Abstract
The multifactorial mechanisms promoting weight loss and improved metabolism following Roux-en-Y gastric bypass (GB) surgery remain incompletely understood. Recent rodent studies suggest that bile acids can mediate energy homeostasis by activating the G-protein coupled receptor TGR5 and the type 2 thyroid hormone deiodinase. Altered gastrointestinal anatomy following GB could affect enterohepatic recirculation of bile acids. We assessed whether circulating bile acid concentrations differ in patients who previously underwent GB, which might then contribute to improved metabolic homeostasis. We performed cross-sectional analysis of fasting serum bile acid composition and both fasting and post-meal metabolic variables, in three subject groups: (i) post-GB surgery (n = 9), (ii) without GB matched to preoperative BMI of the index cohort (n = 5), and (iii) without GB matched to current BMI of the index cohort (n = 10). Total serum bile acid concentrations were higher in GB (8.90 ± 4.84 µmol/l) than in both overweight (3.59 ± 1.95, P = 0.005, Ov) and severely obese (3.86 ± 1.51, P = 0.045, MOb). Bile acid subfractions taurochenodeoxycholic, taurodeoxycholic, glycocholic, glycochenodeoxycholic, and glycodeoxycholic acids were all significantly higher in GB compared to Ov (P < 0.05). Total bile acids were inversely correlated with 2-h post-meal glucose (r = −0.59, P < 0.003) and fasting triglycerides (r = −0.40, P = 0.05), and positively correlated with adiponectin (r = −0.48, P < 0.02) and peak glucagon-like peptide-1 (GLP-1) (r = 0.58, P < 0.003). Total bile acids strongly correlated inversely with thyrotropic hormone (TSH) (r = −0.57, P = 0.004). Together, our data suggest that altered bile acid levels and composition may contribute to improved glucose and lipid metabolism in patients who have had GB.
Introduction
Obesity is occurring at epidemic rates worldwide. The prevalence of adult obesity in the United States in 2003 was 32.2% (ref. 1). Unfortunately, medical management of obesity, including diet and/or medication, has met with limited success.
The increasing prevalence of obesity has led to a parallel rise in bariatric surgery as a treatment for obesity and related comorbid conditions, with an estimated 220,000 procedures in 2008 in the United States (2). Surgical procedures achieve sustained weight reduction of up to 50% of excess body weight in the majority of patients, and are more effective than nonsurgical approaches (3). The most common form of surgery, Roux-en-Y gastric bypass (GB), is particularly effective in producing sustained weight loss. Potential mechanisms contributing to weight loss efficacy include not only gastric restriction leading to distension of the small gastric pouch and early satiety (as also observed with banding procedures), but also mild malabsorption, alterations in neural signals, duodenal exclusion, and early delivery of nutrients to the distal small intestine (4). Collectively, these changes may alter secretion of metabolically active peptides, including ghrelin, glucagon-like peptide-1 (GLP-1), and peptide YY (5–8). Remarkably, GB causes rapid resolution of insulin resistance and improved insulin secretion, even before major weight loss has been achieved (9). However, specific mechanisms mediating GB-induced weight loss and improved diabetes control, and its superiority over purely restrictive procedures, remain incompletely understood.
Bile acids have been long recognized to play a central role in absorption of dietary lipid. As ligands for the farnesoid X receptor (FXR), bile acids also modulate bile acid synthesis and hepatic lipid metabolism. Recently, bile acids have additionally been recognized as important modulators of whole-body metabolism, increasing energy expenditure and preventing obesity, insulin resistance, and hyperglycemia, during high-fat feeding in rodents (10). These effects are largely FXR-independent and are instead mediated by binding to the G-coupled receptor TGR5, leading to cAMP generation and activation of the intracellular type 2 thyroid hormone deiodinase (10). In humans, circulating bile acid levels correlate with measures of insulin sensitivity (11). Moreover, recent human clinical trials indicate that modulation of bile acid homeostasis using the bile acid sequestrant colesevelam improves glycemic control in patients with type 2 diabetes (12,13). Because GB alters upper intestinal tract anatomy potentially affecting enterohepatic circulation of bile acids, we hypothesized that serum bile acid levels might be increased in individuals with a history of GB and potentially contribute to the improved carbohydrate and lipid metabolism observed in this population. As a first step to test this hypothesis, we measured serum bile acid levels in a cross-sectional cohort of healthy patients with a history of GB surgery for obesity and in two groups of control subjects, matched for both preoperative and postoperative BMI, and assessed the relationship between bile acid concentrations and metabolic measures.
