Using an inclusive HCV treatment protocol that encourages prospective engagement in care, HIV-infected patients with and without ongoing barriers to care had similar chances of HCV sustained viral response irrespective of which HCV treatment regimen was used.
Keywords: barriers to care, DAA, HCV, HIV
Abstract
Background. Access to hepatitis C virus (HCV) medications for human immunodeficiency virus (HIV)-infected patients with ongoing barriers to care is restricted by healthcare payers in the absence of HCV treatment outcomes data in the era of direct-acting antivirals (DAA).
Methods. Retrospective analysis of HCV treatment outcomes using interferon (IFN)-free DAA regimens and an inclusive treatment protocol in an urban HIV clinic where ongoing barriers to care (drug or alcohol use, psychiatric disease, and/or unstable housing) are common. Then, using logistic regression analysis, we compared the proportion of HIV-infected patients who achieved HCV sustained viral response (SVR) in the pegylated-IFN plus ribavirin (PEG-IFN/RBV, 2008–2011), pegylated-IFN plus ribavirin and telaprevir (PEG-IFN/RBV/PI, 2011–2013), and IFN-free DAA therapy eras (2014). Results are displayed using forest plots.
Results. The proportion of patients who achieved HCV SVR in the PEG-IFN/RBV, PEG-IFN/RBV/PI, and IFN-free DAA therapy eras increased from 38.4% (95% confidence interval [CI], 23.2–53.7) and 48% (95% CI, 28.4–67.6) to 83.3% (95% CI, 70.0–96.7), respectively. Similar proportions of patients with ongoing barriers to care were treated during the PEG-IFN/RBV (25 of 39 [64%]), PEG-IFN/RBV/PI (14 of 25 [56%]), and IFN-free DAA (16 of 30 [53%]) eras. Hepatitis C virus SVR among patients with ongoing barriers to care improved from 40% (95% CI, 21–59) to 76.5% (95% CI, 56–97) in the PEG-IFN/RBV and IFN-free DAA eras, respectively. After stratification for factors associated with HCV SVR such as HCV genotype and cirrhosis, HCV SVR were similar in patients regardless of the presence of ongoing barriers to care.
Conclusions. Using IFN-free DAA and an inclusive HCV treatment protocol, 76.5% of HIV/HCV-treated patients with ongoing barriers to care achieved HCV SVR.
Chronic hepatitis C virus (HCV) infection is the leading cause of liver-related mortality among human immunodeficiency virus (HIV)-infected people under care [1]. Only 5%–7% of HIV/HCV-coinfected patients achieved HCV sustained viral response (SVR) in the United States in the interferon (IFN)-based era [2, 3]. In the current era of HCV direct-acting antivirals (DAA), most HIV/HCV-coinfected patients can potentially be cured of HCV [4, 5]. Expert guidelines consider treatment of HCV in HIV-infected patients a priority due to an accelerated risk of liver fibrosis progression [6] and an increased risk of HCV transmission, particularly among HIV-infected men who have sex with men (MSM) [7].
There is a high prevalence of ongoing barriers to care (eg, drug or alcohol abuse, neuropsychiatric diseases, and unstable housing) among coinfected patients who are often underinsured, which can affect their overall engagement in care [8]. Although professional guidelines favor treatment of patients with ongoing drug use to prevent risk of HCV forward transmission [7], the main reason for not initiating HCV therapy among those HIV-infected patients who completed HCV clinical staging continues to be ongoing barriers to care (drug or alcohol use, psychiatric disease, and unstable housing) [3].
Recent international recommendations for the management of HCV among people who use drugs highlight that recent drug use at the time of treatment initiation is not associated with reduced response to therapy [9, 10]. However, in the DAA era, many healthcare agencies require that patients with a substance abuse disorder be abstinent from drugs for 6 months before the initiation of HCV treatment [11, 12]. Underlying this recommendation is a concern that people with multiple barriers to care such as ongoing drug or alcohol use may have suboptimal adherence to HCV DAA leading to treatment failure and inappropriate resource utilization.
In 2008, a multidisciplinary HCV coinfection primary care-based program was implemented at the University of California San Diego (UCSD), with an inclusive protocol aimed at increasing HCV treatment uptake among HIV-coinfected patients, including those with ongoing drug and/or alcohol use, neuropsychiatric disease, and unstable housing [13]. Our institution began using IFN-free DAA regimens to treat HCV as part of our standard of care in January 2014. We conducted the present study to describe the following: (1) HCV treatment outcomes in a large academic urban HIV-clinic using IFN-free DAA regimens following their first year of availability, and (2) the proportion achieving HCV SVR among coinfected patients with ongoing barriers to care using DAA therapies in comparison to our prior SVR response rates using IFN-based therapies, and (3) the impact of ongoing barriers to care on treatment completion rates across HCV treatment paradigms (pegylated-IFN and ribavirin era [PEG-IFN/RBV]; pegylated-IFN, ribavirin and telaprevir [PEG-IFN/RBV/PI] era and IFN-free DAA-era).
