Abstract
Thyroid hormone (TH) plays a key role on post-natal bone development and metabolism, while its relevance during fetal bone development is uncertain. To study this, pregnant mice were made hypothyroid and fetuses harvested at embryonic days (E) 12.5, 14.5, 16.5 and 18.5. Despite a marked reduction in fetal tissue concentration of both T4 and T3, bone development, as assessed at the distal epiphyseal growth plate of the femur and vertebra, was largely preserved up to E16.5. Only at E18.5, the hypothyroid fetuses exhibited a reduction in femoral type I and type X collagen and osteocalcin mRNA levels, in the length and area of the proliferative and hypertrophic zones, in the number of chondrocytes per proliferative column, and in the number of hypertrophic chondrocytes, in addition to a slight delay in endochondral and intramembranous ossification. This suggests that up to E16.5, thyroid hormone signaling in bone is kept to a minimum. In fact, measuring the expression level of the activating and inactivating iodothyronine deiodinases (D2 and D3) helped understand how this is achieved. D3 mRNA was readily detected as early as E14.5 and its expression decreased markedly (~ 10-fold) at E18.5, and even more at 14 days after birth (P14). In contrast, D2 mRNA expression increased significantly by E18.5 and markedly (~2.5-fold) by P14. The reciprocal expression levels of D2 and D3 genes during early bone development along with the absence of a hypothyroidism-induced bone phenotype at this time suggest that coordinated reciprocal deiodinase expression keeps thyroid hormone signaling in bone to very low levels at this early stage of bone development.
Keywords: Skeletal development, Congenital hypothyroidism, Deiodinase type 3, Deiodinase type 2, hyroid hormone
Introduction
Thyroid hormone (TH) is essential for a number of metabolic and developmental processes. Its actions occur through binding of triiodothyronine (T3), the main active form of TH, to its nuclear receptors (TRs), which function as hormone inducible transcriptional factors [1]. Inappropriate levels or premature exposure of embryos to TH are deleterious, resulting in abnormal development or death [2–5]. TH is classically known to be important for the development of the central nervous system, where its deficiency promotes severe neurological deficits [6]. In similar ways, bone development, maturation, growth and metabolism have been shown as fundamentally dependent on TH action. The identification of TRα1, TRα2 and TRβ1 in osteoblasts [7–10], osteoclasts [11,12] and chondrocytes [12,13], as well as the responsiveness of bone cells to TH when isolated in cell culture systems are evidence of a direct effect of T3 on bone tissue. Decreased availability of TH during post-natal development results in severe abnormalities in the epiphyseal growth plates (EGP), such as disorganization of the proliferative zone, impaired differentiation of hypertrophic chondrocytes and abnormal synthesis of extra cellular matrix [14–17], resulting in growth arrest and other abnormalities [16,18]. In addition, a generalized delay in endochondral and intramembranous ossification is observed [5,17,19].
The importance of TH to the pre-natal development of the skeleton is less clear. It is well established that during development serum thyroid hormone levels are low and tissue concentration of thyroid hormone can be modified by the iodothyronine deiodinases [20–22]. While the type II deiodinase (D2) activates T4 to T3, the type III deiodinase (D3) inactivates T4 to rT3 and T3 to T2 in a tissue- and time-specific fashion. In various developing structures such as the brain, cochlea, retina, and during tadpole metamorphosis, D2- and D3-controlled thyroid hormone signaling was shown to play an important role [6,20,22,23]. In bone related structures, D2 expression has been shown in multiple bone-derived cell lines such as primary calvaria osteoblastic cells, murine bone marrow-derived stromal ST2 cell line that has the phenotypes of osteoblast- and osteoclast-supporting cells [24], in the MC3T3-E1 mouse osteoblastic cell line, in organ cultures of fetal mouse tibias and ATDC5 cells, a mouse chondrogenic cell line [25,26]. In the developing chicken growth plate, Sonic Hedgehog (Shh)-induced ubiquitin-mediated D2 inactivation along with Dio3 induction creates a relative state of local hypothyroidism and PTHrP secretion that favors chondrocyte proliferation and delays chondrocyte differentiation [27]. A similar scenario was recently described in the skin, with Shh and the deiodinases playing a role in the balance between proliferation and differentiation of keratinocytes [28].
In the present study, we report that marked TH deficiency only minimally affects bone development until 16.5 day of embryonic life (E), indicating that TH signaling in skeleton is minimal up to this developmental stage. Accordingly, D3 expression is high at E14.5 and decreases markedly (~ 10-fold) subsequently while D2 expression profile is the opposite. The higher expression of D3 and the modest effects of TH deficiency in the skeleton until E16.5 suggest that maintaining low levels of TH is critical for early skeleton development.
Materials and methods
Animals and treatment
All experiments were performed under a protocol approved by the Committee of Animal Ethics of the Institute of Biomedical Sciences of the University of Sao Paulo (protocol number 018–29/02). Two-month old mice were obtained from our breeding colony and maintained under controlled conditions of light and temperature (12 h/12 h dark/ light cycle at 25 °C), and access to food and water was ad libitum. For all experiments, mice were bred in-house for 3 h at night and the presence of a vaginal plug was considered as day zero of embryonic development (E0). A total of 65 dams were randomly divided into 2 groups: euthyroid (EUT) and hypothyroid (HYPO). Hypothyroidism was induced by maintaining the pregnant animals on methimazole (MMI; 0,05%) from E1 to E5 and MMI (0,1%) and sodium perchlorate (P; 1%) from E6 to the day of sacrifice. Both drugs were added to the drinking water. The pregnant mice were anesthetized, bled and the fetuses were obtained at E12.5, E14.5, E16.5 and E18.5. Fetuses were dissected out from the uterus, washed in sterile PBS and placed on ice. Length and weight of the fetuses were measured using a digital calliper and an analytic balance, respectively. Some fetuses were frozen in liquid nitrogen and kept at −80 °C. Others were fixed in 95% ethanol or 10% formalin for morphological analysis. Care was taken to avoid the risk of contamination by maternal blood. The post-natal studies were performed at 2, 4, 7,14, 21 and 35 post-natal days (P). After birth, the litter was maintained with the mother in the same cage until the day of sacrifice. The neonatal hypothyroidism was also induced by adding MMl (0,1%) and P (1%) in the drinking water, as both drugs are secreted in the maternal milk.
Determination of T4 and T3 in plasma and tissues
We measured the fetal concentration of T4 and T3 before and after the onset of fetal thyroid activity in E12.5, E14.5, E16.5 and E18.5 fetuses from EUT and HYPO dams by radioimmunoassay (RIA). At the day of sacrifice, maternal blood was collected and the serum was separated by centrifugation and immediately frozen. The protocol used to access total T3 and T4 on fetal tissues was modified from Obregón et al. [29]. Briefly, the whole fetuses were crushed in a steel mortar and pestle set (Fisher Scientific International, Inc, Hampton, NH) precooled in dry ice. The crushed fetuses were transferred to pre-weighted microfuge tubes precooled in ice and 0.15 MNaCl/10−3 M PTU was added in a volume that doubled the sample weight. 10−3 M PTU was added to avoid artifactual deiodinations. The samples were homogenized, kept on ice (15 min) and centrifuged (30 min 2000 rpm, 4 °C). Supernatant aliquots were dried in a Speedvac® at 45 °C (Eppendorf, Hamburg, Germany) and resuspended in 80 µl (E12.5 and E14.5) or 200 µl (E16.5 and E18.5) of 0.15 M NaCl (fetus suspension). In a second set of experiments, animals on P2, P4, P7, P14, P21 and P35 from euthyroid and hypothyroid groups were killed, and the blood was collected and centrifuged for serum separation. The serum and fetus suspension was assayed for total T3 and T4 quantification. Total T4 and T3 levels were measured by commercial RIA kits (RIA-gnost T4 and RIA-gnost T3; CIS bio international, Schering AG, Germany).
