Abstract
The liver hormone hepcidin is the central regulator of systemic iron metabolism. Its increased expression in inflammatory states leads to hypoferremia and anemia. Elucidation of the mechanisms that up-regulate hepcidin during inflammation is essential for developing rational therapies for this anemia. Using mouse models of inflammatory bowel disease, we have shown previously that colitis-associated hepcidin induction is influenced by intestinal microbiota composition. Here we investigate how two commensal bacteria, Bifidobacterium longum and Bacteroides fragilis, representative members of the gut microbiota, affect hepcidin expression. We found that supernatants of a human macrophage cell line infected with either of the bacteria up-regulated hepcidin when added to a human hepatocyte cell line. This activity was abrogated by neutralization of IL-1β. Moreover, purified IL-1β increased hepcidin expression when added to the hepatocyte line or primary human hepatocytes and when injected into mice. IL-1β activated the bone morphogenetic protein (BMP) signaling pathway in hepatocytes and in mouse liver, as indicated by increased phosphorylation of small mothers against decapentaplegic proteins. Activation of BMP signaling correlated with IL-1β-induced expression of BMP2 in human hepatocytes and activin B in mouse liver. Treatment of hepatocytes with two different chemical inhibitors of BMP signaling or with a neutralizing antibody to BMP2 prevented IL-1β-induced up-regulation of hepcidin. Our results clarify how commensal bacteria affect hepcidin expression and reveal a novel connection between IL-1β and activation of BMP signaling. They also suggest that there may be differences between mice and humans with respect to the mechanism by which IL-1β up-regulates hepcidin.
Keywords: bacteria, hepatocyte, inflammation, IL-1, iron metabolism, macrophage, hepcidin
Introduction
Hepcidin is a 25-amino acid peptide hormone that is secreted by the liver and functions as the key regulator of systemic iron homeostasis (1). Its expression in hepatocytes is regulated exclusively at the level of transcription and is sensitive to a number of inputs, including tissue and plasma iron concentrations. Although the exact mechanism by which tissue iron concentrations are detected is not clear, it is known that elevated hepatic iron concentrations lead to increased expression of bone morphogenetic protein 6 (BMP6) (2). BMP6 acts on the BMP3 receptor and its co-receptor hemojuvelin on the hepatocyte surface to activate signals that lead to phosphorylation of the receptor-associated proteins small mothers against decapentaplegic (SMAD) 1, 5, and 8, which then interact with SMAD4. The complex of SMAD4 and the phosphorylated receptor-associated SMAD translocates to the nucleus, binds to specific sites in the hepcidin promoter, and increases transcription (3, 4). Circulating iron levels are sensed by a mechanism involving interactions between the type II transferrin receptor and the hemochromatosis protein HFE, both of which are expressed on the hepatocyte plasma membrane (5). Elevated plasma iron induces the two proteins to associate, leading to increased activation of the BMP signaling pathway and up-regulation of hepcidin transcription. Hepcidin binds to and induces the degradation of the iron transport protein ferroportin (FPN), which is expressed on the plasma membrane of macrophages and the basolateral surface of duodenal enterocytes and is the sole means by which iron recycled from erythrocytes and absorbed from the diet, respectively, enters the circulation (6, 7). Therefore, the effect of the increased expression of hepcidin induced by elevated tissue and plasma iron concentrations is to down-regulate FPN, thereby reducing the amount of iron released into the blood and restoring homeostasis. Conversely, when tissue and plasma iron levels are low, hepcidin expression is decreased, allowing more iron to enter the circulation as a result of increased FPN expression. The hepcidin-FPN axis is therefore a critical component of a negative feedback loop that maintains iron concentrations within a narrow physiologic range (1).
Hepcidin expression is also increased in the context of inflammation, including in conditions such as inflammatory bowel disease (IBD), rheumatoid arthritis, and various infections (8, 9). The abnormally elevated hepcidin levels lead to a drop in plasma iron concentrations because of FPN down-regulation. When this situation persists, it compromises erythropoiesis, resulting in the development of the so-called anemia of inflammation (AI) (9). Because AI can negatively affect quality of life and may require specific treatment beyond management of the underlying inflammatory process, there is a great deal of interest in understanding the factors and mechanisms involved in inflammation-associated up-regulation of hepcidin. In relatively simple experimental models of inflammation, such as those involving the injection of LPS or turpentine, the cytokine IL-6 has been shown to play an important role in hepcidin up-regulation because of its ability to activate signals that phosphorylate STAT3, which then dimerizes and binds to the hepcidin promoter to increase transcription (10–13). However, the factors that influence hepcidin expression in more complex, clinically relevant inflammatory disorders are not very clear. Our studies have tried to shed light on this issue by characterizing hepcidin expression in mouse models of IBD (14–16). We have shown recently that dextran sulfate sodium-induced colitis in wild-type mice was associated with decreased expression of hepcidin, whereas dextran sulfate sodium-induced colitis in IL-10-deficient mice resulted in hepcidin up-regulation. Strikingly, however, when the IL-10-deficient animals were co-caged with wild-type mice for 2 weeks prior to the induction of colitis or were given a wild-type fecal transplant, hepcidin up-regulation was inhibited (16). These results indicate that differences in the commensal bacterial communities that constitute the gut microbiota have a strong influence on inflammation-induced expression of hepcidin. This effect could not be explained by differences in colonic levels of inflammatory cytokines known to alter hepcidin expression, such as IL-6 and TNFα (15, 16). Because there is little or no information on how commensal bacteria might directly or indirectly affect hepcidin expression, we carried out experiments to clarify this issue.
Experimental Procedures
Infection of Differentiated THP-1 Monocyte-Macrophages with Bifidobacterium longum and Bacteroides fragilis
The human monocyte cell line THP-1 was obtained from the American Type Culture Collection and maintained at 37 °C in 5% CO2 in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. Prior to infection, the cells were seeded in tissue culture plates at a density of 106 cells/ml and differentiated into macrophages by treatment with 50 ng/ml of phorbol 12-myristate 13-acetate for 72 h. The differentiated cells were washed twice with PBS and placed in serum- and antibiotic-free medium prior to infection. B. longum and B. fragilis (provided by Dr. Deepak Vijaykumar, Massachusetts General Hospital) were grown anaerobically at 37 °C for 20–22 h in broth cultures of dMan Rogosa Sharpe medium and reinforced clostridial medium, respectively. The dehydrated media were obtained from BD Biosciences and prepared according to the recommendations of the manufacturer. On the basis of the optical density and the results of preliminary titering experiments, appropriate volumes of the B. longum and B. fragilis cultures were centrifuged, and the bacterial pellets were washed with PBS and resuspended in antibiotic- and serum-free RPMI 1640 medium. Fifty million bacteria were added to the differentiated THP-1 cells (multiplicity of infection, 50:1). The infection was allowed to proceed for 1 h at 37 °C, after which the cells were washed twice with PBS and then incubated for a further 4 h at 37 °C in antibiotic- and serum-free medium. The cell supernatants were collected at the end of the 4 h, filter-sterilized by passage through a 0.2-μm filter, and stored in aliquots at −20 °C.
