Abstract
Water-soluble polyrotaxanes have been prepared under heterogeneous conditions from mixtures of β-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin, methyl-β-cyclodextrin, or 6-monoazido-β-cyclodextrin with 4-sulfobutylether-β-cyclodextrin and Pluronic® L81 copolymer modified with cholesterol endcaps. β-CD threading gave polyrotaxane products in modest chemical yield that were reflective of the β–CD feed ratios in the reaction.
Keywords: Polyrotaxane, cyclodextrin, Pluronic, Niemann-Pick Type C, inclusion efficiency, mixed rotaxanation, supromolecule, macrocyclic, amorphous solid
Polyrotaxanes (PR) are self-assemblies obtained from macrocyclic molecules such as cyclodextrins threaded onto polymer axles that non-covalently retain the macrocycles on the polymer core through bulky stoppers that prevent dethreading. These non-covalent constructs have attracted wide interest in supramolecular and polymer chemistry, with particular attention focused on their potential applications as sliding ring gels,1,2 molecular machines,3 and as carriers for drug and gene delivery.4,5 Since the first PRs constructed from a-cyclodextrin (a-CD) and PEG were reported by Harada et al,6,7 many research efforts have been dedicated to the design of various Pluronic®-based PRs using β-cyclodextrin (β-CD) and its derivatives.8–16 Some of these studies have been devoted to understanding the formation of cyclodextrin inclusion compounds, but the forces that drive the complexation, especially for CD-polymer inclusion complex formation are yet to be fully elucidated.17–21 In addition, many reports have described the influence of cyclodextrin modifications on the stability and solubility of the numerous drug:β-CD complexes;22,23 however, none of these contributions have methodically focused on their impact on linear polymer complexes. Tonelli and coworkers have shown, while trying to interchange a-CD between PCL chains and PLLA chains in solution, that the guest-host steric compatibility plays an important role in polymer-CD complex formation.24
We have previously reported the synthesis of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD):Pluronic® copolymer based PRs in heterogeneous conditions as part of our effort to develop substrate reduction agents as potential Niemann-Pick Type C therapeutics.25 This synthetic method is practical for in situ multiple step reactions and allows for optimization of threading of highly water-soluble cyclodextrins. Since the previously reported HP-β-CD:Pluronic® PRs displayed limited solubility in physiological saline solutions, we sought to develop a library of PRs from mixtures of β-CD derivatives with 4-sulfobutylether-β-cyclodextrin (SBE-β-CD) to improve the water solubility of the PR product. SBE-β-CD has been widely utilized to improve the toxicity profile and water solubility of a variety of molecules in drug formulations.26,27 To understand the effect of macrocycle substituents on inclusion complex formation of β-CD and derivatives, we report the synthesis and structural properties of PRs from mixtures of SBE-β-CD with β-CD derivatives including β-CD, HP-β-CD, methyl-β-cyclodextrin (Me-β-CD), 6-deoxy-6-monoazido-β-cyclodextrin (Azido-β-CD). To the best of our knowledge, this is the first report focused on the threading of a Pluronic® surfactant with mixtures of β-cyclodextrin derivatives. We further demonstrate that PR water-solubility can be significantly improved using SBE-β-CD in the β-CD feed mixture.
