inhibitory ability of ALAD, PC-3 and LNCaP cells were transfected with empty vector or myc-tagged ALAD for 48 hours, followed by measurement of proteasomal chymotrypsin (CT)-like activity (Figure 2A). Overexpression of full length ALAD resulted in approximately 30% and 55% inhibition of proteasomal CT-like activity in PC-3 and LNCaP cells, respectively (Figure 2A). Thus, ALAD does exhibit some proteasome inhibitory ability, and importantly, this amount of inhibition has been shown to be sufficient to cause cell growth arrest or death [An et al., 1998; LeBlanc et al., 2002; Lopes et al., 1997].
Figure 2.
ALAD is both an inhibitor and a target of the proteasome. A) ALAD inhibits proteasomal CT-like activity. PC-3 or LNCaP cells were transfected with empty vector or myc-tagged full length ALAD for 48 h followed by measurement of proteasomal CT-like activity. B) ALAD is a proteasomal target protein. PC-3 cells were transfected with empty vector or myc-tagged full length ALAD for 48 h followed by treatment with 1µM MG-132 for 24 h and analysis by Western blot.
To investigate the regulation of ALAD, PC-3 cells were transfected with empty vector or myc-tagged ALAD, followed by treatment with the proteasome inhibitor MG-132. Analysis by Western blot reveals that treatment with 1 µM MG-132 results in an accumulation of both endogenous and tagged ALAD proteins (Figure 2B), indicating that ALAD is a substrate protein of the proteasome.
SAHA treatment enhances the ALAD-proteasome interaction, associated with acetylation of ubiquitinated α2 subunits
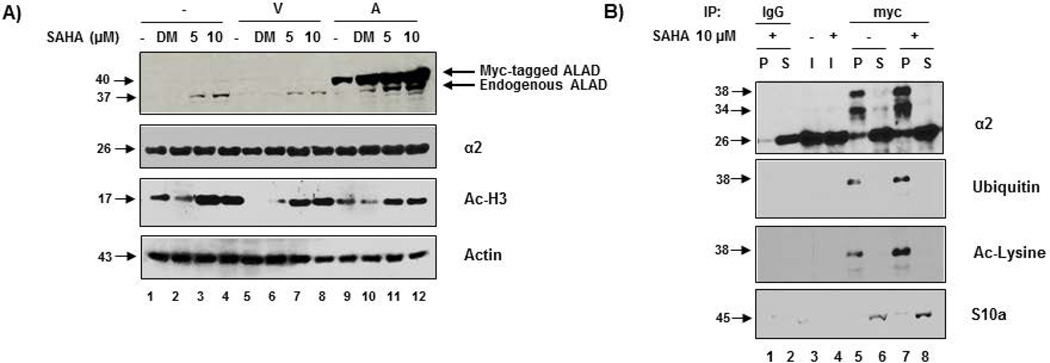
In an attempt to elucidate a role for HDAC inhibition in the regulation of ALAD and the proteasome, we transfected PC-3 cells with empty vector or myc-tagged full length ALAD for 48 h, followed by treatment with 5 or 10 µM SAHA for 24 h. Western blot analysis revealed that SAHA treatment dramatically increases expression levels of both endogenous and transfected ALAD proteins in a dose-dependent manner (Figure 3A). As a control, treatment of these cells with SAHA also increased levels of acetylated histone H3 protein (Figure 3A).
Figure 3.
SAHA treatment enhances the ALAD-proteasome interaction, associated with acetylation of ubiquitinated α2 subunits. A) SAHA treatment enhances ALAD protein levels in a dose-dependent manner. Empty vector or myc-tagged full length ALAD-transfected PC-3 cells were treated with 5 or 10 µM SAHA for 24 h followed by Western blot analysis. B) SAHA enhances the ALAD-proteasome interaction and promotes acetylation of ubiquitinated α2. Myc-tagged full length ALAD-transfected cells were treated with 10 µM SAHA for 24 h followed by immunoprecipitation (500 µg) with anti-myc and analysis by Western blot. Matched IgG immunoprecipitation was performed as a control. Input was loaded at 5% of total protein used for immunoprecipitation. V = Vector-transfected; A = ALAD-transfected; + = SAHA-treatment; − = DMSO treatment; P = Pulldown; S = Supernatant; I = Input.