METHODS
Patients and Study Design
This is a retrospective case series of all coinfected patients treated for HCV using IFN-free DAA-only regimens at the UCSD Owen HCV coinfection clinic between January 1, 2014 and December 31, 2014 (IFN-free DAA era). Our HCV coinfection clinic operates as 1 clinic session per week and is colocated within the UCSD Owen HIV Clinic. Because our coinfection clinic is embedded within our HIV primary care clinic, the only requirement to initiate the referral process is that HIV primary providers place a HCV referral in our electronic medical record. Since the inception of our clinic in 2008, we have used the same clinic protocol for HCV staging and assessment of barriers to care (drug or alcohol use, psychiatric disease, and unstable housing); thus, we decided to compare overall HCV SVR of different HCV treatment regimens with attention to proportion of SVR among patients with and without ongoing barriers to care. We included 2 comparison groups of all coinfected patients treated for HCV in our clinic before 2014: PEG-IFN/RBV (2008–2011) and PEG-IFN/RBV/PI (2011–2013) as previously reported [14]. Hepatitis C virus SVR was defined as having an undetectable HCV viral load after 24 weeks after HCV treatment completion in the PEG-IFN/RBV and PEG-IFN/RBV/PI therapy eras and after 12 weeks in the IFN-free DAA era. This research was approved by the UCSD Human Research Protection Program (Project no. 150 186).
Hepatitis C Virus (HCV) Staging Protocol, Study Definitions, and HCV Treatment Regimens
Our HCV treatment staging protocol has been published elsewhere [15]. In short, in addition to an assessment of control of HIV infection, potential drug interactions, liver fibrosis stage, and concurrent medical comorbidities, we assess for the presence of ongoing barriers to care [16]. Screening for barriers to care includes an extensive evaluation of 3 domains: ongoing drug/alcohol use, psychiatric disease, and/or unstable housing. First, patient's self-reported illicit substance and/or alcohol use within 3 months of HCV treatment initiation by completion of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) instrument [17] and subsequent evaluation by the clinic Substance Abuse Counselor. Second, patients are screened for depression using the Patient Health Questionnaire (PHQ)-9 inventory followed by a baseline formal psychiatric evaluation [18]. Third, patients are assessed for unstable housing by the Substance Counselor, Social Worker, and/or clinicians. The ASSIST and PHQ-9 instruments are administered before every HCV clinical encounter. It is the responsibility of the evaluating clinician to verify that patients complete the screening instruments and print the summary report during clinic visits. Data were stored on a secure intranet server of the clinic.
Our minimal requirements for HCV treatment of patients with ongoing barriers to care (drug/alcohol use, psychiatric disease, and/or unstable housing) are as follows: (1) consistent undetectable HIV viral load; (2) stable concurrent medical comorbidities; (3) favorable assessment by the team's psychiatrist when there is a history of a psychiatric condition that may interfere with treatment; (4) registration in the San Diego needle exchange program in case of ongoing injection drug use (IDU), plus documentation of no missed clinic appointments during the HCV staging process; and (5) alcohol sobriety or controlled drinking (if not completely abstinent) for at least 3 months before HCV treatment initiation.
In 2014, access to HCV IFN-free DAA regimens was limited, and insurance plans often denied approval of IFN-free DAA therapies for HIV-infected patients, hence we frequently used manufacturer-sponsored Patient Assistance Programs. From January to September 2014, patients with genotype 1 received sofosbuvir and simeprevir with or without weight-based ribavirin. We treated ten patients with cirrhosis with 12 weeks of sofosbuvir and simeprevir given no label indication to go to 24 weeks at that time. Sofosbuvir and ribavirin were used for treatment of genotype 2 or 3 and in patients with genotype 1 who were unable to receive simeprevir due to insurmountable drug interactions with a patient's antiretrovirals. After US Food and Drug Administration (FDA) approval, coformulated sofosbuvir/ledipasvir was another option for genotype 1 patients beginning in late October 2014 [19].
On the day of HCV treatment, initiation patients were requested to bring all their medications to clinic, including their HCV regimen, for reconciliation and regimen-specific education. Subsequently, patients were monitored concurrently by medical providers and clinical pharmacist for physical examinations, review of medication side effects, and clinical laboratory testing every 2 weeks for the first month, then monthly for the duration of therapy, unless there was a clinical indication that merited closer clinical or laboratory monitoring. Homeless patients were required to come biweekly for the duration of HCV therapy to promote prospective engagement in care.
The main categories of concurrent medical comorbidities included the following: (1) chronic kidney disease stage 3b or worse (glomerular filtration rate <45 mL per min/1.73 m2); (2) cardiovascular disease, including history of myocardial infarction within the last 6 months, congestive heart failure stage 2 or higher according to the New York Heart Association functional classification system, arrhythmia that required active medical therapy or an implantable device; (3) known neurologic, dermatologic, pulmonary, hematologic, and metabolic conditions where ribavirin-based therapy or medical interactions with DAA could result in aggravation of underlying medical comorbidity.
Statistics
We compared the proportion of coinfected patients who achieved HCV SVR in the PEG-IFN/RBV, PEG-IFN/RBV/PI, and IFN-free DAA treatment periods. We then estimated adjusted SVR proportions in a multiple logistic regression model of treatment period controlling for drug/alcohol use, psychiatric disease, unstable housing, genotype 1, cirrhosis, and presence of severe concurrent medical comorbidities. Two-way interactions between treatment period and the covariates were examined. We used forest plots to display HCV SVR proportions and 95% confidence intervals (CIs) in each treatment era stratified by the covariates. Analysis was performed using Stata version 13.0 (Stata Corp., College Station, TX).
RESULTS
Thirty HIV/HCV-coinfected patients were treated for HCV using DAA regimens in 2014. The main clinical and demographic characteristics of patients during each therapy era are presented in Table 1. Patients treated in IFN-free DAA era were older and had a higher prevalence of cirrhosis, relative to patients treated in the PEG-IFN/RBV and PEG-IFN/RVB/PI periods (Table 1). All patients except 1 elite controller were taking antiretroviral therapy. Eight patients (27%) were antiretroviral treatment-experienced with documented multiclass resistance and required adjustment of their regimens to account for drug interactions with DAA (patients 3, 4, 11, 15, 16, 17, 23, and 29 in Table 2).