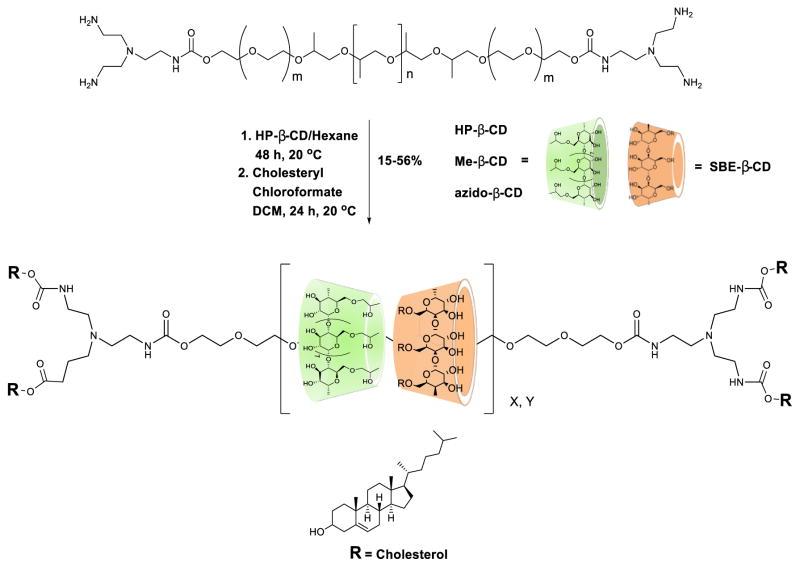
A library of CD:Pluronic® PR compounds was prepared by thoroughly mixing SBE-β-CD and the β-CD derivatives in the solid state by extensive grinding of the two powdered cyclodextrins before initiating the polymer threading and endcapping reactions. The pseudopolyrotaxane intermediates generated by this procedure were endcapped with cholesterol chloroformate to generate the corresponding PR product as depicted in Scheme 1. 1H NMR analysis was used to determine the number of cyclodextrins threaded onto the Pluronic® axle by comparing the integral intensities of the HP-β-CD C1-H (5.05 ppm) and PPG CH3 (1.0 ppm) signals. For compounds that contain SBE-β-CD, the broad peak of the sulfobutyl CH2 moiety at 1.7 ppm was used to determine the number of SBE-β-CD units incorporated. The coverage ratio was calculated based on the assumption that two PPG units are capable of inclusion per CD unit. The estimated number of total cyclodextrins threaded onto each triblock copolymer and the percent CD coverage of eight PR samples corresponding to Pluronic® L81 PRs obtained from β-CD, HP-β-CD, Me-β-CD, and azido-β-CD alone or a 1:1 mixture are shown in Table 1.
Scheme 1.
Synthesis pathway employed for β-CD/SBE-β-CD mixed PR derivatives.
Table 1.
Composition and yield of β-cyclodextrin:Pluronic® L81-based polyrotaxanes.
| Pluronic® L81-baseed PR | βCD/SBE-β-CD Compositiona | Coveragea
|
PR Yieldb | Molecular weight
|
|||
|---|---|---|---|---|---|---|---|
| Tot CD | SBE-βCD | ||||||
|
|
|||||||
| Feed ratio | Total CD Found | (%) | (%) | (%) | Mw, NMR | Mw, GPCc | |
| β-CD-PR | 100 | 18 | 82 | - | 44 | 2.60 x 104 | 8.03 x 104 |
| HP-β-CD-PR | 11 | 50 | - | 13 | 2.17 x 104 | 3.60 x 104 | |
| Me-β-CD-PR | 100 | 8 | 36 | - | 20 | 1.61 x 104 | 1.70 x 104 |
| azido-β-CD-PR | 100 | 8 | 36 | - | 25 | 1.49 x 104 | 2.26 x 104 |
| β-CD/SBE-βCD-PR | 50:50 | 21 | 95 | 41 | 35 | 3.70 x 104 | 5.40 x 104 |
| HP-β-CD/SBE-β-CD-PR | 50:50 | 19 | 86 | 46 | 37 | 3.85 x 104 | 1.50 x 104 |
| Me-β-CD/SBE-β-CD-PR | 50:50 | 19 | 86 | 64 | 27 | 4.00 x 104 | 1.16 x 104 |
| azido-β-CD/SBE-β-CD-PR | 50:50 | 18 | 82 | 64 | 35 | 3.91 x 104 | 5.19 x 104 |
| SBE-β-CD-PR | 100 | 21 | 95 | 100 | 63 | 4.72 x 104 | NA |
percentage of PPG units covered by CD units based on inclusion of two PPG units per CD
ratio of the product mass to the total mass of the feed
GPC values were measured in DMSO at a flow rate of 0.15 mL/min
NA: GPC estimation of Mw of SBE-β-CD-PR is not available
The PR structures were confirmed first by 1H NMR as shown in Figures S1 – S9 (Electronic Supplementary Information). The proton peak at ~1.0 ppm was assigned to the PPG methyl groups on the Pluronic® L81 copolymer, whereas the proton signals in the 3 – 3.5 ppm region were attributed to the methylene units (CH2) of the PEG and most of the cyclodextrin protons. The signal displayed in the 4.5 – 5.0 ppm region is assigned to the cyclodextrin C1-H protons as well as the C6 hydroxyl protons. The broad signal at 1.6 ppm, assigned to the sulfobutyl methylene (CH2) protons, was utilized to determine the number of SBE-β-CD in the complexes.
