Abstract
This study describes a high-throughput fluorescence dilution technique to measure the albumin reflection coefficient (σAlb) of isolated glomeruli. Rats were injected with FITC-dextran 250 (75 mg/kg), and the glomeruli were isolated in a 6% BSA solution. Changes in the fluorescence of the glomerulus due to water influx in response to an imposed oncotic gradient was used to determine σAlb. Adjustment of the albumin concentration of the bath from 6 to 5, 4, 3, and 2% produced a 10, 25, 35, and 50% decrease in the fluorescence of the glomeruli. Pretreatment of glomeruli with protamine sulfate (2 mg/ml) or TGF-β1 (10 ng/ml) decreased σAlb from 1 to 0.54 and 0.48, respectively. Water and solute movement were modeled using Kedem-Katchalsky equations, and the measured responses closely fit the predicted behavior, indicating that loss of albumin by solvent drag or diffusion is negligible compared with the movement of water. We also found that σAlb was reduced by 17% in fawn hooded hypertensive rats, 33% in hypertensive Dahl salt-sensitive (SS) rats, 26% in streptozotocin-treated diabetic Dahl SS rats, and 21% in 6-mo old type II diabetic nephropathy rats relative to control Sprague-Dawley rats. The changes in glomerular permeability to albumin were correlated with the degree of proteinuria in these strains. These findings indicate that the fluorescence dilution technique can be used to measure σAlb in populations of isolated glomeruli and provides a means to assess the development of glomerular injury in hypertensive and diabetic models.
Keywords: kidney, glomerulus, proteinuria, renal hemodynamics, renal disease
proteinuria and microalbuminuria have long been used as markers for early detection of chronic kidney disease (CKD) (2, 3, 5, 11, 14, 52). Elevated excretion of protein may be a consequence of injury to the glomerular protein permeability barrier, defects in tubular reabsorption of filtered protein, or a combination of both (11, 49, 52). However, the relative contributions of changes in renal hemodynamics vs. alterations in the glomerular permeability barrier to protein in the development of proteinuria are difficult to determine in vivo. Thus it is desirable to develop an in vitro model to study the barrier function of glomerulus in the absence of variation in perfusion pressure.
In 1992, Savin et al. (38) described a method to measure the reflection coefficient to albumin (σAlb) in isolated glomeruli and the relative permeability of the glomerulus to albumin (Palb) defined as 1 − σAlb. Changes in the diameter of isolated glomeruli due to water movement across the glomeruli capillaries were found to be proportional to the magnitude of an imposed oncotic gradient (39). Using this approach, Savin and her group demonstrated that numerous mediators including cardiotrophin-like cytokine factor 1 (CLCF1) (43), endothelin (36), transforming growth factor-β (TGF-β) (9, 45), superoxide (41, 44), focal glomerulosclerosis factor (37, 42), tumor necrosis factor-α (TNF-α) (24), and platelet-activating factor (PAF) (46) increase Palb in isolated glomeruli, whereas epoxyeicosatrienoic acids (EETs) (40), 20-hydroxyeicosatetraenoic acid (20-HETE) (25), and nitric oxide (NO) (41, 44) protect the glomerular protein permeability barrier. One limitation of their technique is that the alterations in the diameter of the glomeruli are small (on the order of a few microns) as they are proportional to the cube root of the volume change. Thus the glomeruli have to be imaged at high magnification, which limits the number that can be studied in a given field. This also limits the ability to study populations of glomeruli isolated from different animals, which is necessary when studying hypertensive and diabetic strains with focal glomerular disease since the degree of injury is heterogeneous. Another complication is that the reduction in the compliance of the glomerulus in animals with focal glomerulosclerosis reduces the change in diameter of the glomerular capillaries, leading to an overestimation of the fall in Palb.
The present study describes a modification of the technique of Savin to measure the σAlb in populations of isolated glomeruli. Rats or mice were treated with FITC-dextran (250 kDa) in vivo, and glomeruli were isolated in 6% BSA solution. The changes in the glomerular volume, rather than diameter, were directly measured by the dilution of the fluorescence signal in the glomerular capillaries due to water influx in response to an imposed oncotic gradient. The glomeruli were imaged at low power so that the changes in fluorescence of many glomeruli (20–50) could be simultaneously studied. Alterations in water movement in response to various imposed oncotic gradients were modeled using Kedem-Katchalsky equations (1, 16, 17), and the measured responses fit the predicted behavior. The technique was validated by showing that protamine sulfate (2 mg/ml) and TGF-β1 (10 ng/ml) decreased σAlb as previously reported (9, 38, 39, 45, 51). Moreover, σAlb was reduced in hypertensive and diabetic strains of rats with various degrees of proteinuria.