Importantly, SAHA treatment also enhances the interaction between ALAD and the 20S proteasome in ALAD-transfected PC-3 cells, compared with the solvent control (Figure 3B). Again, anti-myc pulled down ubiquitinated protein(s) at a molecular weight identical to that of the modified α2 band (~38 kDa, Figure 3B, lanes 5, 7), further suggesting that ALAD-associated α2 is ubiquitinated. Furthermore, the level of this α2 species was significantly increased following SAHA treatment (Figure 3B, lanes 7 vs. 5). A specific anti-Ac-lysine antibody also recognized a similar band at ~38 kDa, suggesting that this modified α2 is also acetylated after SAHA treatment (Figure 3B, lanes 5, 7). Again, the 19S subunit S10a was not found in the anti-myc pulldowns (Figure 3B), confirming that there is no 19S proteasome in the ALAD-20S proteasome complexes. These results suggest that in addition to increasing ALAD protein expression (presumably by inhibiting ALAD degradation), SAHA treatment also causes acetylation of ubiquitinated α2 subunits and enhances the ALAD-proteasome interaction.
SAHA treatment promotes nuclear localization of ALAD-proteasome complexes
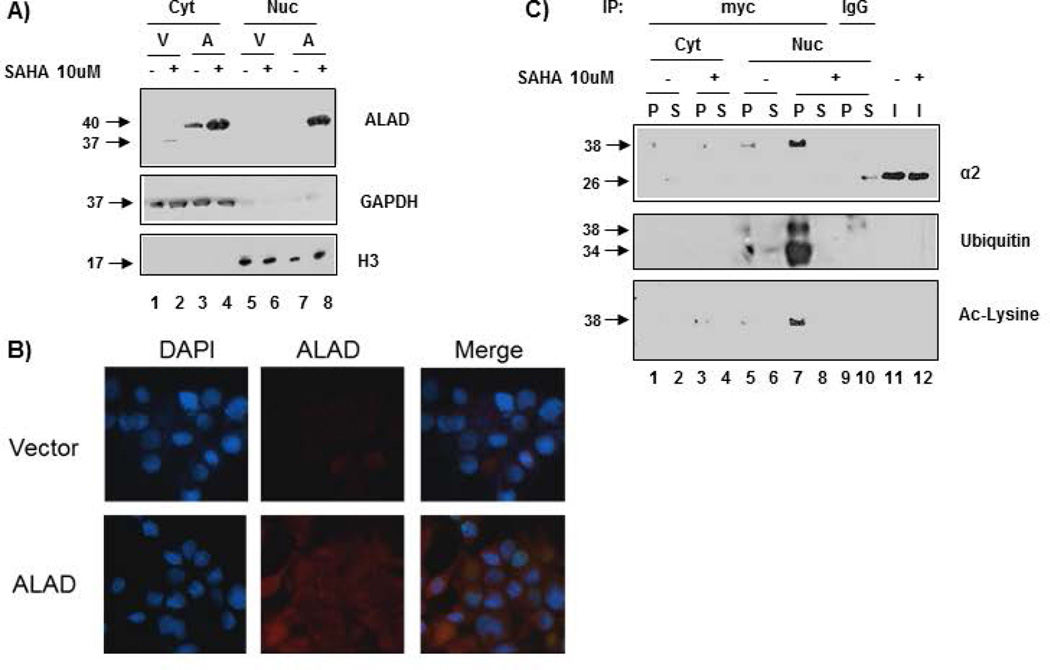
It has been shown that proteasomes exist in both the nucleus and cytosol [Brooks et al., 2000; Tanaka et al., 1989]. To ascertain whether ALAD interacts with cytosolic or nuclear proteasome, or both, ALAD-transfected PC-3 cells were fractionated into cytosolic and nuclear fractions, followed by Western blot analysis (Figure 4A). In these PC-3 cells, ALAD appears to exist only within the cytosol (Figure 4A, lanes 3 vs. 7). Immunofluorescence was also performed and confirmed cytosolic localization of ALAD in untreated ALAD-transfected PC-3 cells (Figure 4B). However, when transfected PC-3 cells were treated with 10 µM SAHA, followed by cellular fractionation, Western blot analysis revealed that SAHA treatment causes nuclear localization of ALAD (Figure 4A, lanes 8 vs. 7); GAPDH and H3 proteins were used as cytosolic and nuclear controls, respectively.
