Abstract
Background and study aims: EndoClot is a novel topical hemostatic powder approved for use in non-variceal upper gastrointestinal bleeding. This study examines its impact as rescue therapy in the management of gastrointestinal bleeding for which standard endoscopic therapy failed to achieve hemostasis.
Methods: This observational study covered a 24-month period. Data were collated from patients treated with EndoClot for comparison with a cohort of patients managed with standard endoscopic therapy. End points of this study included immediate hemostasis, 30-day rebleed rate, 30-day mortality rate, and adverse events.
Results: Between April 1, 2012, and March 31, 2014, gastroscopic procedures were performed in 1009 patients, of whom 173 required endoscopic therapy. EndoClot was used in 21 patients, with immediate hemostasis achieved in all cases, a 30-day rebleed rate of 4.8 % (95 % confidence interval [95 %CI] – 4.34 % to 3.94 %), and a 30-day mortality rate of 19.0 % (95 %CI 2.29 % – 35.91 %). Despite higher risk bleeds in this cohort of patients, Fisher's exact test demonstrated no significant difference between their 30-day mortality rate (P = 0.51) and rebleed rate (P = 0.31) and those of the patients treated with standard endoscopic hemostatic techniques.
Conclusions: This study demonstrates that EndoClot can be used both safely and effectively in the management of non-variceal upper gastrointestinal bleeding.
Introduction
Upper gastrointestinal bleeding remains a significant cause of mortality in the United Kingdom, accounting for 2500 deaths annually 1 2. Although up 70 % of non-variceal bleeds settle with conservative measures, endoscopic therapy is the established method for treating those bleeds for which this is not sufficient. The standard of care is dual therapy with a combination of mechanical, thermal, or injection therapy, which in up to 95 % of patients results in sustained hemostasis 2 3 4. Despite advances and increased expertise in managing upper gastrointestinal bleeding, the associated mortality of up to 15 % has remained unchanged for several years 1 2 5 6. This challenge is likely to increase, given an aging population and the consequent increased burden of cardiovascular disease, for which antiplatelet therapies, anticoagulants, and more recently novel oral anticoagulants are used 1 2 7. Furthermore, not all hospitals in the United Kingdom have comprehensive arrangements for out-of-hours endoscopy, with care often disjointed across specialities 1 2. A simple, effective method to achieve hemostasis is therefore likely to have a significant effect on the outcomes of our patients.
EndoClot (Vitramed, Australia and Malaysia) is a novel topical hemostatic agent that has been approved for use in non-variceal upper gastrointestinal bleeding. It can be administered as a primary hemostatic agent or as an adjunct alongside other modalities 8 9. The exact chemical composition of this product is guarded, with its hemostatic properties attributed to absorbable modified polysaccharides derived from plant starches free of animal or human components 8. EndoClot has been approved for in vivo use since 2011 and has been commercially available in the United Kingdom since 2012 8.
EndoClot consists of a white powder that combines with blood when applied to a bleeding lesion. It draws out water to form a gel matrix that adheres to the mucosa, creating a physical barrier 8. EndoClot claims to increase the local concentration of red blood cells, coagulation factors, and platelets, accelerating the process of clot formation. It is not absorbed or metabolized by the mucosa; instead, it is eliminated from the gastrointestinal tract through a combination of physical forces that cause the particles to slough off and enzymatic degradation by endogenous amylase and glucoamylase 8.
Endoclot is administered through a spray catheter, with particles dispersed over a large field of distribution, making precise deployment unnecessary. The catheter is not required to come directly into contact with the bleeding lesion during application, so that there is no risk of causing further tissue damage or exacerbating bleeding. As a consequence of these features, EndoClot requires minimal technical expertise to use, in comparison with conventional treatment modalities (8 – 10). The exact duration of adherence to the mucosa is unknown but is thought to range between 1 and 48 hours; patient factors and lesion characteristics are likely to be influential.
EndoClot is an addition to a growing variety of commercially available hemostatic powders, which include Hemospray (Cook Medical, Bloomington, Indiana, USA) and Ankaferd Blood Stopper (Ankaferd Health Products, Istanbul, Turkey). These powders have been adopted from military medicine and have been successfully used in emergency situations. Their characteristics and role in the context of upper gastrointestinal bleeding are currently under evaluation, with promising results 9.