Stimulation of HuH7 Hepatocytes
The human hepatoma cell line HuH7 (provided by Dr. Raymond Chung, Massachusetts General Hospital) was maintained at 37 °C in 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. Prior to stimulation, the cells were seeded at 106 cells/ml in 24-well tissue culture plates. After 24 h, the cells were washed twice with PBS and incubated overnight in serum-free medium. In preliminary experiments, the serum-starved cells were stimulated directly with live bacteria in the same way as the THP-1 cells or for 4 h with purified Toll-like receptor (TLR) ligands (5 μg/ml Pam3Cys, 1 μg/ml LPS, and 1 μg/ml flagellin, all from Invivogen, San Diego, CA). In subsequent studies, the serum-starved cells were stimulated for the indicated times in individual experiments with 0.5 ml of supernatant from control or infected THP-1 cells or with 10 ng/ml recombinant human IL-1β (R&D Systems, Minneapolis, MN). In some experiments, the cells were also treated with the BMP signaling inhibitors (17, 18) dorsomorphin (5 μm) and LDN193189 (500 nm) or an equivalent volume of the vehicle DMSO, 5 μg/ml of a monoclonal neutralizing anti-IL-1β antibody (Invivogen), 7.5 μg/ml of a monoclonal anti-BMP2/BMP4-neutralizing antibody (R&D Systems, Minneapolis, MN), or equivalent amounts of the corresponding isotype-matched irrelevant antibodies. Following stimulation under the different conditions, cell supernatants were collected for analysis by ELISA, and the cells were washed with PBS and lysed with TRIzol reagent (Life Technologies) for preparation of RNA or with radioimmune precipitation assay buffer with mixtures of protease and phosphatase inhibitors (19) for protein extraction.
Primary Human Hepatocytes
Primary human hepatocytes were obtained from the Cell Resource Core at Massachusetts General Hospital. The hepatocytes were prepared by the Core from healthy whole livers donated for research purposes using a two-step collagenase perfusion procedure (20). Purity of the isolated hepatocytes was >99%, as assessed by morphology, with 80–90% viability, as measured by trypan blue exclusion. The hepatocytes were cultured overnight in serum-free medium and stimulated as described above for the HuH7 cells, and then TRIzol extracts were prepared. In some experiments, the cells were treated with anti-BMP2/BMP4 or control antibodies as described above.
Mouse Experiments
Six-week-old, female, wild-type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions in the vivarium at Massachusetts General Hospital. Groups of animals were injected intraperitoneally with a single dose of 250 ng of recombinant murine IL-1β (R&D Systems) diluted in PBS or, as a control, with just PBS and then euthanized at the indicated times in the specific experiment. At necropsy, blood was collected in serum separator tubes, and the serum was used for estimation of iron concentrations as described below. Samples of liver were also collected and homogenized in TRIzol reagent or radioimmune precipitation assay buffer with protease and phosphatase inhibitors for preparation of RNA and protein extracts, respectively. All studies with mice were reviewed and approved by the Institutional Animal Care and Use Committee.
Quantitative RT-PCR
Total RNA was prepared from TRIzol extracts according to the recommendations of the manufacturer. It was reverse-transcribed with random hexamers and amplified with gene-specific primers as described in detail previously (21). Expression of the transcript of interest is shown relative to that of actin or GAPDH using the 2−ΔCt method. Primers for amplification of human and mouse hepcidin; mouse BMP2, BMP4, and BMP6; mouse activin B; human actin; and mouse GAPDH have been published previously (2, 15, 21, 22). The primers used for amplification of human BMP2, BMP4, BMP6, and activin B were as follows: BMP2 forward, 5′-ACCCGCTGTCTTCTAGCGT-3′; BMP2 reverse, 5′-TTTCAGGCCGAACATGCTGAG-3′; BMP4 forward, 5′-TGGTCTTGAGTATCCTGAGCG-3′; BMP4 reverse, 5′-GCTGAGGTTAAAGAGGAAACGA-3′; BMP6 forward, 5′-AGCGACACCACAAAGAGTTCA-3′; BMP6 reverse, 5′-GCTGATGCTCCTGTAAGACTTGA-3′; activin B forward, 5′-CGGGTCCGCCTATACTTCTTC-3′; and activin B reverse, 5′-CGTAGGGCAGGAGTTTCAGG-3′.
ELISA
IL-1β and IL-6 concentrations in cell supernatants were measured using kits from eBioscience (San Diego, CA) and Biolegend (San Diego, CA), respectively, and BMP2 was measured in cell lysates and supernatants using the Quantikine ELISA kit from R&D Systems. Protocols provided by the manufacturers were followed in all cases. Cell lysates used for BMP2 estimation were prepared with 0.1% Triton X-100-containing buffer.
Western Blotting
Protein extracts were subjected to SDS-PAGE after normalizing protein concentrations and then transferred to a nitrocellulose membrane by semidry blotting as described in detail previously (15). The membrane was blocked and then incubated overnight with antibodies specific for either phosphorylated SMAD1/5/8 or total SMAD1 (both from Cell Signaling Technology, Beverly, MA). The blot was developed with the relevant fluorescently tagged secondary antibodies, and the signals were analyzed using an Odyssey infrared fluorescence imaging system (Li-Cor Biosciences, Lincoln, NE). The signal intensities of the phosphorylated SMAD1/5/8 bands from each blot were normalized to the intensities of the corresponding total SMAD1 bands by calculating the pSMAD/SMAD1 ratio. The means of the normalized intensities ± S.E. from the blots of multiple experiments are displayed, along with a representative blot from one of the experiments.
Estimation of Serum Iron Concentrations
Serum iron concentrations were measured as described previously (23) using a colorimetric assay kit from Thermo Scientific (Tewksbury, MA).
Statistical Analysis
Macrophage infections and hepatocyte stimulations were carried out in triplicate in each experiment, and each experiment was performed at least twice. Mouse studies involved 3–5 animals/group. The results are displayed as the means ± S.E. on the basis of replicates from a single experiment or pooled from multiple experiments as indicated in the figures. Two-tailed, unpaired Student's t test was used to assess significance, with p < 0.05 considered statistically significant. Statistically significant differences are indicated with asterisks in the figures, whereas the p values and sample numbers (n) are specified in the figure legends.
Results
On the basis of our recent results indicating a clear effect of gut microbiota composition on colitis-associated hepcidin up-regulation (16), we initiated a series of experiments to examine the effects of commensal bacteria on hepcidin expression. Our studies were predicated on the idea that, during colitis, commensal bacteria or their products are translocated across the damaged intestinal epithelium and act on hepatocytes, either directly or indirectly, to induce hepcidin expression. In initial experiments, we exposed the HuH7 hepatocyte cell line to representative live commensal bacteria, the Gram-positive B. longum or the Gram-negative B. fragilis, significant components of the human infant and adult intestinal microbiota, respectively (24). This direct interaction between the hepatocytes and the bacteria did not alter hepcidin expression (Fig. 1A). There was also no change in hepcidin expression when HuH7 cells were treated with bacterial ligands for TLR2 (Pam3Cys), TLR4 (LPS), or TLR5 (flagellin) (Fig. 1B). We then considered the possibility that translocated bacteria or bacterial products might be sensed initially by cells of the innate immune system residing in the colonic lamina propria or liver, which would then secrete molecules that could act on hepatocytes to alter hepcidin expression. Accordingly, we differentiated the THP-1 human monocyte cell line into macrophages with phorbol 12-myristate 13-acetate and then infected the cells with either B. longum or B. fragilis. Cell- and bacteria-free supernatants of the control and infected THP-1 macrophages were then added to HuH7 hepatocytes for 4 h, and the effect on hepcidin expression was measured by quantitative RT-PCR. As shown in Fig. 1C, supernatants of both B. longum- and B. fragilis-infected THP-1 cells induced significant increases in hepcidin expression in HuH7 cells relative to the supernatant of uninfected THP-1 cells.
FIGURE 1.