The molecular weights of the PR estimated by 1H NMR analysis products were then compared with GPC-MALS/RI using DMSO as eluent (Table 1). The molecular weights determined by GPC analysis are in general agreement with the values calculated from the NMR except for β-CD-PR, which appears to be self-aggregating in DMSO based on the appearance of a very high MW peak in the GPC chromatogram (Figure S10).
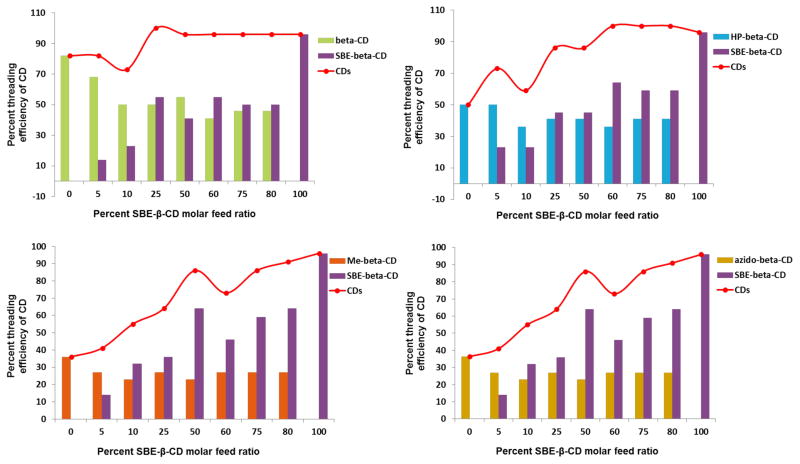
To assess the effect of competition between different CDs in the Pluronic® L81 rotaxanation reaction, four different CD mixtures containing β-CD, HP-β-CD, Me-β-CD, or Azido-β-CD mixed with SBE-β-CD, were prepared at different molar ratios starting with no SBE-β-CD. Solid mixtures of the CDs were suspended in hexane such that the L81 copolymer was afforded ready access to both host CDs in the mixture. As shown Figure 1, where the percent threading efficiency of each CD pair is plotted against the percent molar ratio of SBE-β-CD in the reaction feed, the threading efficiency of the uncharged β-CD derivatives remains roughly constant while the amount of SBE-β-CD threaded onto the polymer axle increases with increasing feed ratio. Since the structural modifications of the CD derivatives vary significantly across this family of β-CD derivatives, we infer from these findings that the CD-entrapped hexane solvent is more readily displaced from the SBE-β-CD cavity by the PEG ends of the polymer strand than the cavities of the other less polar, non-ionic CD derivatives.
Figure 1.
Threading efficiency of β-CD and SBE-β-CD mixtures using the heterogeneous threading reaction for PR synthesis (bars for individual CD and red lines for combined CD sums). The percent coverage was determined as the ratio of found CDs to the whole PPG units of L81 ÷ 2. Top left: β-CD + SBE-β-CD. Top right: HP-β-CD + SBE-β-CD. Bottom left: Me-β-CD + SBE-β-CD. Bottom right: Azido-β-CD + SBE-β-CD.
FTIR was used to analyze the eight PR compounds (Figure S12) and the spectra compared to those of the pure β-CD precursors and the Pluronic® L81 polymer core. The peak corresponding to the cholesterol endcap carbamate appears at 1672 cm−1 and the C-O-C and C-N-C stretching vibrations of carbonyl group in carbamate linkage emerge at 1109 cm−1 and 1230 cm−1, respectively, for all PRs. The broad absorption between 3100 – 3500 cm−1 corresponds to the CD hydroxyl stretching vibrations.