MATERIALS AND METHODS
General
Experiments were performed in male C57BL/6 mice (The Jackson Laboratory), Sprague-Dawley (SD; Charles River Laboratories, Wilmington, MA), Dahl salt-sensitive (Dahl SS), type 2 diabetic nephropathy (T2DN) (26), and fawn hooded hypertensive (FHH) rats that were obtained from inbred colonies maintained at the University of Mississippi Medical Center (UMMC). The animals were housed in the Animal Care Facility at UMMC that is approved by the American Association for the Accreditation of Laboratory Animal Care. The mice and rats had free access to food and water throughout the study. All protocols received approval by the Institutional Animal Care Committee at UMMC.
Protocol 1: Glomerular Isolation and Imaging
The rats or mice were anesthetized with 2% isoflurane. FITC-dextran (250 kDa; Sigma-Aldrich, St. Louis, MO) at a dose of 75 mg/kg in a 0.9% NaCl solution was injected into the femoral vein. Other fluorescently labeled Cy3, Cy5, and rhodamine high-molecular weight dextrans work equally as well. After 3–5 min, the kidneys were harvested and placed in ice-cold isotonic HBSS (Life Technologies, Grand Island, NY) containing 6% BSA (Sigma-Aldrich) and 10 mM HEPES (Sigma-Aldrich), pH 7.4. Glomeruli were isolated as previously described (38, 39). Briefly, the kidney was hemisected on a sagittal plane and the renal cortex was separated from the medulla, chopped into fine pieces, and passed through stainless steel filters with decreasing pore sizes from 150 to 106 μm (USA standard sieve No. 100 and No. 140, respectively, Thermo Fisher Scientific, Waltham, MA) into a petri dish. The glomeruli were captured on a 70-μm cell strainer (BD Bioscience, San Jose, CA) and washed off of the sieve with ice-cold Hank's solution containing 6% BSA. The mixture was transferred to a 15-ml tube and stored on ice before the experiment.
A small aliquot (50–100 μl) of the isolated glomeruli was loaded onto a coverslip, precoated with poly-l-lysine hydrobromide (10 mg/ml, Sigma-Aldrich), which formed the bottom of a fluid exchange chamber (RC-24, Warner Instruments, Hamden, CT). The inflow line to the chamber was attached to two peristaltic pumps so that the bath could be rapidly exchanged in less than 10 s. The effluent was collected through a vacuum line. The isolated glomeruli were imaged using a fluorescent microscope (Nikon TS-100, Nikon Instruments, Melville, NY) equipped with a high-sensitivity camera and a 175-Wt Xenon Arc Lamp (Intracellular Imaging, Cincinnati, OH) and filter wheel (Excitation/emission: 480/510–550 μm). The glomeruli were observed using a low-numerical aperture ×5 lens (Nikon Instruments, Melville, NY) with a large depth of field (>50 μm) so that the fluorescence signal from the entire glomerulus could be collected. Approximately 30 glomeruli/field were selected for study based on their morphological appearance. A recording area slightly larger than the circumference of the glomeruli was defined using Image 1 fluorescent imaging software (Incyte, Cincinnati, OH) so that all of the label remains in the recorded volume when the glomeruli expand following a reduction in the oncotic pressure of the bath. We excluded glomeruli that had intact Bowman's capsules, visible arteriolar fragments, attached proximal tubules, or were deformed or torn (39), as well as those were poorly labeled or too bright with intensities near the saturation level of the camera.
Fluorescent intensities were individually recorded, and the values are expressed as the percentage of the control intensity measured at time 0 for each glomerulus. There was more variation in the time course of the changes in fluorescent intensity between glomeruli than between rats of a given strain, so we typically averaged all the individual data and expressed the results as number of glomeruli studied per strain or group rather than calculating the group mean from the average value recorded from each rat.