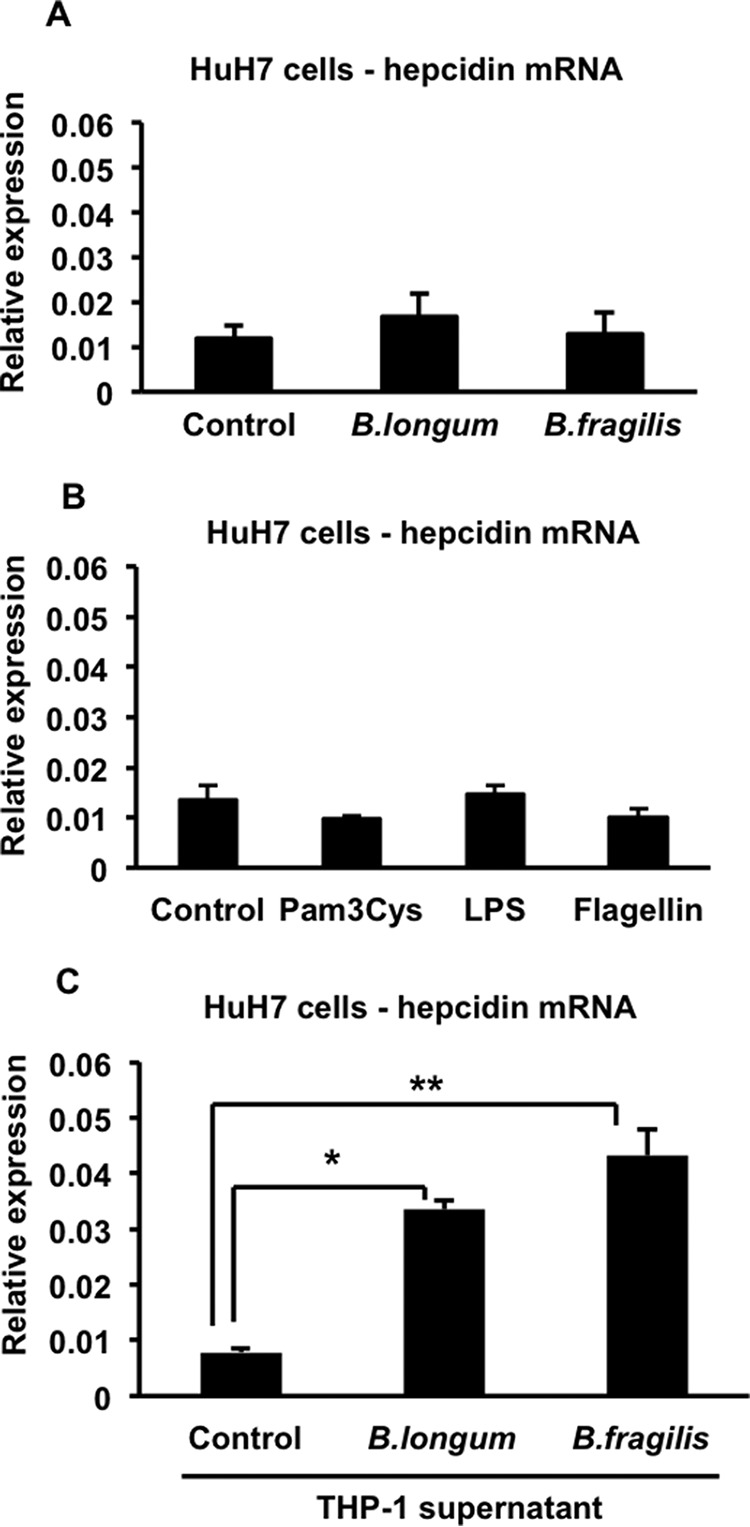
Effects of direct or indirect bacterial stimulation of HuH7 cells on hepcidin expression. A, HuH7 cells were infected with B. longum or B. fragilis for 1 h, and then they were washed and incubated for another 4 h. Total RNA was prepared and used to assess hepcidin expression by quantitative RT-PCR. n = 5–6 samples/group from two experiments. B, HuH7 cells were treated for 4 h with 5 μg/ml of Pam3Cys, 1 μg/ml of LPS, or 1 μg/ml of flagellin. Total RNA was prepared and used to assess hepcidin expression by quantitative RT-PCR. n = 3 samples/group from one of two similar experiments. C, HuH7 cells were treated with control supernatant or supernatants of B. longum- or B. fragilis-infected THP-1 cells for 4 h. Total RNA was prepared from HuH7 cells and used to assess hepcidin expression by quantitative RT-PCR. *, p = 0.0001; **, p = 0.0001; n = 10–13 samples/group from multiple experiments.
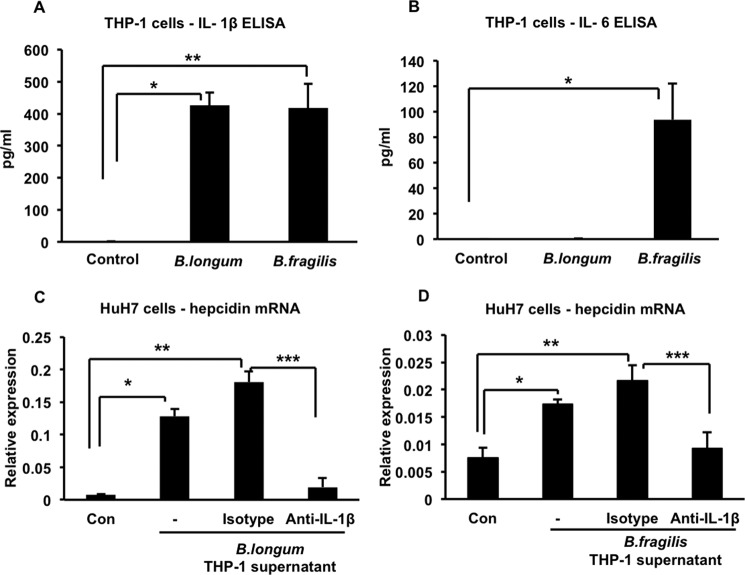
We examined the supernatants of the infected THP-1 cells for the presence of cytokines that might influence hepcidin expression and found that infection with both B. longum and B. fragilis induced the production of significant amounts of IL-1β (Fig. 2A). IL-6, the cytokine that has been associated most often with hepcidin up-regulation (10–13), was induced only by B. fragilis (Fig. 2B), indicating that it could not explain the hepcidin up-regulating activity present in the supernatant of B. longum-infected THP-1 cells. Importantly, we found that treating the supernatant of B. longum-infected THP-1 cells with 5 μg/ml of a monoclonal anti-IL-1β-neutralizing antibody significantly inhibited its ability to up-regulate hepcidin in HuH7 cells, whereas an equivalent amount of an isotype-matched irrelevant antibody did not have this effect (Fig. 2C). Similar results were obtained when neutralizing IL-1β in the supernatant of B. fragilis-infected THP-1 cells (Fig. 2D). These results indicate that B. longum- or B. fragilis-induced IL-1β secreted by THP-1 cells is required for up-regulation of hepcidin in HuH7 cells (even in the presence of IL-6 in the case of B. fragilis).
FIGURE 2.
IL-1β is involved in up-regulating hepcidin expression in HuH7 cells. A, supernatants of control, B. longum-, and B. fragilis-infected THP-1 cells were analyzed for the presence of IL-1β by ELISA. *, p = 0.0001; **, p = 0.005; n = 5–9 samples/group from multiple experiments. B, supernatants of control, B. longum-, and B. fragilis-infected THP-1 cells were analyzed for the presence of IL-6 by ELISA. *, p = 0.008; n = 9–12 samples/group from multiple experiments. C, HuH7 cells were treated for 4 h with the supernatant of B. longum-infected THP-1 cells (−) or with the supernatant in the presence of 5 μg/ml of either a neutralizing anti-IL-1β antibody or an irrelevant isotype-matched control antibody. Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.008; **, p = 0.04; ***, p = 0.008; n = 3 samples/group from one of two similar experiments. Con, control. D, HuH7 cells were treated for 4 h with the supernatant of B. fragilis-infected THP-1 cells (−) or with the supernatant in the presence of 5 μg/ml of either a neutralizing anti-IL-1β antibody or an irrelevant isotype-matched control antibody. Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.003; **, p = 0.01; ***, p = 0.01; n = 3 samples/group from one of two similar experiments.