Methods And Procedures
Subjects
The Joslin Diabetes Center Institutional Review Board approved the study and written informed consent was obtained from all participants. Subjects who had undergone uncomplicated GB 2–4 years previously and severely obese individuals being evaluated for bariatric surgery were recruited from bariatric clinics, and overweight, nonseverely obese subjects from newspaper advertisement. All 24 subjects were weight stable for 6 months and had no history of diabetes or known glucose intolerance. Exclusion criteria included current or past heart failure, chronic liver or kidney disease, malignancy, acute infection or injury, current pregnancy, and use of medications known to affect insulin sensitivity.
Subjects were instructed to consume a diet containing at least 200 g carbohydrate for 3 days before each visit. Severely obese and overweight groups underwent 2-h 75-g oral glucose tolerance tests and were found to be nondiabetic using National Diabetes Data Group criteria (14). On a subsequent day, height and weight were measured using a wall-mounted stadiometer (Holtain, Crymych, UK) and electronic scale (Model 0501; ACME, San Leandro, CA), respectively, and fasting blood samples obtained before a liquid mixed meal tolerance test (Ensure, 9 g protein, 40 g carbohydrate, 6 g fat). Additional blood samples were collected at 10, 20, 30, 60, and 120 min for glucose, insulin, and GLP-1.
Assays
Glucose was measured by glucose oxidation, fasting cholesterol, and high-density lipoprotein (HDL) by cholesterol esterase assay, triglycerides via hydrolysis to glycerol and free fatty acid (Synchron CX3delta and CX9; Beckman Coulter, Brea, CA), and hemoglobin A1c by high-performance liquid chromatography (Tosoh 2.2; Tosoh Bioscience, San Francisco, CA). Immunoassays were performed in duplicate on fasting serum by commercial assay, including radioimmunoassay for insulin and C-peptide (Diagnostic Systems Laboratories, Webster, TX) and adiponectin (Linco Research, St Charles, MO), and enzyme-linked immunosorbent assay for leptin (Linco Research). Thyroid function was analyzed using the Centaur Immunoassay platform (Bayer Diagnostics, Tarrytown, NY).
Plasma GLP-1 was measured after extraction of serum with 70% ethanol (vol/vol) (15), using antiserum (code 89390) specific for the amidated C-terminus of GLP-1 and therefore primarily reactive with GLP-1 of intestinal origin. (The assay reacts equally with intact GLP-1 and with GLP-1 3–36 amide, the primary metabolite.) Assay sensitivity was below 1 pmol/l and intra-assay coefficient of variation below 6% at 20 pmol/l. Plasma FGF21 was measured by specific radioimmunoassay (Phoenix Pharmaceuticals, Burlingame, CA) (16). Plasma FGF19 was measured by enzyme-linked immunosorbent assay (Human Kit; R&D Systems, Minneapolis, MN); output was normalized to plasma recovery of 94%.
Bile acid measurements
Fasting serum bile acids were measured by high-performance liquid chromatography tandem mass spectrometry and quantified using deuterium-labeled internal standards (17).
Statistical analysis
Demographic characteristics are presented as mean ± s.d.; results are presented as mean ± s.e. Multivariate analysis of bile acid fractions was performed using General Linear Model multivariate analysis (SPSS version 11; SPSS, Chicago, IL) using all bile acids as dependent variables and study groups as a fixed factor. One-way ANOVA, with between-group post hoc t-testing, Pearson correlation, and simple and bivariate regression analyses were performed using StatView (SAS Institute, Cary, NC). Independent variables were assessed after logarithmic transformation for skewed distribution. Two-tailed P values of <0.05 were considered significant.
Results
Demographic and metabolic characteristics are summarized in Table 1. No subject had diabetes. The nine subjects who had GB for morbid obesity a mean of 2.7 years earlier had substantial weight loss (preoperative BMI 50.2 ± 11.4 kg/m2 vs. current BMI 29.9 ± 4.5 kg/m2, P = 0.0004). To control for potential effects related to preoperative obesity magnitude, as well as for current BMI, 5 morbid obese subjects similar for preoperative BMI, and 10 obese subjects similar for current BMI (overweight) were also studied. As expected, postoperative subjects had significantly lower waist circumference, fasting triglyceride, fasting and 2-h glucose, and fasting insulin levels, and increased HDL and adiponectin levels as compared with severely obese controls (all P < 0.05). Likewise, significant differences between post-bypass subjects and overweight subjects were observed for triglyceride, HDL, fasting, and 2-h glucose, and adiponectin levels, consistent with improved lipid and carbohydrate metabolism in post-bypass subjects. Severely obese subjects had significantly higher fasting leptin and insulin levels than overweight subjects. All post-GB subjects were taking multivitamin supplements, and six of nine subjects were taking antidepressant medications, as compared with 3 of 15 nonsurgical subjects (P < 0.02 by χ2-analysis); there were no other differences in medication profiles between groups. Although no overweight subjects had a history of cholecystectomy, 7 of 14 subjects with morbid obesity had prior cholecystectomy; the frequency did not differ between post-GB (4/9) and severely obese subject groups (3/5).