Figure 4.
SAHA-treatment promotes nuclear localization of ALAD and modified α2. A) ALAD is a cytosolic protein that is localized to the nucleus after treatment with SAHA. Vector or myc-tagged full length ALAD-transfected PC-3 cells were treated with 10 µM SAHA for 24 h followed by subcellular fractionation and analysis by Western blot. B) ALAD is a cytosolic protein. ALAD-transfected PC-3 cells were visualized by immunofluorescence microscopy. The lack of purple color in the nuclei indicates that ALAD exists only in the cytosol under normal conditions. C) SAHA-treatment promotes nuclear localization of acetylated/ubiquitinated α2. PC-3 cells transfected with myc-tagged full length ALAD for 48 h were treated with 10 µM SAHA for 24 h, followed by subcellular fractionation, immunoprecipitation (500 µg) with anti-myc, and Western blot analysis. Matched IgG immunoprecipitation was performed as a control. Cyt = Cytosolic fraction; Nuc = Nuclear fraction; V = Vector-transfected; A = ALAD-transfected; + = SAHA-treatment; − = DMSO treatment; P = Pulldown; S = Supernatant; I = Input.
Immunoprecipitation with anti-myc following transfection and SAHA-treatment demonstrated that SAHA treatment also causes nuclear localization of ALAD-associated ubiquitinated α2 (Figure 4C, lane 7). Anti-myc antibody pulled down ubiquitinated α2 in the nuclear, but not the cytosolic fraction of ALAD-overexpressing PC-3 cells after SAHA treatment, compared with DMSO-treated cells (Figure 4C). Additionally, nuclear ubiquitinated α2 is also acetylated in the SAHA-treated (compared with DMSO-treated) ALAD-overexpressing cells (Figure 4C, lane 7). Together, these results suggest that acetylation may function to localize ALAD-associated ubiquitinated α2-containing proteasome complexes to the nucleus.
Discussion
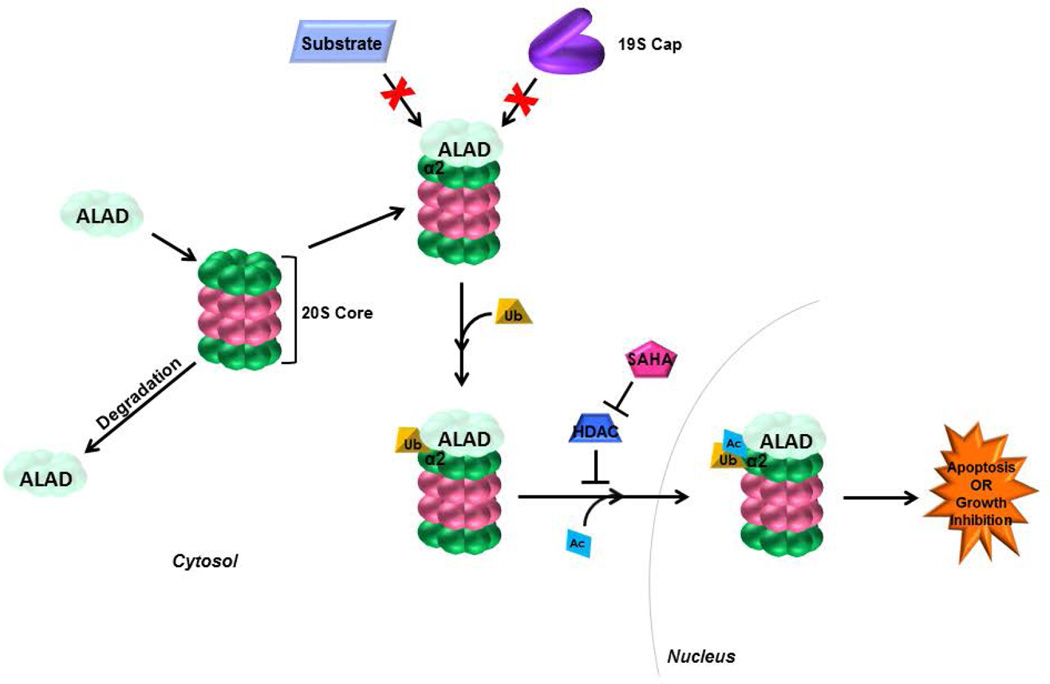
Many proteins are known to interact with the proteasome to carry out various functions. Some examples include Rad23 which is a carrier molecule for proteins destined for the proteasome [Elsasser et al., 2002], HSP70 which aids in the unfolding of protein substrates prior to their degradation by the proteasome [Bercovich et al., 1997] and ALAD, which has been reported to inhibit the proteasome [Guo et al., 1994] upon its direct binding to the proteasome [Bardag-Gorce and French, 2011]. Additionally, the proteasome has been reported to exist in several forms, including the constitutive 26S proteasome, which is the most common form consisting of the 20S core and two 19S regulatory caps. The 20S core can be unbound, the predominant species in mature ALAD-expressing erythrocytes [Neelam et al., 2011], or it can contain immune-specific catalytic subunits