Skeletal preparations and histology
Fetuses on E12.5, E14.5, E16.5 and E18.5 and mice on P2, P4, P7, P14, P21 and P35 from EUT and HYPO groups were measured and weighted immediately after euthanasia. For histological and whole mount preparations, mice were eviscerated and had the skin removed. After a 48 h fixation in 95% ethanol, specimens were stained with alcian blue for cartilage and alizarin red for calcified tissue as previously described [16]. For histological analysis, the samples were fixed in 10% formalin for 24 h–72 h at 4 °C. Fetuses on E18.5 and all the newborns were demineralized in 10% formalin and 10% formic acid solution at room temperature for 48 h. Samples were dehydrated and embedded in Paraplast (Oxford, St Louis, MO, USA). The fetuses of all ages studied were sectioned sagittally for analysis of vertebral column morphology. The vertebral column and right femur of five animals of each group were serially sectioned (5 µm) onto Poly-l-Lysine (EMS, Hatfield, PA, USA) coated slides, deparaffinized in xylene, rehydrated, and stained with alcian blue 8GX (pH 2,5), floxin and hematoxylin. The sections were photographed under light microscopy for analyses. Femurs were analyzed morphometrically. Morphometric measurements were performed using the image analyzer system Image-pro Plus (Media Cybernetics, Silver Spring, MD, USA).
Semi-quantitative real-time PCR
Under a stereomicroscope, femurs were dissected and then crushed in a steel mortar and pestle set (Fisher Scientific International, Inc, Hampton, NH) precooled in dry ice. The crushed bones were transferred to microfuge tubes precooled in ice and total RNA was extracted using Trizol (Invitrogen, Calbard, CA, USA) following the manufacturer’s instructions. Reverse transcription was performed with RevertAid-H-Minus M-MuLV Reverse Transcriptase (Fermentas, Hanover, MD, USA) and 1 µg of total RNA. The mRNA expression of TRα1, TRβ1, type X collagen (Col10), type I collagen (Col1), osteocalcin (OC), D1, D2 and D3 were determined by semi-quantitative real-time PCR as described previously [30,31]. mRNA levels of 18SrRNA (18S) and cyclophilin A (Cyclo A) were used as internal controls. The relative levels of mRNA of the tested genes were estimated by real-time PCR by measuring the fluorescence quantified with the ABI Prism 7500 sequence detector (Applied Biosystems) comparing all samples and controls in duplicates. The real-time PCR reactions were performed in a total volume of 25 µl containing different amounts of template and primers, depending on the gene evaluated. D2 and D3 relative expression analysis was performed using 50 ng of template and 300 nM of specific primers. For all the other genes evaluated in this study 20 ng of template and 450 nM of specific primers were used. All primers were designed with the aim of the Primer Express software (Invitrogen) and were synthesized (Integrated DNA Technologies, Coralville, IA, USA) with annealing temperature between 58 and 60 °C, 18 and 30 base pairs and keeping a percentage of 40–60% of G and C bases. The PCR amplifications were performed in duplicates with cDNA diluted in a reaction buffer containing SYBR Green PCR master mix (Applied Biosystems) and specific primers (forward and reverse) in the following cycling conditions: 1 cycle at 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All Ct values were normalized using an internal control (18S or Cyclo A mRNA) and the results were expressed as fold-induction relative to the expression of the control, the calibrator sample, arbitrarily set to 1 as previously described by Livak [30]. A complete list of primers used in this study is listed in Table 1.
Table 1.
Oligonucleotide primers
| Gene | Forward primer | Reverse primer | Gene bank # |
|---|---|---|---|
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | M11188 |
| CycloA | GCCGATGACGAGCCCTTG | TGCCGCCAGTGCCATTATG | NM008907.1 |
| Dio1 | CCACCTTCTTCAGCATCC | AGTCATCTACGAGTCTCTTG | NM007860 |
| Dio2 | TTCTCCAACTGCCTCTTCCTG | CCCATCAGCGGTCTTCTCC | NM010050 |
| Dio3 | CGCTCTCTGCTGCTTCAC | CTCCTCGCCTTCACTGTTG | NM172119 |
| COL10a1 | CCTTTCTGCTGCTAATGTTCTTGA | GCGAAGGCAACAGTCGCT | NM009925.3 |
| COL1a1 | GCGAAGGCAACAGTCGCT | CTTGGTGGTTTTGTATTCGATGAC | NM007742 |
| OC | CTCACAGATGCCAAGCCCA | CCAAGGTAGCGCCGGAGTCT | U11542 |
| TRα1 | GCTGTGCTGCTAATGTCAACAGA | GCCTCCTGACTCTTCTCGATCTT | NM178060 |
| TRβ1 | AAGCCACAGGGTACCACTATCG | GGAGACTnTCTGAATGGTTCTTCTAA | NM009380 |
Sequence of primers designed for evaluation of the relative mRNA expression by real-time PCR.
Determination of D2 and D3 activity
D2 and D3 activities were measured in the tibia and femur of fetuses of mice at E18.5. Accordingly to Gouveia et al. [[25]], femur and tibia were dissected out from frozen E18.5 fetuses (n = 6), crushed in a steel mortar and pestle set (Fisher Scientific International) precooled in dry ice, and sonicated in 100 µl of PE buffer (0.1 potassium phosphate, 1 mM EDTA, pH6.9) containing 0.25 sucrose and 10 mM dithiothreitol (DTT). As previously described by Christoffolete et al. [31] and Steinsapir et al. [32], with minor modifications, bone homogenates (15 µg of protein) were assayed for D2 deiodination of freshly purified 2 nM 125I–labeled T4, in the presence of 20 mM DTT and unlabeled T4 in 0.1 nM and 100 nM, a nonsaturating and saturating T4 concentration of D2 activity, respectively. The incubation was performed for 16 h at 37 °C At the end of the assay, 200 µl of horse serum was added, and protein was precipitated by the addition of 100 µl 50% trichloroacetic acid followed by centrifugation at 12,000 ×g for 3 min. Finally, 360 µl of the supernatant containing 125I generated was counted in a δ-counter (Cobra II; Packard, Meriden CT). Accordingly, to da-Silva et al. [33] and Christoffolete et al. [31], specific T4-to-T3 conversion by D2 was calculated by subtracting nonspecific deiodination (determined by the saturating concentration of T4 — 100 nM) from specific D2 deiodination (determined by the nonsaturating concentration of T4 — 0.1 nM). Deiodinase activity was expressed as femtomoles T4 per hour per milligram protein (fmol/h*mg protein) [20,34]. The assays consumed less than 70% of the substrate.
D3 deiodination of 125l-labeled T3 was performed with 4 µg bone homogenate in the presence of 0.1 nM unlabeled T3, 10 mM PTU and 100 nM DTT for 24 h at 37 °C. The samples were analyzed for D3 deiodinase products by high pressure liquid chromatography system (HPLC Waters 1525, Waters Corporation, MA, USA) [35]. The results were reported as femtomoles T3 per hour per milligram protein (fmol/h*mg protein).