Table 1.
Demographics of study cohorts
| Post-GB | Overweight | Morbid obese | |
|---|---|---|---|
| Age (year) | 48 ± 9 | 48 ± 8 | 41 ± 16 |
| Gender (male/female) | 2/7 | 6/4 | 0/5 |
| Preoperative BMI (kg/m2) | 50.2 ± 11.4 | NA | NA |
| BMI (kg/m2) | 29.9 ± 4.5 | 31.5 ± 4.0 | 52.0 ± 6.4††,‡‡ |
| Waist (cm) | 95 ± 17 | 102 ± 17 | 143 ± 19††,‡‡ |
| HbA1c (%) | 5.2 ± 0.5 | 5.6 ± 0.3 | 5.5 ± 0.4 |
| Chol (mg/dl) | 176 ± 27 | 188 ± 27 | 189 ± 36 |
| TG (mg/dl) | 51 ± 12 | 132 ± 110* | 127 ± 71†† |
| HDL (mg/dl) | 71 ± 25 | 42 ± 11** | 39 ± 8† |
| SBP (mm Hg) | 124 ± 16 | 120 ± 6 | 126 ± 13 |
| DBP (mm Hg) | 73 ± 7 | 73 ± 7 | 73 ± 8 |
| Fasting glucose (mg/dl) | 84 ± 7 | 94 ± 6** | 96 ± 9† |
| 2-h Glucose (mg/dl) | 70 ± 9 | 91 ± 13** | 99 ± 13†† |
| Fasting insulin (µU/ml) | 7.9 ± 10.2 | 15.2 ± 10.3 | 28.1 ± 5.8††,‡ |
| Leptin (ng/ml) | 12.9 ± 5.1 | 17.6 ± 10.5 | 70.8 ± 19.6††,‡‡ |
| Adiponectin (µg/dl) | 26.4 ± 10.3 | 13.6 ± 8.3** | 8.0 ± 2.8†† |
Data are presented as mean ± s.d.
chol, cholesterol; DBP, diastolic blood pressure; GB, gastric bypass; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; SBP, systolic blood pressure; TG, triglyceride.
P < 0.05.
P < 0.01 for comparison of GB vs. overweight.
P < 0.05.
P < 0.01 for comparison of post-GB vs. morbid obesity.
P < 0.05.
P < 0.01 for comparison of overweight vs. morbid obesity.
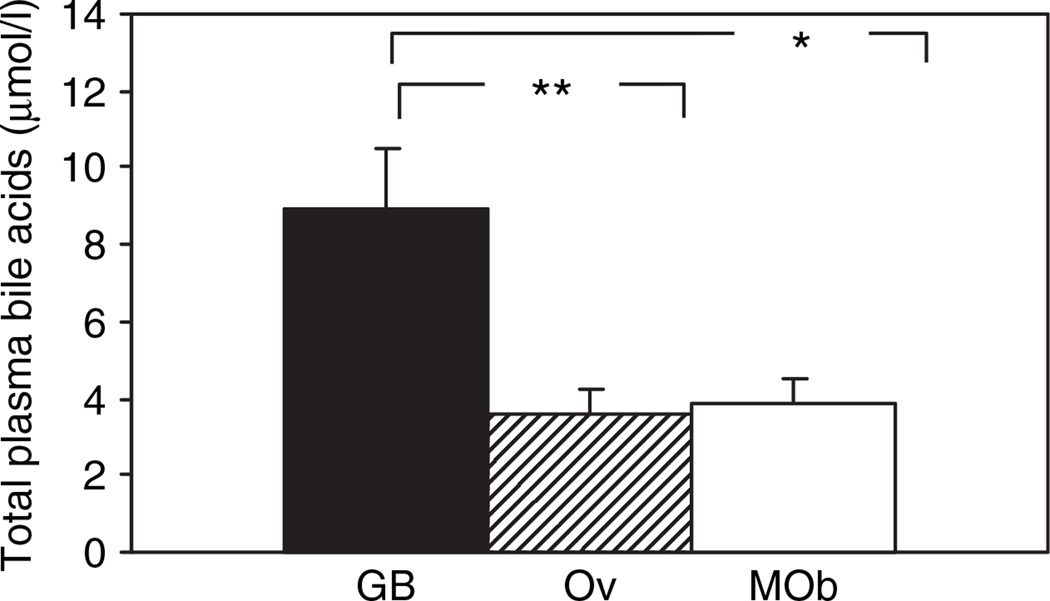
Total bile acids differed significantly between groups (P < 0.007, ANOVA) and were increased by 2.5-fold in post-bypass subjects compared with overweight subjects (P < 0.001), and by 2.3-fold compared with the severely obese group (P = 0.045) (Figure 1). Bile acid constituents were examined to determine which specific components accounted for the increase in total bile acids. Levels of taurodeoxycholic, glycocholic, glycochenodeoxycholic, and glycodeoxycholic acids differed across the three study groups in multivariate analysis (Supplementary Table S1, column 1, all P < 0.05), and were significantly increased in GB as compared with both overweight and severely obese subjects.