Additional studies were performed to determine the distribution of the labeled dextran in the glomerular capillaries following intravenous administration. The glomeruli were labeled in vivo with FITC-dextran as described above. The kidneys were harvested and immediately placed in 10% neutral buffered formalin. Paraffin sections (3 μm) were prepared and counterstained with Evans blue (0.001% for 5 min) that exhibits red fluorescence (12). Images were obtained using a fluorescent microscope (Olympus BH-2) equipped with a highly sensitivity Q-Imaging digital camera (×400 magnification, W. Nuhsbaum, McHenry, IL). Overlaid images were created to visualize the localization of the dyes by using FITC (excitation/emission: 480/510–550 μm) and rhodamine (excitation/emission: 550/610 μm) filters, respectively.
Protocol 2: Response to Different Oncotic Gradients
Glomeruli were isolated from SD rats or C57BL/6 mice as described above. After selection of the glomeruli to be studied, the chamber was perfused at 0.5 ml/min with a solution containing 6% BSA, and baseline fluorescent signals were recorded at a rate of 1 image/s for 60 s. The solution was switched to one containing 5, 4, 3, or 2% BSA, and the fluorescent signals from the glomeruli were recorded at 1-s intervals for an additional 180 s. The initial fluorescence value (time 0) was converted to 100%, and the subsequent values are expressed as a percentage of the initial value.
The percent change in the intensity of glomerular fluorescence signal at equilibrium was plotted against the percent change in the protein concentration of the bath that is proportional to the magnitude of the oncotic pressure gradient. In addition, the results were fit to a model of water movement across the glomerular capillaries using the following equations (1, 16, 17):
| (1) |
| (2) |
where dV/dt = the change in glomerular volume per unit time; Jv = net fluid movement; LpA = filtration coefficient; A = glomerular area; σAlb = reflection coefficient for albumin; R = a modified gas constant expressed in mmHg; T = the bath temperature in degrees Kelvin; q/v = concentration of BSA in the glomeruli; C0(t) = concentration of BSA in the bath; 1 − σAlb = inverse of the reflection coefficient; and k = diffusional permeability of capillary wall to solute. These equations assume that the initial hydrostatic pressure gradient across the isolated glomerulus is negligible and the reflection coefficient for small solutes is zero. The nonlinear differential equations were solved using the finite difference least squares method using a MATLAB computing environment, and the three parameters (LpA, σAlb, and k) were estimated by an iterative best fit of the experimental data.
Protocol 3: Reversibility of Water Movement
To test whether the changes in water movement and the fluorescence of the glomeruli is bidirectional, glomeruli were isolated from two groups of 12-wk-old normal SD rats. In one group, the glomeruli were isolated in 6% BSA and the fluorescence was recorded for 20–40 s to obtain a baseline value. An oncotic gradient was imposed by changing the BSA concentration in the bath from 6 to 4% for 5 min, and then back to 6% for another 5 min. In the second group, the glomeruli were isolated in 4% BSA and the oncotic pressure was increased by changing BSA in the bath from 4 to 6% for 5 min, and then back to 4% for another 5 min.
Protocol 4: Effect of Temperature and Storage Time on Measurement of σAlb
Glomeruli were isolated in 6% BSA and stored at room temperature or on ice for various times, and σAlb was measured at room temperature. The data were recorded for 20–40 s to obtain the baseline value. An oncotic gradient was imposed by rapidly changing BSA in the medium from 6 to 4% for 3 min. An aliquot of the isolated glomeruli was studied every 15 min for up to 90 min after isolation.
Protocol 5: Response to Protamine Sulfate and TGF-β1
Male SD rats at age of 12 wk were anesthetized with isoflurane and received a 50 mg/kg intravenous injection of protamine sulfate or vehicle, which has been shown to increase glomerular permeability to albumin both in vivo and in vitro (38, 39, 51). After 15 min, the glomeruli were labeled by an intravenous injection of FITC-dextran (75 mg/kg) and were isolated in 6% BSA containing 2 mg/ml protamine sulfate as described above. The change in fluorescence in response to a decrease in the BSA concentration of the bath from 6 to 4% was recorded. Additional experiments were performed with glomeruli isolated from 12-wk-old SD rats. These glomeruli were incubated in vitro with TGF-β1 (10 ng/ml, Sigma-Aldrich) or vehicle for 15 min at 37°C that also has been reported to increase Palb (34, 38, 45).
Protocol 6: Relationships Between σAlb and Protein Excretion in Hypertensive and Diabetic Rats
Hypertensive models.
