Abstract
The ubiquitous efflux transporter ABCC5 (ATP-binding cassette subfamily C member 5) is present at high levels in the blood-brain barrier, neurons, and glia, but its in vivo substrates and function are not known. Using untargeted metabolomic screens, we show that Abcc5−/− mice accumulate endogenous glutamate conjugates in several tissues, but brain in particular. The abundant neurotransmitter N-acetylaspartylglutamate was 2.4-fold higher in Abcc5−/− brain. The metabolites that accumulated in Abcc5−/− tissues were depleted in cultured cells that overexpressed human ABCC5. In a vesicular membrane transport assay, ABCC5 also transported exogenous glutamate analogs, like the classic excitotoxic neurotoxins kainic acid, domoic acid, and NMDA; the therapeutic glutamate analog ZJ43; and, as previously shown, the anti-cancer drug methotrexate. Glutamate conjugates and analogs are of physiological relevance because they can affect the function of glutamate, the principal excitatory neurotransmitter in the brain. After CO2 asphyxiation, several immediate early genes were expressed at lower levels in Abcc5−/− brains than in wild type brains, suggesting altered glutamate signaling. Our results show that ABCC5 is a general glutamate conjugate and analog transporter that affects the disposition of endogenous metabolites, toxins, and drugs.
Keywords: brain, drug transport, glutamate, neurotoxin, neurotransmitter transport, MRP5, blood-brain barrier
Introduction
ABCC5 (ATP-binding cassette subfamily C member 5), also known as MRP5 (multidrug resistance-associated protein 5), is a member of the C branch of the superfamily of ATP-binding cassette transporters, which use the energy provided by the hydrolysis of ATP to transport substrates across the plasma membrane (1, 2). ABCC5 is ubiquitously expressed, but levels in brain and muscle are especially high (3–5). In the brain, ABCC5 is present in brain capillary endothelial cells, neurons and glia (6–8). In most cells, ABCC5 routes to the basolateral plasma membrane (1), but in the brain capillary endothelial cells that form the blood-brain barrier, it appears to reside in the apical membrane (7, 9, 10). Based on this location, ABCC5 is considered to be a part of the blood-brain barrier by many (11), but its actual function has remained a mystery. In terms of drug resistance, ABCC5 is best known for its ability to transport nucleotide analogs (12, 13) and antifolates like methotrexate (14) but elevated ABCC5 levels have not been linked to clinical drug resistance (1). Although ABCC5 transports endogenous metabolites like cyclic nucleotides (15), folic acid (14), and the recently identified N-lactoyl-amino acids (16) in vitro, the physiological relevance of this transport is unclear. Abcc5−/− mice do not have an overt phenotype and do not provide clues about the in vivo substrates of ABCC5 either (12). ABCC5 has recently been associated with primary angle closure glaucoma (17), osteoclast formation in bone metastases (18), and heme homeostasis (19), but the ABCC5 substrates responsible for these phenotypes are not known. Because the substrates and function of ABCC5 are still largely unknown, we have exploited the Abcc5−/− mice to find additional endogenous substrates of ABCC5, as we have previously done for Abcc2−/− (20), Abcc3−/− (21), and Abcg2−/− (22) mice. Here we present the results of an untargeted metabolic screen detecting metabolites that accumulate in tissues of Abcc5−/− mice.
Experimental Procedures
Chemicals
β-Citryl glutamate was kindly provided by the group of van Schaftingen (23), whereas succinylaminoimidazolecarboxamide riboside (SAICAr)2 was obtained from Maria Zikánová (24). The peptides Asp-Gly-Glu, NAAG2, and Val-Asp-Gly-Glu were from Thermo Fisher Scientific, whereas (−)-(α)-kainic acid was from Cayman Chemical, and domoic acid was from Abcam Biochemicals. NAAG was obtained from Sigma or Bachem. Radiolabeled l-[2,3,4–3H]glutamic acid and l-[2,3–3H]aspartic acid were from American Radiolabeled Chemicals (St. Louis, MO). All other chemicals and reagents were from Sigma, unless stated otherwise. A library containing 20 N-acetylated dipeptides containing a C-terminal glutamate (see Fig. 7) was synthesized by the peptide facility of the Netherlands Cancer Institute using Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry with triethylsilane as scavenger (solid phase peptide synthesis, preloaded Wang resin). After lyophilization, the crude peptide library was used without further purification.
FIGURE 7.
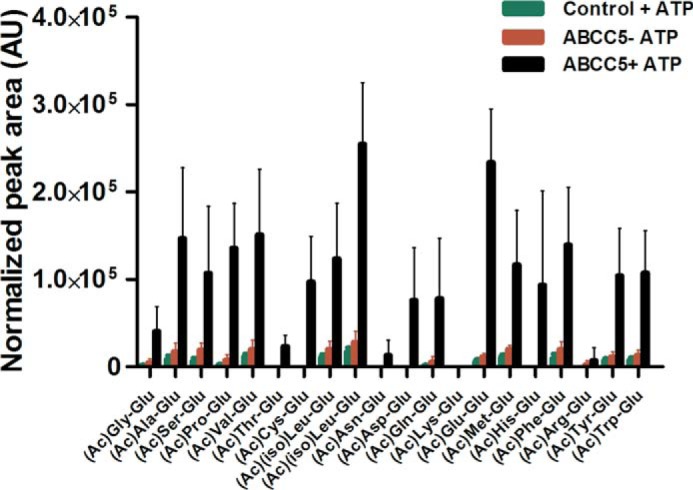
Vesicular transport of an N-acetylated dipeptide library shows that ABCC5 is a general C-terminal glutamate dipeptide transporter. Inside-out membrane vesicles with or without ABCC5 were incubated (10 min, 37 °C) with a library of 20 N-acetylated, C-terminal glutamate dipeptides (∼10 μm per peptide) in the presence and absence of 5 mm ATP. The relative amount of each peptide in the vesicles was determined using LC-MS and normalized on the peptide signal in the original peptide library. Poor LC-MS sensitivity for (Ac)Lys-Glu resulted in a low signal in the input library and undetectable levels in vesicles. We were unable to discriminate (Ac)Ile-Glu and (Ac)Leu-Glu because of their identical mass. Hence the uptakes of both of these dipeptides are labeled (Ac)(iso)Leu-Glu. The data are presented as means plus S.D. (n = 4). AU, arbitrary units.
Animals
Abcc5−/− mice on a FVB background (25) and wild type FVB mice were housed in constant temperature rooms with a 12-hour light/12-hour dark cycle and received food and water ad libitum. All experiments were reviewed and approved by our Institutional Animal Care and Use Committee. Approximately 16-week-old male mice (n = 5 per genotype) were sacrificed by CO2-asphyxiation after which the various tissues were collected and frozen on dry ice. Whole blood was collected by cardiac puncture and kept on ice until separation of plasma and erythrocytes by centrifugation (10 min, 5,000 × g, 4 °C). The plasma layer was collected, and the buffy coat containing peripheral blood mononuclear cells was discarded from the erythrocyte pellet, which was subsequently washed with 1 ml of cold PBS. All samples were stored at −20 °C until further processing.
Automated Home Cage Monitoring
Automated home cage monitoring was performed by Sylics (Amsterdam, the Netherlands) on six wild type and six Abcc5−/− mice, as described (26).
Phencyclidine-induced Locomotor Activation
Mice were housed in Phenotyper cages (Noldus, Wageningen, The Netherlands) to automatically track animal behavior. The first 2 days, the mice were allowed to adapt to their new environment. On the third day, spontaneous behavior was determined (dark phase only). 5, 8, and 11 days after being placed in the Phenotyper cages, mice got 5, 15, and 0 (saline) mg of PCP/kg of body weight, respectively, all administered intraperitoneally. Locomotor activity was subsequently followed automatically and presented as distance moved/5 min.
Cell Lines and Culture Conditions
Cells were cultured in DMEM as described (27). The generation of the hABCC5-overexpressing cell line HEK293/5i has been described elsewhere (13). HEK293 cells were transfected with pEF6Myc-His constructs encoding ribosomal modification proteins RIMKLA and RIMKLB (rimK-like family members A and B) (kindly provided by van Schaftingen and co-workers (23) using calcium phosphate precipitation. Clones resistant to blasticidin (Invitrogen) were analyzed by LC-MS, and the clones with the highest levels of NAAG (RIMKLA clones) or BCG (RIMKLB clones) were supertransfected with Gateway compatible pQCXIP vectors encoding GFP or hABCC5. Clones that were resistant to puromycin (2 μg/ml; Invitrogen) were selected and tested for GFP and hABCC5 expression on Western blot. Cells were seeded in 6-well plates (Corning Costar, Lowell, MA) at a density of 7.5 × 105 cells/well and allowed to grow overnight. At this point, the medium was replaced with 2 ml of fresh medium, and the cells were incubated for 3 days. Culture medium was collected on ice, whereas cultured cells were washed with cold PBS and lysed in 1 ml of 70% cold MeOH.