Figure 1.
Total bile acids are increased following Roux-en-Y gastric bypass surgery (GB) as compared with control subjects matched for postoperative BMI (overweight (Ov)) and for preoperative BMI (morbid obesity (MOb)). Data are presented as mean ± s.e. P (ANOVA) = 0.007. *Indicates P < 0.05, **P < 0.01 for comparison of GB vs. overweight.
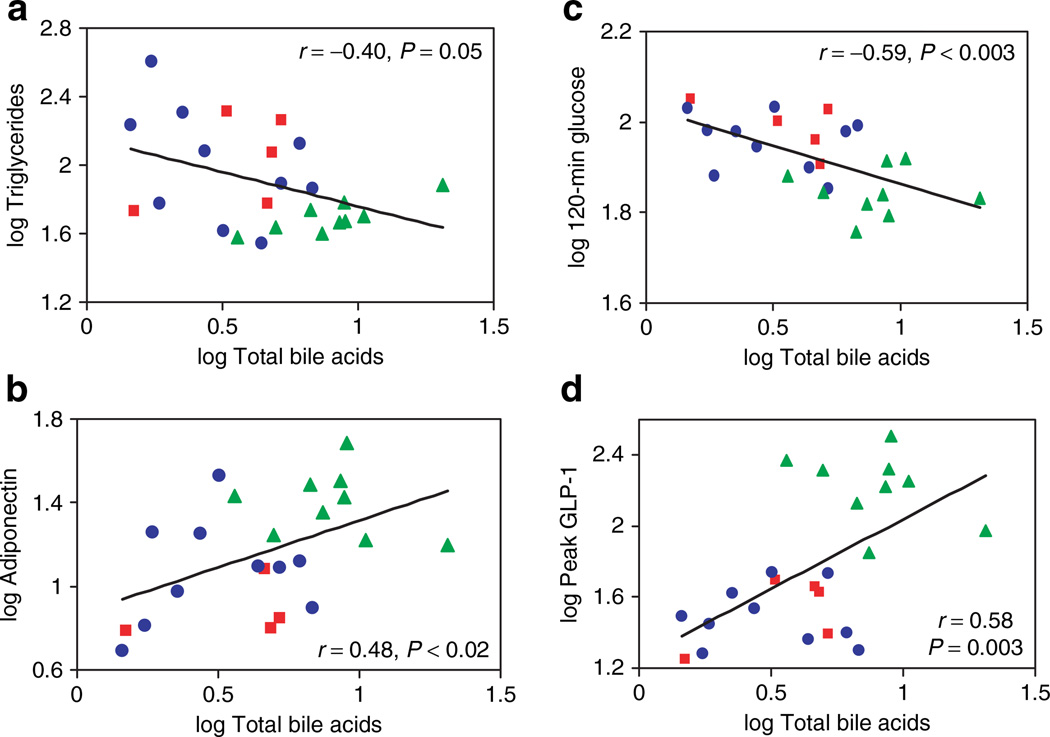
Bile acids play an established role in dietary lipid absorption and cholesterol homeostasis and are known ligands of the nuclear hormone receptor FXR. Increased transhepatic bile acid flux results in reduced triglycerides (18). In accord, we find that serum bile acid levels correlated inversely with triglycerides (r = −0.40, P = 0.05) (Figure 2a). Although HDL levels were significantly increased in GB patients, bile acid levels did not correlate significantly with HDL. Although FGF15/19 has been recently linked to regulation of bile acid synthesis (19), we found no significant differences in FGF15/19 between groups. Similarly, there were no differences in FGF21, a regulator of hepatic lipid metabolism (16) between groups and no correlation with bile acids (not shown).
Figure 2.
Linear regression relationships between serum bile acid levels and (a) fasting triglycerides, (b) plasma adiponectin, (c) glucose level at 2-h post-mixed meal, and (d) peak GLP-1 level post-mixed meal in all subject groups. Morbid obesity (MOb) subjects are designated by the red squares, overweight (Ov) by blue circles, and post-GB (GB) by the green triangles.