These experiments were performed in 12- to 15-wk-old male FHH and Dahl SS rats. The FHH rats begin to develop progressive proteinuria as early as 9 wk of age and chronic kidney disease by 21 wk of age (10, 19–23, 28, 29, 35). They were maintained on a normal rodent diet containing 0.4% NaCl. The Dahl SS rat is a genetic model of salt-sensitive hypertension that rapidly develops proteinuria and glomerulosclerosis when fed a high-salt (HS) diet (6–8, 30, 32, 33, 48). Two groups of Dahl SS rats were studied: one group was fed a low-salt (LS) diet containing 0.4% NaCl from birth, while the other was switched to a HS diet containing 8% NaCl starting at 9 wk of age for 3 wk. Before measurement of σAlb, the FHH and Dahl SS rats were placed in metabolic cages and urine was collected to measure protein excretion. Then, the rats were anesthetized, the glomeruli were labeled and isolated, and σAlb was measured as described above.
Diabetic models.
Nine-week-old male Dahl SS rats were treated with streptozotocin (STZ; 50 mg/kg, ip) to induce type I diabetes and were studied 3 wk later (13, 15, 31, 50). Six-month-old rats with type II diabetes and diabetic nephropathy (T2DN) rats were used as a model of type II diabetes that develops proteinuria (26, 54). The rats were placed in metabolic cages and urine was collected to measure protein excretion. Glomeruli were harvested, and σAlb was measured as described above.
Statistics
Data are presented as means ± SE. The significance of the differences in mean values between two groups of rats or treatments was evaluated using a paired or unpaired t-test. The significance of the differences in mean values between and within multiple groups over time was tested using an ANOVA for repeated measures and a Holm-Sidak test for preplanned comparisons. A P value <0.05 was considered to be statistically significant.
RESULTS
Protocol 1: Glomerular Isolation and Imaging
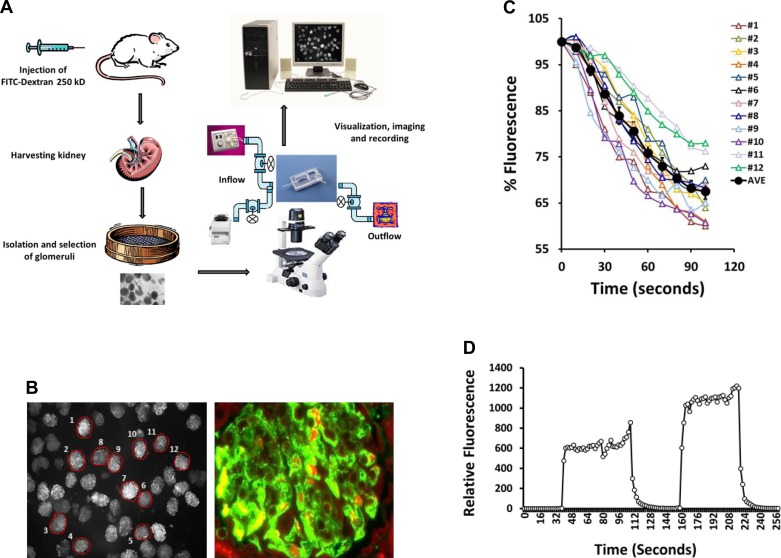
A schematic summary of the fluorescence dilution technique for measurement of σAlb is presented in Fig. 1A. Rats or mice are injected with a high-molecular weight FITC-dextran that remains in the vasculature. The kidneys are collected, and glomeruli are isolated by passing the tissue through a series of sieves. The glomeruli are placed in a rapid exchange recording chamber connected to two inflow pumps to allow for rapid exchange of the bathing media. The effluent is collected by vacuum, and the glomeruli are imaged on a fluorescent inverted microscope equipped with a ×5 (0.3 NA) fluorescent lens with a large depth of field (>50 μm). The glomeruli are visualized, and the fluorescence in an area encompassing each glomerulus is defined using Image 1 fluorescent imaging software.
Fig. 1.