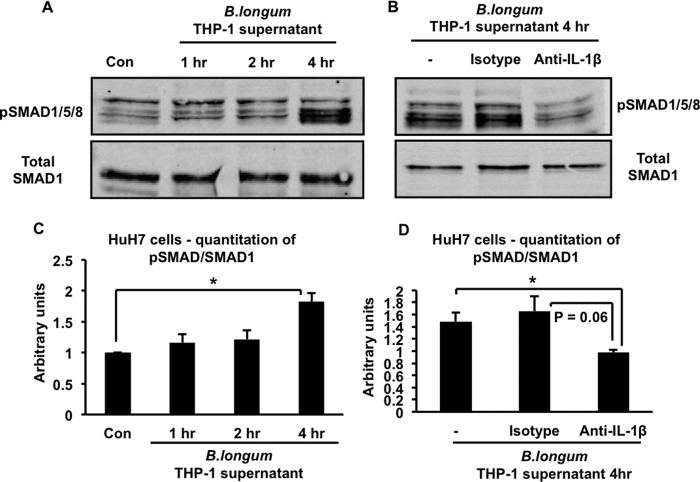
We proceeded to carry out experiments to clarify the mechanisms involved in the IL-1β-induced up-regulation of hepcidin. We were particularly interested in the potential involvement of the BMP signaling pathway because our previous experiments have shown that inhibition of this pathway prevented hepcidin up-regulation in the context of both infectious and non-infectious intestinal inflammation in vivo, including in multiple mouse models of IBD (14, 21). Therefore, we used immunoblotting with antibodies specific for phosphorylated SMAD1/5/8 and total SMAD1 to examine the effect of the supernatant of B. longum-infected THP-1 macrophages on phosphorylation of the BMP receptor-associated SMADs in HuH7 cells (4). As shown in the representative Western blot in Fig. 3A and the corresponding quantitation of several such blots in Fig. 3C, the supernatant induced a time-dependent increase in the phosphorylation of the receptor-associated SMADs. Neutralizing IL-1β in the supernatant of B. longum-infected THP-1 cells inhibited this increase in SMAD1/5/8 phosphorylation (Fig. 3B, the corresponding quantitation of several such blots is shown in Fig. 3D). In keeping with the requirement for IL-1β in the ability of the supernatants of infected THP-1 cells to increase hepcidin expression and activate BMP signaling, we found that treatment of HuH7 cells with 10 ng/ml of recombinant IL-1β was sufficient to induce a significant up-regulation of hepcidin at 2 and 4 h (Fig. 4A). Moreover, IL-1β at either 1 or 10 ng/ml increased phosphorylation of SMAD1/5/8 (Fig. 4B). Quantitation of immunoblots from several such experiments indicated that the increase in SMAD1/5/8 phosphorylation induced by 10 ng/ml of IL-1β was detectable by 2 h and became significant at 4 h (Fig. 4C). The inability to detect a significant increase in SMAD1/5/8 phosphorylation at the 2-h time point (when hepcidin is increased significantly) is probably related to the lower sensitivity of Western blotting in detecting changes in SMAD1/5/8 phosphorylation relative to the ability of quantitative RT-PCR to detect changes in hepcidin expression.
FIGURE 3.
IL-1β is involved in activating the BMP signaling pathway in HuH7 cells. A, HuH7 cells were treated with the supernatant of B. longum-infected THP-1 cells for the indicated times. Cell lysates were analyzed by SDS-PAGE and immunoblotting with antibodies to either phosphorylated SMAD1/5/8 (pSMAD1/5/8) or total SMAD1. Con, control. B, HuH7 cells were treated for 4 h with the supernatant of B. longum-infected THP-1 cells (−) or with the supernatant in the presence of 5 μg/ml of either a neutralizing anti-IL-1β antibody or an irrelevant isotype-matched control antibody. Cell lysates were analyzed by SDS-PAGE and immunoblotting with antibodies to either phosphorylated SMAD1/5/8 (pSMAD1/5/8) or total SMAD1. C, quantitation of Western blots corresponding to A. The intensities of the phosphorylated SMAD1/5/8 and total SMAD1 bands in blots from multiple experiments were determined by Li-Cor imaging, and the ratio was calculated. *, p = 0.004; n = 3–5 samples/group from multiple experiments. D, quantitation of Western blots corresponding to B. The intensities of the phosphorylated SMAD1/5/8 and total SMAD1 bands in blots from multiple experiments were determined by Li-Cor imaging, and the ratio was calculated. *, p = 0.006; n = 3–4 samples/group from multiple experiments.
FIGURE 4.
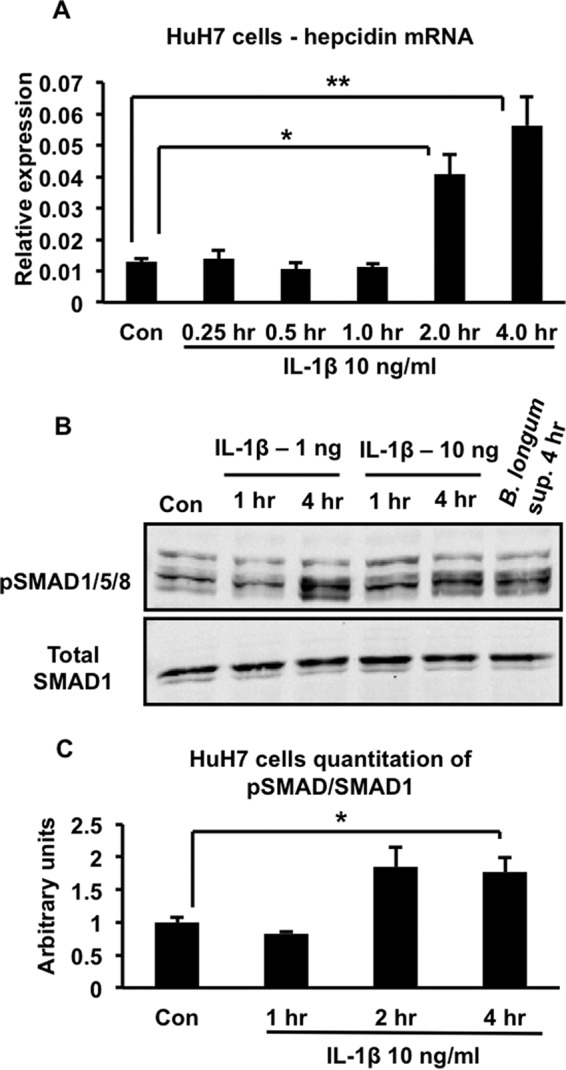
Effects of IL-1β on hepcidin expression and BMP signaling in HuH7 cells. A, HuH7 cells were treated with 10 ng/ml of IL-1β for the indicated times, and hepcidin expression was analyzed by quantitative RT-PCR. *, p = 0.04; **, p = 0.03; n = 3 samples/group from one of two similar experiments. Con, control. B, HuH7 cells were treated with 1 or 10 ng/ml of IL-1β for different times from 1–4 h or with the supernatant of B. longum-infected THP-1 cells for 4 h. Cell lysates were analyzed by SDS-PAGE and immunoblotting with antibodies to either phosphorylated SMAD1/5/8 (pSMAD1/5/8) or total SMAD1. C, quantitation of Western blots corresponding to B. The intensities of the phosphorylated SMAD1/5/8 and total SMAD1 bands in blots from multiple experiments were determined by Li-Cor imaging, and the ratio was calculated. *, p = 0.01; n = 3–5 samples/group from multiple experiments.