Table 1.
Demographics and Clinical Characteristics of Patients Treated During the HCV Treatment Eras
| Clinic Characteristic | IFN-Free DAA (n = 30) | PEG-IFN/RBV/PI (n = 24) | PEG-IFN/RBV (n = 39) |
|---|---|---|---|
| Median age, years (range) | 54 (43–69) | 47 (28–55) | 49 (19–61) |
| Sex: Male (%) | 27 (90) | 21 (88) | 31 (80) |
| Race: Non-White (%) | 8 (27) | 9 (38) | 13 (33) |
| Ethnicity: Hispanic (%) | 4 (13) | 6 (25) | 7 (18) |
| HIV risk | |||
| MSM/bisexual (%) | 8 (27) | 8 (33) | 13 (33) |
| Heterosexual (%) | 2 (7) | 1 (4) | 3 (8) |
| Hemophilia (%) | 1 (3) | 3 (13) | 0 |
| MSM and intravenous drug use | 11 (36) | 8 (33) | 13 (33) |
| Heterosexual and intravenous drug use (%) | 8 (27) | 5 (21) | 10 (26) |
| Median T CD4+ cell count, cells/mm3 (range) | 395 (87–1094) | 650 (100–1360) | 494 (130–1142) |
| Detectable HIV viral load: >40 copies/mL (%) | 1 (3) | 0 | 10 (26) |
| Hepatitis C genotype | |||
| 1a | 23 | 24 | 30 |
| 2 | 3 | 0 | 5 |
| 3 | 2 | 0 | 3 |
| 4 | 2 | 0 | 1 |
| Liver fibrosis scoresa | |||
| F0–2 (%) | 5 (17) | 8 (38) | 11 (55) |
| F3–6 (%) | 25 (83) | 13 (72) | 9 (45) |
| Median HCV load-log10 copies/mL | 6.46 (4.2–7.7) | 6.38 (4.9–7.9) | 6.21 (3.0–7.5) |
| Prior HCV treatment exposure | 14 (47) | 10 (42) | 7 (18) |
| Interferon intolerant | 3 | 1 | 2 |
| Relapsed | 5 | 7 | 4 |
| Null responders | 3 | 2 | 1 |
| Failed interferon/ribavirin/telaprevir | 3 | 0 | 0 |
| Failed Sofosbuvir/daclastavirb | 1 | 0 | 0 |
Abbreviations: DAA, direct-acting antivirals; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IFN, interferon; MSM, men who have sex with men; PEG-IFN/RBV, pegylated-interferon and ribavirin era (2008–2011); PEG-IFN/RBV/PI, pegylated-interferon, ribavirin and HCV protease inhibitor (telaprevir) era (2011–2013).
a Using the modified Ishak histological score system in those patients with available biopsy results.
b Patient failed HCV therapy provided through a clinical trial.
Table 2.
Demographic and Main Clinical Characteristics of 30 HIV-Infected Patients Treated Using Interferon-Free Direct-Acting Antivirals Against Hepatitis C at the UCSD Owen Hepatitis Clinic in 2014
| Patient Number | Age/Sex | Race | Barriers to Care | Other Illness | GT | Livera Fibrosis | Prior HCV Treatment | HAART | Prior Liver Instability | CTP Score | CD4 | HIV RNA | HCV Regimen-Weeks | HCV Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53/m | Hispanic | Alcohol | Severe PCT | 1a | F0 | Naive | FTC/TDF + DRV/r | n/a | n/a | 520 | UD | SOF/LDV-12 | SVR 12 |
| 2 | 63/m | White | No | No | 2 | F1 | Viral relapse | FTC/TDF/EFV | n/a | n/a | 476 | UD | SOF + RBV-12 | SVR 12 |
| 3 | 55/m | White | Drugs | None | 1a | F1 | Naive | FTC/TDF/RPV + DTG | n/a | n/a | 813 | UD | SIM + SOF-12 | SVR 12 |
| 4 | 62/m | White | Psych | Polycythemia | 1a | F1 | Naive | FTC/TDF/RPV + DTG | n/a | n/a | 246 | UD | SIM + SOF-12 | SVR 12 |
| 5 | 50/m | Hispanic | Alcohol | Psych | 2 | F2 | Naive | 3TC/ABC + RAL | n/a | n/a | 690 | UD | SOF + RBV-12 | Relapse |
| 6 | 61/f | White | No | COPD | 1a | F3 | Viral relapse | None | n/a | n/a | 1094 | UD | SIM + SOF-12 | Dead |
| 7 | 54/m | White | Psych/ drugs | No | 3 | F3 | Naive | FTC/TDF + DRV/r | n/a | n/a | 469 | UD | SOF + RBV-24 | SVR 12 |
| 8 | 52/m | White | Psych/ drugs | No | 3 | F3 | Interferon intolerant | FTC/TDF + FAP/r | n/a | n/a | 395 | UD | SOF + RBV-24 | SVR 12 |
| 9 | 54/m | White | Psych | Chronic pain | 1a | F5 | Failed telaprevir | FTC/TDF + ATV/r | No | A5 | 356 | UD | SOF/LDV-24 | SVR 12 |
| 10 | 64/m | AA | No | DM2, SZS | 1a | F5 | Null response | FTC/TDF + RAL | No | A5 | 698 | UD | SIM + SOF + RBV-12b | SVR 12 |
| 11 | 57/m | AA | No | DM2, HTN, A-fib | 1a | F5 | Null response and failed SOF + DCL | FTC/TDF/RPV + DTG + MVC | No | A5 | 509 | UD | SIM + SOF + RBV-24 | SVR 12 |