Patients and methods
This study evaluated the impact of EndoClot in the management of non-variceal upper gastrointestinal bleeding in a large district general hospital serving a population of more than 500 000 people. Although large randomized controlled trials will ultimately be required to advocate the widespread use of this product, at present there is paucity of even preliminary evidence to guide the effective use of EndoClot. Rather than a formal trial designed to demonstrate superiority, we present here our early clinical experience of EndoClot, comparing outcomes with those of patients treated with the more established techniques.
This was a single-center retrospective observational study conducted over a 24-month period. A pragmatic approach to the use of EndoClot was adopted because even in the event of proven benefit, the cost of this product in comparison with that of standard hemostatic techniques would likely preclude its routine use as first-line therapy. To reflect realistic clinical practice, EndoClot was applied at the endoscopist’s discretion only if conventional endoscopic treatment with dual or triple therapy had failed to accomplish complete hemostasis or was technically impossible. The product was not used as monotherapy, primary therapy, or prophylaxis. All procedures were performed or supervised by consultant gastroenterologists with appropriate experience in the management of gastrointestinal bleeding. Again, to reflect daily clinical practice, patients with non-variceal upper gastrointestinal bleeding of all etiologies were considered for EndoClot treatment and included in this analysis.
The EndoClot application system consists of 2 g of powder within a mixing chamber, which is applied through a 2300-mm delivery catheter fed through the working channel of an endoscope. An external air compressor creates the sustained force required to drive the powder from within the chamber through the catheter, resulting in the multidirectional distribution of the product onto the mucosa (Fig. 1, Fig. 2). Given the wide field of distribution, an en face view of the point of bleeding is not essential; this is particularly useful for lesions that are notoriously difficult to access, such as ulcers on the posterior duodenal wall. Both the endoscopists and the endoscopy nurses underwent a brief training session in the use of this system before its introduction.
Fig. 1.
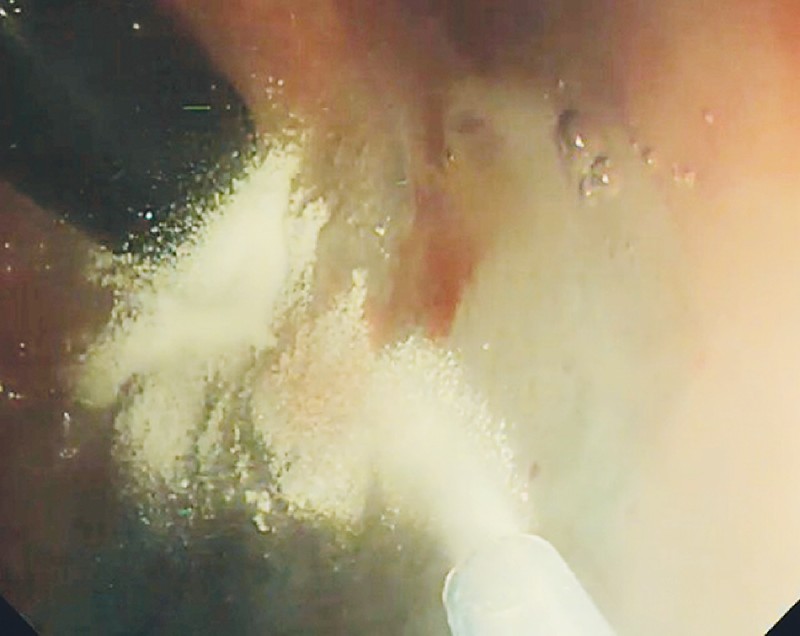
Endoscopic application of EndoClot.
Fig. 2 a, b.
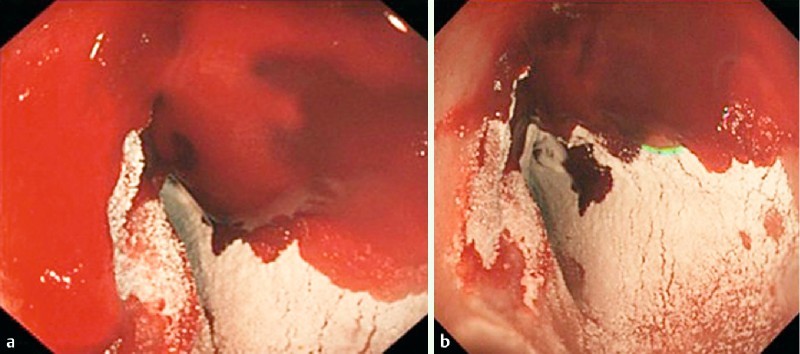
Endoscopic appearance of EndoClot when applied to a bleeding lesion.