We observed a strong peak at 2103 cm−1 in the Azido-β-CD:Pluronic® L81 PR and Azido-β-CD/SBE-β-CD:Pluronic® L81 PR compounds, corresponding to the azide stretching mode, indicating that the PR isolated from these reactions has significant Azido-β-CD content. The PR products were further characterized using wide-angle X-ray scattering (WAXS), differential scanning calorimetry (DSC), and thermal gravimetric analysis (TGA). Figure S16 shows that for all the PRs synthesized, a unique peak is observed near 2θ = 20°; this peak is different from those found in the corresponding pure cyclodextrins or Pluronic® L81. Amorphous HP-β-CD, Me-β-CD, SBE-β-CD, and β-CD samples show peaks at 12.04°, 14.36°, 18.24°, and 21.76°. Taken together, these data suggest the presence of a channel-type crystalline structure for all β-CD PRs generated in this study, similar to the channel-like structures that were previously reported by Harada et al.28
The inclusion of the CDs was further supported by the DSC and TGA analysis. As depicted in Figure S13A, the DSC thermograms of pure cyclodextrins (except Azido-β-CD), as well as the unmodified Pluronic® L81 polymer, do not give rise to any significant endothermic transitions. This is likely due to disassembly of the β-CD microcrystalline powder during the first heating phase, producing an amorphous solid that has no melting transitions. For Azido-β-CD, the peak at 140 °C can be attributed to extrusion of N2(g).29 On the other hand, clear endothermic transitions are observed for the PR in Figure S13B. This corresponds to the aggregation of rod-like segments of the nearest neighbor PR species into microcrystalline domains that melt at elevated temperatures. It can be seen that the enthalpy exchanges corresponding to all SBE-β-CD-based PRs are smaller than those of single CD and mixed PR materials. Presumably, the charge repulsion caused by the sulfonate groups prevents these molecules from packing into ordered structures.
The thermal stability of the PRs was also evaluated by TGA. Figure S14A shows that the pure cyclodextrins with the exception of Azido-β-CD and SBE-β-CD, had a single thermal decomposition event. Azido-β-CD produces a different profile with a sharp weight loss at 440 °C indicating the decomposition of the azide group and loss of N2(g) from the CD.
The multiple weight losses seen for SBE-β-CD are likely due to dehydration, extrusion and/or cracking (e.g. loss of H2O, SO2 and butanesultone) processes of the CD modifications. In the case of the PRs, the thermograms showed two or more steps in the thermal degradation process. The first step is attributed to the thermal decomposition of the corresponding cyclodextrins, while the second one is related to the Pluronic® L81 triblock copolymer degradation. Apparently, the weight losses of the inclusion polymer complexes are slower than those for the free CDs, suggesting that the cyclodextrins bound to the PPG block confer greater thermal stability for the CDs than the monomeric CDs species. In addition, the decomposition temperatures of the Pluronic® L81 during the second phase of PR degradation increases relative to that of the free polymer. We infer from these observations that inclusion of the PPG chains within the CD cavities affords greater thermal stability for the triblock copolymer. These findings, i.e., that the thermal stability of both the β-CD species and the Pluronic® L81 triblock copolymer are increased upon rotaxanation, are strongly suggestive that the polymer is included within the hydrophobic CD cavity.
Using negative-stain TEM microscopy, we were able to observe the microstructures of the PR dispersions. TEM micrographs were obtained for HP-β-CD:Pluronic® L81 PR and HP-β-CD/SBE-β-CD:Pluronic® L81 PR, which were chosen as models for all PR prepared (Figure S11). Both compounds show self-aggregation to produce spherical particles. As illustrated in Figure S11(A), HP-β-CD:Pluronic® L81 PR gave rise to nanoparticles with diameters of 10 – 88 nm, whereas under the same conditions, HP-β-CD/SBE-β-CD:Pluronic® L81 PR aggregated into more regular and small particles that were 6 – 12 nm in diameter as shown in Figure S11B. The net difference seen between these two samples can be explained by electrostatic charge repulsion between the sulfonated CDs, thereby preventing more extensive aggregation of the PRs in the case of HP-β-CD/SBE-β-CD:Pluronic® L81 PR.