Statistical analysis
Student’s t-test was used to determine significant differences between groups and was performed using Prism 3.0 (GraphPad Software, San Diego, CA, USA). Multiple comparisons were performed by ANOVA followed by the Student–Newman–Keuls test.
Results
Serum levels and whole body T4 and T3 concentrations in mice fetuses
HYPO dams showed significantly lower serum levels of T4 (62–89%) and T3 (61–91%) in all gestational ages evaluated, when compared to EUT group (Figs. 1A, B). In addition, HYPO dams had a prolonged gestation and reduction in litter size, due to fetal and neonatal mortality of approximately 30% (data not shown). Figs. 1C and D show the concentration of T4 and T3 in whole body homogenates of E12.5 to E18.5 fetuses. While T4 was undetectable in E12.5 EUT fetus homogenates (Fig. 1C), it reached the detection level at E14.5 and increased significantly until E18.5 (7-fold vs. E14.5). In the HYPO group, T4 levels were still undetectable at E14.5 and were significantly lower than in EUT fetuses at E16.5 (54%) and E18.5 (53%). T3 was also undetectable in EUT and HYPO fetuses at E12.5, and from E14.5 to E18.5, T3 was 66–90% lower in HYPO vs. EUT fetuses. Accordingly, serum levels of T3 and T4 were significantly lower in MM1 P-treated mice from P2 to P35 (Figs. 1E and F).
Fig. 1.
Concentrations of total T4 and T3 in the serum of dams and pups and in the whole body homogenates of the fetuses. (A and B) Maternal serum levels of T4 and T3, respectively. (C and D) Fetal whole body concentrations of T4 and T3, respectively. (E and F) Post-natal serum levels of T4 and T3, respectively. Maternal serum levels and fetal concentrations of T4 and T3 were determined on the following gestational days: 12.5, 14.5, 16.5 and 18.5. Post-natal serum levels were determined at 2, 4, 7, 14, 21 and 35 days after birth. Hypothyroidism (HYPO) was induced by adding sodium perchlorate and methimazole to the drinking water of the dams. Euthyroid (EUT) dams, fetuses and pups are untreated animals. Values represent the mean±SEM (n = 4–12). *p<0.05, ** p<0.01 and ***p<0.001 vs. EUT by Student t-test.
Morphological analysis
Crown–rump length increased progressively from E12.5 to E18.5 in all fetuses, but those fetuses from HYPO dams had significant shorter crown–rump length (6–13%) and weight (9–26%) than those from EUT dams (Figs. 2A and B, respectively). In addition, it is noteworthy that after birth, especially from P7 to P35, the increase in body length and weight were higher in EUT than in HYPO mice. The measurement of the length of the humerus, femur and tibia (Figs. 2C–E) showed that differences between EUT and HYPO groups were observed only from E18.5 on, and that these differences increased after birth, from 6 to 16% at P2 to 25 to 30 % at P35. This indicates that the growth delay caused by TH deficiency occurs almost exclusively after birth.
Fig. 2.
Effect of hypothyroidism on body length and weight during pre- and post-natal development. (A) Crown–rump length, (B) weight and (C, D and E) mean length of humerus, femur and tibia, respectively, from E12.5 to P35. Results are expressed as mean±SEM of 13–45 samples. *p<0.05, **p<0.01 and ***p<0.001 vs. EUT by Student t-test.
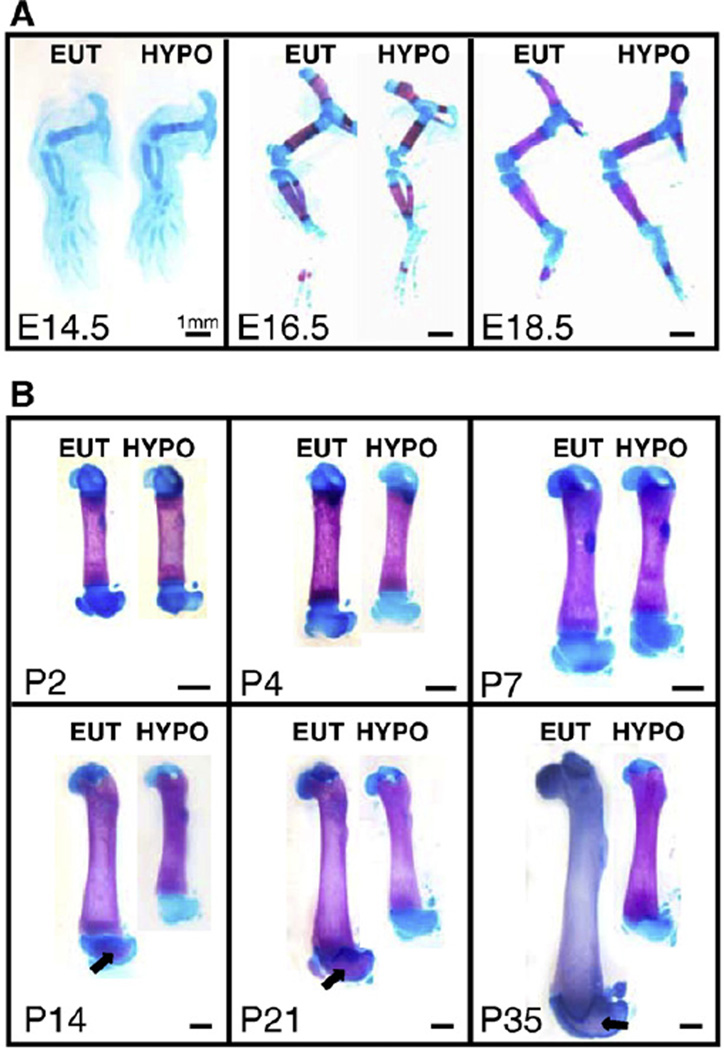
The evaluation of ossification by alcian blue and alizarin red staining shows slight but detectable differences between EUT and HYPO groups regarding the intramembranous ossification. More precisely, the closure of the anterior and posterior fontanelles and the ossification of the squamosal and sagittal suture are delayed in the HYPO mice at E18.5, but not at E16.5 (Fig. 3A). On the other hand, this analysis did not show clear differences in endochondral ossification throughout the skeleton between fetuses from EUT and HYPO groups. As can be observed in Fig. 4A, no differences of ossification could be detected in the hind limbs of E14.5, E16.5 and E18.5 fetuses. As expected, hypothyroidism resulted in an important delay in intramembranous and endochondral ossification during post-natal development (Figs. 3B and 4B). In the femur, for example, there is a clear delay in the secondary ossification from P14 to P35 (Fig. 4B).
Fig. 3.
Intramembranous ossification during pre- and post-natal development. Analysis of intramembranous ossification by alizarin red and alcian blue staining in the (A) fetal skull (1,2× magnification) and in the (B) skull of mice on the 14th post-natal day (0,8× magnification). (C) Diagram of the anatomy of the skull bones, sutures and fontanelles. EUT: euthyroid mice; HYPO: hypothyroid mice; E: embryonic age; P: post-natal days; PI: posterolateral fontanelle; Sq: squamosal suture; arrow: delayed closure of sutures; asterisk: delayed closure of fontanelles.
Fig. 4.
Endochondral ossification during pre- and post-natal development. Analysis of endochondral ossification by alizarin red and alcian blue staining in the (A) fetal hind limb and in the (B) femur of mice during the post-natal development. EUT: euthyroid mice; HYPO: hypothyroid mice; E: embryonic age; P: post-natal days; arrow: secondary ossification. Scale bar: 1 mm for all.