Sample Processing
Collected tissues were weighed, transferred to 2 ml of Eppendorf vials on ice containing 70% methanol, and lysed for 10 min at 50 Hz using the TissueLyser LT (Qiagen). For plasma, erythrocytes and culture medium a volume of 200 μl of sample was mixed with 600 μl of cold methanol, vortexed, and left on ice for 30 min. After centrifugation (10 min, 20,800 × g, 4 °C) of the extracts and cell lysates, the supernatant was transferred to a second Eppendorf vial and evaporated to dryness in a SpeedVac at room temperature. The dried extracts were reconstituted in mobile phase A (5 mm N,N-dimethylhexylamine in water:methanol 95:5 (v/v) adjusted to pH 7.0), centrifuged (10 min, 20,800 × g, 4 °C), and analyzed by untargeted LC-MS metabolomics.
Untargeted Metabolomics
Untargeted metabolomics was performed using ion pair liquid chromatography coupled to an LTQ-Orbitrap Discovery (Thermo Fisher Scientific) operated in the negative ionization mode, as previously described (27), with a scan range of m/z 50–1500. Global metabolite profiles of wild type and Abcc5−/− mice were first compared per organ using XCMS (28). From these data we selected the features that were significantly (p < 0.05) higher (>2-fold) in Abcc5−/− mice in at least two tissue types using metaXCMS (29). The retention time and peak shape of each feature were compared to manually filter out features associated with the same metabolite. The accurate mass of the differentially present compounds was used for searches in the METLIN (30), HMDB (31), and MassBank (32) databases. Additionally, high resolution fragmentation spectra were obtained as described (27). The fragmentation spectra and isotope distribution pattern were used to determine the elemental composition with Sirius2 software (33, 34).
Vesicular Uptake
Inside-out membrane vesicles were prepared from HEK293 cells transiently transduced with hABCC5 or GFP (control) as described (35). Vesicular uptake of radiolabeled substrates was assessed in samples containing 16 μg of protein using a rapid filtration technique, followed by liquid scintillation counting (36). Transport of nonradiolabeled substrates was assessed in samples containing 75 μg of protein as described (20). Filtered vesicles were lysed, evaporated to dryness, and reconstituted in 50 μl of mobile phase A before analysis by LC-MS (see “Untargeted Metabolomics”). Extracted ion chromatograms corresponding to the tested substrates, their sodium adduct (NAAG), or in-source fragment (domoic acid, m/z 222.15) were manually integrated using XCalibur software (Thermo Fisher Scientific).
Gene Expression Profiling
Whole brains were collected from CO2-asphyxiated wild type and Abcc5−/− mice and immediately frozen on dry ice. After homogenization in TRIzol (Invitrogen), RNA was extracted according to the manufacturer's protocol. Sequencing libraries were generated according to the manufacturer's instructions (TruSeq RNA Sample Preparation v2 Guide; Illumina; catalog no. 15026495 Rev. F). Briefly, polyadenylated RNA from intact total RNA was purified using oligo(dT) beads. Following purification the RNA was fragmented, random primed, and reverse transcribed using SuperScript II reverse transcriptase (Invitrogen, catalog no. 18064-014). Second strand synthesis was performed using polymerase I and RNaseH. The resulting cDNA was converted to blunt end fragments using an end repair mix (Illumina), followed by 3′ end adenylation and ligated to Illumina paired end sequencing adapters and subsequently amplified by 16 cycles of PCR. The libraries were analyzed on a 2100 Bioanalyzer (Agilent) using a 7500 chip (Agilent) and diluted to 10 nm. The samples were single-end sequenced with a length of 51 bp on a HiSeq2000 machine. The reads were aligned using Tophat (Version 2.0.6) against the Mouse reference (build 37). Tophat was supplied with a known set of gene models using a GTF file (Ensembl version 66). HTSeq-count was used to generate a list with the number of uniquely mapped reads for each gene. All samples were combined into one data set, and genes that have no expression across all samples in the data set were removed. To determine the differentially expressed genes between groups, the R package EdgeR and Limma were used. The voom function was used to transform the count data to log2 counts per million and estimate the variance across the samples. Different groups were defined, and the differentially expressed genes were determined using lmFit and eBayes.
Results
Endogenous Glutamate Conjugates Accumulate in Abcc5−/− Tissues
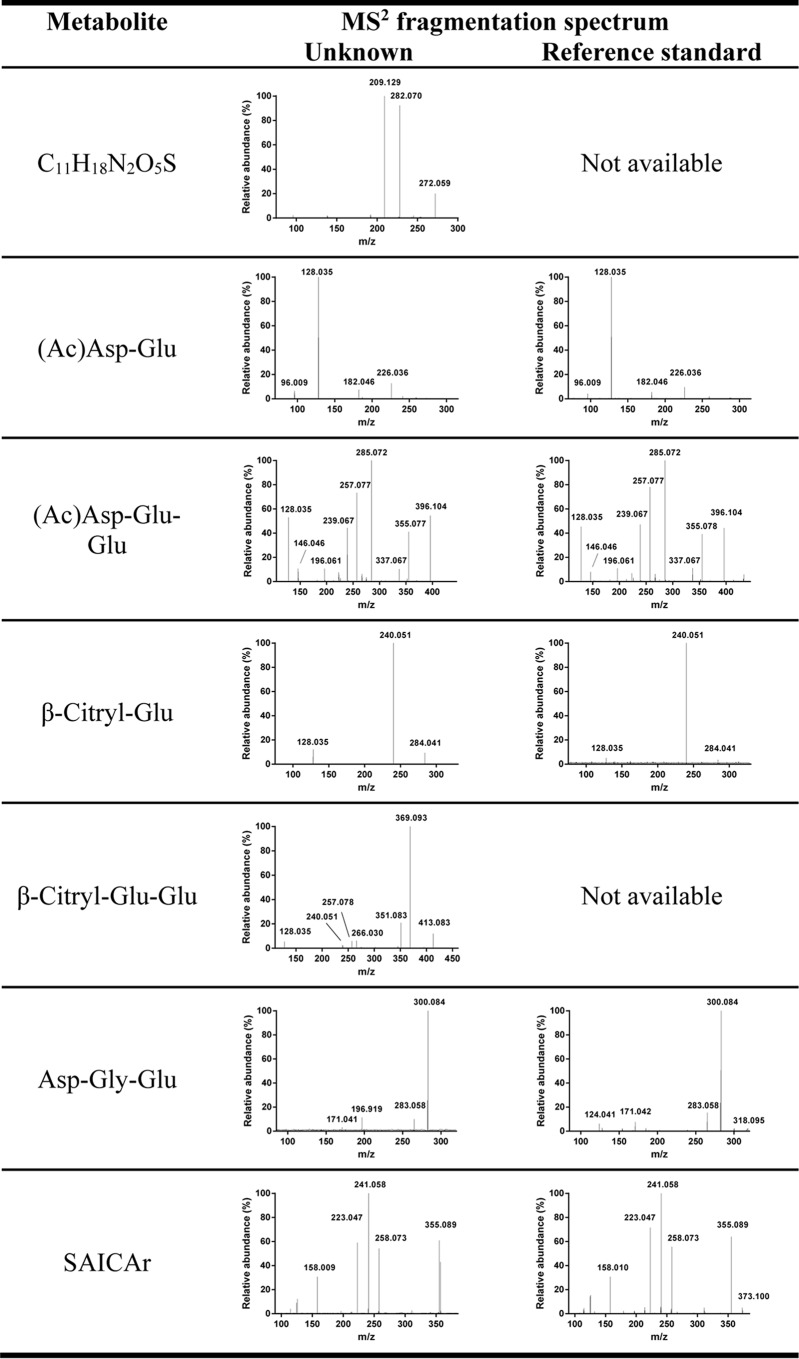
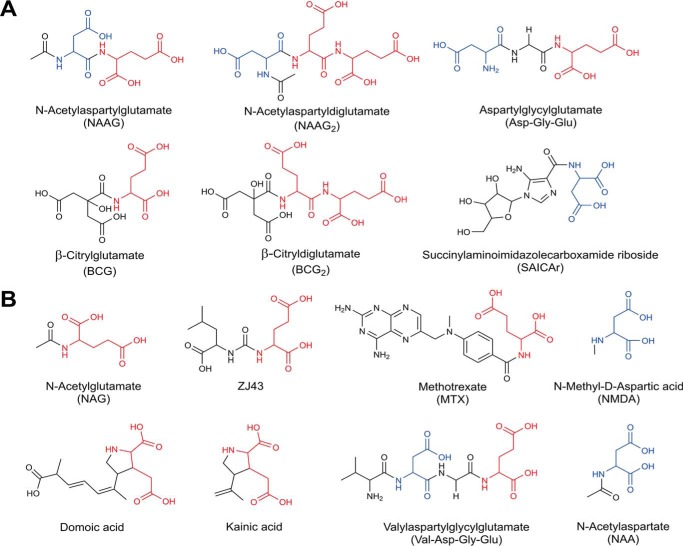
Using LC-MS untargeted metabolomics, we generated global metabolite profiles of various tissues collected from wild type and Abcc5−/− mice (accessible via the MetaboLights repository (37), MTBLS197). We compared these profiles using (meta)XCMS (28, 38) to detect metabolites that were more abundant in Abcc5−/− tissue. Nine metabolites were significantly increased in multiple Abcc5−/− tissues, which made them potential ABCC5 substrates (Table 1). Using accurate mass-based metabolite database searches, MS-MS fragmentation spectra, and reference standards (Fig. 1), we were able to identify six of these metabolites (Table 1). Two of the identified accumulating metabolites, β-citryldiglutamate (BCG2) and Asp-Gly-Glu, have never been described as endogenous metabolites before. Strikingly, five of the identified metabolites contain a C-terminal glutamate moiety, whereas the sixth contains a C-terminal aspartate moiety, very similar to glutamate (Fig. 2A).