| 12 | 47/m | White | No | Chronic pancytopenia | 1a | F6 | Interferon intolerant | 3TC/ABC + RAL | Yes | B7 | 166 | UD | SIM + SOF-12 | SVR 12 |
| 13 | 50/m | White | No | None | 2 | F6 | Interferon intolerant | FTC/TDF + DRV/r | No | A5 | 389 | UD | SOF + RBV-12 | SVR 12 |
| 14 | 51/m | White | Alcohol | None | 1a | F6 | SOF + RBV intolerant | FTC/TDF + RAL | Yes | C10 | 256 | UD | SIM + SOF-12 | Dead week 6 |
| 15 | 53/m | AA | Drugs | HBV, CKD, stroke | 1a | F6 | Relapsed on telaprevir | 3TC + DTG + DRV/r | Yes | A5 | 474 | UD | SOF + RBV-24 | SVR 12 |
| 16 | 57/m | AA | No | CKD and new stroke, DM, HTN | 1a | F6 | Naive | 3TC/ABC + ETV + DTG + DRV/r | No | A6 | 341 | 54 | SOF + RBV-24 | SVR 12 |
| 17 | 56/m | White | Drugs | CKD | 1a | F6 | Viral relapse | 3TC/ABC + RPV + DTG | Yes | B7 | 230 | UD | SIM + SOF-12 | Relapsed |
| 18 | 53/m | AA | Psych/ Alcohol and Drugs | Pysch | 1a | F6 | Viral relapse | FTC/TDF + DTG | No | A5 | 344 | UD | SIM + SOF-12 | SVR 12 |
| 19 | 69/m | White | Drugs | A-fib, chronic pain | 1a | F6 | Naive | 3TC/ABC + DTG | No | A6 | 288 | UD | SIM + SOF-12 | SVR 12 |
| 20 | 68/m | Hispanic | No | CKD | 1a | F6 | Naive | 3TC/ABC + DTG | No | A5 | 719 | UD | SIM + SOF-12 | SVR 12 |
| 21 | 43/m | Hispanic | No | None | 1a | F6 | Naive | FTC/TDF + RAL | Yes | C11 | 87 | UD | SIM + SOF + RBV-12 | SVR 12 |
| 22 | 58/m | White | Drugs | Pulmonary HTN | 1a | F6 | Naive | FTC/TDF + DTG | No | A6 | 230 | UD | SIM + SOF-24 | SVR 12 |
| 23 | 50/m | White | Psych/ Alcohol | Hyperthyroidism | 1a | F6 | Naive | FTC/TDF/RPV + DTG | No | A5 | 256 | UD | SOF/LDV-12 | SVR 12 |
| 24 | 44/f | AA | No | Severe psoriasis | 1a | F6 | Failed telaprevir | FTC/TDF/EFV | No | A5 | 563 | UD | SOF/LDV-24 | SVR 12 |
| 25 | 50/m | White | Unstable housing/ psych | DM | 4 | F6 | Null response | FTC/TDF + RAL | Yes | C10 | 174 | UD | SIM + SOF + RBV-12 | SVR 12 |
| 26 | 54/m | AA | Psych | None | 1a | F6 | No | 3TC/ABC + DTG | Yes | C11 | 251 | UD | SIM + SOF-12 | Relapse |
| 27 | 61/m | AA | No | DM, CHF | 1a | F6 | No | 3TC/ABC + DTG | Yes | B8 | 538 | UD | SIM + SOF-12 | SVR 12 |
| 28 | 53/m | White | No | Psych | 1a | F6 | No | FTC/TDF + DTG | Yes | C10 | 256 | UD | SIM + SOF-24 | SVR 12 |
| 29 | 46/m | White | No | CKD | 1a | F6 | No | 3TC/ABC + DTG + RPV | Yes | A6 | 796 | UD | SIM + SOF-24 | SVR 12 |
| 30 | 54/f | Hispanic | No | None | 4 | F6 | Viral relapse | FTC/TDF/RPV | No | A6 | 657 | UD | SIM + SOF-24 | SVR 12 |
Abbreviations: AA, African American; A-fib, atrial fibrillation; ATV/r, ritonavir-boosted atazanavir; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CTP, Child-Turcotte-Pugh; DM, diabetes mellitus; DRV/r, ritonavir-boosted darunavir; DTG, dolutegravir; ETV, etravirine; f, female; FAP/r, ritonavir-boosted fosamprenavir; FTC/TDF, fixed-dose combination tenofovir/emtricitabine; FTC/TDF/EFV, fixed-dose combination tenofovir/emtricitabine/efavirenz; GT, hepatitis C genotype; HAART, highly active antiretroviral therapy; HBV, chronic active hepatitis B; HTN, hypertension; INF, interferon; m, male; MVC, maraviroc; n/a, not applicable; PCT, porphyria cutanea tarda; RAL, raltegravir; RBV, ribavirin; RLP/FTC/TDF/RLP, fixed-dose combination tenofovir/emtricitabine/rilpivirine; RPV, rilpivirine; SIM, simeprevir; SOF, sofosbuvir; SOF/LDV, fixed-dose combination sofosbuvir/ledipasvir; SVR12, sustained viral response after 12 weeks of HCV treatment completion; SZS, seizures; UCSD, University of California at San Diego; UD, undetectable; 3TC, lamivudine; 3TC/ABC, fixed-dose combination lamivudine/abacavir.
a By liver biopsy using the modified Ishak histological score with Stage 5 or greater equaling cirrhosis and/or abdominopelvic computer tomography showing indirect evidence of advanced liver disease (nodular liver) and/or portal hypertension.
b Discontinued ribavirin after 5 weeks due to gastrointestinal side effects and moderate anemia.