and interact with two 11S regulatory subunits to form the immunoproteasome. In the current study we have confirmed that the proteasome exists in an ALAD-bound state, in which ALAD binds in place of the 19S regulatory cap(s) (Figures 1B–E). It is possible that when ALAD is bound, substrate proteins are unable to enter the catalytic core, thus inhibiting its activity (Figure 5). Consistently, transfection of prostate cancer cells with full length ALAD did result in decreased proteasomal activity (Figure 2). However, further studies should be completed to confirm this molecular mechanism.
Figure 5.
Proposed Mechanism. ALAD binds to the 20S proteasomal core in place of the19S proteasome and is associated with ubiquitination of α2 subunits (and potentially other α subunits). ALAD binding to the 20S core hinders binding of the 19S regulatory cap(s), thus inhibiting entry of substrate proteins into the catalytic core. Upon HDAC inhibition, ALAD levels are enhanced, ubiquitinated α2 is acetylated and the ALAD-proteasome (with acetylated/ubiquitinated-α2) complex translocates into the nucleus, likely resulting in induction of apoptosis or cell growth inhibition in tumor cells.
We have discovered that ALAD is associated with ubiquitination of 20S proteasomal α2 (and possibly other α) subunits (Figures 1B–E). Post-translational modifications are important for the proper assembly [Satoh et al., 2001] and function [Iwafune et al., 2002; Kimura et al., 2000; Satoh et al., 2001; Zhang et al., 2003] of the 26S proteasome, and studies have shown that these post-translational modifications include phosphorylation, myristoylation, glycosylation and acetylation [Kikuchi et al., 2010]. Monoubiquitination, unlike polyubiquitination, does not serve as a signal for degradation, rather it can be a signal for fates like receptor internalization, vesicle sorting, DNA repair, gene silencing and subcellular localization [Gregory et al., 2003; Johnson, 2002]. Additionally, monoubiquitination of the 19S subunit Rpn10/S5a has been reported to inhibit its binding to ubiquitinated protein substrates by blocking its ubiquitin-interacting motif [Isasa et al., 2010]. However, ubiquitination of 20S subunits has not been reported. Thus, we have described a previously unreported post-translational modification to a proteasomal 20S subunit, which may serve to alter its function. The monoubiquitination of proteasomal α subunits (Figure 1B–E) may directly inhibit function, or may serve as a docking point for ALAD, which when bound, inhibits proteasome activity (Figure 2A). The exact function for this ubiquitination, as well as potential ubiquitination of other 20S core proteins must also be explored.