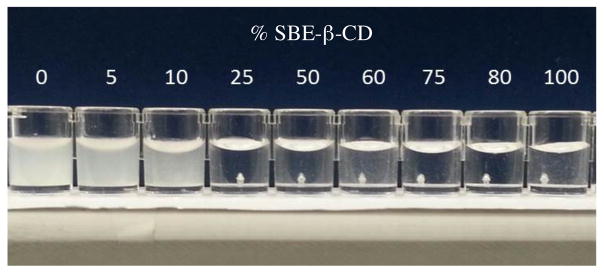
Our long-term objective of increasing the solubilities of HP-β-CD PRs for Niemann Pick Type C therapeutics was then tested. We anticipated that the negative charges introduced by the SBE-β-CD units in the PR complexes would reduce their aggregation and improve their water solubility. To test this assumption, the samples obtained from HP-β-CD/SBE-β-CD mixtures were dissolved in nanopure water and the appearance of the solutions monitored (Figure 2). Our findings show that increasing the amount of SBE-β-CD in the PR structure leads to a significant improvement in their water solubility, particularly for the samples with SBE-β-CD molar ratios of ≥ 25%. It is well known that CD-based PRs are generally insoluble in water due to the aggregation of their rod-like structures.30,31 The incorporation of SBE-β-CD, a highly water soluble material, leads to weaker intermolecular interactions and improved water solubilities of the mixed PR products.
Figure 2.
Water solubility of the HP-β-CD/SBE-β-CD:Pluronic® L81 polyrotaxanes at 50 mg/mL.
Conclusions
In summary, we report an efficient synthetic method for the construction of mixed CD based PRs using a heterogeneous strategy. The competition between β-CD monomers during inclusion complex formation of PRs is likely dictated by a balance between electrostatic repulsion between neighboring intrinsic SBE-β-CD units, the intermolecular hydrogen bonding capability of the various CD derivatives, and their relative solubilities in the organic solvent used during the heterogeneous threading reaction. A cooperative process of the CDs during the rotaxanation reaction is inferred, leading to threading of mixed CDs on the polymer core. Inclusion of SBE-β-CD increased as a function of its increasing molar ratio in the feed. Interestingly, the water solubilities of the PRs were significantly improved due to the presence of SBE-β-CD units in the PR product. The synthesis of water soluble PRs with defined units of azide may open possibilities for controlled and orthogonal post-modifications for a wide variety of applications.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the Ara Paraseghian Medical Research Foundation/Smith Family BReaKThru Fund and the Indiana Clinical and Translational Sciences Institute Core Pilot Funding Grant #UL1TR001108. NMR and MS data were acquired in the Purdue University Center for Cancer Research Interdepartmental NMR Facility and Campus-Wide Mass Spectrometry Center, both supported by NCI CCSG CA23168 to the Purdue Center for Cancer Research.
Abbreviations
- Azido-β-CD
6-deoxy-6-monoazido-β-cyclodextrin
- CD
cyclodextrin
- β-CD
β-cyclodextrin
- DSC
differential scanning calorimetry
- GPC
gel permeation chromatography
- HP-β-CD
2-hydroxypropyl-β-cyclodextrin
- MALS/RI
multiangle light scattering/refractive index detection
- Me-β-CD
methyl-β-cyclodextrin
- PR
polyrotaxane
- SBE-β-CD
4-sulfobutylether-β-cyclodextrin
- TGA
thermal gravimetric analysis
- WAXS
wide-angle X-ray scattering
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
1H NMR, GPC, DSC, TGA, FTIR, and TEM data of the PR products are available in Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fleury G, Schlatter G, Brochon C, Travelet C, Lapp A, Lindner P, Hadziioannou G. Topological Polymer Networks With Sliding Cross-link Points: The “Sliding Gels”. Relationship Between Their Molecular Structure and the Viscoelastic as Well as the Swelling Properties. Macromolecules. 2007;40:535–543. [Google Scholar]