Glomerular isolation and imaging. A: schematic of the fluorescence dilution albumin reflection coefficient (σAlb) measurement technique. Rats were injected with FITC-dextran (250 kDa). The kidneys were harvested, and the cortex was separated and passed through filters into a petri dish. The glomeruli were collected using a 70-μm nylon filter in ice-cold HBSS containing 6% BSA and were loaded onto a fast fluid exchange electrophysiological chamber and imaged using an inverted fluorescent microscope. B, left: typical low-power image of glomeruli (mouse) filled with FITC-dextran. Twelve glomeruli in the field were selected for study (circled), and the fluorescent intensity was individually recorded. Right: intrarenal localization of FITC-dextran in a paraffin renal section that was counterstained with Evans blue. FITC-dextran was confined to the glomerular capillaries. C: typical traces from the 12 circled mouse glomeruli as indicated in B, left. The average value for this group of glomeruli is plotted in a bold black line. D: typical trace showing the changes in fluorescence intensity in the bath when the perfusion solution was changed from media containing FITC-dextran to one without indicates that the bath is fully exchanged in a few seconds.
The left panel of Fig. 1B presents a representative field of view of the fluorescently labeled isolated mouse glomeruli. The field contains >30 glomeruli, and most are well filled with FITC-dextran. Twelve glomeruli were selected for study (circled), and the fluorescent intensity was individually recorded. The right panel of Fig. 1B presents a renal section that was counterstained with Evans blue and indicates that the FITC-dextran was confined to the glomerular capillaries. Figure 1C presents typical traces from the 12 circled mouse glomeruli as indicated in Fig. 1B, left panel. The average value for this group of glomeruli is plotted as a bold black line. Figure 1D presents the time course of changes in the fluorescence in the bath following switching of the bath solution between a control solution and one that contains FITC-dextran. These data demonstrate that the bath can be completely exchanged within seconds.
Protocol 2: Response to Different Oncotic Gradients
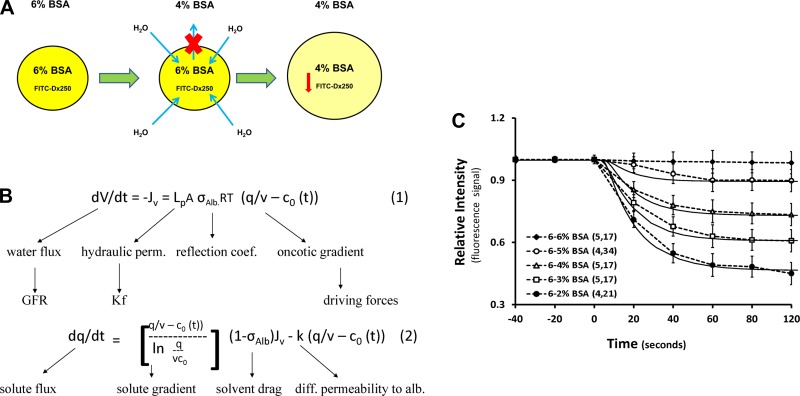
A simplified schematic of the theoretical basis of the fluorescence dilution technique is presented in Fig. 2A. Glomeruli were isolated in an isotonic HBSS solution containing 6% BSA. Following a rapid step change in the concentration of the bath from 6 to 4%, an oncotic gradient of ∼9 mmHg is generated which drives water into the capillaries. As a result, the concentration and fluorescence intensity of the FITC-dextran in the glomerular capillaries is reduced. Assuming the glomerulus was impermeable to albumin and the reflection coefficient is 1, a reduction in the protein concentration of the media from 6 to 4% would reduce the oncotic pressure in the bath by 33% and should increase the volume of the glomerulus and reduce the fluorescent signal by a similar amount.
Fig. 2.
Theoretical basis of the fluorescence dilution σAlb measurement. A: mechanism of water flux after changing oncotic gradient from 6 to 4% BSA. Rapid exchange of medium to a lower BSA concentration imposes an oncotic gradient across the capillary wall that drives water into the capillary and dilutes the fluorescent intensity. B: Kedem-Katchalsky equations for water and solute flux across the glomerular capillaries. Water and solute (BSA) flux are described in Eqs. 1 and 2, respectively. C: validation of the fluorescence dilution technique. The dotted lines are experimental results with FITC-dextran-labeled glomeruli isolated from Sprague-Dawley (SD) rats using our new fluorescence dilution technique. The solid lines are of a best fit of the experimental data based on a computer model of water movement in isolated glomeruli using the Kedem-Katchalsky equations previously described by Beard et al. (1). Values are means ± SE. Numbers in parentheses indicate the number of rats and glomeruli studied in each group.