Because GB surgery is so effective in normalizing insulin sensitivity and reversing hyperglycemia, we assessed the relationship between serum bile acid levels and mediators of glucose homeostasis. Bile acid levels did not correlate with BMI, either in the entire cohort (r = 0.15, P = 0.5) or the nonsurgical subjects only (r = 0.08, P = 0.8). However, total bile acids correlated positively with plasma adiponectin, a marker of insulin sensitivity (r = 0.48, P < 0.02) (Figure 2b). Furthermore, total bile acid concentrations strongly and inversely correlated with 2-h post-meal glucose concentrations (r = −0.59, P < 0.003) (Figure 2c); similar trends were observed for hemoglobin A1c (r = −0.38, P = 0.07) and fasting glucose (r = −0.40, P < 0.06). Although there was a trend between total bile acid concentration with post-meal peak insulin concentrations (r = 0.38, P < 0.07), interestingly, there were robust correlations between bile acid levels and post-meal peak GLP-1 concentrations (r = 0.58, P < 0.003) (Figure 2d). Although the sample size limits full multiple regression analysis, we fit bivariate models to explore relationships between total bile acids, peak GLP-1, and 2-h post-meal glucose. Significant interactions were demonstrated for both peak GLP-1 (P = 0.03) and total bile acids (P < 0.05). However, when considering effects of adiponectin and total bile acids on 2-h post-meal glucose, only adiponectin remained significant (P = 0.01), and bile acids were only of borderline significance (P < 0.07).
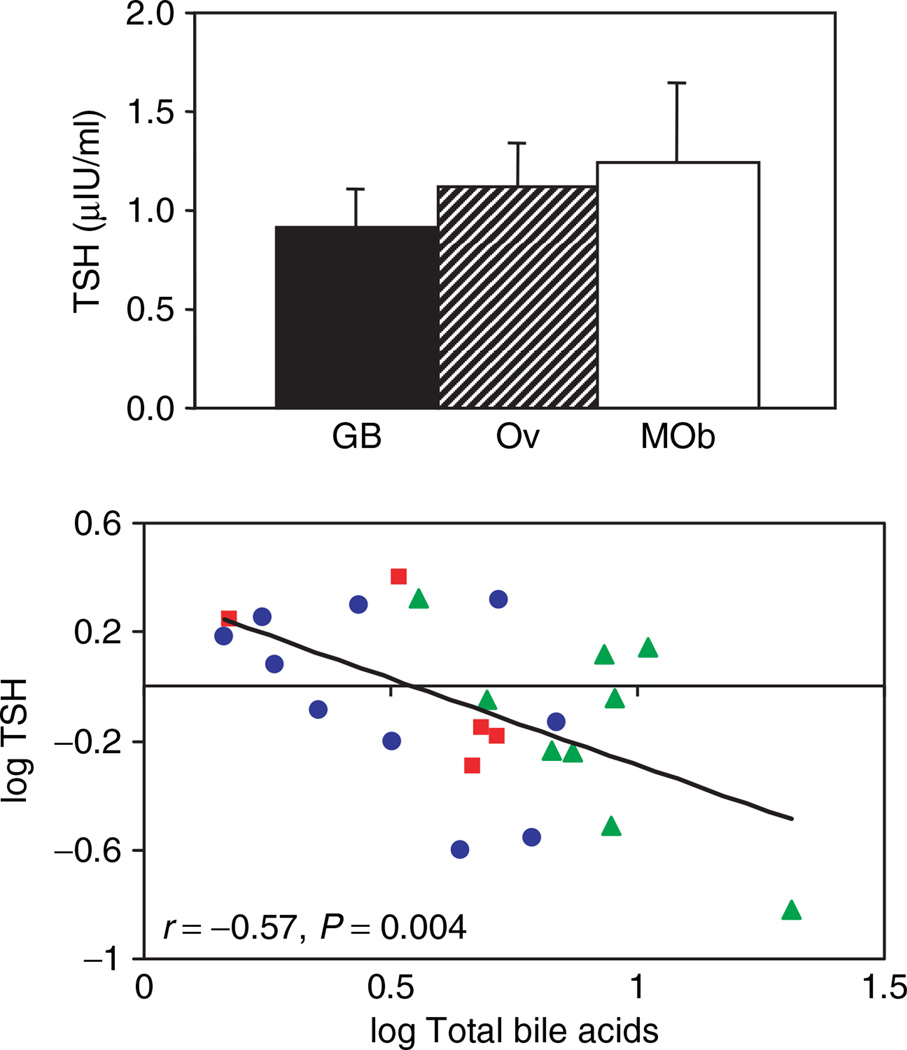
Metabolic effects of bile acids may also be mediated via TGR5-dependent pathways leading to increased intracellular type 2 deiodinase (D2) activity and consequently, to increased intracellular thyroid hormone availability (20). We therefore assessed serum thyroid hormone levels in post-bypass patients and controls. Total T4, T3, and the T4/T3 ratio did not differ between groups (not shown); similarly, thyrotropic hormone (TSH) was in the normative range for all participants and did not differ between groups (Figure 3a). However, TSH concentrations were strongly and inversely correlated with total bile acid levels (r = −0.57, P = 0.004) (Figure 3b).
Figure 3.