- 2.Okumura Y, Ito K. Polyrotaxane Gel: A Topological Gel by Figure-of-Eight Cross-links. Adv Mater. 2001;13:485–487. [Google Scholar]
- 3.Ooya T, Eguchi M, Yui N. Supramolecular Design for Multivalent Interaction: Maltose Mobility along Polyrotaxane Enhanced Binding with Concanavalin A. J Am Chem Soc. 2003;125:13016–13017. doi: 10.1021/ja034583z. [DOI] [PubMed] [Google Scholar]
- 4.Ooya T, Yui N. Multivalent Interactions Between Biotin-Polyrotaxane Conjugates and Streptavidin as a Model of New Targeting for Transporters. J Controlled Release. 2002;80:219–228. doi: 10.1016/s0168-3659(02)00030-5. [DOI] [PubMed] [Google Scholar]
- 5.Kulkarni A, DeFrees K, Schuldt RA, Hyun SH, Wright KJ, Yerneni CK, VerHeul R, Thompson DH. Cationic α-Cyclodextrin:Poly(ethylene glycol) Polyrotaxanes for siRNA Delivery. Mol Pharmaceutics. 2013;10:1299–1305. doi: 10.1021/mp300449t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada A, Li J, Kamachi M. The Molecular Necklace - A Rotaxane Containing Many Threaded α-Cyclodextrins. Nature. 1992;356:325–327. [Google Scholar]
- 7.Harada A, Li J, Kamachi M. Double-strained Inclusion Complexes of Cyclodextrin Threaded on Poly(ethylene glycol) Nature. 1994;370:126–128. [Google Scholar]
- 8.Fujita H, Ooya T, Kurisawa M, Mori H, Terano M, Yui N. Thermally Switchable Polyrotaxane as a Model of Stimuli-responsive Supramolecules for Nano-scale Devices. Macromol Rapid Commun. 1996;17:509–515. [Google Scholar]
- 9.Fujita H, Ooya T, Yui N. Synthesis and Characterization of a Polyrotaxane Consisting of β-Cyclodextrins and a Poly(ethylene glycol) Poly(propylene glycol) Triblock Copolymer. Macromol Chem Phys. 1999;200:706–713. [Google Scholar]
- 10.Fujita H, Ooya T, Yui N. Thermally Induced Localization of Cyclodextrins in a Polyrotaxane Consisting of β-Cyclodextrins and Poly(ethylene glycol)-Poly(propylene glycol) Triblock Copolymer. Macromolecules. 1999;32:2534–2541. [Google Scholar]
- 11.Gaitano GG, Brown W, Tardajos G. Inclusion Complexes Between Cyclodextrins and Triblock Copolymers in Aqueous Solution: A Dynamic and Static Light Scattering Study. J Phys Chem B. 1997;101:710–719. [Google Scholar]
- 12.Joseph J, Dreiss CA, Cosgrove T, Pedersen JS. Rupturing Polymeric Micelles with Cyclodextrins. Langmuir. 2006;23:460–466. doi: 10.1021/la061850g. [DOI] [PubMed] [Google Scholar]
- 13.Loethen S, Ooya T, Choi HS, Yui N, Thompson DH. Synthesis, Characterization and pH-Triggered Dethreading of α-Cyclodextrin Polyethylene Glycol Polyrotaxanes Bearing Cleavable Stoppers. Biomacromolecules. 2006;7:2501–2506. doi: 10.1021/bm0602076. [DOI] [PubMed] [Google Scholar]
- 14.Collins CJ, McCauliff LA, Hyun SH, Zhang Z, Paul LN, Kulkarni A, Zick K, Wirth M, Storch J, Thompson DH. Synthesis, Characterization, and Evaluation of Pluronic-based β-Cyclodextrin Polyrotaxanes for Mobilization of Accumulated Cholesterol from Niemann-Pick Type C Fibroblasts. Biochemistry. 2013;52:3242–3253. doi: 10.1021/bi3010889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castiglione F, Valero M, Dreiss CA, Mele A. Selective Interaction of 2,6-Di-O-methyl-β-cyclodextrin and Pluronic F127 Micelles Leading to Micellar Rupture: A Nuclear Magnetic Resonance Study. J Phys Chem B. 2011;115:9005–9013. doi: 10.1021/jp203753r. [DOI] [PubMed] [Google Scholar]