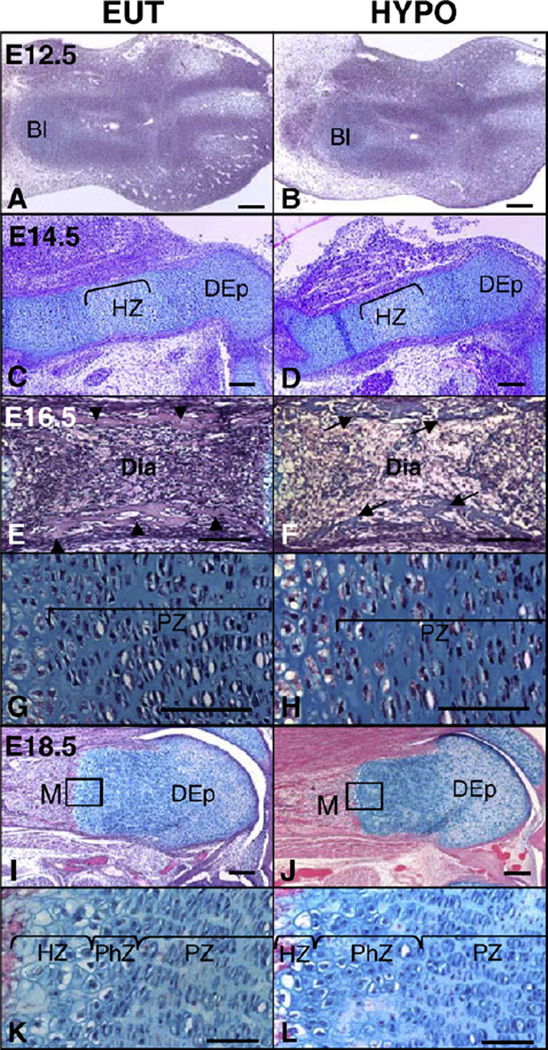
To further evaluate the effects of hypothyroidism on the fetal development of the skeleton, we analyzed the morphology of the developing vertebra and femur at E12.5, E14.5, E16.5 and E18.5 (Figs. 5–6, respectively). It is noticeable that at E12.5 and E14.5 there were no clear morphological differences in these developing skeletal sites in fetuses from HYPO and EUT dams (Figs. 5A–D; Figs. 6A–D), except for slight differences observed in the spine of E14.5 fetuses. At this embryonic age, the vertebral bodies were found to be well developed and the chondrocytes were distinctly organized in two different regions in the EUT fetuses (Fig. 5C). In the central region of the vertebral body, the chondrocytes were randomly distributed, whereas around the notochord, at the extremities of the vertebral body, chondrocytes were more condensed, which was not observed in the HYPO fetuses (Fig. 5D). At E16.5, mild differences in the morphology of the spine and femur were observed between EUT and HYPO fetuses. Both groups presented well developed vertebral bodies with distinguished hypertrophic and proliferative zones (Figs. 5E and F). The only difference between the two groups was a slight disorganization in the proliferative zone in HYPO fetuses (Fig. 5F). In the femur, both groups presented primary ossification and well established reserve, proliferative and hypertrophic zones (Figs. 6E–H). However, through this analysis, we could see that the ossification was slightly delayed by hypothyroidism, as evidenced by the presence of cartilaginous matrix in the diaphysis of HYPO fetuses (Figs. 6E and F–>). Additionally, the HYPO group, showed a statistically significant decrease in the number of hypertrophic chondrocytes, compared to EUT, and disorganization of the proliferative columns (Table 2 and Figs. 6G and H).
Fig. 5.
Effect of hypothyroidism on the vertebral morphology during pre-natal development (A and B) Mouse sample of fetus at embryonic day (E) 12.5, (C and D) E14.5, (E and F) E16.5 and (G and H) E18.5. Samples from (A, C, E and G) euthyroid (EUT) and (B, D, F and H) hypothyroid (HYPO) fetuses. as: anterior sclerotome; ps: posterior sclerotome; n: notochord; V: vertebral body; PZ: proliferative zone; HZ: hypertrophic zone; O: ossification center; arrow: cartilage extracellular matrix. Scale bar: 50 µm for all.
Fig. 6.
Effect of hypothyroidism on the femoral morphology during pre-natal development. (A and B) Mouse sample of fetus at embryonic day (E) 12.5, (C and D) E14.5, (E–H) E16.5 and (I–L) E18.5. Samples from (A, C, E, G, I and K) euthyroid (EUT) and (B, D, F, H, J and L) hypothyroid (HYPO) fetuses. K and L are details from I and J, respectively. Bl: blastema of the femur. HZ: hypertrophic zone; DEp: distal epiphysis; Dia: diaphysis; PZ: proliferative zone; M: metaphysis; PhZ: pre-hypertrophic zone; arrow head: cortical bone; arrow: cartilage extracellular matrix. Scale bar: 100 µm (A, B, C, D, I and J) and 50 µm (E, F, G, H, K and L).
Table 2.
Morphometry of distal EGP from fetal femur
| E16.5 |
E18.5 |
|||
|---|---|---|---|---|
| EUT | HYPO | EUT | HYPO | |
| PZ | ||||
| Area (mm2) | 124.1±1.7 | 107.9±12.4 | 175.7±12.3 | 147.0±9.5* |
| Length (mm) | 286.0±50.2 | 252.3±54.0 | 352.1±19.8 | 315.3±7.8* |
| 1PC/column | +//0 | +//0 | 10.6±1.1 | 7.8±0.8* |
| HZ | ||||
| Area (mm2) | 78.5±8.3 | 72.3±3.4 | 70.1±11.9 | 53.7±5.9* |
| Length (mm) | 174.5±4.8 | 167.9±7.7 | 141.8±5.4 | 136.2±5.2 |
| 1HC | 104.9±2.6 | 91.5±5.4* | 99.6±7.4 | 84.8±6.8* |
Measurements of distal EGP from E16.5 and E18.5 fetal femur. EUT: euthyroid; HYPO: hypothyroid; PZ: proliferative zone; HZ: hypertrophic zone; 1PC: number of proliferative chondrocytes; 1HC: number of hypertrophic chondrocytes. +//0: absence of proliferative columns. The results are expressed as mean±SEM, n=3–5.
p<0.05 by Student t-test.
At E18.5, more clear differences in the morphology of the femur and spine were observed between EUT and HYPO fetuses. At this fetal age, both groups presented developed primary ossification centers in the femur and vertebra (Figs. 5G and H; Figs. 6I–L). However, whereas residual cartilage extracellular matrix was notable in the vertebra of HYPO fetuses, it was barely observed in EUT fetuses (Figs. 5G and H). Moreover, HYPO fetuses presented a moderate disorganization in the proliferative zones of the femur and vertebra when compared to EUT fetuses. The morphometric analysis of the femur showed that the area, longitudinal length and number of chondrocytes per column in the proliferative zone were significantly lower in the HYPO vs. EUT group (p<0.05). In addition, the area of the hypertrophic zone and the number of hypertrophic chondrocytes were significantly decreased in HYPO vs. EUT fetuses (Table 2) (p<0.05). Fig. 7 illustrates the expected and striking effects of TH deficiency on the femur during post-natal development, from P7 to P35, which includes an important delay in the endochondral ossification, a reduction of the hypertrophic zone and a clear disorganization of the proliferative zone of the EGP (Fig. 7).
Fig. 7.