Potential links between bile acids and thyroid hormone action. (a) Thyrotropic hormone (TSH) levels do not differ in post-GB patients (GB) as compared with BMI-matched controls (morbid obese (MOb) and overweight (Ov)), but (b) correlate inversely with total bile acid levels. MOb subjects are designated by the red squares, Ov by blue circles, and post-GB by the green triangles.
Discussion
Despite wide implementation and therapeutic success of GB surgery, our understanding of mechanisms mediating improved metabolic homeostasis and long-term weight loss remains incomplete. Although postsurgical alterations in satiety, intestinal anatomy, neural signaling, secretion of gastrointestinal peptides, and nutrient absorption together contribute to weight loss and associated improvements in systemic metabolism, we propose that increased serum concentrations of bile acids may also contribute to improved carbohydrate, lipid, and energy metabolism post-GB.
We now demonstrate that total serum bile acid concentrations are more than twofold higher in individuals 2–4 years after GB surgery than in overweight or severely obese individuals who have not had bariatric surgery. This pattern of bile acid increase encompassed multiple subfractions, including both primary and secondary bile acids, and reached statistical significance for glycochenodeoxycholic (more than fourfold increase in this most abundant fraction), glycodeoxycholic, glycocholic, and taurodeoxycholic acids.
One limitation of our study is that we measured peripheral levels of bile acids. Serum bile acid levels are correlated with portal venous concentrations (21) but provide only surrogate information about the complex enterohepatic circulation of bile acids, including synthesis and secretion by the liver, both active and passive reabsorption from the small intestine (predominantly the terminal ileum), return to the liver via the portal vein, and efficient hepatic uptake. Although mechanisms responsible for increased serum bile acid levels cannot be determined from this study, the observed differential bile acid distribution, with prominent increases in both primary (e.g., glycochenodeoxycholic acid) and secondary (e.g., glycodeoxycholic acid) conjugated bile acids post-GB, is more suggestive of increased intestinal uptake of bile acids with resultant spillover into the systemic circulation, as absorption patterns differ for distinct bile acid species (22). Post-GB anatomic or functional adaptive changes, including altered dietary patterns, intestinal motility, mucosal hyperplasia, or gut flora, could each contribute to increased postprandial bile acid absorption. Although bile acid absorption has not been evaluated post-GB, it is interesting to note that absorption is enhanced in rats following ileal transposition, a procedure which also exposes ileum “prematurely” to nutrients and bile salts (23).
Increases in bile acid production or elimination could also contribute to the observed patterns in post-bypass subjects. We are unable to evaluate hepatic expression of CYP7A1, the rate-limiting enzyme of bile acid biosynthesis. Although we observed no significant differences in circulating FGF21, a mediator of hepatic lipid metabolism (16), or FGF15/19, a regulator of bile acid synthesis, released from the ileum in an FXR-dependent manner (24), we recognize that our sample size is small. Whereas cholecystectomy could also potentially alter bile acid synthesis (25), previous studies have found no significant effects of cholecystectomy on pool size in humans (26). In our cohort, it is unlikely that cholecystectomy was responsible for increased bile acids in post-GB subjects, as the frequency of cholecystectomy did not differ in post-GB compared to severely obese subjects, and we observed no differences in bile acids as a function of cholecystectomy status in these groups. Finally, we cannot exclude the possibility that hepatic bile acid elimination is altered in post-GB patients. Whereas conjugation is the dominant pathway, oxidative metabolism, via CYP3A4-dependent pathways, may play a minor role (27) and thus be potentially susceptible to CYP3A4-inhibitory effects of some selective serotonin reuptake inhibitor antidepressants. Although these medications were more common in post-GB patients, we observed no differences in bile acids in selective serotonin reuptake inhibitor treated vs. untreated post-GB subjects. To fully address all of these potential mechanisms, detailed analysis of prandial bile acid profiles and bile acid turnover will be required (24). Finally, as our study is cross-sectional, we cannot confirm that the concentrations of bile acids are changed by the surgery itself; future longitudinal studies will be important.
Given that bile acids are now recognized as signaling hormones with endocrine effects, it is interesting that total bile acid levels in the post-bypass state also correlated significantly with several key parameters of both lipid and glucose metabolism. It is not surprising that serum bile acid levels inversely relate to triglycerides—perhaps reflecting bile acid activation of hepatic FXR with inhibition of triglyceride synthesis (18,28)—or have direct effects to promote insulin signaling and glycogen synthase activation (29). More striking was the robust inverse correlation between bile acid levels and serum glucose at 2 h following mixed meal. The association between bile acids and markers of improved glucose metabolism in our study may likewise reflect GB-mediated improvements in insulin sensitivity (as suggested by the positive correlation with adiponectin), increased incretin-mediated insulin secretion (as indicated by the positive correlation with peak GLP-1), or a combination of these processes. It should also be noted that none of our subjects had diabetes before GB; thus, although insulin resistance and impaired incretin responsiveness are hallmarks of type 2 diabetes mellitus, we do not yet know whether similar patterns may contribute to GB-mediated resolution of type 2 diabetes.