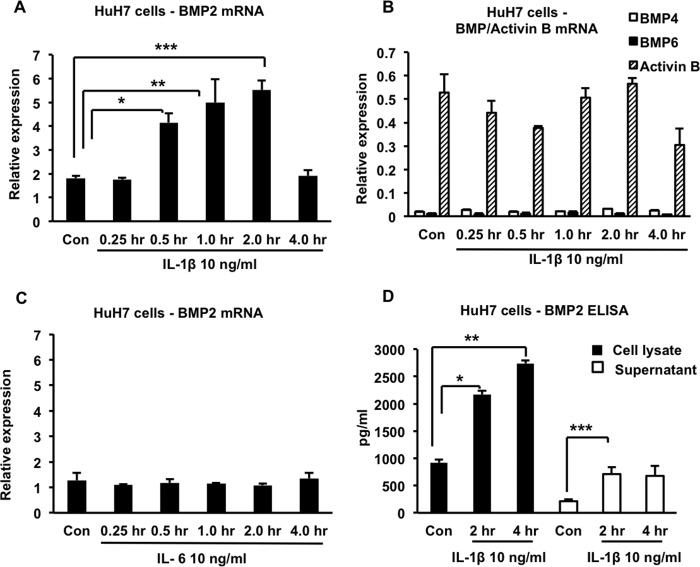
Because IL-1β is not known to activate the BMP signaling pathway directly (25), we considered the possibility that the cytokine induced the expression of another molecule that mediated this activation in an autocrine or paracrine fashion. We focused on BMPs 2, 4, 5, 7, and 9, which have been shown previously to up-regulate hepcidin expression in vitro in hepatocyte cell lines; BMP6, which up-regulates hepcidin in vitro and is also required for hepcidin expression in vivo; and activin B, a member of the TGFβ superfamily that has been implicated in phosphorylation of SMAD1/5/8 and up-regulation of hepcidin in vitro and in vivo (22, 23, 26, 27). Using quantitative RT-PCR, we found that treatment of HuH7 cells with 10 ng/ml of recombinant IL-1β for at least 0.5 h led to a significant increase in BMP2 expression, whereas the expression of BMPs 4, 5, 6, 7, and 9 and activin B did not show any significant changes (Fig. 5, A and B, and data not shown). This effect was specific to IL-1β because treatment of HuH7 cells with 10 ng/ml of IL-6 failed to increase BMP2 expression (Fig. 5C). We confirmed by ELISA that IL-1β increased levels of BMP2 protein in the cell lysates and supernatants of HuH7 cells (Fig. 5D).
FIGURE 5.
Effects of IL-1β and IL-6 on BMP expression in HuH7 cells. A, HuH7 cells were treated with 10 ng/ml of IL-1β for the indicated times, and BMP2 expression was analyzed by quantitative RT-PCR. *, p = 0.02; **, p = 0.03; ***, p = 0.008. Con, control. B, the same RNA samples as in A were analyzed by quantitative RT-PCR for expression of BMP4, BMP6, and activin B. C, HuH7 cells were treated with 10 ng/ml of IL-6 for the indicated times, and BMP2 expression was analyzed by quantitative RT-PCR. D, HuH7 cells were treated with 10 ng/ml of IL-1β for the indicated times, and BMP2 concentrations in the cell lysates and cell supernatants were determined by ELISA. *, p = 0.002; **, p = 0.004; ***, p = 0.04. In all cases, n = 3 samples/group from one of two similar experiments.
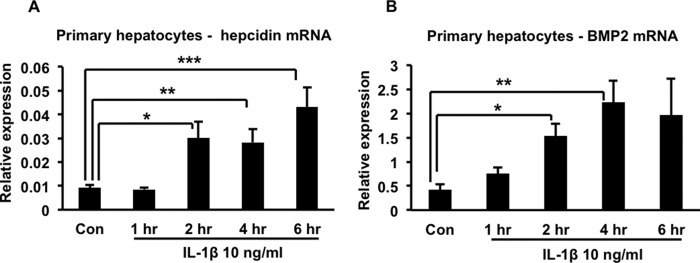
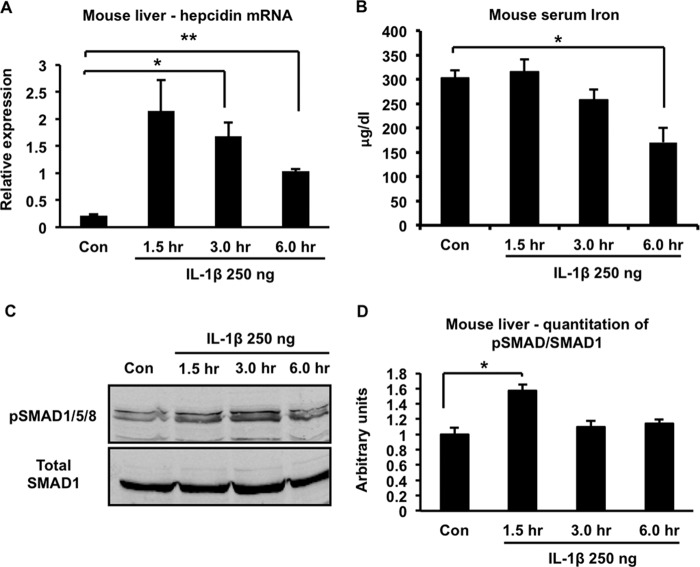
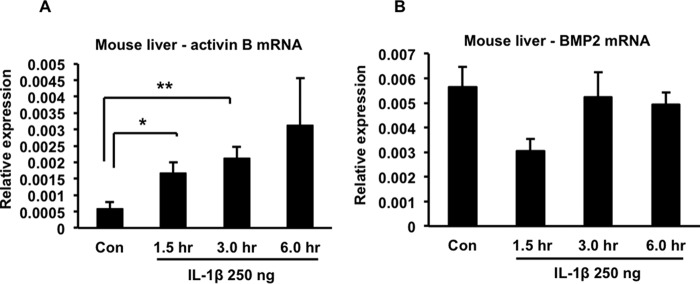
To extend our observations beyond the HuH7 cell line, we studied primary human hepatocytes and confirmed that 10 ng/ml of IL-1β also induced up-regulation of both hepcidin and BMP2 in these cells (Fig. 6, A and B). We then injected mice intraperitoneally with 250 ng of recombinant murine IL-1β per animal and found that it induced a significant increase in liver hepcidin expression that peaked at about 1.5 h after the injection (Fig. 7A). Correspondingly, a significant decline in serum iron concentration was observed 6 h after the injection (Fig. 7B). The IL-1β-induced up-regulation of hepcidin in mouse liver was associated with increased phosphorylation of SMAD1/5/8, consistent with activation of the BMP signaling pathway (Fig. 7C, corresponding quantitation of several such blots is shown in Fig. 7D). Interestingly, and in contrast to our observations with HuH7 cells and primary human hepatocytes, we found that IL-1β induced a significant increase in expression of activin B in mouse liver (Fig. 8A), whereas BMP2 expression displayed a transient decline (that was not statistically significant) before returning to baseline (Fig. 8B). Therefore, there appears to be a difference between the murine and human systems with respect to the molecule that mediates IL-1β-induced activation of the BMP signaling pathway.
FIGURE 6.