The decision to use EndoClot was made at the time of endoscopy and so did not affect pre-endoscopy treatment, including appropriate resuscitation. There was no limit on the quantity that could be used to achieve cessation of bleeding. Failure of treatment was defined as the inability to achieve hemostasis on completion of the endoscopic procedure. Following treatment of the bleeding lesion, standard medical therapy was continued with inpatient observation and, for ulcer bleeds, a 72-hour infusion of a proton pump inhibitor. Patients did not routinely undergo a second-look endoscopy unless clinical or biochemical evidence of recurrent bleeding was noted.
We evaluated all patients with non-variceal upper gastrointestinal bleeding requiring endoscopic therapy over a 24-month period between April 1, 2012, and March 31, 2014. The demographic and clinical data of those treated with EndoClot were collated for comparison with the data of the cohort of patients managed with standard endoscopic therapy. The primary outcome measure of this study was successful hemostasis, defined as the cessation of active bleeding as visualized by the endoscopist at the time of the procedure. Secondary outcomes included 30-day rebleed rate, 30-day mortality rate, and adverse events directly related to the use of EndoClot. Observed group differences were statistically analysed with Fisher’s exact test.
Results
During the 24-month study period, 1009 patients underwent endoscopic procedures for non-variceal upper gastrointestinal bleeding, of whom 173 patients required endoscopic therapy (Fig. 3, Table 1). Those who received treatment had a median age of 77 years (range 18 – 101). The male-to-female ratio was 2 : 1. The majority of the endoscopic procedures were performed during the working day within 12 hours of presentation; however, 3 patients underwent endoscopy out of hours in an operating theatre, and 10 patients required stabilization in intensive care with intubation. The lesions accounting for active bleeding included the following: duodenal ulcer (n = 96), gastric ulcer (n = 23), esophageal ulcer (n = 16), malignancy (n = 11), Mallory – Weiss tear (n = 9), gastric antral vascular ectasia (n = 5), sphincterotomy bleeding after endoscopic retrograde cholangiopancreatography (n = 4), angiodysplasia (n = 3), Dieulafoy lesion (n = 3), nonspecific oozing (n = 1), aneurysm (n = 1), and bleeding after biopsy (n = 1). There were 24 deaths within 30 days (13.9 %; 95 %CI 7.74 % – 17.66 %) in patients with a median age of 81 years; duodenal ulcers were the underlying pathology in the majority of cases.
Fig. 3.
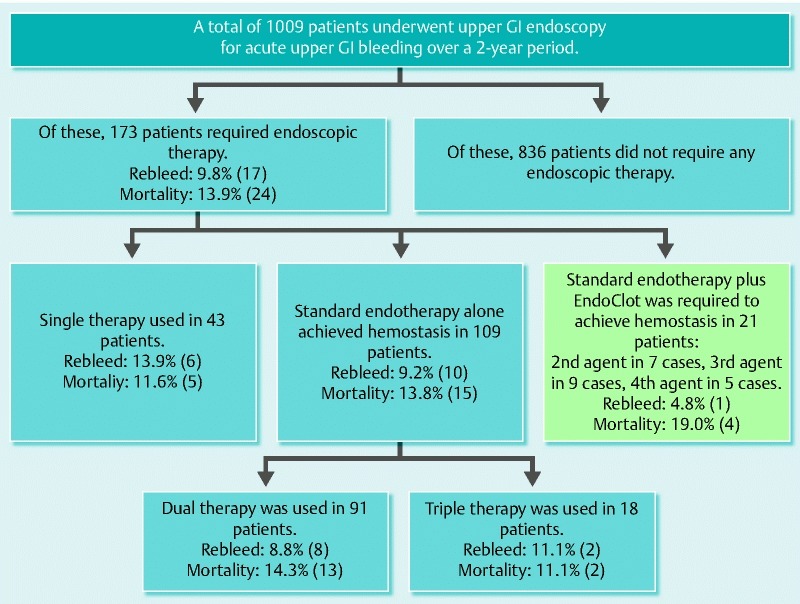
Schematic summarizing the management of non-variceal upper gastrointestinal bleeding and the cohort of patients treated with EndoClot.