A mathematic model derived from Kedem-Katchalsky equations (1, 16, 17) that describes the expected movement of water and solute (BSA) in response to an oncotic pressure gradient is presented in Fig. 2B. In this model, water flux (Eq. 1) is equal to the product of LpA (hydraulic permeability), σAlb (reflection coefficient), and sum of the oncotic and hydrostatic pressure gradients across the glomerular capillaries. The oncotic pressure gradient is defined by the difference in the concentration of albumin in the glomerular capillaries and the bath. Equation 2 describes the diffusion and filtration of albumin across the glomerular capillaries. If we assume that the initial hydrostatic pressure gradient, at least at time 0, is negligible, the albumin reflection coefficient (σAlb) can be determined by the measuring change in volume of the glomerulus, whereas σAlb = the observed % change in Δv/the predicted % change in volume which should be equal to the percentage change in oncotic pressure determined by the imposed protein concentration gradient. The range of values for σAlb is 0 and 1. A relative Palb can be calculated as 1 − σAlb.
In the current study, a high molecular weight of fluorescent FITC-dextran which is impermeable to the glomeruli capillaries was used as a volume marker. Under these conditions, the fluorescence is the glomerular capillaries is directly proportional to the concentration of FITC-dextran and inversely proportional to the glomerular volume. Therefore, we substituted the percent change in fluorescence for Δv and defined the σAlb = observed % change fluorescence/expected % change in volume that is equal to the % change in the albumin concentration of the bath.
The time course of the change in fluorescent intensity in FITC-dextran-labeled glomeruli isolated from 9-wk-old young SD rats in response to different oncotic gradients imposed by changes of BSA concentration from 6 to 5, 4, 3, and 2% is presented in Fig. 2C. These data were iteratively fit to a mathematical models of Eqs. 1 and 2 as described in detail previously by Beard et al. (1) by first assuming that the glomerular capillaries were impermeable to albumin (σAlb = 1) to obtain the best fit estimates for Lp A=3.8 dl/mg × s and K = 0.05 s. We then varied σAlb to 0.83 to obtain the best fit for the magnitude of the responses. Lowering σAlb decreases the magnitude of the response, whereas altering LpA affects the shape of the curve and how rapidly equilibrium is attained. The model was relatively insensitive to changes in K, as would be expected since diffusive loss of protein across the capillaries is negligible relative to the rapid movement of water.
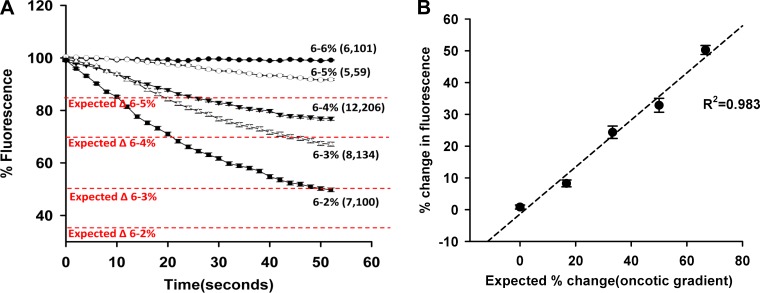
Additional glomeruli were isolated from 9-wk-old young SD rats to better understand the changes in fluorescent intensity in response to different oncotic gradients imposed by changes in BSA concentration as presented in Fig. 3A. The expected changes in the fluorescence intensity of the glomeruli in response to changing the bath from 6% BSA to 4, 3, and 2% are 33.3, 50, and 67.7%, respectively. The experimental results indicated the measured changes in fluorescent signal were 27.9, 33.5, and 50.2%. The relationship between % change in fluorescence and the expected % change in fluorescence was linear, with a slope of 0.992 and an adjusted R2 of 0.983 (Fig. 3B).
Fig. 3.
Response to different oncotic gradients. A: fluorescence dilution curves. Changes in the fluorescent intensity in FITC-dextran-labeled glomeruli isolated from SD rats exposed to different oncotic gradients are presented. The expected fall in fluorescence based on the change in oncotic pressure is presented in red. B: relationship between the measured change in fluorescence vs. the % fall in oncotic pressure. The % change in fluorescence intensity in the isolated glomeruli is linearly related to the % change in oncotic pressure. Values are means ± SE. Numbers in parentheses indicate the number of rats and glomeruli studied per group.