Twenty-three patients (76%) were infected with HCV genotype 1a, 5 patients (17%) were infected with genotype 2 or 3, and 2 patients (7%) were infected with genotype 4. Twenty-two patients (73.3%) had cirrhosis, 10 (45%) of whom had prior liver decompensation (ascites 9, hepatic encephalopathy 7, and bleeding esophageal varices 3). Eight patients with cirrhosis had Child-Pugh-Turcotte scores B8 or higher. Nearly half of participants had failed prior HCV treatment, including 3 patients with genotype 1a who had prior null response to PEG-IFN/RBV (patients 10, 11, and 25 in Table 2), 3 patients treated with PEG-IFN/RBV/PI, and 1 who failed sofosbuvir and daclatasvir in a clinical trial (Table 2). Of note, 25 patients (83.3%) who were treated in our clinic in 2014 did not qualify for enrollment in any clinical trial either due to unstable medical conditions (prior liver decompensation and/or severe concurrent medical comorbidity), insurmountable antiretroviral drug interactions, ongoing barriers to care, or different combinations of these 3 factors.
Simeprevir and sofosbuvir was used in 19 patients including 2 with HCV genotype 4 (patients 25 and 30 in Table 2). Seven patients received sofosbuvir and ribavirin, 2 of which were genotype 1a with advanced cirrhosis and unable to receive simeprevir (patient 15 and 16 in Table 2), and 4 patients with HCV genotype 1a received coformulated sofosbuvir/ledipasvir. By insurance status, 22 patients were enrolled in Medicare, 1 patient was enrolled in Ryan White, 1 patient with hemophilia was enrolled in a genetic handicap insurance program administered by the State of California, and 6 patients whose insurance denied HCV medication coverage received HCV therapy through manufacturer-sponsored patient assistance programs (they were insured through different health maintenance organizations affiliated through the California Department of Health Care Services or Medi-Cal).
Overall, 25 patients (83.3%) achieved HCV SVR and remain alive, 19 patients with HCV genotype 1a (19 of 23 or 82.6%), 3 patients with HCV genotype 3 (3 of 3 or 100%), 2 patients with HCV genotype 2 (2 of 3 or 66.6%), and the 2 patients with HCV genotype 4 (100%). Three patients relapsed after discontinuing HCV therapy (patients 5, 17, and 26 in Table 2) and 2 died. One patient died due to spontaneous bacterial peritonitis while on week 6 of simeprevir and sofosbuvir, and the other patient died 2 weeks after finishing HCV therapy; she was found dead at home of a suspected opiate overdose (patient 6 and 14, respectively, in Table 2).
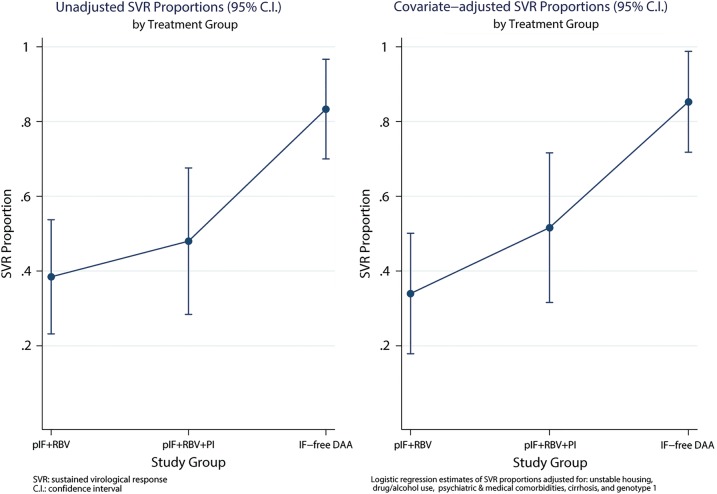
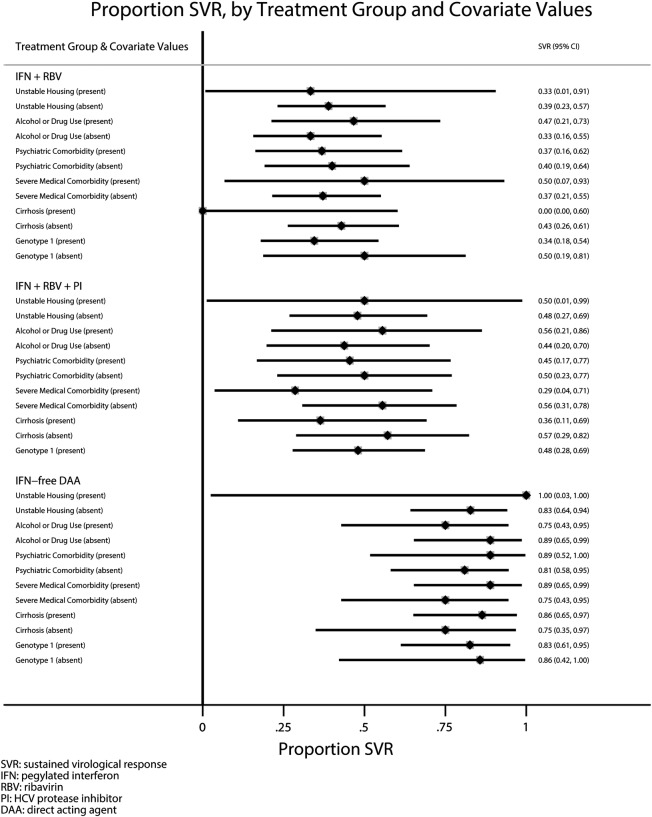
More than half of our patients treated with IFN-free DAA regimens had significant ongoing barriers to care (n = 16, 53%). Some patients (n = 12) had ongoing drug or alcohol use, and others had ongoing active neuropsychiatric disease (n = 9), and 1 patient was homeless. The proportion of patients with ongoing barriers to care treated in our clinic during the PEG-IFN/RBV (25 of 39 [64%]) and PEG-IFN/RBV/PI (14 of 25 [56%]) therapy eras were similar to the group of patients treated in 2014 as previously reported [13, 14]. The unadjusted chances of HCV SVR in our clinic in the PEG-IFN/RBV, PEG-IFN/RBV/PI, and IFN-free DAA era increased from 38.4% (95% CI, 23.2–53.7) and 48% (95% CI, 28.4–67.6) to 83.3% (95% CI, 70.0–96.7), respectively (Figure 1, left panel). Covariate adjusted SVR proportions were as follows: 33.8% (95% CI, 17.9–49.7), 50.1% (95% CI, 30.0–70.1), and 86.0% (95% CI, 73.2–98.8), respectively (Figure 1, right panel). None of the covariates included in the multiple logistic regression model (drug/alcohol use, psychiatric disease, unstable housing, severe medical comorbidity, HCV genotype 1, and cirrhosis) were independent (P < .05) predictors of treatment response. There were no statistically significant (P < .20) interactions between treatment period and any of the modeled covariates. Patients with ongoing drug/alcohol use, psychiatric disease, and/or unstable housing who fulfill our HCV treatment eligibility protocol had similar chances of SVR during each HCV treatment era as patients