Table 1. Baseline characteristics of the populations treated with conventional hemostasis vs. EndoClot.
| Patient characteristics | Dual therapy | Triple therapy | Standard therapy plus EndoClot | ||
| Mean age, y | 73.2 | 66.7 | 72.7 | ||
| Male-to-female ratio | 2.5 : 1 | 2 : 1 | 2 : 1 | ||
| Patients, n | 91 | 18 | 21 | ||
| Underlying pathology | |||||
| Duodenal ulcer | 66 | 14 | 14 | ||
| Gastric ulcer | 17 | 4 | 2 | ||
| Esophageal ulcer | 5 | – | 1 | ||
| Mallory-Weiss tear | 3 | – | 2 | ||
| Other | – | – | 2 | ||
| 30-day rebleed rate, % | 8.8 | 11.1 | 4.8 | ||
| 30-day mortality rate, % | 14.3 | 11.1 | 19.0 | ||
A single endoscopic modality was used in 43 patients; this cohort consisted mainly of patients with low risk lesions, such as known malignant lesions with oozing, angiodyplasia, and gastric antral vascular ectasia. Given the more indolent course of these lesions, the patients in this group were deemed inadequate for comparison and therefore excluded from our analysis.
Dual endotherapy was required in 91 patients, the most common combination being epinephrine with gold probe. This resulted in a rebleed rate of 8.8 % (95 % confidence interval [95 %CI] 2.98 % – 14.62 %) at 30 days, with 1 patient requiring surgery to achieve definitive hemostasis. There was a 30-day mortality of 14.3 % (95 %CI 7.11 % – 21.49 %). There were 18 patients who had bleeding lesions requiring triple therapy with gold probe, epinephrine injection, and clips. The associated 30-day rebleed rate was 11.1 % (95 %CI – 3.41 % to 25.61 %), and the 30-day mortality was 11.1 % (95 %CI – 3.41 % to 25.61 %). No patient receiving dual or triple therapy required surgery.
EndoClot was used in a total of 21 patients; this was as a second agent in 7 cases, a third agent in 9 cases, and the fourth form of endoscopic therapy in 5 cases. The lesions treated included duodenal ulcer (n = 14), Mallory-Weiss tear (n = 2), gastric ulcer (n = 2), malignancy (n = 1), esophageal ulcer (n = 1), and nonspecific gastric oozing (n = 1). Of the treated lesions, 5 were characterized as 1a and 16 as 1b per the Forrest classification 11. EndoClot was used by 7 different endoscopists, giving a median experience with this product of 3 applications per operator (range 1 – 5).
Immediate hemostasis was achieved in all patients treated with EndoClot. A single patient underwent endoscopy because of rebleeding; although the second gastroscopy demonstrated active bleeding, this was from a newly diagnosed Mallory-Weiss tear rather than the originally treated duodenal ulcer and therefore is not representative of a failure of EndoClot therapy. Another patient, in whom a large malignant duodenal ulcer was diagnosed, demonstrated biochemical and clinical evidence of rebleeding; however, it was deemed inappropriate to perform a second endoscopy. The cohort treated with EndoClot had a 30-day rebleed rate of 4.8 % (95 %CI – 4.34 % to 13.94 %). The 30-day mortality was 19.0 % (95 %CI 2.29 % – 35.91 %), although only 1 death was attributable to upper gastrointestinal bleeding (this was the patient with the aforementioned malignant ulcer, for whom surgery was not an option because of significant co-morbidities). There was no significant difference between the 30-day mortality rate and rebleed rate of the group of patients treated with EndoClot and the rates of those successfully treated with dual or triple endoscopic therapy alone (P = 0.51 and P = 0.31, respectively, with Fisher’s exact test).
In no instance did the use of the EndoClot system complicate or potentiate bleeding. In two cases, the administration of treatment was hampered by occlusion of the spray catheter with activated particles. There were no reported side effects in association with the use of EndoClot. Two patients underwent another endoscopy following the use of EndoClot for suspected rebleeding; both procedures occurred within 24 hours of the initial gastroscopy. Residual EndoClot was not seen in either patient.