Protocol 3: Reversibility of Water Movement
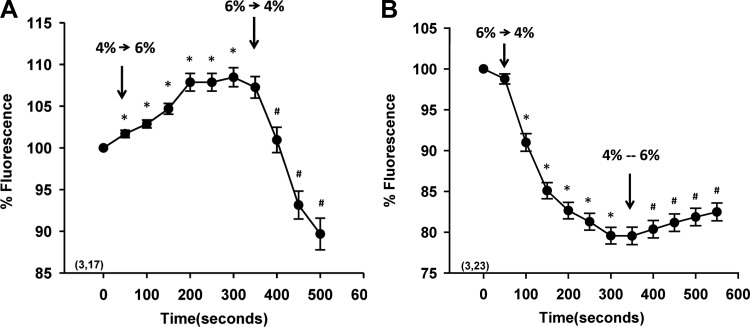
The results of experiments to determine whether water movement in the glomerulus was bidirectional are presented in Fig. 4A. The fluorescence intensity increased by 8% (P < 0.05) in FITC-dextran-labeled glomeruli isolated from SD rats in 4% BSA after the bath was replaced with 6% BSA. It decreased by 20% (P < 0.05) after medium was switched back to 4% BSA. Similarly, Fig. 4B demonstrates that changing the medium containing 6% BSA to 4% BSA resulted in a decrease in fluorescence intensity of 20% (P < 0.05) and increased by 4% (P < 0.05) after medium was changed back to 6% BSA.
Fig. 4.
Reversibility of water movement. A: time course of changes in fluorescence in labeled glomeruli after changing of the albumin concentration of the bath from 4 to 6% and then back to 4% BSA. B: time course of the changes in fluorescence of labeled glomeruli after changing of the albumin concentration of the bath from 6 to 4% and then back to 6% BSA. Values are means ± SE. Numbers in parentheses indicate the number of rats and glomeruli studied per group. *Significant difference (P < 0.05) compared with the time 0 control point. #Significant difference (P < 0.05) compared with the switch in the oncotic pressure gradient.
Protocol 4: Effect of Temperature and Storage Time on Measurement of σAlb
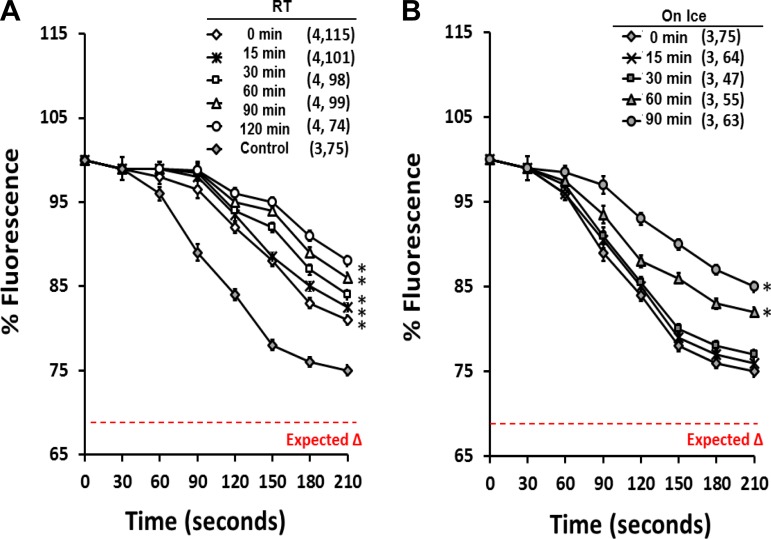
These studies were performed to determine the effect of temperature and storage time on the measurement of σAlb. FITC-dextran-labeled glomeruli were isolated from young 9-wk-old SD rats and stored at room temperature or on ice. The intensity of the fluorescence signal of the glomeruli was recorded every 15 min for 90 min after glomerular isolation. As displayed in Fig. 5, there was a time-dependent decrease in the magnitude of the σAlb response in glomeruli isolated and stored at room temperature (Fig. 5A) relative to glomeruli isolated with cold media and studied immediately at room temperature (Fig. 5B). Glomeruli isolated and stored on ice exhibited a better sigmoid curve, and the fall in fluorescence intensity ranged from 25 ± 1% at time 0 in control glomeruli vs. only 20 ± 1 and 15 ± 1 in glomeruli isolated and stored at room temperature for 0 and 90 min. The relative intensity of the fluorescence of glomeruli isolated and stored on ice at time 0 most closely matched the expected change (75 vs. 67%). There is no significant difference in the response of glomeruli isolated and stored on ice for 15 or 30 min, but the response was diminished when glomeruli were stored on ice for longer periods (60–90 min).