without barriers to care or other known predictors of HCV treatment response (Figure 2). However, chances of SVR among patients with ongoing drug/alcohol use, psychiatric disease, and unstable housing improved considerably in DAA era, from 40% (95% CI, 21–59) to 76.5% (95% CI, 56–97) in the PEG-IFN/RBV and IFN-free DAA eras, respectively (Figure 2). Few patients interrupted HCV therapy or were lost to follow up due to ongoing barriers to care in the PEG-IFN/RBV and PEG-IFN/RBV/PI therapy eras (3 of 39 [8%] and 2 of 25 [8%], respectively). In the IFN-free DAA era, no patients interrupted HCV therapy or were lost to follow up (Table 2). Since 2008, 2 patients became reinfected with HCV after achieving SVR: 1 patient through IDU and the other through probable sexual transmission. Both patients were treated in the PEG-IFN/RBV era. The observed rates of HCV reinfection was 3.31 per 100 person-years of follow up in the PEG-IFN/RBV therapy era, and no HCV reinfections have been observed in the PEG-IFN/RBV/PI and IFN-free eras after 24.1 and 17.8 person-years of follow up, respectively.
Figure 1.
Error bars plot depicting proportion of hepatitis C virus sustained viral response (SVR) achieved during each treatment era in an unadjusted model (left panel) and adjusted multiple logistic regression model of treatment period controlling for barriers to care (drug/alcohol abuse, psychiatric disease, unstable housing), genotype 1, cirrhosis, and presence of severe concurrent medical comorbidities (right panel). Abbreviations: C.I., confidence interval; DAA, direct-acting antivirals; pIF/RBV/PI, pegylated-interferon, ribavirin and HCV protease inhibitor (telaprevir) era.
Figure 2.
Forest plot that displays hepatitis C virus (HCV) sustained viral response (SVR) proportions and 95% confidence intervals in each treatment era stratified by barriers to care (drug/alcohol abuse, psychiatric disease, unstable housing), genotype 1, cirrhosis, and presence of severe concurrent medical comorbidities.
DISCUSSION
The availability of a consistent protocol for assessment and staging of ongoing barriers to care (drug/alcohol use, psychiatric disease, and/or unstable housing) since the creation of our HIV/HCV hepatitis coinfection clinic allows us to quantify the individual effect of ongoing barriers to care on HCV treatment response, despite rapid changes in the HCV treatment landscape in the last decade. We observed that in the DAA era, the proportion of patients who achieved HCV SVR was 83.3%, even in harder-to-treat populations of inner-city HIV clinics. Furthermore, HCV treatment and cure of HIV patients with drug or alcohol use, psychiatric disease, and/or unstable housing is feasible, when they fulfill criteria of engagement in care such as consistent undetectable HIV viral loads and compliance with scheduled clinic visits irrespective of ongoing drug use.
Unlike other reports of HCV treatment outcomes using DAA in HIV-infected patients [20–22], one third of our treated population had a history of liver decompensation. There were no serious adverse events or treatment interruptions attributable to IFN-free DAA regimens, a significant contrast with our prior experience using PEG-IFN/RBV/PI [14]. The cause of death for the 2 patients who died was likely unrelated to HCV therapy. One death was attributed to patient noncompliance with his secondary antibiotic prophylaxis to prevent spontaneous bacterial peritonitis, and the other patient died after successfully finishing HCV therapy due to suspected opiate overdose in the context of severe underlying pulmonary disease. It could be rightfully argued that the HCV relapse in the 2 cirrhotic patients with HCV genotype 1a who were treated with 12 weeks of simeprevir and sofosbuvir was due to short duration of therapy [7]. However, at the time they were treated, an FDA-approved indication for this combination did not exist, so extending therapy beyond 12 weeks was not an option supported by payors or Patient Assistance Programs during first half of 2014. This report demonstrates that IFN-free DAA regimens are safe and result in a high HCV SVR rate in this high-risk HIV population.