Effects of IL-1β on hepcidin and BMP2 expression in primary human hepatocytes. A, primary human hepatocytes were treated with 10 ng/ml of IL-1β for the indicated times. Total RNA was prepared and used for quantitative RT-PCR analysis of hepcidin expression. *, p = 0.01; **, p = 0.01; ***, p = 0.02. Con, control. B, the same RNA samples as in A were analyzed by quantitative RT-PCR for BMP2 expression. *, p = 0.003; **, p = 0.005. In both cases, n = 3–7 samples/group from two separate experiments.
FIGURE 7.
Effects of IL-1β on iron homeostasis in mice. IL-1β was injected intraperitoneally at a dose of 250 ng/mouse. Groups of animals (n = 3/time point) were sacrificed at the indicated times after injection, and various parameters were analyzed. A, liver hepcidin expression. *, p = 0.02; **, p = 0.0002. Con, control. B, serum iron concentrations. *, p = 0.02. C, immunoblot of liver lysates with antibodies to phosphorylated SMAD1/5/8 (pSMAD1/5/8) or total SMAD1. D, quantitation of Western blots corresponding to C. The intensities of the phosphorylated SMAD1/5/8 and total SMAD1 bands in blots from multiple experiments were determined by Li-Cor imaging, and the ratio was calculated. *, p = 0.008; n = 3 samples/group (except for the 6-h time point, where n = 2) from multiple experiments.
FIGURE 8.
Effects IL-1β on activin B and BMP2 expression in mouse liver. IL-1β was injected intraperitoneally at a dose of 250 ng/mouse. Groups of animals (n = 3/time point) were sacrificed at the indicated times after injection. Liver RNA was prepared and used for estimation of activin B and BMP2 expression by quantitative RT-PCR. A, liver activin B expression. *, p = 0.05; **, p = 0.03. Con, control. B, liver BMP2 expression. The results shown are from one of two similar experiments.
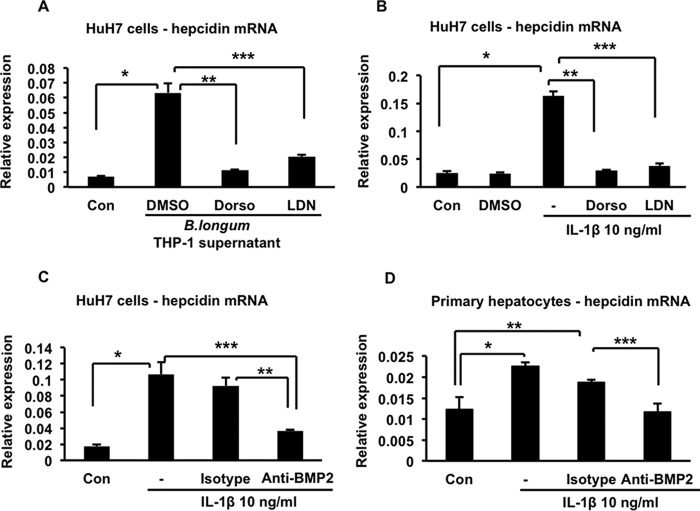
To determine whether activation of the BMP signaling pathway is required for IL-1β-induced up-regulation of hepcidin, we treated HuH7 cells with two different inhibitors of this pathway, dorsomorphin or LDN193189 (17, 18), before stimulating them with either the supernatant of B. longum-infected THP-1 macrophages or IL-1β itself. In both cases, treatment with dorsomorphin or LDN193189 significantly inhibited hepcidin up-regulation, whereas the vehicle, dimethyl sulfoxide, did not have this effect (Fig. 9, A and B). In addition, we found that treatment of HuH7 cells with 7.5 μg/ml of a monoclonal anti-BMP2/BMP4-neutralizing antibody significantly inhibited IL-1β-induced hepcidin up-regulation, whereas an equivalent amount of an isotype-matched irrelevant antibody did not (Fig. 9C). Although the anti-BMP2 antibody used in this experiment cross-reacts with BMP4 (a close structural relative of BMP2), it is unlikely that its inhibitory effect on hepcidin expression is due to BMP4 neutralization. BMP4 is expressed at about 1/100th of the level of BMP2 and, unlike BMP2, is not up-regulated by IL-1β (Fig. 5, A and B). We also confirmed that BMP2 neutralization significantly inhibited IL-1β-induced up-regulation of hepcidin in primary human hepatocytes (Fig. 9D).
FIGURE 9.
Effects of BMP signaling inhibitors on hepcidin up-regulation. A, HuH7 cells were treated for 4 h with the supernatant of B. longum-infected THP-1 cells in the presence of the inhibitors dorsomorphin (Dorso, 5 μm) or LDN193189 (LDN, 500 nm) or an equivalent volume of the vehicle dimethyl sulfoxide (DMSO). Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.007; **, p = 0.009; ***, p = 0.01; n = 3 samples/group from one of two similar experiments. Con, control. B, HuH7 cells were treated for 4 h with 10 ng/ml of IL-1β by itself (−) or in the presence of the inhibitors dorsomorphin (5 μm) or LDN193189 (500 nm). Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.001; **, p = 0.003; ***, p = 0.001; n = 3 samples/group from one of two similar experiments. C, HuH7 cells were treated for 4 h with 10 ng/ml of IL-1β by itself (−) or in the presence of 7.5 μg/ml of either a neutralizing anti-BMP2 antibody or an irrelevant isotype-matched control antibody. Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.02; **, p = 0.02; ***, p = 0.03; n = 3 samples/group from one of two similar experiments. D, primary human hepatocytes were treated for 4 h with 10 ng/ml of IL-1β by itself (−) or in the presence of 7.5 μg/ml of either a neutralizing anti-BMP2 antibody or an irrelevant isotype-matched control antibody. Hepcidin expression was measured by quantitative RT-PCR. *, p = 0.001; **, p = 0.01; ***, p = 0.005; n = 4 samples/group from one of two similar experiments.
Discussion
The data presented here indicate that the interaction between commensal bacteria and monocyte-macrophages leads to the secretion of IL-1β, which is then able to induce hepcidin expression in hepatocytes, findings that confirm and extend observations reported by other investigators (28–30). In addition, we demonstrate that IL-1β is able to up-regulate hepcidin in the mouse liver and cause a corresponding decline in serum iron concentration in vivo. We also show, for the first time to our knowledge, that IL-1β up-regulates hepcidin by an unexpected mechanism involving increased expression of BMP2 or activin B and consequent activation of the BMP signaling pathway. This is in contrast to earlier studies that have implicated the transcription factors NF-κB and C/EBPβ in IL-1β-induced hepcidin up-regulation (29, 30). Although our experiments do not exclude involvement of these transcription factors, they highlight the importance of the BMP signaling pathway and its target transcription factor SMAD4 as intermediaries between inflammation and increased hepcidin expression. This idea has also been suggested earlier by data showing that activin B, which is able to up-regulate hepcidin in hepatocytes in vitro, is induced in mouse liver following systemic administration of LPS (22). In fact, our findings make it very likely that this effect of LPS is the result of its ability to induce the secretion of IL-1β by macrophages and other cells. Our results identify BMP2 as another member of the BMP/TGFβ superfamily that is responsive to inflammatory mediators, as well as a potential target of interventions directed at controlling hepcidin expression in the context of inflammation. Together with an earlier work on activin B (22), the findings reported here broaden the role of the BMP signaling pathway in regulating hepcidin expression beyond simply responding to changes in tissue and circulating iron levels (4). The ability of IL-1β to activate the BMP signaling pathway could help to explain the previously noted synergistic up-regulation of hepcidin by the combination of IL-1β and IL-6 (31). IL-6-induced signals activate the transcription factor STAT3, which binds to the proximal region of the promoter of the hepcidin gene (12). Immediately adjacent to the STAT3-binding site is the so-called BMP-responsive element 1, which encompasses a binding site for the complex of SMAD4 and phosphorylated SMAD1 (32). Because we now know that IL-1β can activate the BMP signaling pathway by increasing the expression of BMP2 or activin B, it would be reasonable to speculate that the synergistic effect of IL-1β and IL-6 on hepcidin expression is the outcome of cooperative interactions between STAT3 and the SMAD1-SMAD4 complex. Along with work published previously (28–31), our observations suggest that it may be necessary to reconsider the dominant role generally assigned to IL-6 and the STAT3 pathway in inflammation-associated hepcidin up-regulation. Mediators such as IL-1β that are able to activate BMP signaling are also likely to contribute significantly to increased hepcidin expression in inflammatory diseases.