TABLE 1.
Summary of metabolites that are more than 2-fold higher in multiple Abcc5−/− tissues compared to wild type tissues
m/z indicates mass to charge ratio. Rt indicates retention time.
| m/z | Rt | Elemental composition | Identity |
|---|---|---|---|
| min | |||
| 320.062 | 17.4 | C11H15NO10 | β-Citrylglutamic acid (β-citryl-Glu; BCG) |
| 449.105 | 21.0 | C16H22N2O13 | β-Citryldiglutamic acid (β-citryl-Glu-Glu; BCG2)a |
| 303.083 | 11.9 | C11H16N2O8 | N-Acetylaspartylglutamic acid ((Ac)Asp-Glu; NAAG) |
| 432.125 | 17.3 | C16H23N3O11 | N-Acetylaspartyldiglutamic acid ((Ac)Asp-Glu-Glu; NAAG2) |
| 318.095 | 5.0 | C11H17N3O8 | Asp-Gly-Glu |
| 373.010 | 9.0 | C13H18N4O9 | Succinylaminoimidazolecarboxamide riboside (SAICAr) |
| 289.086 | 3.7 | C11H18N2O5S | Unknown; contains sulfate moiety |
| 421.055 | 18.5 | Unknown | Unknown |
| 456.175 | 13.5 | Unknown | Unknown |
a Putative identification; reference standard is not available.
FIGURE 1.
MS2 fragmentation spectra of unknowns and available reference standards resulted in the identification of most of the accumulating metabolites. The neutral loss of 79.957 in the spectrum of C11H18N2O5S indicates the presence of a sulfate moiety. The spectrum of putative BCG2 contains fragments (m/z 128.035 and 240.051) that are identical to that of BCG, strengthening its putative identification. The peak at m/z 196.919 in the spectrum of Asp-Gly-Glu is a nonspecific background peak that we commonly observe in low abundant MS2 spectra. The unknowns with m/z 421.0557 and 456.1745 did not yield any database hits, nor could we accurately determine the elemental composition because of the relatively high mass and low abundance.
FIGURE 2.
Chemical structures of glutamate conjugates and analogs found to be transported by ABCC5. A, in vivo substrates found through comparative metabolomics of wild type and Abcc5−/− mouse tissues. B, additional ABCC5 substrates identified through in vitro vesicular transport studies. MTX transport was shown by Wielinga et al. (14). Glutamate moieties are highlighted in red, whereas aspartate moieties are highlighted in blue.
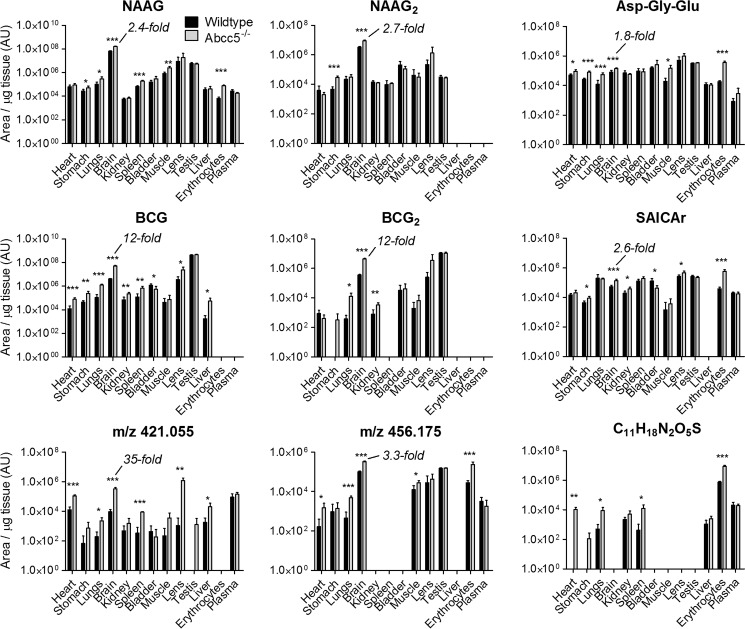
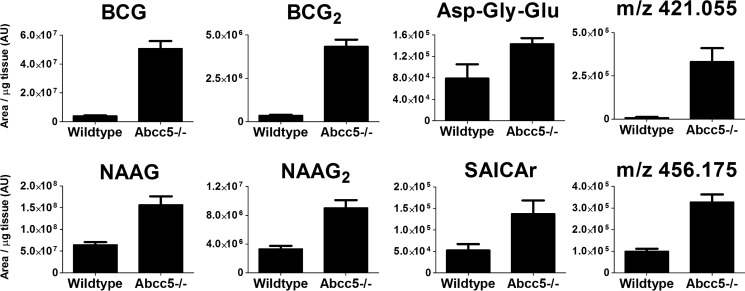
The tissue levels of the accumulating metabolites are represented on a logarithmic scale in Fig. 3. Although each metabolite accumulated differently, all metabolites strongly accumulated in brain, as depicted on a linear scale in Fig. 4. Compared with other tissues, the concentrations of the accumulating glutamate conjugates were particularly high in brain as well. These results point to a prominent role for ABCC5 in the brain. It was unclear, however, whether the metabolite changes were directly or indirectly related to ABCC5 expression.
FIGURE 3.
Nine metabolites accumulated in Abcc5−/− tissues. Tissues of wild type and Abcc5−/− mice were analyzed using untargeted LC-MS metabolomics, after which the data sets were compared using the XCMS online platform. Only metabolites that were significantly (p < 0.05) and over 2-fold increased in at least two Abcc5−/− tissues are shown here. The elemental composition could be determined for a single unknown, whereas two other unknowns could not be identified beyond their mass over charge ratio (m/z). The metabolite concentrations in brains were relatively high and showed clear ABCC5 dependence. The data are presented on a log scale as means plus S.D. (n = 5 per genotype). AU, arbitrary units. p values were calculated using the Student's t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FIGURE 4.
The metabolites detected in our screen strongly accumulated in Abcc5−/− brain. Tissues of wild type and Abcc5−/− mice were analyzed using untargeted LC-MS metabolomics, after which the data sets were compared using the XCMS online platform. Of the nine metabolites that were significantly (p < 0.05) and over 2-fold increased in at least two Abcc5−/− tissues, eight accumulated in Abcc5−/− brain. Two unknowns could not be identified beyond their mass over charge ratio (m/z). The data are presented as means plus S.D. (n = 5 per genotype). AU, arbitrary units.