Discussion
Upper gastrointestinal bleeding remains a common medical emergency, with endoscopic hemostasis the treatment of choice in patients with high risk lesions. The annual incidence of upper gastrointestinal bleeding requiring endoscopic therapy in our population was 8.6 per 100 000 patients. Our institution offers a daily endoscopy list for upper gastrointestinal bleeding within the endoscopy department 7 days a week and an out-of-hours on-call service on weekends. During the week, there is an ad hoc arrangement, with out-of-hours endoscopy performed in the operating theatre with theatre nurses and anesthetic support if required. This setup is not atypical and makes the availability of an effective hemostatic agent desirable 1 2 9 10. Our data support the main advantages of EndoClot – namely, efficacy comparable with that of the more established therapeutic modalities, ease of use, ability to overcome challenging anatomy, and the lack of complications 8 9 10.
By definition, the cohort receiving EndoClot therapy in our study included many of the patients at highest risk because they were the patients in whom standard endotherapy failed to achieve hemostasis. It is therefore reassuring to note that the addition of EndoClot therapy resulted in no statistical difference between the 30-day mortality rate (P = 0.51) and rebleeding rate (P = 0.31) of these patients and the rates of the patients in whom hemostasis was achieved with standard dual or triple therapy alone.
It was possible to use EndoClot despite limited experience, with a median of three applications per operator. There was no discernible difference in outcomes between those with the least experience and those with greater experience with this system. On two occasions, EndoClot was used outside the endoscopy department, where ease of use was demonstrated; these procedures were assisted by operating theatre nurses who had no specific training in the use of EndoClot.
A difficult anatomical position of the lesion was the indication for EndoClot use in the majority of cases, most commonly in the duodenum. Diffuse bleeding was seen in one patient; this is likely to be an increasing problem with the advent of novel oral anticoagulants, for which at present there is no clear consensus regarding endoscopic management.
In our study, no complications were associated with the use of EndoClot. Theoretical risks include allergic reaction, embolization, and intestinal obstruction. Limited data have been published on the use of EndoClot, so it is unclear how frequent these complications are likely to be; greater experience is required, in addition to vigilance for their occurrence.
Of the available hemostatic powders, Hemospray has perhaps the most evidence behind its use, with safety and ease of use demonstrated in case reports and several small case series. Our preliminary data compare favorably with the immediate success rate of Hemospray of 88.5 % and rebleed rate of 16.2 %, described in a recent review of 234 pooled patients 12 13 14. Hemospray has been tested in a wide range of clinical scenarios, with growing evidence for its use in bleeding associated with portal hypertension or ischemic colitis, bleeding after endoscopic mucosal resection, and even diverticular bleeding 15 16 17 18. Randomized controlled trials are awaited.
Ankaferd is licensed for use in Turkey but is not currently available in the United Kingdom. Several small case series have been published, with heterogeneity in the pathological conditions and populations studied. One of the larger series, including 27 patients, demonstrated immediate hemostasis in 73 % with a rebleed rate of 20 % 19. No studies have compared the available hemostatic agents in regard to efficacy, although with increasing interest and their greater position in the marketplace, head-to-head studies are likely to follow.
The main limitations of this study are its observational nature and the relatively small number of patients who received EndoClot therapy. EndoClot was used only as rescue therapy in the study, so it is difficult to speculate on its potential efficacy as primary monotherapy or as part of conventional dual therapy. We are unable to compare usefully the efficacy of EndoClot with that of other commercially available hemostatic agents. However, the study has demonstrated the successful use of EndoClot in a range of pathological conditions, with promising results. Greater experience is required to fully understand the potential of topical hemostatic agents, such as EndoClot. It is unlikely that conventional endoscopic techniques will be discontinued in favor of these; however, EndoClot is clearly a useful tool in the armamentarium of treatments for non-variceal upper gastrointestinal bleeding.
Acknowledgment
Images were provided by Vitramed.