Due to previous success with the combination of the HDAC inhibitor SAHA and proteasome inhibitors like bortezomib, we investigated the effects of SAHA on ALAD and its interaction with the 20S proteasome. Interestingly, SAHA treatment enhances ALAD protein levels as well as the ALAD-proteasome interaction (Figure 3A, B. In addition to its enhancement of ALAD protein levels, we also found that SAHA treatment is associated with acetylation (Figure 3B) and nuclear localization (Figure 4C) of the ubiquitinated proteasomal α2 species. While protein acetylation has only recently been heavily explored, many functions have been suggested for the cytosolic acetylation of proteins. Several studies have revealed at least one-hundred proteins that are acetylated for purposes like cytoskeletal regulation and transport along the cytoskeleton, translation, membrane transport, and subcellular localization [Sadoul et al., 2011]. Interestingly, protein acetylation has also been described in other cellular organelles including the mitochondria, ER and Golgi apparatus [Sadoul et al., 2011]. When acetylation serves as a marker for localization, it enhances the nuclear localization of some proteins and the cytosolic localization of others [Sadoul et al., 2011]. While proteasomes are expressed ubiquitously throughout the cell [Brooks et al., 2000; Tanaka et al., 1989], ALAD is a cytosolic protein [Lim and Sassa, 1993], as expected, since its major role is in heme biosynthesis. We have confirmed cytosolic localization of ALAD (Figure 4A, B, and have also discovered that treatment with the HDAC inhibitor SAHA results in translocation of proteasome-bound ALAD into the nucleus (Figure 4A). Therefore it is possible that acetylation of ALAD-bound α2 subunits functions to cause nuclear import of proteasomes, which might have an important function, for example, inducing tumor cell death or growth inhibition (Figure 5), which must be further investigated.
Taken together, our data suggest the following mechanism (Figure 5): i) ALAD binds to the proteasomal α ring in place of the 19S regulatory cap, associated with ubiquitination of α2 (and possibly other α subunits), and ii) upon HDAC inhibition, ALAD levels are enhanced, ALAD-bound-, ubiquitinated α2 is acetylated and the ALAD-proteasome (with acetylated/ubiquitinated-α2) complex translocates into the nucleus. Further understanding of ALAD-proteasome complexes may have a clinical impact in improving proteasome inhibitor or HDAC inhibitor-based anti-cancer therapies.
Acknowledgments
Funding
This research was supported partly by the Thomas C. Rumble Graduate Fellowship (to S.M. Schmitt), the Wayne State University President’s Research Enhancement Program award (to C. Neslud-Dudas), NIH/NCI (1R01CA20009, 5R01CA127258-05 and R21CA184788 to Q.P. Dou; NIH P30 CA22453 to Karmanos Cancer Institute)
References
- An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, French SW. Delta-aminolevulinic dehydratase is a proteasome interacting protein. Exp Mol Pathol. 2011;91:485–489. doi: 10.1016/j.yexmp.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem. 1997;272:9002–9010. doi: 10.1074/jbc.272.14.9002. [DOI] [PubMed] [Google Scholar]
- Berlin A, Schaller KH. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974;12:389–390. [PubMed] [Google Scholar]
- Brooks P, Fuertes G, Murray RZ, Bose S, Knecht E, Rechsteiner MC, Hendil KB, Tanaka K, Dyson J, Rivett J. Subcellular localization of proteasomes and their regulatory complexes in mammalian cells. Biochem J. 2000;346(Pt 1):155–161. [PMC free article] [PubMed] [Google Scholar]
- Dou QP, Li B. Proteasome inhibitors as potential novel anticancer agents. Drug Resist Updat. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Muller B, Feng MT, Tubing F, Dittmar GA, Finley D. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol. 2002;4:725–730. doi: 10.1038/ncb845. [DOI] [PubMed] [Google Scholar]