Endogenous Glutamate Conjugates Are Effluxed from HEK293/ABCC5 Cells
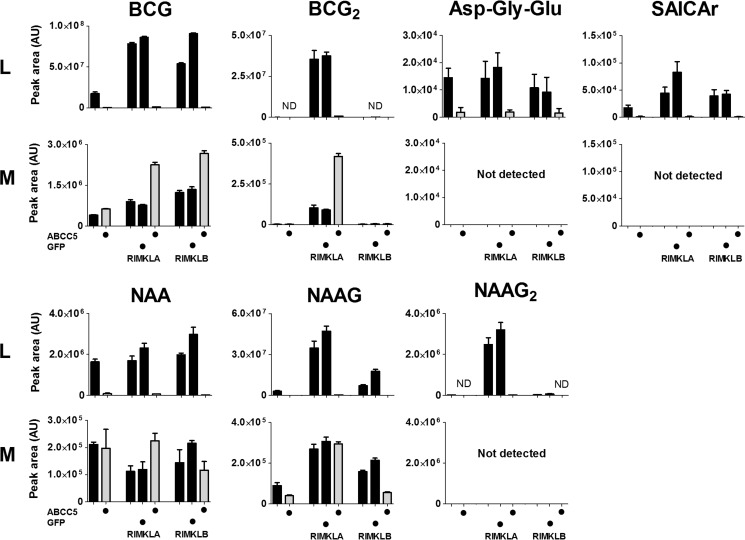
To confirm our in vivo results, we determined the levels of the putative ABCC5 substrates in cell lysate and culture medium of parental HEK293 cells and of HEK293 cells overexpressing human ABCC5 (hABCC5). Although some of the putative substrates were expected to be present in parental HEK293 cells, we also expressed hABCC5 in HEK293 cells that stably overexpressed ribosomal modification proteins RIMKLA and RIMKLB, the enzymes that synthesize NAAG, N-acetylaspartyldiglutamate (NAAG2), and β-citrylglutamate (BCG) (23, 39).
As expected, RIMKLA and RIMKLB overexpression resulted in increased levels of NAAG, NAAG2, and BCG in the cells (Fig. 5). The concentration of the newly discovered metabolite BCG2 was also strongly increased in RIMKLA-overexpressing cells, indicating that RIMKLA ligates a second glutamate not only to NAAG (39), but also to BCG.
FIGURE 5.
Metabolites that accumulate in Abcc5−/− organs in vivo are also effluxed from HEK293 cells by human ABCC5 in vitro. HEK293 parental cells and HEK293 cells stably overexpressing RIMKLA or RIMKLB in combination with ABCC5 (depicted in gray) or GFP (depicted in black) were cultured for 3 days. Cell lysate (L) and culture medium (M) were analyzed using untargeted LC-MS metabolomics. The data are presented as means plus S.D. (n = 3). AU, arbitrary units; ND, not detected.
In line with the in vivo results, overexpression of hABCC5 resulted in a strong decrease in intracellular levels of the metabolites that accumulated in Abcc5−/− mice. As anticipated for hABCC5 substrates, the levels of BCG and BCG2 in medium increased upon hABCC5 overexpression. It was unclear whether the same holds true for NAAG2, SAICAr, and Asp-Gly-Glu, because their levels were below the limit of detection in culture medium.
Unexpectedly, the extracellular levels of NAAG were not affected by hABCC5 overexpression. A potential mechanism that explains the reduced intracellular NAAG and NAAG2 levels without the expected extracellular increase is that their precursor N-acetylaspartate (NAA) is an ABCC5 substrate. NAA levels were indeed strongly decreased in cell lysates of hABCC5-overexpressing cells, suggesting that NAA is also an ABCC5 substrate (Fig. 5). Medium NAA levels were not altered, however, and the tissue levels of NAA were not significantly different between wild type and Abcc5−/− mice. Apparently NAA levels are also affected by mechanisms other than ABCC5. The three unidentified metabolites were not detected in lysate or medium samples. Taken together, these results show that the putative mouse ABCC5 (mABCC5) substrates identified through our metabolomics screen in vivo are also transported by human ABCC5 in cultured cells in vitro.
ABCC5 Is a General Glutamate Conjugate and Analog Transporter
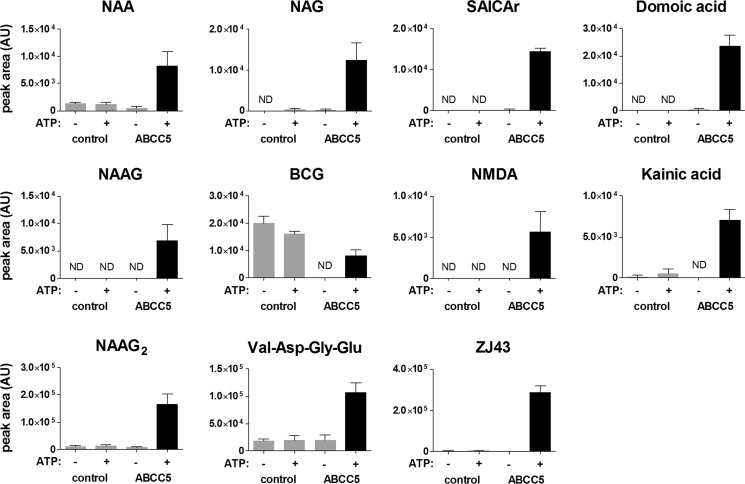
To verify whether the differentially present metabolites are genuine ABCC5 substrates, we determined the transport of available metabolites into ABCC5-containing inside-out membrane vesicles. NAA, NAAG, NAAG2, and SAICAr were transported into inside-out membrane vesicles in an ABCC5- and ATP-dependent fashion, confirming that these metabolites are bona fide ABCC5 substrates (Fig. 6). BCG was also transported into ABCC5-containing vesicles in an ATP-dependent fashion, but the background levels of BCG were much higher in control vesicles than in ABCC5-containing vesicles. This difference in background levels must be due to the difference in intracellular BCG levels in the HEK293 cells transduced with control or ABCC5 virus that were used to prepare the inside-out membrane vesicles. Vesicular transport of Asp-Gly-Glu could not be verified because the levels of this metabolite inside the vesicles were below our LC-MS detection limits. Reference BCG2 was not available.
FIGURE 6.
Vesicular transport assays show that ABCC5 transports glutamate conjugates and analogs. Inside-out membrane vesicles without (control) or with ABCC5 (ABCC5) were incubated (10 min, 37 °C) with test substrates (100 μm) in the presence and absence of 5 mm ATP. The relative amount of test substrate in the vesicles was determined using LC-MS. The data are presented as means plus S.D. (n = 3). AU, arbitrary units; ND, not detected.
A striking feature of the chemical structure of all novel substrates identified here, and the previously identified ABCC5 substrates folate, methotrexate, and their polyglutamylated metabolites (14), is the presence of a glutamate or aspartate moiety (Fig. 2A). To test whether other compounds with glutamate or aspartate moieties (Fig. 2B) are also ABCC5 substrates, we tested these potential ABCC5 substrates in vesicular transport assays. We found that N-acetylglutamate; the tetrapeptide Val-Asp-Gly-Glu; the excitotoxic glutamate analogs NMDA, kainic acid, and domoic acid; and the therapeutic glutamate analog ZJ43 were all transported into vesicles in an ABCC5- and ATP-dependent fashion (Fig. 6). Additionally, we synthesized a library containing 20 N-acetylated dipeptides, all containing a C-terminal glutamate. When incubated with membrane vesicles, these dipeptides were transported into the membrane vesicles by ABCC5 (Fig. 7). Glutamic acid and aspartic acid were not transported themselves, however (data not shown). Note that the relative transport of the dipeptides in Fig. 7 more likely reflects the relative affinity of the peptides for the transporter than the maximal rate of transport of each peptide, because there will be competition for the transporter by the dipeptide mix.
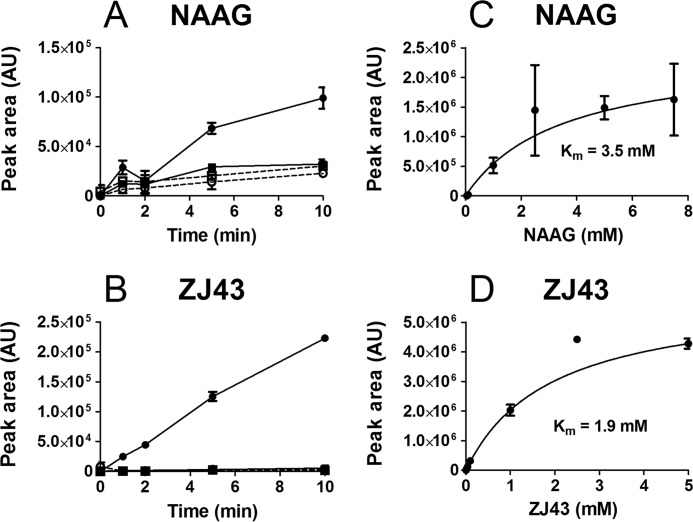
For the endogenous glutamate conjugate NAAG and the therapeutic glutamate analog ZJ43, we investigated the ABCC5-mediated transport in more detail. Both NAAG and ZJ43 were taken up into ABCC5-containing vesicles in a time- and ATP-dependent fashion (Fig. 8A). The concentration-dependent transport fits Michaelis-Menten kinetics with a comparable Km for NAAG (3.5 mm) and ZJ43 (1.9 mm) (Fig. 8B).