Effect of hypothyroidism on the femoral morphology during post-natal development (A–D) Pups on the 7th post-natal day (P). (E–H) P14 pups. (I–L) P35 mice. The squares at G, H, K and L indicate the area of the detail in each picture. PZ: proliferative zone; HZ: hypertrophic zone; EGP: epiphyseal growth plate; arrow: developed secondary ossification center; arrow head: beginning of secondary ossification. Scale bar: 100 µm (A–D; G, H, K and L) and 200 µm (E, F, I and J).
The mRNA expression of Col1, OC Col10 and TRs in the fetal skeleton
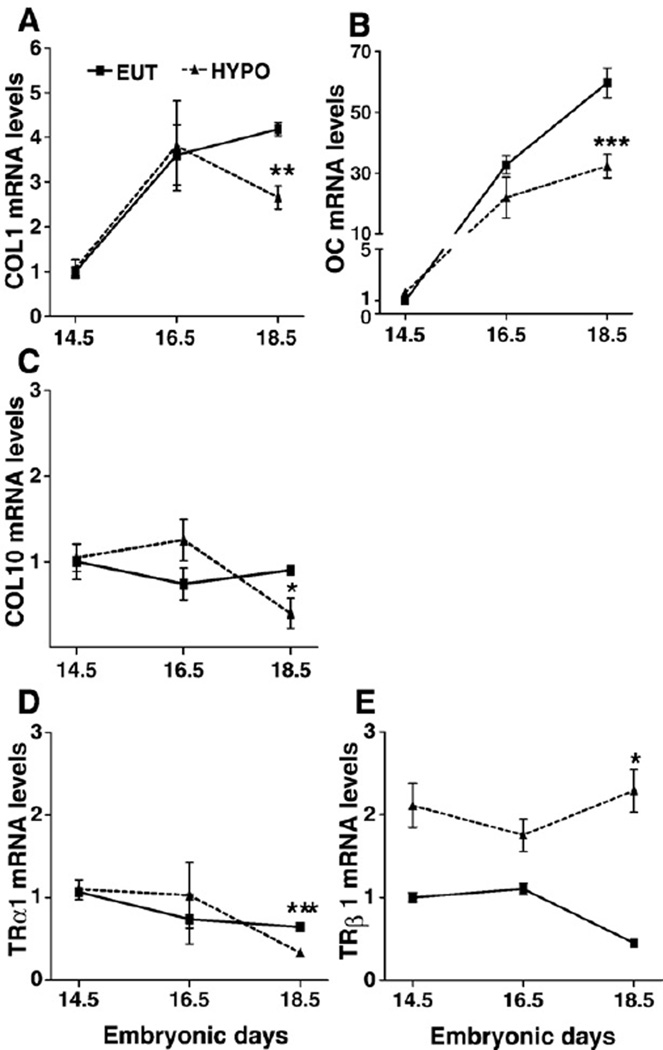
In order to better characterize the effects of TH on the pre-natal development of the skeleton, we also evaluated the expression of two markers of osteoblast phenotype, Col1 and OC; a marker of chondrocyte differentiation, Col10; and the expression of TRα1 and TRβ1 in the femur during fetal life. Due to technical limitations, E12.5 fetuses were excluded. The relative mRNA expression of Col1 and OC in the femur increased as a function of fetal development in EUT and HYPO fetuses (Figs. 8A and B, respectively). Col1 and OC expression increased 3.6 (p<0.01) and 32 (p<0.001) times from E14.5 to E16.5. From E16.5 to E18.5, Col1 expression did not change and the OC expression increased 1.9-fold (p < 0.001) in EUT fetuses. Col10 expression did not change as a function of development from E14.5 to E18.5 (Fig. 8C). It is interesting to note that TH deficiency significantly decreased the expression of Col1, Col10 and OC only at E18.5 (Figs. 8A–C). TRα1 and TRβ1 were shown to be expressed in the femur in all fetal ages studied, from E14.5 to E18.5, and their expressions were not dependent on the developmental stage or thyroid status until E16.5. At E18.5, however, TRβ1 expression was significantly reduced and TRβ1 expression was significantly increased in TH deficient fetuses (Figs. 8D and E).
Fig. 8.
Relative mRNA expression of collagen type 1 (Col1), osteocalcin (OC), collagen type X (Col10), TRαl and TRβ1 in the femur of fetuses. mRNA expression of (A) Col1, (B) OC, (C) Col10, (D) TRα1, and (E) TRβ1. The mRNA expression was analyzed by real-time PCR and the relative gene expression was determined by designating EUT group expression at E14.5 to 1. E: embryonic days. Values are the mean±SEM of 3–5 samples. *p<0.05, ** p<0.01, ***p<0.001 vs. EUT by Student t-test.
Relative expression of Dio2 and Dio3 mRNA in bone tissue
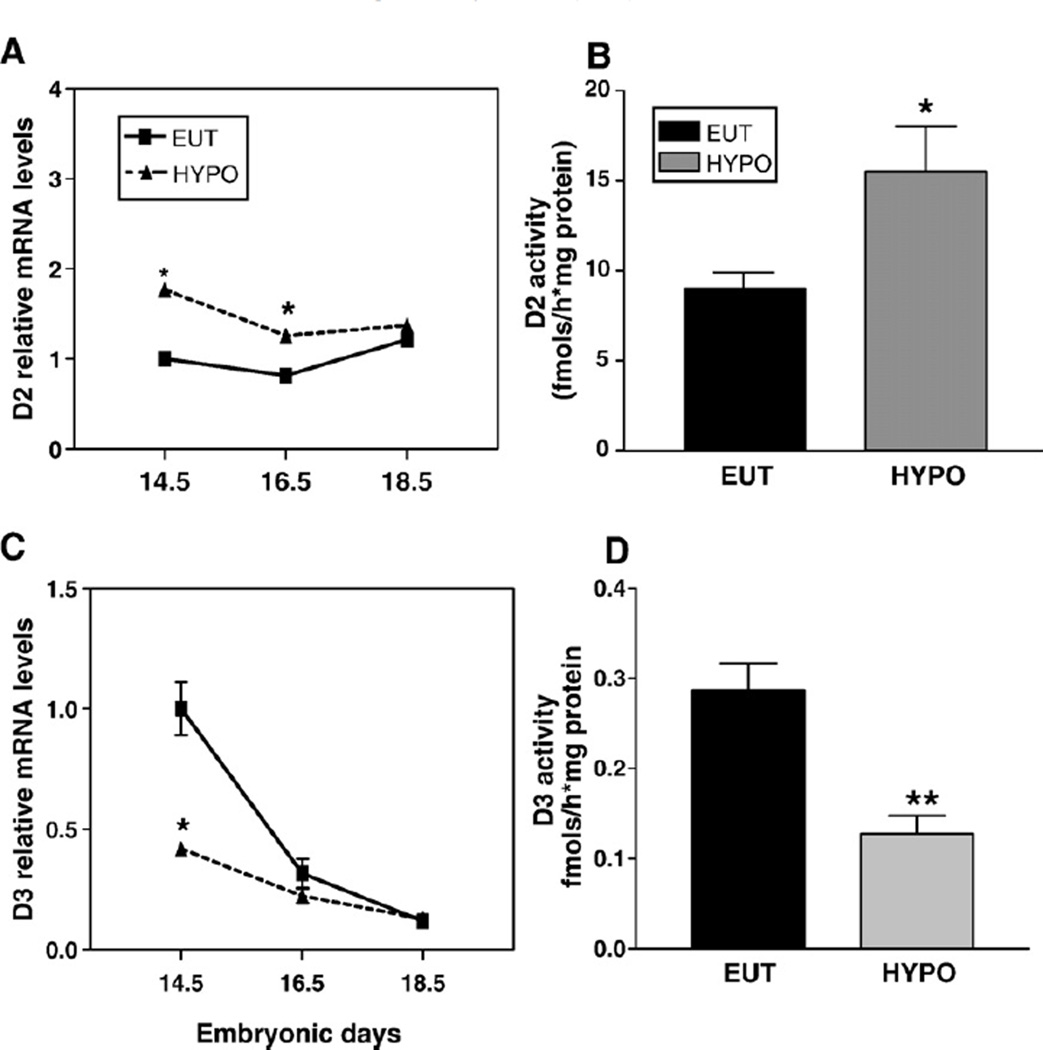
The relative mRNA expression of D2 and D3 was measured using real-time PCR in the femur during the pre- and post-natal development of mice (Figs. 9A and B, respectively). Fig. 9A shows a marked and gradual decrease in D3 expression during E14.5 to E18.5, when D3 mRNA levels decreased 10-fold. The decrease in D3 expression continued during post-natal development: D3 expression decreased 3-fold from P4 to P14, reaching undetectable levels at P35 (Fig. 9B). D2 mRNA showed a stable expression during pre- and post-natal development, while reaching its highest expression levels at P14 (~3-fold). Fig. 10 shows the modulation of deiodinases mRNA expression by thyroid status. The relative expression of D2 was significantly increased at E14.5 (1.7-fold; p<0.05) and E16.5 (1.6-fold; p<0.05) in HYPO vs. EUT fetuses but not at E18.5 (Fig. 10A). D3 expression was decreased by hypothyroidism at E14.5 (2.5-fold; p<0.05), but was not different from EUT group at E16.5 and E18.5 (Fig. 10C).