Bile acids have recently been recognized as positive regulators of whole-body energy metabolism in rodents. Oral administration of cholic acid, a major bile acid constituent, increases bile acid pool size and serum bile acid levels, and in parallel prevents obesity, insulin resistance, and glucose intolerance during high-fat feeding (10). These effects of bile acids are largely mediated by bile acid activation of the TGR5 receptor and increased expression of D2 (refs. 10,20). D2 converts inactive thyroxine (T4) to the transcriptionally active triiodothyronine (T3), leading to increased thyroid hormone receptor transcriptional activation. Given the robust inverse correlation between bile acids and TSH in our clinically euthyroid population, we speculate that increased bile acids could also increase D2 activity and intracellular T3, and thus contribute to downregulation of TSH in thyrotroph cells. Similar activation of the TGR5-D2 pathway by cholic acid conjugates or other bile acids with affinity for the TGR5 receptor (30) in other tissues could, in turn, increase whole-body oxidative metabolism. Interestingly, a recent study demonstrated an inverse correlation between fasting insulin and postprandial elevations in glycochenodeoxycholic acid (11), a bile acid constituent we found to be robustly upregulated in GB patients. This concept is of particular interest as oxidative gene expression and function are impaired in humans with type 2 diabetes (31,32). Analysis of systemic energy expenditure, D2 activity, and expression of genes involved in muscle oxidative metabolism or energy homeostasis would be required to further test this hypothesis.
Bile acid activation of TGR5 may also be linked to secretion of GLP-1, a key incretin hormone regulating glucose-dependent insulin secretion. In the murine enteroendocrine cell line STC-1, lithocholic acid and deoxycholic acid both enhance GLP-1 secretion via TGR5 and cAMP-dependent mechanisms (33). Similarly, direct infusion of deoxycholate into the colon can also increase secretion of enteroglucagon (proglucagon-derived peptide products of the L cell including GLP-1) in humans (34). Animal studies of procedures that increase “premature” exposure of more distal intestine to bile acids such as internal biliary diversion (diversion of bile to the jejunum) (35), ileal transposition (surgical placement of ileum to a more proximal location), and GB, all improve glucose tolerance, perhaps via bile acid-mediated increases in enteroglucagon/ GLP-1 secretion (36,37). In this context, it is interesting that we find serum bile acid levels were highly correlated in humans in vivo with peak GLP-1 levels following mixed meal challenge. It will also be interesting to examine whether bile acids are similarly altered in humans who have undergone biliopancreatic diversion for obesity, a procedure also potentially altering nutrient and bile acid delivery to the distal gut and associated with improvements in systemic metabolism and resolution of type 2 diabetes (38).
We do not yet know whether a specific constituent of bile acids is largely responsible for these findings. Differing affinity of bile acid species for the TGR5 receptor (31) may confer endocrine signaling potency. A recent study demonstrated an inverse correlation between fasting insulin and postprandial elevations in glycochenodeoxycholic acid (11), suggesting that bile acid signaling may be related to insulin sensitivity in humans. Interestingly, this same bile acid constituent was robustly upregulated in our GB subjects.
Emerging data indicate that manipulation of bile acid levels therapeutically may also yield positive metabolic effects in humans. Bile acid sequestrants, which reduce enterohepatic circulation of bile acids, increase bile acid synthesis from cholesterol and thus lower serum cholesterol. In parallel, bile acid sequestrants also produce substantial improvements in glycemic control. For example, administration of colesevelam, a high-capacity bile acid binding polymer, to humans with type 2 diabetes reduced not only cholesterol and low-density lipoprotein, as expected, but also decreased hemoglobin A1c (12,13). In rodents, colestimide treatment prevents both diet-induced obesity and hyperglycemia (19). These effects of bile acid sequestrants may also be related to increased circulating levels of cholic acid, as demonstrated following cholestyramine administration (39), altered secretion of FGF15/19 (ref. 40), or reduced expression of small heterodimer partner (19). Whether bile acid sequestrants also enhance TGR5 activation and D2 activity, or incretin hormone secretion, in humans remains unknown.
In summary, we demonstrate that serum bile acid levels are over twofold higher in persons who previously had GB surgery, as compared to those without GB similar to either preoperative or current BMI. These effects were most prominent for glycochenodeoxycholic, glycodeoxycholic, glycocholic, and taurodeoxycholic acids. Moreover, bile acid concentrations correlate with key metabolic parameters, including inverse relationships with 2-h post-meal glucose, triglyceride, and TSH levels, and positive correlations with adiponectin and peak post-meal GLP-1 levels. Our current cross-sectional analysis cannot establish causality. For example, it is also possible that improved insulin sensitivity following bypass-induced weight loss itself contributes to a relative increase in bile acid synthesis and/or absorption. Nevertheless, these data in humans are consistent with rodent data suggesting increased serum bile acid levels may contribute to improvements in insulin sensitivity, incretin secretion, and postprandial glycemia. Further studies of glucose, lipid, and energy metabolism, as influenced by pharmacologic modulation of bile acids in humans, will be required to test these mechanisms.