FIGURE 8.
NAAG and ZJ43 are transported into inside-out membrane vesicles by ABCC5. A and B, 100 μm NAAG (A) or 100 μm ZJ43 (B) was incubated with control vesicles (squares) and ABCC5-containing vesicles (circles) at 37 °C, in the presence (solid line) and absence (dashed line) of 5 mm ATP. At the indicated time points, samples containing 75 μg of protein were taken. After washing over a filter, the vesicular content was analyzed by LC-MS (n = 3). C and D, various concentrations of NAAG (C) or ZJ43 (D) were incubated with control vesicles and ABCC5-containing vesicles for 10 min at 37 °C, in the presence of 5 mm ATP (n = 4). ABCC5-dependent transport was calculated and fitted to Michaelis-Menten kinetics (solid line) using GraphPad Prism. The data are presented as means ± S.E. because rare outliers resulted in a high S.D. that required a scale on which the data were not interpretable. AU, arbitrary units.
Immediate Early Genes Are Down-regulated in Abcc5−/− Mouse Brain
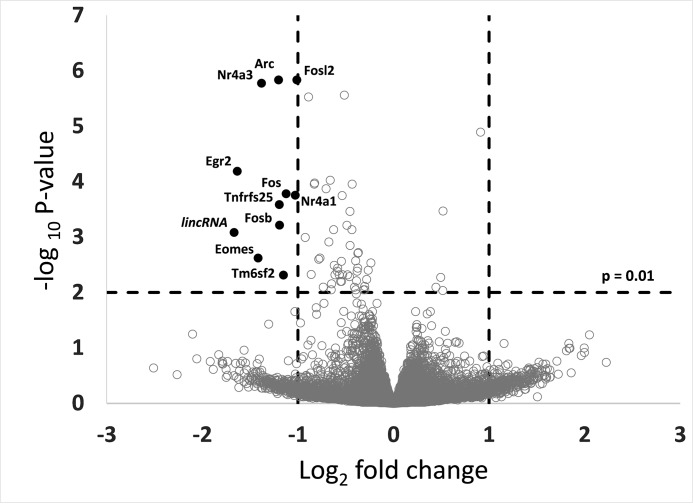
To assess the potential effects of the altered metabolite distribution, we performed RNA sequencing on brains of wild type and Abcc5−/− mice (GEO accession number GSE68628) after CO2 asphyxiation. Interestingly, many of the most differentially transcribed genes (Fig. 9) were immediate early genes that are known to be activated through glutamate receptors (40, 41). Immediate early genes represent markers for neuronal activity (40) that can be induced rapidly in response to diverse stimuli like stress or ischemia (42, 43). The decreased induction of these genes in Abcc5−/− mice might indicate attenuated glutamate excitotoxicity in response to acute ischemia.
FIGURE 9.
Expression levels of several immediate early genes are down-regulated in Abcc5−/− mouse brains. mRNA of whole brains collected from wild type and Abcc5−/− mice was sequenced (n = 5 single brains/group), as described under “Experimental Procedures.”
Discussion
Thus far only a handful of ABCC5 substrates were known. Using untargeted metabolomics, we have now identified over 30 additional substrates, demonstrating that ABCC5 is a general glutamate conjugate and analog transporter. Glutamate is the principal excitatory neurotransmitter in the brain. It is essential for everyday brain functions like learning and memory but causes excitotoxic damage in traumatic brain injury and stroke (44). Glutamate levels in the brain are therefore highly regulated, and glutamate-like compounds can strongly affect glutamate neurotransmission (45).
The endogenous glutamate conjugate NAAG, for example, attenuates glutamate neurotransmission and has been implicated in schizophrenia and inhibition of glutamate excitotoxicity (46–48). Several exogenous glutamate analogs like NMDA, domoic acid and kainic acid can, in contrast, act as potent excitotoxins. To protect the brain against such neurotoxic glutamate analogs, the blood-brain barrier strongly limits their brain penetration (49, 50). Interestingly, we found that ABCC5 transports NAAG, as well as NMDA, domoic acid, and kainic acid. Although structurally similar, the functions of the metabolites we identified are diverse, and only some have known functions in the brain, as described below.
NAAG is mainly present in peripheral and central nervous tissue where it is the third most prevalent neurotransmitter, reaching millimolar levels (51). NAAG is transported into synaptic vesicles by sialin (SLC17A5) (52), and these vesicles are released into the synaptic cleft upon depolarization (53). Our data indicate that NAAG is also constitutively released from cells by ABCC5, a completely different mechanism of release. NAAG is reported to be an NMDA receptor-ligand and an agonist at the metabotropic glutamate receptor 3 (46, 47). Activated metabotropic glutamate receptor 3 inhibits subsequent glutamate and glycine release (47).
Extracellular NAAG is hydrolyzed to NAA and glutamate by glutamate carboxypeptidase 2 (GCP2) (54, 55). Pharmacological GCP2 inhibition increases NAAG levels while at the same time reducing glutamate levels. GCP2 inhibitors, like ZJ43, have initially been tested as neuroprotective agents in ischemic brain injury (56) but were also shown to be effective in animal models for traumatic brain injury, schizophrenia, and neuropathic pain (57). NAAG is formed from NAA and glutamate by RIMKLA, which is also known as NAAG synthase 2. It was recently shown that the same enzyme can also ligate a second glutamate to NAAG, forming NAAG2 (39). NAAG2 also accumulated in Abcc5−/− brain, but its function, if any, is currently not known.
Analogous to NAAG, BCG is formed by RIMKLB and hydrolyzed back to citrate and glutamate by GCP3 (58), which is ubiquitously expressed in mouse brain (59). We now show that RIMKLA can also ligate a second glutamate to BCG, forming the previously unknown BCG2. The intriguing metabolite BCG was first identified in newborn rat brain, where it is present up to low millimolar levels (60). It is present at particularly high levels in the developing brain of many species but decreases to high micromolar levels with age (61). Despite these extremely high brain concentrations, the function of BCG is elusive. Thus far, BCG has been suggested to be related to cell differentiation (62–65) and neuronal protection by metal chelation (66–68). Because we are the first to report the existence of BCG2, its function, if any, is unclear. Likewise, Asp-Gly-Glu was not known to be present in animals, and the source and function of this tripeptide remain to be determined.
Finally, SAICAr is a dephosphorylated purine synthesis intermediate that reaches high brain concentrations in patients lacking adenylosuccinate lyase, who show severe psychomotor delay (69). SAICAr can induce neuronal damage, but at levels that are much higher than the 2.6-fold increase found in the brains of our Abcc5−/− mice (70).
Regardless of their diverse functions, all metabolites were transported into ABCC5-containing vesicles. Vesicular transport of NAAG and ZJ43 shows that ABCC5 has a relatively low affinity for glutamate conjugates, which is comparable to the Km reported for folic acid (1.3 mm) (14) and N-lactoyl-phenylalanine (∼1 mm) (16). As the physiological levels of NAAG and BCG can reach millimolar concentrations in the brain, these substrates can still be transported by ABCC5 at considerable rates.
Unexpectedly, metabolites from known endogenous ABCC5 substrate classes (cyclic nucleotides, folates, and N-lactoyl-amino acids) were not found in this screen. The basal levels of the cyclic nucleotides were likely too low to be detected reproducibly. Moreover, cyclic nucleotide transport by mouse ABCC5 is doubtful, because the transporter did not contribute to cGMP transport in mouse erythrocyte vesicles (25). Folate accumulated in Abcc5−/− tissues, but this accumulation was nonsignificant in all tissues except brain. The lack of difference in levels of N-lactoyl-amino acids can be explained by the fact that it is in intracellular equilibrium with the levels of lactate and amino acids (16).