Fig. 9.
D2 and D3 mRNA relative expression pattern in the femur during pre and post-natal development (A) Fetuses at embryonic days 14.5, 16.5 and 18.5 and (B) mice at the post-natal days (P) 4, 7, 14 and 35. The mRNA expression was analyzed by real-time PCR and the relative gene expression was determined by designating the EUT group expression at E14.5 to 1 for each gene, separately. The values are the mean±SEM (n = 5). ap<0.05, E14.5 vs. E16.5; bp<0.05, E14.5 vs. E18.5; cp<0.01, E18.5 vs. all fetal ages; dp<0.05, P4 vs. P7; ep<0.01, P14 vs. all post-natal ages.
Fig. 10.
Effect of thyroid status on D2 and D3 mRNA expression and activity in the femur of mice during pre-natal development. (A) D2 mRNA expression. (B) D2 activity. (C) D3 mRNA expression. (C) D3 activity. The mRNA expression was analyzed by real-time PCR and the relative gene expression was determined by designating the EUT group expression at E14.5 to 1 (A and C). D2 and D3 enzyme activities were measured in the bones of E 18.5 fetus. E: embryonic days. Values represent the mean ± SEM of determinations on three independent tissue preparations. *p<0.05, **p<0.01 vs. EUT by Student t-test.
D2 and D3 activity in bone tissue
D2 and D3 activities were measured in the femur of E18.5 fetuses. Hypothyroidism resulted in a significant increase in D2 activity (70% vs. EUT) while D3 activity was reduced (52% vs. EUT). These changes in deiodinase activity took place even though their mRNA levels at this fetal age were not significantly affected by changes in thyroid status (Figs. 10B and D), indicating post-translational regulation of enzyme activity.
Discussion
Thyroid hormone deficiency after birth severely impairs bone growth and maturation [15,16,18]. While in human newborns with congenital hypothyroidism (CHT), the absence of secondary ossification is one of the hallmarks exhibited at birth [36], the importance of TH during the pre-natal development of the skeleton is not well established. We attempted to clarify this question by inducing maternal and fetal hypothyroidism (congenital) and evaluating the morphological aspects of bone development as well as the expression of genes related to bone maturation. Our data indicate that early fetal bone development takes place while thyroid hormone signaling is minimal, which is achieved at least in part via coordinated reciprocal expression of the activating and inactivating deiodinases.
Both methimazole (MMI) and sodium perchlorate (P) used in the present study cross the placental barrier and impair fetal thyroid function [37,38]. The reduced maternal serum levels and fetal concentrations of T3 and T4 in MMI P-treated animals (Fig. 1) confirm that this model of CHT was effective. That T3 and T4 were not detectable at E12.5 in both groups and that T4 was not detectable at E14.5 in HYPO animals can be explained by the sensitivity of the detection assay, i.e. 1 ng/ml for T4 and 0.01 ng/ml for T3. There is evidence that both T3 and T4 are present at very low levels in embryos before the onset of the fetal thyroid gland activity, that occurs at E16.5 to E17.5 in mice [39], and that both have maternal origin [4,40]. In fact, more sensitive RIAs were able to detect T3 and T4 in several E9 rat embryonic tissues in rats [3,6,40–43],
The crown–rump length and weight of fetuses were significantly reduced by hypothyroidism, but not severely impaired. In fact, the longitudinal growth and weight gain during fetal development did not differ between EUT and HYPO groups (Figs. 2A and B). TH deficiency leads to a reduction in the length of the humerus, femur and tibia (Figs. 2C, D and E), but only at the end of fetal development (at E18.5). The same pattern was observed in the morphological analysis of the femur and vertebra. They showed that hypothyroidism only mildly affected the fetal skeleton up to E16.5, but increasing bone hypothyroid-induced alterations were obvious between E18.5 and P35 (Figs. 5–7). From E12.5 to E16.5, slight morphological defects were detected in vertebra and femur of HYPO fetuses, compared to EUT fetuses (Figs. 5A–D). At E16.5, the exposure of fetuses to low levels of TH barely delayed endochondral ossification, reduced the number of hypertrophic chondrocytes and affected negatively the organization of the proliferative columns in the vertebral EGPs (Figs. 5E and F) and femur (Figs. 6E–H; Table 2). At E18.5, which is the end of the gestational period, alterations in the morphology of the femur and spine were clearly observed in HYPO fetuses. Not only was the number of hypertrophic chondrocytes reduced, but also the hypertrophic zone area, the number of proliferative chondrocytes per column and the area and length of the proliferative zone (Figs. 5G and H; Figs. 6I–L; Table 2). Notably, low levels of TH only significantly decreased the mRNA expression of Col1 and OC, two markers of osteoblast phenotype, and Col10, a marker of chondrocyte differentiation, at E18.5, respectively (Fig. 8). This is in agreement with the more evident morphological alterations of the skeleton at the end of the fetal development. In spite of the presence of TRs (TRα1 and TRβ1) in the skeleton of all fetal ages evaluated (E14.5 to E18.5), it is noteworthy that the hypothyroidism-induced defects in fetal bone morphology and gene expression profile of OC, Col1 and Col10 were only clearly detected at late gestation, at E18.5. This strongly suggests that TH signaling assumes a more important role in the skeletal development only at the end of the fetal life and during the post-natal development, when the expected and striking effects of TH deficiency were easily identified (Figs. 4 and 7).