Our HCV treatment experience may be reflective of ongoing realities in many HIV clinics in the United States, characterized by a large proportion of HIV patients with an urgent need for HCV therapy, many of whom have ongoing barriers to care and are underinsured [2, 23, 24]. Our observed rate of HCV SVR in coinfected patients with ongoing drug/alcohol use, psychiatric disease, and/or unstable housing was similar to patients without barriers to care or other known predictors of HCV outcomes such as cirrhosis and HCV genotype, and it was independent of the HCV treatment era. Many patients will test positive in urine drug tests but maintain durable HIV viral load suppression and compliance with scheduled clinic visits. Using IFN-free DAA regimens, 3 of 4 individuals with ongoing barriers to care including those with ongoing drug use achieved HCV SVR. Hepatitis C virus reinfection is a valid concern among patients with ongoing drug use [25–27]. Among 94 patients treated in our clinic from 2008 to the present, we have observed only 2 HCV reinfection: 1 through IDU and the other likely through MSM sexual transmission. In light of these findings, the protocols of third-party payors that require patients to be sober for a minimum of 6 months and document negative urine toxicology tests as an absolute criterion for HCV treatment eligibility should be revised [11]. Because of these restrictions, 20% of our patients would not have accessed HCV therapy in the absence of Patient Assistance Programs.
We recognize several limitations to our study. First, due to challenges with accessing DAA in 2014 in a clinical setting, our sample size was small (N = 30). Our goal is to report a successful HCV treatment experience in an HIV population commonly encountered in clinical practice but often excluded from HCV clinical trials [28, 29]. Second, our patients with ongoing drug/alcohol use, psychiatric disease, and/or, unstable housing were carefully selected to demonstrate engagement in care before HCV treatment initiation. Thus, our results may not generalize to all patients with similar barriers to care. We proposed a practical strategy to increase HCV treatment uptake in HIV-infected patients with ongoing barriers to care. Our protocol relied on frequent clinical assessments to promote prospective engagement in care. If patients were not immediately eligible for HCV therapy, we worked with them toward improving their treatment eligibility based on their individual needs. This strategy is a departure from conventional cross-sectional HCV clinical assessments with distant follow ups [13]. Our approach allowed us to treat HCV in a population who otherwise would have been considered ineligible for HCV therapy in many conventional hepatology models of care [13]. Third, due to the small sample size, we may lack power to detect a statistically significant difference that could show that coinfected patients with ongoing drug/alcohol use, psychiatric disease, and/or unstable housing have lower HCV SVR rates than patients without barriers. However, the proportion of patients with HCV SVR was not significantly different from those without barriers to care and other known predictors of HCV treatment response. This trend was observed across the PEG-IFN/RBV, PEG-IFN/RBV/PI, and IFN-free DAA therapy eras in our clinic, supporting the validity of the described HCV treatment protocol. Fourth, we lack formal quantitative data on HCV medication adherence during our study periods. However, based on the observed proportion of HCV SVR among patients with ongoing drug/alcohol use, psychiatric disease, and/or unstable housing, we believe that this population can be successfully treated for their HCV. Furthermore, HCV treatment in the DAA era is safer and more effective [5, 30]. Finally, our treatment protocols may not be generalizable to other populations with different cultural perceptions and health system access [31, 32]. We recognize that there are multiple venues and strategies to treat HCV in HIV patients with ongoing barriers to care [33]. Our intention is to highlight that to scale up HCV treatment and reduce the risk of HCV forward transmission in our communities, we need interventions and healthcare policies that include rather exclude vulnerable populations with ongoing barriers to care.
CONCLUSIONS
In summary, using INF-free DAA regimens and an inclusive HCV treatment protocol, the proportion of patients achieving HCV cure was high in an HIV-infected population with advanced liver disease, concurrent severe medical comorbidities, and multiple ongoing barriers to care including active drug use. These results may help to provide quantitative estimates for third-party payors and health policy makers.
Acknowledgments
We thank Susan McQuillen for providing administrative assistance since the inception of the Owen Hepatitis C Virus coinfection clinic and our patients whose resilience and perseverance continue to inspire us to improve our clinical services.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was funded in part by the Clinical Investigation Core of the University of California San Diego Center for AIDS Research (grant AI036214), the CFAR Network of Integrated Clinical Systems (CNICS) (grant R24 AI067039-01A1), and the Pacific AIDS Education and Training Center.