Although Abcc5−/− mice were found to be healthy and fertile (13), one would expect the observed changes in metabolite levels to result in a (subtle) phenotype. The accumulation of glutamate conjugates and altered gene expression in Abcc5−/− brains indicate that this phenotype could be of neurological nature. We did not find major differences in the spontaneous behavior of wild type and Abcc5−/− mice using an automated home cage monitoring system that monitors various aspects of behavior (26), however. Zuo et al. (71) previously showed that pharmacological inhibition of GCP2 results in increased NAAG levels and reduced phencyclidine-induced locomotor activity in an animal model for schizophrenia. Such a difference was not found between our wild type and Abcc5−/− mice, however (data not shown). Dedicated behavior assays should be performed to find more subtle neurological phenotypes, such as the reduced social interaction found in GCP2+/− mice (72) and the reduced susceptibility to traumatic brain injury found in GCP2−/− mice (73). Behavioral phenotypes would likely be more obvious in humans, but ABCC5-deficient humans have never been described, despite the likely existence of null alleles (74).
Many drugs that target glutamate neurotransmission are glutamate analogs and therefore potential ABCC5 substrates. Interestingly, one of the major problems in the development of GCP2 inhibitors is that they do not reach the brain (75). Our results indicate that part of the poor brain penetration of these glutamate analogs might be due to the presence of ABCC5 in the blood-brain barrier.
Our data suggest that ABCC5 can also protect the brain against the prototypical neurotoxins kainate and NMDA. When administered outside the brain, kainate only becomes toxic at a relatively high dose, underlining the important role of the blood-brain barrier in the protection against toxic glutamate analogs (49). Many similar excitotoxic glutamate analogs, like domoic acid, can be present in food and represent potential ABCC5 substrates (76–78). Further studies are required to delineate the role of ABCC5 in the protection of the brain against domoic acid and other toxic glutamate analogs. In conclusion, our results show that ABCC5 is a general glutamate conjugate and analog transporter that can limit the brain levels of endogenous metabolites, drugs, and toxins.
Author Contributions
R. S. J. designed and performed most of the experiments, analyzed the data, and drafted the paper. S. M. performed vesicular transport experiments, and M. D. H. made DNA constructs. K. V. D. W. handled the laboratory animals, designed the research, supervised the project, and wrote the paper with P. B. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank François Collard and Emile van Schaftingen (de Duve Institute, Brussels, Belgium) for providing DNA plasmids and BCG reference material and Maria Zikánová (Institute for Inherited Metabolic Disorders, Prague, Czech Republic) for the SAICAr reference material. We thank Henk Hilkmann and Dris el Atmioui (Netherlands Cancer Institute, Amsterdam, The Netherlands) for the synthesis of the peptide library and Alfred Schinkel (Netherlands Cancer Institute) for critical reading of our manuscript. We are grateful to Iris de Rink (Netherlands Cancer Institute) for the analysis of the gene expression data. We thank Maarten Loos (Sylics, Amsterdam, The Netherlands) for performing the behavior tests. The raw LC-MS metabolomics data are accessible via the MetaboLights repository (37), MTBLS197. Raw RNA-seq data is available through the GEO repository (accession number GSE68628).
The authors declare that they have no conflicts of interest with the contents of this article.
- SAICAr
- succinylaminoimidazolecarboxamide riboside
- NAA
- N-acetylaspartate
- NAAG
- N-acetylaspartylglutamate
- NAAG2
- N-acetylaspartyldiglutamate
- BCG
- β-citrylglutamate
- BCG2
- β-citryldiglutamate
- GCP2
- glutamate carboxypeptidase 2.
References
- 1.Borst P., de Wolf C., and van de Wetering K. (2007) Multidrug resistance-associated proteins 3, 4, and 5. Pflügers Arch. 453, 661–673 [DOI] [PubMed] [Google Scholar]
- 2.Dean M., and Allikmets R. (2001) Complete characterization of the human ABC gene family. J. Bioenerg. Biomembr. 33, 475–479 [DOI] [PubMed] [Google Scholar]
- 3.Belinsky M. G., Bain L. J., Balsara B. B., Testa J. R., and Kruh G. D. (1998) Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J. Natl. Cancer Inst. 90, 1735–1741 [DOI] [PubMed] [Google Scholar]
- 4.Kool M., de Haas M., Scheffer G. L., Scheper R. J., van Eijk M. J., Juijn J. A., Baas F., and Borst P. (1997) Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 57, 3537–3547 [PubMed] [Google Scholar]
- 5.McAleer M. A., Breen M. A., White N. L., and Matthews N. (1999) pABC11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J. Biol. Chem. 274, 23541–23548 [DOI] [PubMed] [Google Scholar]
- 6.Dallas S., Miller D. S., and Bendayan R. (2006) Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol. Rev. 58, 140–161 [DOI] [PubMed] [Google Scholar]
- 7.Nies A. T., Jedlitschky G., König J., Herold-Mende C., Steiner H. H., Schmitt H.-P., and Keppler D. (2004) Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience 129, 349–360 [DOI] [PubMed] [Google Scholar]
- 8.Ashraf T., Kao A., and Bendayan R. (2014) Functional expression of drug transporters in glial cells: potential role on drug delivery to the CNS. Adv. Pharmacol. 71, 45–111 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Schuetz J. D., Elmquist W. F., and Miller D. W. (2004) Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J. Pharmacol. Exp. Ther. 311, 449–455 [DOI] [PubMed] [Google Scholar]
- 10.Soontornmalai A., Vlaming M. L., and Fritschy J.-M. (2006) Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience 138, 159–169 [DOI] [PubMed] [Google Scholar]
- 11.International Transporter Consortium, Giacomini K. M., Huang S.-M., Tweedie D. J., Benet L. Z., Brouwer K. L., Chu X., Dahlin A., Evers R., Fischer V., Hillgren K. M., Hoffmaster K. A., Ishikawa T., Keppler D., Kim R. B., Lee C. A., Niemi M., Polli J. W., Sugiyama Y., Swaan P. W., Ware J. A., Wright S. H., Yee S. W., Zamek-Gliszczynski M. J., and Zhang L. (2010) Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid G., Wielinga P., Zelcer N., De Haas M., Van Deemter L., Wijnholds J., Balzarini J., and Borst P. (2003) Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol. Pharmacol. 63, 1094–1103 [DOI] [PubMed] [Google Scholar]
- 13.Wijnholds J., Mol C. A., van Deemter L., de Haas M., Scheffer G. L., Baas F., Beijnen J. H., Scheper R. J., Hatse S., De Clercq E., Balzarini J., and Borst P. (2000) Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc. Natl. Acad. Sci. U.S.A. 97, 7476–7481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wielinga P., Hooijberg J. H., Gunnarsdottir S., Kathmann I., Reid G., Zelcer N., van der Born K., de Haas M., van der Heijden I., Kaspers G., Wijnholds J., Jansen G., Peters G., and Borst P. (2005) The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res. 65, 4425–4430 [DOI] [PubMed] [Google Scholar]
- 15.Jedlitschky G., Burchell B., and Keppler D. (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J. Biol. Chem. 275, 30069–30074 [DOI] [PubMed] [Google Scholar]