Footnotes
Competing interests: None
References
- 1.Hearnshaw S A, Logan R F, Lowe D. et al. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59:1022–1029. doi: 10.1136/gut.2008.174599. [DOI] [PubMed] [Google Scholar]
- 2.Hearnshaw S A, Logan R F, Lowe D. et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. doi: 10.1136/gut.2010.228437. [DOI] [PubMed] [Google Scholar]
- 3.Rockall T A, Logan R F, Devlin H B. et al. Variation in outcome after acute upper gastrointestinal haemorrhage. The National Audit of Acute Upper Gastrointestinal Haemorrhage, Lancet. 1995;346:346–350. doi: 10.1016/s0140-6736(95)92227-x. [DOI] [PubMed] [Google Scholar]
- 4.Rockall T A, Logan R F, Devlin H B. et al. Influencing the practice and outcome in acute upper gastrointestinal haemorrhage. Steering Committee of the National Audit of Acute Upper Gastrointestinal Haemorrhage. Gut. 1997;41:606–611. doi: 10.1136/gut.41.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE. National Institute for Health and Care Excellence Acute upper gastrointestinal bleeding: management. NICE guideline CG141 https://www.nice.org.uk/guidance/cg141/resources[Accessed August 15, 2015; Published June 2012]
- 6.SIGN. Scottish Intercollegiate Guidelines Network Management of acute upper and lower gastrointestinal bleeding: a national clinical guideline. SIGN guideline 105 http://www.sign.ac.uk/pdf/sign105.pdf[Accessed August 15, 2015; Published September 2008]
- 7.Holster I L, Kuipers E J, Tjwa E TTL. Hemospray in the treatment of upper gastrointestinal hemorrhage in patients on antithrombotic therapy. Endoscopy. 2013;45:63–66. doi: 10.1055/s-0032-1325793. [DOI] [PubMed] [Google Scholar]
- 8.Vitramed Revolutionary haemostasis system. EndoClot www.vitramed.com.au/EndoClot.aspx[Accessed August 15, 2015; Published 2011]
- 9.Bustamante-Balén M, Plumé G. Role of hemostatic powders in the endoscopic management of gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5:284–292. doi: 10.4291/wjgp.v5.i3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weusten B, Bergman J J. A hemostatic spray: the easy way out for upper gastrointestinal bleeding? Endoscopy. 2011;43:343–344. doi: 10.1055/s-0030-1256302. [DOI] [PubMed] [Google Scholar]
- 11.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet 1974; 2: 394–397 [Google Scholar]
- 12.Changela K, Papafragkakis H, Ofori E. et al. Hemostatic powder spray: a new method for managing gastrointestinal bleeding. Therap Adv Gastroenterol. 2015;8:125–135. doi: 10.1177/1756283X15572587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung J, Luo D, Wu C. et al. Early clinical experience of the safety and effectiveness of Hemospray in achieving hemostasis in patients with acute peptic ulcer bleeding. Endoscopy. 2011;43:291–295. doi: 10.1055/s-0030-1256311. [DOI] [PubMed] [Google Scholar]
- 14.Babiuc R D, Purcarea M, Sadagurschi R. et al. Use of Hemospray in the treatment of patients with acute UGIB – short review. J Med Life. 2013;6:117–119. [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim M Lemmers A Devière J Novel application of Hemospray to achieve hemostasis in post-variceal banding esophageal ulcers that are actively bleeding Endoscopy 201446UCTNE263. [DOI] [PubMed] [Google Scholar]
- 16.Curcio G, Granata A, Traina M. Hemospray for multifocal bleeding following ultra-low rectal endoscopic submucosal dissection. Dig Endosc. 2014;26:606–607. doi: 10.1111/den.12301. [DOI] [PubMed] [Google Scholar]
- 17.Granata A Curcio G Barresi L et al. Hemospray rescue treatment of severe refractory bleeding associated with ischemic colitis: a case series Int J Colorectal Dis2015 May 6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Dietrich C Hochdörffer R Fuchs E S et al. Successful use of Hemospray to control refractory duodenal diverticular bleeding Endoscopy 201446UCTNE605–E606. [DOI] [PubMed] [Google Scholar]
- 19.Gungor G, Goktepe M H, Biyik M. et al. Efficacy of Ankaferd blood stopper application on non-variceal upper gastrointestinal bleeding. World J Gastrointest Endosc. 2012;4:556–560. doi: 10.4253/wjge.v4.i12.556. [DOI] [PMC free article] [PubMed] [Google Scholar]