It will be important to clarify the mechanisms that contribute to the induction of IL-1β by B. longum and B. fragilis, particularly because there is little information on how commensal bacteria activate the inflammasome, the multiprotein complex that is involved in the processing and secretion of IL-1β (33). One of the few studies that addressed this issue has shown recently that a commensal strain of Proteus mirabilis activated the NLRP3 inflammasome in inflammatory monocytes via the production of a hemolysin (34). How more typical commensals such as B. longum and B. fragilis induce IL-1β secretion remains mysterious because they do not express flagellins, type 3 secretion system components, or pore-forming toxins, molecules that have been implicated in inflammasome activation by other bacteria (33). We are currently investigating this question, and our preliminary results from studies with B. longum suggest that phagocytosis of the bacteria is required, possibly to generate degradation products that can activate the inflammasome.4
An intriguing aspect of our results is the apparent dichotomy between humans and mice with respect to the specific activator of BMP signaling that is induced by IL-1β: BMP2 in human hepatocytes and activin B in murine liver. It is not clear from our data whether this difference represents a real biological distinction or simply reflects the technical characteristics of the experimental systems used. Importantly, our in vivo mouse studies involved whole liver, which consists of a number of different cell types besides hepatocytes, whereas our in vitro human experimental system made use of a hepatocyte-derived cell line or purified primary hepatocytes. On the basis of studies in the rat, hepatic stellate cells appear to be the major source of both BMP2 and activin B, with hepatocytes being a relatively minor source of these molecules (35, 36). This pattern of expression may help to explain why we did not observe IL-1β-induced up-regulation of activin B in HuH7 cells and primary human hepatocytes but does not satisfactorily account for our failure to detect increased BMP2 expression in the livers of mice injected with IL-1β. It is worth mentioning in this connection that the primers that we used for quantitative RT-PCR are able to amplify both of the murine BMP2 mRNA isoforms that are recorded in the National Center for Biotechnology Information nucleotide database: the canonical transcript (reference sequence NM_007553.3) and the predicted, alternately spliced variant X1 transcript (reference sequence XM_006498619.1). Therefore, our results suggest that neither isoform is induced by IL-1β. Further work will be required to clarify the roles of BMP2 and activin B in IL-1β-induced up-regulation of hepcidin in vivo. However, our findings raise the possibility that what we learn from the mouse in this regard may not necessarily apply to humans.
Although we have not addressed the issue of in vivo significance in this study, there is evidence to support the relevance of our findings to the commensal bacteria-dependent intestinal inflammation that is the central abnormality of both human IBD and mouse models of this disease. Firstly, IL-1β has been implicated in the pathogenesis of IBD by several observations. Increased production of the cytokine has been seen in mucosal mononuclear cells from patients with Crohn colitis and has also been associated with genetic variants that heighten the risk of Crohn disease (37, 38). Moreover, IL-1 receptor blockade led to clinical and laboratory improvement in two patients with colitis associated with chronic granulomatous disease, a condition that resembles Crohn colitis (39). Similar results have been obtained in corresponding mouse models, and a recent study of dextran sulfate sodium-induced colitis has shown that commensal bacteria induced high levels of IL-1β secretion by inflammatory monocytes recruited to the colon (34, 39, 40). Secondly, there is reason to believe that IL-1β is involved in hepcidin up-regulation in inflammatory states. In our studies of dextran sulfate sodium-induced colitis in wild-type and IL-10 deficient mice, we found that colonic IL-1β levels were generally higher in the latter strain,5 correlating with its significantly higher hepcidin expression, whereas colonic IL-6 was similar in both strains (16). Similarly, in a mouse model of Mycoplasma arthritidis-induced inflammation, hepcidin up-regulation occurred even in the absence of changes in serum IL-6 and correlated with marked increases in circulating IL-1β (41). Taken together, these observations indicate that IL-1β should be investigated as an important contributor to hepcidin up-regulation and the pathogenesis of AI in inflammatory conditions such as IBD.
Specific treatment of AI can be difficult because of the relatively poor absorption of oral iron supplements in this condition (9). There is also some concern that oral iron may alter the gut microbiota and worsen intestinal inflammation in IBD (42–45). Therefore, there is a need to develop rational therapeutic strategies for AI on the basis of inhibiting hepcidin expression or function. Several such strategies are in various stages of development and testing and include the use of blocking antibodies directed against hepcidin, FPN and IL-6, BMP sequestering agents, and inhibitors of BMP signaling (1, 46). The results presented here indicate that reagents to specifically inhibit IL-1β, BMP2, and activin B should also be considered for this type of intervention.
Author Contributions
N. K. N. S. and K. C. designed and executed experiments, acquired and analyzed the data, and assisted with writing the manuscript. B. J. C. conceived the study, interpreted the results, and wrote the manuscript.
Acknowledgments
We thank Sinan Ozer and Korkut Uygun of the Cell Resource Core at Massachusetts General Hospital for the primary human hepatocytes used in this study.
This work was supported by National Institutes of Health Grant R01 AI089700 (to B. J. C.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
K. Chen and B. J. Cherayil, unpublished data.
N. K. N. Shanmugam and B. J. Cherayil, unpublished data.
- BMP
- bone morphogenetic protein
- SMAD
- small mothers against decapentaplegic
- FPN
- ferroportin
- IBD
- inflammatory bowel disease
- AI
- anemia of inflammation
- TLR
- Toll-like receptor.