- 16.Jansen R. S., Addie R., Merkx R., Fish A., Mahakena S., Bleijerveld O. B., Altelaar M., IJlst L., Wanders R. J., Borst P., and van de Wetering K. (2015) N-Lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl. Acad. Sci. U.S.A. 112, 6601–6606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nongpiur M. E., Khor C. C., Jia H., Cornes B. K., Chen L.-J., Qiao C., Nair K. S., Cheng C.-Y., Xu L., George R., Tan D., Abu-Amero K., Perera S. A., Ozaki M., Mizoguchi T., Kurimoto Y., Low S., Tajudin L.-S. A., Ho C.-L., Tham C. C. Y., Soto I., Chew P. T. K., Wong H.-T., Shantha B., Kuroda M., Osman E. A., Tang G., Fan S., Meng H., Wang H., Feng B., Yong V. H. K., Ting S. M. L., Li Y., Wang Y.-X., Li Z., Lavanya R., Wu R.-Y., Zheng Y.-F., Su D. H., Loon S.-C., Yong V. K. Y., Allingham R. R., Hauser M. A., Soumittra N., Ramprasad V. L., Waseem N., Yaakub A., Chia K.-S., Kumaramanickavel G., Wong T. T., How A. C., Chau T. N. B., Simmons C. P., Bei J.-X., Zeng Y.-X., Bhattacharya S. S., Zhang M., Tan D. T., Teo Y.-Y., Al-Obeidan S. A., Hon D. N., Tai E.-S., Saw S.-M., Foster P. J., Vijaya L., Jonas J. B., Wong T.-Y., John S. W. M., Pang C.-P., Vithana E. N., Wang N., and Aung T. (2014) ABCC5, a gene that influences the anterior chamber depth, is associated with primary angle closure glaucoma. PLoS Genet. 10, e1004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourskaia A. A., Amir E., Dong Z., Tiedemann K., Cory S., Omeroglu A., Bertos N., Ouellet V., Clemons M., Scheffer G. L., Park M., Hallett M., Komarova S. V., and Siegel P. M. (2012) ABCC5 supports osteoclast formation and promotes breast cancer metastasis to bone. Breast Cancer Res. 14, R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korolnek T., Zhang J., Beardsley S., Scheffer G. L., and Hamza I. (2014) Control of metazoan heme homeostasis by a conserved multidrug resistance protein. Cell Metab. 19, 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krumpochova P., Sapthu S., Brouwers J. F., de Haas M., de Vos R., Borst P., and van de Wetering K. (2012) Transportomics: screening for substrates of ABC transporters in body fluids using vesicular transport assays. FASEB J. 26, 738–747 [DOI] [PubMed] [Google Scholar]
- 21.van de Wetering K., Feddema W., Helms J. B., Brouwers J. F., and Borst P. (2009) Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 137, 1725–1735 [DOI] [PubMed] [Google Scholar]
- 22.van de Wetering K., and Sapthu S. (2012) ABCG2 functions as a general phytoestrogen sulfate transporter in vivo. FASEB J. 26, 4014–4024 [DOI] [PubMed] [Google Scholar]
- 23.Collard F., Stroobant V., Lamosa P., Kapanda C. N., Lambert D. M., Muccioli G. G., Poupaert J. H., Opperdoes F., and Van Schaftingen E. (2010) Molecular identification of N-acetylaspartylglutamate synthase and β-citrylglutamate synthase. J. Biol. Chem. 285, 29826–29833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zikánová M., Krijt J., Hartmannová H., and Kmoch S. (2005) Preparation of 5-amino-4-imidazole-N-succinocarboxamide ribotide, 5-amino-4-imidazole-N-succinocarboxamide riboside and succinyladenosine, compounds usable in diagnosis and research of adenylosuccinate lyase deficiency. J. Inherit. Metab. Dis. 28, 493–499 [DOI] [PubMed] [Google Scholar]
- 25.de Wolf C. J., Yamaguchi H., van der Heijden I., Wielinga P. R., Hundscheid S. L., Ono N., Scheffer G. L., de Haas M., Schuetz J. D., Wijnholds J., and Borst P. (2007) cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 274, 439–450 [DOI] [PubMed] [Google Scholar]
- 26.Loos M., Koopmans B., Aarts E., Maroteaux G., van der Sluis S., Verhage M., Smit A. B., and Neuro-BSIK Mouse Phenomics Consortium (2014) Sheltering behavior and locomotor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLoS One 9, e108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen R. S., Küçükosmanoglu A., de Haas M., Sapthu S., Otero J. A., Hegman I. E., Bergen A. A., Gorgels T. G., Borst P., and van de Wetering K. (2013) ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. U.S.A. 110, 20206–20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith C. A., Want E. J., O'Maille G., Abagyan R., and Siuzdak G. (2006) XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 [DOI] [PubMed] [Google Scholar]
- 29.Patti G. J., Tautenhahn R., and Siuzdak G. (2012) Meta-analysis of untargeted metabolomic data from multiple profiling experiments. Nat. Protoc. 7, 508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith C. A., O'Maille G., Want E. J., Qin C., Trauger S. A., Brandon T. R., Custodio D. E., Abagyan R., and Siuzdak G. (2005) METLIN: a metabolite mass spectral database. Ther. Drug Monit. 27, 747–751 [DOI] [PubMed] [Google Scholar]
- 31.Wishart D. S., Knox C., Guo A. C., Eisner R., Young N., Gautam B., Hau D. D., Psychogios N., Dong E., Bouatra S., Mandal R., Sinelnikov I., Xia J., Jia L., Cruz J. A., Lim E., Sobsey C. A., Shrivastava S., Huang P., Liu P., Fang L., Peng J., Fradette R., Cheng D., Tzur D., Clements M., Lewis A., De Souza A., Zuniga A., Dawe M., Xiong Y., Clive D., Greiner R., Nazyrova A., Shaykhutdinov R., Li L., Vogel H. J., and Forsythe I. (2009) HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res. 37, D603–D610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horai H., Arita M., Kanaya S., Nihei Y., Ikeda T., Suwa K., Ojima Y., Tanaka K., Tanaka S., Aoshima K., Oda Y., Kakazu Y., Kusano M., Tohge T., Matsuda F., Sawada Y., Hirai M. Y., Nakanishi H., Ikeda K., Akimoto N., Maoka T., Takahashi H., Ara T., Sakurai N., Suzuki H., Shibata D., Neumann S., Iida T., Tanaka K., Funatsu K., Matsuura F., Soga T., Taguchi R., Saito K., and Nishioka T. (2010) MassBank: a public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 45, 703–714 [DOI] [PubMed] [Google Scholar]
- 33.Böcker S., Letzel M. C., Lipták Z., and Pervukhin A. (2009) SIRIUS: decomposing isotope patterns for metabolite identification. Bioinformatics 25, 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasche F., Svatos A., Maddula R. K., Böttcher C., and Böcker S. (2011) Computing fragmentation trees from tandem mass spectrometry data. Anal. Chem. 83, 1243–1251 [DOI] [PubMed] [Google Scholar]
- 35.El-Sheikh A. A., van den Heuvel J. J, Koenderink J. B., and Russel F. G. (2007) Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J. Pharmacol. Exp. Ther. 320, 229–235 [DOI] [PubMed] [Google Scholar]
- 36.van de Wetering K., Zelcer N., Kuil A., Feddema W., Hillebrand M., Vlaming M. L., Schinkel A. H., Beijnen J. H., and Borst P. (2007) Multidrug resistance proteins 2 and 3 provide alternative routes for hepatic excretion of morphine-glucuronides. Mol. Pharmacol. 72, 387–394 [DOI] [PubMed] [Google Scholar]
- 37.Haug K., Salek R. M., Conesa P., Hastings J., de Matos P., Rijnbeek M., Mahendraker T., Williams M., Neumann S., Rocca-Serra P., Maguire E., González-Beltrán A., Sansone S.-A., Griffin J. L., and Steinbeck C. (2013) MetaboLights: An open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 41, D781–D786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tautenhahn R., Patti G. J., Kalisiak E., Miyamoto T., Schmidt M., Lo F. Y., McBee J., Baliga N. S., and Siuzdak G. (2011) metaXCMS: second-order analysis of untargeted metabolomics data. Anal. Chem. 83, 696–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodder-Gadaczek J., Becker I., Gieselmann V., Wang-Eckhardt L., and Eckhardt M. (2011) N-Acetylaspartylglutamate synthetase II synthesizes N-acetylaspartylglutamylglutamate. J. Biol. Chem. 286, 16693–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuno H. (2011) Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neurosci. Res. 69, 175–186 [DOI] [PubMed] [Google Scholar]
- 41.Lerea L. S. (1997) Glutamate receptors and gene induction: signalling from receptor to nucleus. Cell Signal. 9, 219–226 [DOI] [PubMed] [Google Scholar]
- 42.Soriano M. A., Tessier M., Certa U., and Gill R. (2000) Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J. Cereb. Blood Flow Metab. 20, 1045–1055 [DOI] [PubMed] [Google Scholar]
- 43.Cullinan W. E., Herman J. P., Battaglia D. F., Akil H., and Watson S. J. (1995) Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505 [DOI] [PubMed] [Google Scholar]
- 44.Danbolt N. C. (2001) Glutamate uptake. Prog. Neurobiol. 65, 1–105 [DOI] [PubMed] [Google Scholar]
- 45.Meldrum B. S. (2000) Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 130, 1007S–15S [DOI] [PubMed] [Google Scholar]