- Gregory RC, Taniguchi T, D'Andrea AD. Regulation of the Fanconi anemia pathway by monoubiquitination. Semin Cancer Biol. 2003;13:77–82. doi: 10.1016/s1044-579x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Guo GG, Gu M, Etlinger JD. 240-kDa proteasome inhibitor (CF-2) is identical to delta-aminolevulinic acid dehydratase. J Biol Chem. 1994;269:12399–12402. [PubMed] [Google Scholar]
- Isasa M, Katz EJ, Kim W, Yugo V, Gonzalez S, Kirkpatrick DS, Thomson TM, Finley D, Gygi SP, Crosas B. Monoubiquitination of RPN10 regulates substrate recruitment to the proteasome. Mol Cell. 2010;38:733–745. doi: 10.1016/j.molcel.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafune Y, Kawasaki H, Hirano H. Electrophoretic analysis of phosphorylation of the yeast 20S proteasome. Electrophoresis. 2002;23:329–338. doi: 10.1002/1522-2683(200202)23:2<329::AID-ELPS329>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jaffe EK, Martins J, Li J, Kervinen J, Dunbrack RL., Jr The molecular mechanism of lead inhibition of human porphobilinogen synthase. J Biol Chem. 2001;276:1531–1537. doi: 10.1074/jbc.M007663200. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Ubiquitin branches out. Nat Cell Biol. 2002;4:E295–E298. doi: 10.1038/ncb1202-e295. [DOI] [PubMed] [Google Scholar]
- Kikuchi J, Iwafune Y, Akiyama T, Okayama A, Nakamura H, Arakawa N, Kimura Y, Hirano H. Co- and post-translational modifications of the 26S proteasome in yeast. Proteomics. 2010;10:2769–2779. doi: 10.1002/pmic.200900283. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Takaoka M, Tanaka S, Sassa H, Tanaka K, Polevoda B, Sherman F, Hirano H. N(alpha)-acetylation and proteolytic activity of the yeast 20 S proteasome. J Biol Chem. 2000;275:4635–4639. doi: 10.1074/jbc.275.7.4635. [DOI] [PubMed] [Google Scholar]
- Kumatori A, Tanaka K, Inamura N, Sone S, Ogura T, Matsumoto T, Tachikawa T, Shin S, Ichihara A. Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci U S A. 1990;87:7071–7075. doi: 10.1073/pnas.87.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci U S A. 2000;97:3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Sassa S. The Porphyirias. In: Lim HW, Scoter NA, editors. Clinical Photomedicine. New York, NY: Marcel Dekker, Inc; 1993. pp. 241–267. [Google Scholar]
- Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- Lopes UG, Erhardt P, Yao R, Cooper GM. p53-dependent induction of apoptosis by proteasome inhibitors. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- Neelam S, Kakhniashvili DG, Wilkens S, Levene SD, Goodman SR. Functional 20S proteasomes in mature human red blood cells. Exp Biol Med (Maywood) 2011;236:580–591. doi: 10.1258/ebm.2011.010394. [DOI] [PubMed] [Google Scholar]
- Sadoul K, Wang J, Diagouraga B, Khochbin S. The tale of protein lysine acetylation in the cytoplasm. J Biomed Biotechnol. 2011;2011:970382. doi: 10.1155/2011/970382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K, Sasajima H, Nyoumura KI, Yokosawa H, Sawada H. Assembly of the 26S proteasome is regulated by phosphorylation of the p45/Rpt6 ATPase subunit. Biochemistry. 2001;40:314–319. doi: 10.1021/bi001815n. [DOI] [PubMed] [Google Scholar]
- Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL. Docking of the proteasomal ATPases' carboxyl termini in the 20S proteasome's alpha ring opens the gate for substrate entry. Mol Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kumatori A, Ii K, Ichihara A. Direct evidence for nuclear and cytoplasmic colocalization of proteasomes (multiprotease complexes) in liver. J Cell Physiol. 1989;139:34–41. doi: 10.1002/jcp.1041390107. [DOI] [PubMed] [Google Scholar]
- Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]