Acknowledgments
Supported by NIH DK062948 (M.-E.P), DK060837 (M.-E.P and A.B.G), Juvenile Diabetes Research Foundation (M.-E.P and A.B.G), K23-DK02795 and American Diabetes Association Career Development Award 06-CD-07 (A.B.G), DK65055 (Bianco), Picower Institute (M.K.B and E.M.-F), M01 RR001032 (General Clinical Research Center), and DK36836 (Diabetes and Endocrinology Research Center, Joslin Diabetes Center). Dr Auwerx thanks CNRS, INSERM, Université Louis Pasteur and NIH (DK 067320) for grant support. Dr Holst has been supported by the Danish Medical Research Council and the Novo Nordisk Foundation. We appreciate the technical expertise of Henk Overmars, Lone Bagger Thielsen, Petr Jarolim, and assistance of Stanley Vance, and Rebecca Friedrichs.
Footnotes
Supplementary Material
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
Disclosure
The authors declared no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Metabolic and Bariatric Surgery. Fact Sheet: Metabolic and Bariatric Surgery. 2008 < http://www.asbs.org/Newsite07/media/fact-sheet1_bariatric-surgery.pdf>. [Google Scholar]
- 3.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 4.Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:153–158. doi: 10.1097/MED.0b013e3282f88a0a. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 6.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 7.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 8.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 11.Shaham O, Wei R, Wang TJ, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–1540. doi: 10.1001/archinte.168.14.1531. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–1484. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 15.Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 16.Badman MK, Pissios P, Kennedy AR, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Argmann CA, Houten SM, Champy MF, Auwerx J. Lipid and bile acid analysis. In: Albright LM, Borowsky ML, Coen DM, et al., editors. Current Protocols in Molecular Biology. John Wiley & Sons; 2006. pp. 29B.2.1–29B.2.24. [DOI] [PubMed] [Google Scholar]
- 18.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi M, Ikegami H, Fujisawa T, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56:239–247. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- 20.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–2579. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelin B, Einarsson K, Hellström K. Evidence for the absorption of bile acids in the proximal small intestine of normo- and hyperlipidaemic subjects. Gut. 1976;17:420–425. doi: 10.1136/gut.17.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol. 1996;271:G681–G691. doi: 10.1152/ajpgi.1996.271.4.G681. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Sauter GH, Moussavian AC, Meyer G, et al. Bowel habits and bile acid malabsorption in the months after cholecystectomy. Am J Gastroenterol. 2002;97:1732–1735. doi: 10.1111/j.1572-0241.2002.05779.x. [DOI] [PubMed] [Google Scholar]
- 26.Kullak-Ublick GA, Paumgartner G, Berr F. Long-term effects of cholecystectomy on bile acid metabolism. Hepatology. 1995;21:41–45. doi: 10.1002/hep.1840210109. [DOI] [PubMed] [Google Scholar]
- 27.Deo AK, Bandiera SM. Identification of human hepatic cytochrome p450 enzymes involved in the biotransformation of cholic and chenodeoxycholic acid. Drug Metab Dispos. 2008;36:1983–1991. doi: 10.1124/dmd.108.022194. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han SI, Studer E, Gupta S, et al. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology. 2004;39:456–463. doi: 10.1002/hep.20043. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Macchiarulo A, Thomas C, et al. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831–1841. doi: 10.1021/jm7015864. [DOI] [PubMed] [Google Scholar]
- 31.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 34.Adrian TE, Ballantyne GH, Longo WE, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–1224. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manfredini G, Ermini M, Scopsi L, Bonaguidi F, Ferrannini E. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol. 1985;249:G519–G527. doi: 10.1152/ajpgi.1985.249.4.G519. [DOI] [PubMed] [Google Scholar]
- 36.Ohneda A, Tsuchiya T, Naito H, et al. Increased plasma glucagon-like immunoreactivity in dogs with ileojejunal transposition. Tohoku J Exp Med. 1990;162:95–108. doi: 10.1620/tjem.162.95. [DOI] [PubMed] [Google Scholar]
- 37.Strader AD, Vahl TP, Jandacek RJ, et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 38.Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–2143. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 39.Angelin B, Björkhem I, Einarsson K, Ewerth S. Cholestyramine treatment reduces postprandial but not fasting serum bile acid levels in humans. Gastroenterology. 1982;83:1097–1101. [PubMed] [Google Scholar]
- 40.Lundasen T, Galman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–536. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]