Potential conflicts of interest. D. W. Research funds have been paid to the University of California San Diego for conduct of clinical trials: Gilead, AbbVie, Vertex, and Merck; Consultant: Janssen, AbbVie, Bristol Myers Squibb. A. K. received research and grant support from Gilead and is on the advisory board for Gilead. R. S. received research funds support from Gilead Sciences.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Data Collection on Adverse Events of Anti-HIV Drugs Study Group; Smith C, Sabin CA, Lundgren JD et al. . Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 2010; 24:1537–48. [DOI] [PubMed] [Google Scholar]
- 2.Adeyemi OM, Go B, Vibhakar S et al. . The CORE HCV cascade a decade later: looking ahead to an IFN-free era. 21st Conference on Retroviruses and Opportunistic Infections Boston, MA, March 3–6, 2014. (Abstract 669). [Google Scholar]
- 3.Cachay ER, Hill L, Wyles D et al. . The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS One 2014; 9:e102883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano V, Vispo E, Fernandez-Montero JV et al. . Update on HIV/HCV coinfection. Curr HIV/AIDS Rep 2013; 10:226–34. [DOI] [PubMed] [Google Scholar]
- 5.Rockstroh JK. Optimal therapy of HIV/HCV co-infected patients with direct acting antivirals. Liver Int 2015; 35:51–5. [DOI] [PubMed] [Google Scholar]
- 6.Konerman MA, Mehta SH, Sutcliffe CG et al. . Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology 2014; 59:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AASLD/IDSA/IAS—USA: Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org. Accessed 12 June 2015.
- 8.Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013; 207:S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robaeys G, Grebely J, Mauss S et al. . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Clin Infect Dis 2013; 57:S129–37. [DOI] [PubMed] [Google Scholar]
- 10.Grebely J, Robaeys G, Bruggmann P et al. . Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int J Drug Policy 2015; 26:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Available at: http://www.dhcs.ca.gov/pages/hepatitisc.aspx Accessed 13 April 2015.
- 12.Barua S, Greenwald R, Grebely J et al. . Restrictions for Medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 13.Cachay ER, Hill L, Ballard C et al. . Increasing hepatitis C treatment uptake among HIV-infected patients using an HIV primary care model. AIDS Res Ther 2013; 10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cachay ER, Wyles DL, Torriani FJ et al. . High incidence of serious adverse events in HIV-infected patients treated with a telaprevir-based hepatitis C virus treatment regimen. AIDS 2013; 27:2893–7. [DOI] [PubMed] [Google Scholar]
- 15.Cachay ER, Wyles DL, Goicoechea M et al. . Reliability and predictive validity of a hepatitis-related symptom inventory in HIV-infected individuals referred for hepatitis C treatment. AIDS Res Ther 2011; 8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cachay ER. The forgotten component in the staging and management of HIV/hepatitis C virus-coinfected patients. Clin Infect Dis 2014; 59:320–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002; 97:1183–94. [DOI] [PubMed] [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA approves first combination pill to treat hepatitis C. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm418365.htm Accessed 16 October 2014.
- 20.Del Bello DB, Levine C, Cha A et al. . Real-world data on HIV-positive patients with HCV treated with sofosbuvir and/or simeprevir. 22nd Conference on Retroviruses and Opportunistic Infections Seattle, WA, February 23–26, 2015. (Abstract: 647). [Google Scholar]
- 21.Gilmore JL, Breen D, Trooskin D et al. . Effectiveness of sofosbuvir/simeprevir for HIV/HCV patients in clinical practice. 22nd Conference on Retroviruses and Opportunistic Infections Seattle, WA, February 23–26, 2015. (Abstract 645). [Google Scholar]
- 22.Grant J, Stosor V, Palella F et al. . Successful treatment with direct acting antivirals in HIV/HCV patients. 22nd Conference on Retroviruses and Opportunistic Infections Seattle, WA, February 23–26, 2015. (Abstract 649). [Google Scholar]
- 23.Vellozzi C, Buchacz K, Baker R et al. . Treatment of hepatitis C virus (HCV) infection in patients coinfected with HIV in the HIV Outpatient Study (HOPS), 1999–2007. J Viral Hepat 2011; 18:316–24. [DOI] [PubMed] [Google Scholar]
- 24.Cacoub P, Dabis F, Costagliola D et al. . Burden of HIV and hepatitis C co-infection: the changing epidemiology of hepatitis C in HIV-infected patients in France. Liver Int 2015; 35:65–70. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham EB, Applegate TL, Lloyd AR et al. . Mixed HCV infection and reinfection in people who inject drugs--impact on therapy. Nat Rev Gastroenterol Hepatol 2015; 12:218–30. [DOI] [PubMed] [Google Scholar]
- 26.Aspinall EJ, Corson S, Doyle JS et al. . Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis 2013; 57:S80–9. [DOI] [PubMed] [Google Scholar]
- 27.Martin TC, Martin NK, Hickman M et al. . Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS 2013; 27:2551–7. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski MS, Sherman KE, Dieterich DT et al. . Combination therapy with telaprevir for chronic hepatitis C virus genotype 1 infection in patients with HIV: a randomized trial. Ann Intern Med 2013; 159:86–96. [DOI] [PubMed] [Google Scholar]
- 29.Shafran SD. HIV coinfected have similar SVR rates as HCV monoinfected with DAAs: it's time to end segregation and integrate HIV patients into HCV trials. Clin Infect Dis 2015; 61:1127–34. [DOI] [PubMed] [Google Scholar]
- 30.Sulkowski MS. Management of acute and chronic HCV infection in persons with HIV coinfection. J Hepatol 2014; 61:S108–19. [DOI] [PubMed] [Google Scholar]
- 31.Yu ML, Yeh ML, Tsai PC et al. . Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: a nationwide survey in Taiwan. Medicine (Baltimore) 2015; 94:e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh N, Maher L. HIV and HCV among people who inject drugs in Central Asia. Drug Alcohol Depend 2013; 132:S37–40. [DOI] [PubMed] [Google Scholar]
- 33.Graham CS, Swan T. A path to eradication of hepatitis C in low and middle income countries. Antiviral Res 2015; 119:89–96. [DOI] [PubMed] [Google Scholar]