These observations are partially supported by studies of different TR knockout (KO) mice models that present a bone phenotype of hypothyroidism. These animals commonly do not show reduction in the crown–rump length and a delay in bone development at late gestation and/or at birth, and their bone phenotype becomes more severe and evident during post-natal development [5,17,19]. Interestingly, a TR knock-in mouse model of resistance to thyroid hormone that presents a skeletal specific congenital hyperthyroidism shows a very distinct phenotype. These mice present a severe reduction in body length and weight at E17.5 due to advanced growth in uterus and massively advanced endochondral and intramenbranous ossification. After birth, the advanced bone age is identified by post-natal growth retardation, premature closure of the growth plates, with increased bone mineralization and craniosynostosis [5,19]. Taken together, these findings support our hypothesis that TH is more involved with the late gestation and post-natal bone development. They also suggest that decreased TH signaling is a normal mechanism during early fetal bone development, given that increased levels of TH seem to be more deleterious than its deficiency during fetal bone development [5,19].
D3, which inactivates T4 and T3, exhibited the highest mRNA expression at E14.5 and this expression decreased approximately 5-fold by E16.5 and 3-fold more between E16.5 and E18.5. The high expression of D3 and the lack of effects of thyroid hormone deficiency on bone at E14.5 also suggest that low levels of T3 are needed at this stage for normal development. Thus, D3 activity is likely to have an essential role during fetal skeleton development, keeping T3 levels to a minimum in the bone tissue. Our observations corroborate other studies that illustrate the role played by D3 in different fetal tissues and not only in the uteroplacental unit [20,44–46]. In fact, mice with targeted disruption of the D3 gene (Dio3) have severe growth retardation and impaired fertility and viability [40]. On the other hand, the ~ 10-fold decrease in D3 expression observed between E14.5 and E18.5, associated with the first evidence of hypothyroidism-induced defects in the skeleton during this period, and the increasing concentrations of T3 and T4 during fetal development, suggest a progressively more important role of thyroid hormone with the advancement of fetal age. It is also interesting to note that between P4 and P35, when thyroid hormone assumes an essential role for skeletal development, D3 expression decreases significantly (~3-fold), not even being detected at P35. This takes place while D2 expression is detected in all post-natal ages studied and is significantly increased, particularly at P14 (~3-fold), which is the period of widespread secondary ossification (Fig. 9B). Remarkably, the femoral mRNA expression of both D2 and D3 were regulated by TH status (Fig. 10).
In summary, we showed that severe TH deficiency only minimally affects bone development until E16.5. The expression pattern of D3 and the lack of effects of TH deficiency in the skeleton before this fetal stage, indicate that thyroid hormone signaling during fetal bone development is kept to a minimum thanks to D3 expression. While D3 expression decreases during E16.5 and E18.5, increased thyroid hormone signaling seems to be more critical, as hypothyroid-induced morphological defects can be observed. Moreover, D2 mRNA expression increases to reach much higher levels at post-natal ages, which highlights the importance of D2 in the local regulation of thyroid hormone signaling during skeleton development. To our knowledge, this is the first in vivo demonstration that D2 and D3 mRNA exhibit a temporal expression pattern in the fetal bone. This supports the concept that both deiodinases are likely to have an important role during pre-natal development of the skeleton, fine tuning the appropriate skeletal levels of TH that ensure normal fetal bone development.
Acknowledgments
We thank Dr. Anselmo Sigari Moriscot for helping to establish the dissection technique of the fetuses and Alessandra Crescenzi, Michele Mulcahey, Wagner Seixas da Silva and Marcelo Christoffolete for the assistance in the D3 and D2 activity determinations. This work was supported by a grant (CHAG) from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (04/01833-7). EHB and LPC were recipients of fellowships from CAPES and FAPESP (03/07327-3), respectively.
References
- 1.Brent GA, Moore DD, Larsen PR. Thyroid hormone regulation of gene expression. Annu Rev Physiol. 1991;53:17–35. doi: 10.1146/annurev.ph.53.030191.000313. [DOI] [PubMed] [Google Scholar]
- 2.Morreale de Escobar G, Obregon MJ, Calvo R, Escobar del Rey F. Maternal thyroid hormones during pregnancy: effects on the fetus in congenital hypothyroidism and in iodine deficiency. Adv Exp Med Biol. 1991;299:133–156. doi: 10.1007/978-1-4684-5973-9_6. [DOI] [PubMed] [Google Scholar]
- 3.Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Maternal-fetal thyroid hormone relationships and the fetal brain. Acta Med Austriaca. 1988;15(Suppl 1):66–70. [PubMed] [Google Scholar]
- 4.Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci U S A. 2004;101:3474–3479. doi: 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14:356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 6.Galton VA. The roles of the iodothyronine deiodinases in mammalian development. Thyroid. 2005;15:823–334. doi: 10.1089/thy.2005.15.823. [DOI] [PubMed] [Google Scholar]
- 7.Rizzoli R, von Tscharner V, Fleisch H. Increase of adenylate cyclase catalytic-unit activity by dexamethasone in rat osteoblast-like cells. Biochem J. 1986;237:447–454. doi: 10.1042/bj2370447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato K, Han DC, Fujii Y, Tsushima T, Shizume K. Thyroid hormone stimulates alkaline phosphatase activity in cultured rat osteoblastic cells (ROS 17/2.8) through 3,5,3 c-triiodo L-thyronine nuclear receptors. Endocrinology. 1987;120:1873–1881. doi: 10.1210/endo-120-5-1873. [DOI] [PubMed] [Google Scholar]
- 9.LeBron BA, Pekary AE, Mirell C, Hahn TJ, Hershman JM. Thyroid hormone 5 ‘-deiodinase activity, nuclear binding, and effects on mitogenesis in UMR-106 osteoblastic osteosarcoma cells. J Bone Miner Res. 1989;4:173–178. doi: 10.1002/jbmr.5650040207. [DOI] [PubMed] [Google Scholar]
- 10.Kasono K, Sato K, Han DC, Fujii Y, Tsushima T, Shizume K. Stimulation of alkaline phosphatase activity by thyroid hormone in mouse osteoblast-like cells (MC3T3-E1): a possible mechanism of hyperalkaline phosphatasia in hyperthyroidism. Bone Miner. 1988;4:355–363. [PubMed] [Google Scholar]
- 11.Allain TJ, Yen PM, Flanagan AM, McGregor AM. The isoform-specific expression of the tri-iodothyronine receptor in osteoblasts and osteoclasts. Eur J Clin Invest. 1996;26:418–425. doi: 10.1046/j.1365-2362.1996.160289.x. [DOI] [PubMed] [Google Scholar]
- 12.Abu EO, Bord S, Horner A, Chatterjee VK, Compston JE. The expression of thyroid hormone receptors in human bone. Bone. 1997;21:137–142. doi: 10.1016/s8756-3282(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 13.Robson H, Siebler T, Stevens DA, Shalet SM, Williams GR. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology. 2000;141:3887–3897. doi: 10.1210/endo.141.10.7733. [DOI] [PubMed] [Google Scholar]