References
- 1.Ganz T. (2013) Systemic iron homeostasis. Physiol. Rev. 93, 1721–1741 [DOI] [PubMed] [Google Scholar]
- 2.Kautz L., Meynard D., Monnier A., Darnaud V., Bouvet R., Wang R. H., Deng C., Vaulont S., Mosser J., Coppin H., and Roth M. P. (2008) Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1 and Atoh8 in the mouse liver. Blood 112, 1503–1509 [DOI] [PubMed] [Google Scholar]
- 3.Parrow N. L., and Fleming R. E. (2014) Bone morphogenetic proteins as regulators of iron metabolism. Annu. Rev. Nutr. 34, 77–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Core A. B., Canali S., and Babitt J. L. (2014) Hemojuvelin and bone morphogenetic protein (BMP) signaling in iron homeostasis. Front. Pharmacol. 5, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worthen C. A., and Enns C. A. (2014) The role of hepatic transferrin receptor 2 in the regulation of iron homeostasis in the body. Front. Pharmacol. 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemeth E., Tuttle M. S., Powelson J., Vaughn M. B., Donovan A., Ward D. M., Ganz T., and Kaplan J. (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306, 2090–2093 [DOI] [PubMed] [Google Scholar]
- 7.Ward D. M., and Kaplan J. (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim. Biophys. Acta 1823, 1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drakesmith H., and Prentice A. M. (2012) Hepcidin and the iron-infection axis. Science 338, 768–772 [DOI] [PubMed] [Google Scholar]
- 9.Nemeth E., and Ganz T. (2014) Anemia of inflammation. Hematol. Oncol. Clin. North Am. 28, 671–681, vi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemeth E., Rivera S., Gabayan V., Keller C., Taudorf S., Pedersen B. K., and Ganz T. (2004) IL-6 mediates hypoferremia of inflammation by inducing synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 113, 1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wrighting D. M., and Andrews N. C. (2006) Interleukin-6 induces hepcidin expression through STAT3. Blood 108, 3204–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verga Falzacappa M. V., Vujic Spasic M., Kessler R., Stolte J., Hentze M. W., and Muckenthaler M. U. (2007) STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109, 353–358 [DOI] [PubMed] [Google Scholar]
- 13.Pietrangelo A., Dierssen U., Valli L., Garuti C., Rump A., Corradini E., Ernst M., Klein C., and Trautwein C. (2007) STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology 132, 294–300 [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Trebicka E., Fu Y., Ellenbogen S., Hong C. C., Babitt J. L., Lin H. Y., and Cherayil B. J. (2012) The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shanmugam N. K., Ellenbogen S., Trebicka E., Wang L., Mukhopadhyay S., Lacy-Hulbert A., Gallini C. A., Garrett W. S., and Cherayil B. J. (2012) Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS ONE 7, e38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanmugam N. K., Trebicka E., Fu L. L., Shi H. N., and Cherayil B. J. (2014) Intestinal inflammation modulates expression of the iron-regulating hormone hepcidin depending on erythropoietic activity and the commensal microbiota. J. Immunol. 193, 1398–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D., and Peterson R. T. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuny G. D., Yu P. B., Laha J. K., Xing X., Liu J. F., Lai C. S., Deng D. Y., Sachidanandan C., Bloch K. D., and Peterson R. T. (2008) Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett. 18, 4388–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanmugam N. K., and Cherayil B. J. (2013) Serum-induced up-regulation of hepcidin expression involves the bone morphogenetic protein signaling pathway. Biochem. Biophys. Res. Commun. 441, 383–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn J. C., Tompkins R. G., and Yarmush M. L. (1991) Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol. Prog. 7, 237–245 [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Harrington L., Trebicka E., Shi H. N., Kagan J. C., Hong C. C., Lin H. Y., Babitt J. L., and Cherayil B. J. (2009) Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J. Clin. Invest. 119, 3322–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besson-Fournier C., Latour C., Kautz L., Bertrand J., Ganz T., Roth M. P., and Coppin H. (2012) Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 120, 431–439 [DOI] [PubMed] [Google Scholar]
- 23.Babitt J. L., Huang F. W., Xia Y., Sidis Y., Andrews N. C., and Lin H. Y. (2007) Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Invest. 117, 1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozupone C. A., Stombaugh J. I., Gordon J. I., Jansson J. K., and Knight R. (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen P. (2014) The TLR and IL-1 signaling network at a glance. J. Cell Sci. 127, 2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meynard D., Kautz L., Darnaud V., Canonne-Hergaux F., Coppin H., and Roth M.-P. (2009) Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 41, 478–481 [DOI] [PubMed] [Google Scholar]
- 27.Andriopoulos B. Jr., Corradini E., Xia Y., Faasse S. A., Chen S., Grgurevic L., Knutson M. D., Pietrangelo A., Vukicevic S., Lin H. Y., and Babitt J. L. (2009) BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 41, 482–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee P., Peng H., Gelbart T., Wang L., and Beutler E. (2005) Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. U.S.A. 102, 1906–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer F., Torzewski J., Kamenz J., Veit K., Hombach V., Dedio J., and Ivashchenko Y. (2008) Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NF-κB- and C/EBPβ-dependent autocrine interleukin-6 loop. Mol. Immunol. 45, 2678–2689 [DOI] [PubMed] [Google Scholar]
- 30.Matak P., Chaston T. B., Chung B., Srai S. K., McKie A. T., and Sharp P. A. (2009) Activated macrophages induce hepcidin expression in HuH7 hepatoma cells. Haematologica 94, 773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bode J. G., Albrecht U., Häussinger D., Heinrich P. C., and Schaper F. (2012) Hepatic acute phase proteins: regulation by IL-6 and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur. J. Cell Biol. 91, 496–505 [DOI] [PubMed] [Google Scholar]
- 32.Truksa J., Lee P., and Beutler E. (2009) Two BMP responsive elements, STAT and bZIP/HNF4/COUP motifs of the hepcidin promoter are critical for BMP, SMAD1, and HJV responsiveness. Blood 113, 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanaja S. K., Rathinam V. A., and Fitzgerald K. A. (2015) Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 25, 308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo S. U., Kamada N., Muñoz-Planillo R., Kim Y. G., Kim D., Koizumi Y., Hasegawa M., Himpsl S. D., Browne H. P., Lawley T. D., Mobley H. L., Inohara N., and Núñez G. (2015) Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity 42, 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang A. S., Anderson S. A., Wang J., Yang F., DeMaster K., Ahmed R., Nizzi C. P., Eisenstein R. S., Tsukamoto H., and Enns C. A. (2011) Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase in matriptase-2 protein. Blood 117, 1687–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bleser P. J., Niki T., Xu G., Rogiers V., and Geerts A. (1997) Localization and cellular sources of activins in normal and fibrotic rat liver. Hepatology 26, 905–912 [DOI] [PubMed] [Google Scholar]
- 37.Mahida Y. R., Wu K., and Jewell D. P. (1989) Enhanced production of IL-1β by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut 30, 835–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plantinga T. S., Crisan T. O., Oosting M., van de Veerdonk F. L., de Jong D. J., Philpott D. J., van der Meer J. W., Girardin S. E., Joosten L. A., and Netea M. G. (2011) Crohn's disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut 60, 1229–1235 [DOI] [PubMed] [Google Scholar]
- 39.de Luca A., Smeekens S. P., Casagrande A., Iannitti R., Conway K. L., Gresnigt M. S., Begun J., Plantinga T. S., Joosten L. A., van der Meer J. W., Chamilos G., Netea M. G., Xavier R. J., Dinarello C. A., Romani L., and van de Veerdonk F. L. (2014) IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc. Natl. Acad. Sci. U.S.A. 111, 3526–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B. G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., and Akira S. (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 [DOI] [PubMed] [Google Scholar]
- 41.Koening C. L., Mu H. H., Van Schelt A., Lo E., Ward D. M., Kaplan J., and De Domenico I. (2009) Hepcidin is elevated in mice injected with Mycoplasma arthritidis. J. Inflamm. 10.1186/1476-9255-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulnigg S., and Gasche C. (2006) Systematic review: managing anemia in Crohn's disease. Aliment. Pharmacol. Ther. 24, 1507–1523 [DOI] [PubMed] [Google Scholar]
- 43.Erichsen K., Ulvik R. J., Nysaeter G., Johansen J., Ostborg J., Berstad A., Berge R. K., and Hausken T. (2005) Oral ferrous fumarate or intravenous iron sucrose for patients with inflammatory bowel disease. Scand. J. Gastroenterol. 40, 1058–1065 [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann M. B., Chassard C., Rohner F., N'goran E. K., Nindjin C., Dostal A., Utzinger J., Ghattas H., Lacroix C., and Hurrell R. F. (2010) The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am. J. Clin. Nutr. 92, 1406–1415 [DOI] [PubMed] [Google Scholar]
- 45.Werner T., Wagner S. J., Martínez I., Walter J., Chang J. S., Clavel T., Kisling S., Schuemann K., and Haller D. (2011) Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut. 60, 325–333 [DOI] [PubMed] [Google Scholar]
- 46.Sun C. C., Vaja V., Babitt J. L., and Lin H. Y. (2012) Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am. J. Hematol. 87, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]