- 16.Dreiss CA, Nwabunwanne E, Liu R, Brooks NJ. Assembling and de-Assembling Micelles: Competitive Interactions of Cyclodextrins and Drugs with Pluronics. Soft Matter. 2009;5:1888–1896. [Google Scholar]
- 17.Liu L, Guo QX. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J Inclusion Phenom Macrocyclic Chem. 2002;42:1–14. [Google Scholar]
- 18.Harada A. Construction of Supramolecular Structures from Cyclodextrins and Polymers. Carbohydr Polym. 1997;34:183–188. [Google Scholar]
- 19.Harada A. Cyclodextrin-based Molecular Machines. Acc Chem Res. 2001;34:456–464. doi: 10.1021/ar000174l. [DOI] [PubMed] [Google Scholar]
- 20.Ceccato M, LoNostro P, Baglioni P. α-Cyclodextrin/Polyethylene Glycol Polyrotaxane: A Study of the Threading Process. Langmuir. 1997;13:2436–2439. [Google Scholar]
- 21.Okumura Y, Ito K, Hayakawa R. Theory on Inclusion Behavior Between Cyclodextrin Molecules and Linear Polymer Chains in Solution. Polym Adv Technol. 2000;11:815–819. [Google Scholar]
- 22.Muller BW, Brauns U. Hydroxypropyl-β-Cyclodextrin Derivatives - Influence of Average Degree of Substitution on Complexing Ability and Surface Activity. J Pharm Sci. 1986;75:571–572. doi: 10.1002/jps.2600750609. [DOI] [PubMed] [Google Scholar]
- 23.Buvári-Barcza Á, Barcza L. Influence of the Guests, the Type and Degree of Substitution on Inclusion Complex Formation of Substituted β-Cyclodextrins. Talanta. 1999;49:577–585. doi: 10.1016/s0039-9140(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 24.Rusa CC, Fox J, Tonelli AE. Competitive Formation of Polymer-Cyclodextrin Inclusion Compounds. Macromolecules. 2003;36:2742–2747. [Google Scholar]
- 25.Mondjinou YA, McCauliff LA, Kulkarni A, Paul L, Hyun SH, Zhang Z, Wu Z, Wirth M, Storch J, Thompson DH. Synthesis of 2-Hydroxypropyl-β-Cyclodextrin and Pluronic Based Polyrotaxanes in Heterogeneous Reactions for Niemann-Pick Type C Therapy. Biomacromolecules. 2013;14:4189–4197. doi: 10.1021/bm400922a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gopinathan S, O’Neill E, Rodriguez LA, Champ R, Phillips M, Nouraldeen A, Wendt M, Wilson AGE, Kramer JA. In Vivo Toxicology of Excipients Commonly Employed in Drug Discovery in Rats. J Pharmacol Toxicol Methods. 2013;68:284–295. doi: 10.1016/j.vascn.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Uehata K, Anno T, Hayashida K, Motoyama K, Hirayama F, Ono N, Pipkin JD, Uekama K, Arima H. Effect of Sulfobutyl Ether-β-Cyclodextrin on Bioavailability of Insulin Glargine and Blood Glucose Level After Subcutaneous Injection to Rats. Int J Pharm. 2011;419:71–76. doi: 10.1016/j.ijpharm.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Harada A, Kamachi M. Complex Formation Between Poly(ethylene glycol) and α-Cyclodextrin. Macromolecules. 1990;23:2821–2823. [Google Scholar]
- 29.Potvin H, Back MH. Study of Decomposition of Sodium Azide Using Differential Thermal Analysis. Can J Chem. 1973;51:183–186. [Google Scholar]
- 30.Araki J, Ito K. Recent Advances in the Preparation of Cyclodextrin-based Polyrotaxanes and Their Applications to Soft Materials. Soft Matter. 2007;3:1456–1473. doi: 10.1039/b705688e. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Loh XJ. Cyclodextrin-based Supramolecular Architectures: Syntheses, Structures, and Applications for Drug and Gene Delivery. Adv Drug Delivery Rev. 2008;60:1000–1017. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.