- 14.Lewinson D, Harel Z, Shenzer P, Silbermann M, Hochberg Z. Effect of thyroid hormone and growth hormone on recovery from hypothyroidism of epiphyseal growth plate cartilage and its adjacent bone. Endocrinology. 1989;124:937–945. doi: 10.1210/endo-124-2-937. [DOI] [PubMed] [Google Scholar]
- 15.Stevens DA, Hasserjian RP, Robson H, Siebler T, Shalet SM, Williams GR. Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of parathyroid hormone-related peptide and its receptor during endochondral bone formation. J Bone Miner Res. 2000;15:2431–2442. doi: 10.1359/jbmr.2000.15.12.2431. [DOI] [PubMed] [Google Scholar]
- 16.Freitas FR, Capelo LP, O’Shea PJ, Jorgetti V, Moriscot AS, Scanlan TS, et al. The thyroid hormone receptor beta-specific agonist GC-1 selectively affects the bone development of hypothyroid rats. J Bone Miner Res. 2005;20:294–304. doi: 10.1359/JBMR.041116. [DOI] [PubMed] [Google Scholar]
- 17.Bassett JH, Nordstrom K, Boyde A, Howell PG, Kelly S, Vennstrom B, et al. Thyroid status during skeletal development determines adult bone structure and mineralization. Mol Endocrinol. 2007;21:1893–1904. doi: 10.1210/me.2007-0157. [DOI] [PubMed] [Google Scholar]
- 18.Allain TJ, McGregor AM. Thyroid hormones and bone. J Endocrinol. 1993;139:9–18. doi: 10.1677/joe.0.1390009. [DOI] [PubMed] [Google Scholar]
- 19.O’Shea PJ, Bassett JH, Sriskantharajah S, Ying H, Cheng SY, Williams GR. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor alphal or beta. Mol Endocrinol. 2005;19:3045–3059. doi: 10.1210/me.2005-0224. [DOI] [PubMed] [Google Scholar]
- 20.Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140:844–851. doi: 10.1210/endo.140.2.6537. [DOI] [PubMed] [Google Scholar]
- 21.Obregon MJ, Calvo RM, Del Rey FE, de Escobar GM. Ontogenesis of thyroid function and interactions with maternal function. Endocr Dev. 2007;10:86–98. doi: 10.1159/000106821. [DOI] [PubMed] [Google Scholar]
- 22.Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA. Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology. 2000;141:2490–2500. doi: 10.1210/endo.141.7.7571. [DOI] [PubMed] [Google Scholar]
- 23.Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA. The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeicma tadpoles. Endocrinology. 1997;138:2989–2997. doi: 10.1210/endo.138.7.5272. [DOI] [PubMed] [Google Scholar]
- 24.Miura M, Tanaka K, Komatsu Y, Suda M, Yasoda A, Sakuma Y, et al. A novel interaction between thyroid hormones and l,25(OH)(2)D(3) in osteoclast formation. Biochem Biophys Res Commun. 2002;291:987–994. doi: 10.1006/bbrc.2002.6561. [DOI] [PubMed] [Google Scholar]
- 25.Gouveia CH, Christoffolete MA, Zaitune CR, Dora JM, Harney JW, Maia AL, et al. Type 2 iodothyronine selenodeiodinase is expressed throughout the mouse skeleton and in the MC3T3-E1 mouse osteoblastic cell line during differentiation. Endocrinology. 2005;146:195–200. doi: 10.1210/en.2004-1043. [DOI] [PubMed] [Google Scholar]
- 26.Miura M, Tanaka K, Komatsu Y, Suda M, Yasoda A, Sakuma Y, et al. Thyroid hormones promote chondrocyte differentiation in mouse ATDC5 cells and stimulate endochondral ossification in fetal mouse tibias through iodothyronine deiodinases in the growth plate. J Bone Miner Res. 2002;17:443–454. doi: 10.1359/jbmr.2002.17.3.443. [DOI] [PubMed] [Google Scholar]
- 27.Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7:698–705. doi: 10.1038/ncb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, et al. Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci U S A. 2007;104:14466–14471. doi: 10.1073/pnas.0706754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obregon MJ, Morreale de Escobar G, Escobar del Rey F. Concentrations of triiodo-L-thyronine in the plasma and tissues of normal rats, as determined by radioimmunoassay: comparison with results obtained by an isotopic equilibrium technique. Endocrinology. 1978;103:2145–2153. doi: 10.1210/endo-103-6-2145. [DOI] [PubMed] [Google Scholar]
- 30.Livak K. ABI Prism 7700 sequence detection system, user bulletin 2. Applied biosystem. 1997 [Google Scholar]
- 31.Christoffolete MA, Ribeiro R, Singru R, Fekete C, da Silva WS, Gordon DF, et al. Atypical expression of type 2 iodothyronine deiodinase in thyrotrophs explains the thyroxine-mediated pituitary thyrotropin feedback mechanism. Endocrinology. 2006;147:1735–1743. doi: 10.1210/en.2005-1300. [DOI] [PubMed] [Google Scholar]
- 32.Steinsapir J, Harney J, Larsen PR. Type 2 iodothyronine deiodinase in rat pituitary tumor cells is inactivated in proteasomes. J Clin Invest. 1998;102:1895–1899. doi: 10.1172/JCI4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da-Silva WS, Harney JW, Kim BW, Li J, Bianco SD, Crescenzi A, et al. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007;56:767–776. doi: 10.2337/db06-1488. [DOI] [PubMed] [Google Scholar]
- 34.Galton VA, Wood ET, St Germain EA, Withrow CA, Aldrich G, St Germain GM, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148:3080–3088. doi: 10.1210/en.2006-1727. [DOI] [PubMed] [Google Scholar]
- 35.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 36.Senecal J, Grosse MC, Vincent A, Simon J, Lefreche JN. Bone maturation in the fetus and newborn infant. Arch Fr Pediatr. 1977;34:424–438. [PubMed] [Google Scholar]
- 37.Marchant B, Brownlie BE, Hart DM, Horton PW, Alexander WD. The placental transfer of propylthiouracil, methimazole and carbimazole. J Clin Endocrinol Metab. 1977;45:1187–1193. doi: 10.1210/jcem-45-6-1187. [DOI] [PubMed] [Google Scholar]
- 38.Mandel SJ, Cooper DS. The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab. 2001;86:2354–2359. doi: 10.1210/jcem.86.6.7573. [DOI] [PubMed] [Google Scholar]
- 39.Morreale de Escobar G, Pastor R, Obregon MJ, Escobar del Rey F. Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinology. 1985;117:1890–1900. doi: 10.1210/endo-117-5-1890. [DOI] [PubMed] [Google Scholar]
- 40.Obregon MJ, Mallol J, Pastor R, Morreale de Escobar G, Escobar del Rey F. L-thyroxine and 3,5,3’-triiodo-L-thyronine in rat embryos before onset of fetal thyroid function. Endocrinology. 1984;114:305–307. doi: 10.1210/endo-114-1-305. [DOI] [PubMed] [Google Scholar]
- 41.Morreale de Escobar G, Obregon MJ, Ruiz de Ona C, Escobar del Rey F. Transfer of thyroxine from the mother to the rat fetus near term: effects on brain 3,5,3’-triiodothyronine deficiency. Endocrinology. 1988;122:1521–1531. doi: 10.1210/endo-122-4-1521. [DOI] [PubMed] [Google Scholar]
- 42.Calvo R, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. The rat placenta and the transfer of thyroid hormones from the mother to the fetus. Effects of maternal thyroid status. Endocrinology. 1992;131:357–365. doi: 10.1210/endo.131.1.1612015. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz de Ona C, Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregon MJ. Thyroid hormones and 5’-deiodinase in the rat fetus late in gestation: effects of maternal hypothyroidism. Endocrinology. 1991;128:422–432. doi: 10.1210/endo-128-1-422. [DOI] [PubMed] [Google Scholar]
- 44.Huang TS, Chopra IJ, Boado R, Soloman DH, Chua Teco GN. Thyroxine inner ring monodeiodinating activity in fetal tissues of the rat. Pediatr Res. 1988;23:196–199. doi: 10.1203/00006450-198802000-00014. [DOI] [PubMed] [Google Scholar]
- 45.Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103:979–987. doi: 10.1172/JCI6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. doi: 10.1172/JCI26240. [DOI] [PMC free article] [PubMed] [Google Scholar]