- 46.Bergeron R., and Coyle J. T. (2012) NAAG, NMDA receptor and psychosis. Curr. Med. Chem. 19, 1360–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neale J. H., Olszewski R. T., Zuo D., Janczura K. J., Profaci C. P., Lavin K. M., Madore J. C., and Bzdega T. (2011) Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J. Neurochem. 118, 490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowland L. M., Kontson K., West J., Edden R. A., Zhu H., Wijtenburg S. A., Holcomb H. H., and Barker P. B. (2013) In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr. Bull. 39, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berger M. L., Lefauconnier J. M., Tremblay E., and Ben-Ari Y. (1986) Limbic seizures induced by systemically applied kainic acid: how much kainic acid reaches the brain? Adv. Exp. Med. Biol. 203, 199–209 [DOI] [PubMed] [Google Scholar]
- 50.Preston E., and Hynie I. (1991) Transfer constants for blood-brain barrier permeation of the neuroexcitatory shellfish toxin, domoic acid. Can. J. Neurol. Sci. 18, 39–44 [DOI] [PubMed] [Google Scholar]
- 51.Neale J. H., Bzdega T., and Wroblewska B. (2000) N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J. Neurochem. 75, 443–452 [DOI] [PubMed] [Google Scholar]
- 52.Lodder-Gadaczek J., Gieselmann V., and Eckhardt M. (2013) Vesicular uptake of N-acetylaspartylglutamate is catalysed by sialin (SLC17A5). Biochem. J. 454, 31–38 [DOI] [PubMed] [Google Scholar]
- 53.Williamson L. C., and Neale J. H. (1988) Calcium-dependent release of N-acetylaspartylglutamate from retinal neurons upon depolarization. Brain Res. 475, 151–155 [DOI] [PubMed] [Google Scholar]
- 54.Coyle J. T. (1997) The nagging question of the function of N-acetylaspartylglutamate. Neurobiol. Dis. 4, 231–238 [DOI] [PubMed] [Google Scholar]
- 55.Sácha P., Zámecník J., Barinka C., Hlouchová K., Vícha A., Mlcochová P., Hilgert I., Eckschlager T., and Konvalinka J. (2007) Expression of glutamate carboxypeptidase II in human brain. Neuroscience 144, 1361–1372 [DOI] [PubMed] [Google Scholar]
- 56.Slusher B. S., Vornov J. J., Thomas A. G., Hurn P. D., Harukuni I., Bhardwaj A., Traystman R. J., Robinson M. B., Britton P., Lu X. C., Tortella F. C., Wozniak K. M., Yudkoff M., Potter B. M., and Jackson P. F. (1999) Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat. Med. 5, 1396–1402 [DOI] [PubMed] [Google Scholar]
- 57.Zhou J., Neale J. H., Pomper M. G., and Kozikowski A. P. (2005) NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat. Rev. Drug Discov. 4, 1015–1026 [DOI] [PubMed] [Google Scholar]
- 58.Collard F., Vertommen D., Constantinescu S., Buts L., and Van Schaftingen E. (2011) Molecular identification of β-citrylglutamate hydrolase as glutamate carboxypeptidase 3. J. Biol. Chem. 286, 38220–38230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T.-M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H.-W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K.-R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., and Jones A. R. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 [DOI] [PubMed] [Google Scholar]
- 60.Miyake M., Kakimoto Y., and Sorimachi M. (1978) Isolation and identification of β-citryl-l-glutamic acid from newborn rat brain. Biochim. Biophys. Acta 544, 656–666 [DOI] [PubMed] [Google Scholar]
- 61.Miyake M., and Kakimoto Y. (1981) Developmental changes of N-acetyl-l-aspartic acid, N-acetyl-α-aspartylglutamic acid and β-citryl-l-glutamic acid in different brain regions and spinal cords of rat and guinea pig. J. Neurochem. 37, 1064–1067 [DOI] [PubMed] [Google Scholar]
- 62.Miyake M., Kume S., and Kakimoto Y. (1982) Correlation of the level of β-citryl-l-glutamic acid with spermatogenesis in rat testes. Biochim. Biophys. Acta 719, 495–500 [DOI] [PubMed] [Google Scholar]
- 63.Miyake M., and Morino H. (1992) Developmental changes in β-citryl-l-glutamate concentration and its synthetic and hydrolytic activities in neuronal cells cultured from chick embryo optic lobes. J. Neurochem. 59, 1654–1660 [DOI] [PubMed] [Google Scholar]
- 64.Narahara M., Tachibana K., Kurisu N., Kanazawa M., and Miyake M. (2000) Immunohistochemical and chemical changes of β-citryl-l-glutamate in the differentiation of bovine lens epithelial cells into lens fiber cells. Biol. Pharm. Bull. 23, 704–707 [DOI] [PubMed] [Google Scholar]
- 65.Tsumori M., Asakura M., Narahara M., Ogawa T., Nakae M., Nakagawa S., Kawai Y., Morino H., Hama T., and Miyake M. (1995) Presence of β-citryl-l-glutamic acid in the lens: its possible role in the differentiation of lens epithelial cells into fiber cells. Exp. Eye Res. 61, 403–411 [DOI] [PubMed] [Google Scholar]
- 66.Hamada-Kanazawa M., Kouda M., Odani A., Matsuyama K., Kanazawa K., Hasegawa T., Narahara M., and Miyake M. (2010) β-Citryl-l-glutamate is an endogenous iron chelator that occurs naturally in the developing brain. Biol. Pharm. Bull. 33, 729–737 [DOI] [PubMed] [Google Scholar]
- 67.Hamada-Kanazawa M., Narahara M., Takano M., Min K.-S., Tanaka K., and Miyake M. (2013) Nitric oxide promotes survival of cerebral cortex neurons with simultaneous addition of [Fe(II)(β-citryl-l-glutamate)] complex in primary culture. Biol. Pharm. Bull. 36, 1068–1079 [DOI] [PubMed] [Google Scholar]
- 68.Narahara M., Hamada-Kanazawa M., Kouda M., Odani A., and Miyake M. (2010) Superoxide scavenging and xanthine oxidase inhibiting activities of copper-β-citryl-l-glutamate complex. Biol. Pharm. Bull. 33, 1938–1943 [DOI] [PubMed] [Google Scholar]
- 69.Van den Berghe G., Vincent M. F., and Jaeken J. (1997) Inborn errors of the purine nucleotide cycle: adenylosuccinase deficiency. J. Inherit. Metab. Dis. 20, 193–202 [DOI] [PubMed] [Google Scholar]
- 70.Stone T. W., Roberts L. A., Morris B. J., Jones P. A., Ogilvy H. A., Behan W. M., Duley J. A., Simmonds H. A., Vincent M. F., and van den Berghe G. (1998) Succinylpurines induce neuronal damage in the rat brain. Adv. Exp. Med. Biol. 431, 185–189 [DOI] [PubMed] [Google Scholar]
- 71.Zuo D., Bzdega T., Olszewski R. T., Moffett J. R., and Neale J. H. (2012) Effects of N-acetylaspartylglutamate (NAAG) peptidase inhibition on release of glutamate and dopamine in prefrontal cortex and nucleus accumbens in phencyclidine model of schizophrenia. J. Biol. Chem. 287, 21773–21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han L., Picker J. D., Schaevitz L. R., Tsai G., Feng J., Jiang Z., Chu H. C., Basu A. C., Berger-Sweeney J., and Coyle J. T. (2009) Phenotypic characterization of mice heterozygous for a null mutation of glutamate carboxypeptidase II. Synapse 63, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao Y., Xu S., Cui Z., Zhang M., Lin Y., Cai L., Wang Z., Luo X., Zheng Y., Wang Y., Luo Q., Jiang J., Neale J. H., and Zhong C. (2015) Mice lacking glutamate carboxypeptidase II develop normally, but are less susceptible to traumatic brain injury. J. Neurochem. 134, 340–353 [DOI] [PubMed] [Google Scholar]
- 74.1000 Genomes Project Consortium, Abecasis G. R., Auton A., Brooks L. D., DePristo M. A., Durbin R. M., Handsaker R. E., Kang H. M., Marth G. T., and McVean G. A. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Plechanovová A., Byun Y., Alquicer G., Skultétyová L., Mlčochová P., Němcová A., Kim H.-J., Navrátil M., Mease R., Lubkowski J., Pomper M., Konvalinka J., Rulíšek L., and Bařinka C. (2011) Novel substrate-based inhibitors of human glutamate carboxypeptidase II with enhanced lipophilicity. J. Med. Chem. 54, 7535–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Cairasco N., Moyses-Neto M., Del Vecchio F., Oliveira J. A., dos Santos F. L., Castro O. W., Arisi G. M., Dantas M., Carolino R. O., Coutinho-Netto J., Dagostin A. L., Rodrigues M. C., Leão R. M., Quintiliano S. A., Silva L. F. Jr., Gobbo-Neto L., and Lopes N. P. (2013) Elucidating the neurotoxicity of the star fruit. Angew. Chem. Int. Ed. Engl. 52, 13067–13070 [DOI] [PubMed] [Google Scholar]
- 77.Krogsgaard-Larsen P., and Hansen J. J. (1992) Naturally-occurring excitatory amino acids as neurotoxins and leads in drug design. Toxicol. Lett. 64–65, 409–416 [DOI] [PubMed] [Google Scholar]
- 78.Olney J. W. (1994) Excitotoxins in foods. Neurotoxicology 15, 535–544 [PubMed] [Google Scholar]