Fig. 5.
Effect of temperature and storage time. A: change in fluorescence intensity in glomeruli isolated and stored at room temperature (RT) compared with glomeruli isolated on ice and studied at time 0 (control). B: change in fluorescence intensity in glomeruli isolated and stored on ice for various lengths of time. All glomeruli were isolated in isotonic HBSS solution with 6% BSA. The change in fluorescence intensity in an aliquot of the stored glomeruli in response to a fall in the albumin concentration of the bath from 6 to 4% was recorded every 15 min up to 90 min after glomerular isolation. The expected change in fluorescence based on the fall in oncotic pressure of the bath is plotted in red. Values are means ± SE. Numbers in parentheses indicate the number of SD rats and glomeruli studied per group. *Significant difference (P < 0.05) compared with the change in fluorescence of glomeruli isolated on ice and studied immediately after isolation at time 0.
Protocol 5: Response to Protamine Sulfate and TGF-β1
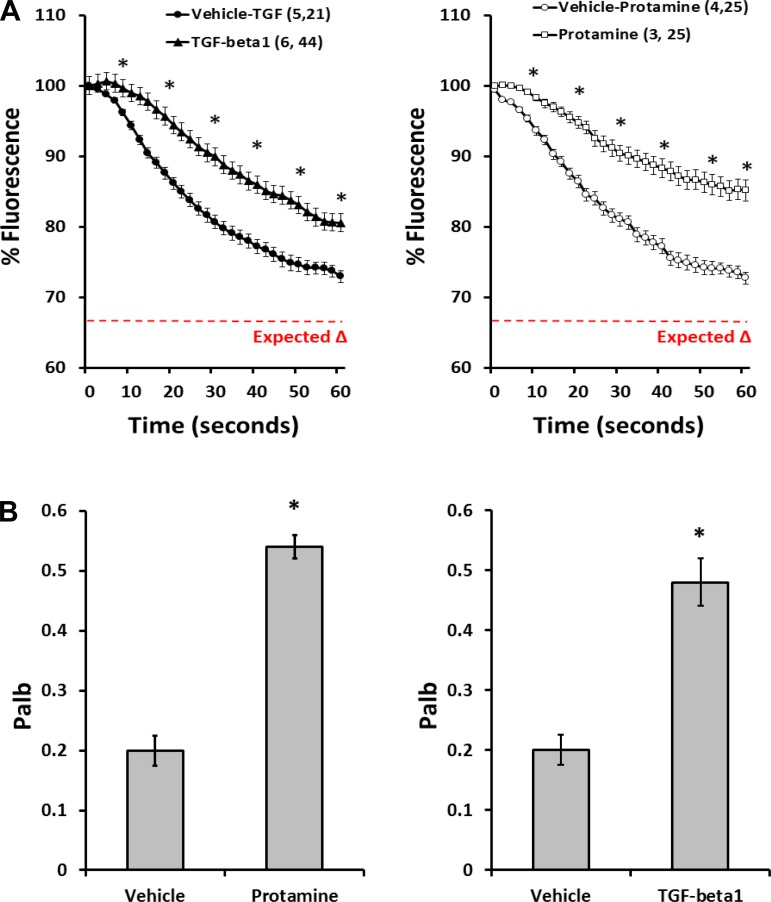
Previous studies reported that protamine sulfate and TGF-β1 increase the albumin permeability of the glomerulus (38, 45); therefore, experiments were performed to determine whether the fluorescence dilution assay can detect the expected changes in σAlb. The results are presented in Fig. 6. The fluorescence of the glomeruli decreased by ∼25% in response to a decrease in protein concentration of the bath from 6 to 4% in glomeruli isolated from control rats. The magnitude of the response was significantly reduced in glomeruli pretreated with protamine sulfate (Fig. 6A, right) or TGF-β1 (Fig. 6A, left) in vitro. Correspondingly, Palb increased from 0.20 ± 0.03 to 0.54 ± 0.02 in glomeruli treated with protamine sulfate (Fig. 6B, right) and to 0.48 ± 0.04 in the TGF-β1 (Fig. 6B, left)-treated group.
Fig. 6.
Response to protamine sulfate and transforming growth factor-β1 (TGF-β1). A: response to protamine sulfate and TGF-β1. Time course of changes in fluorescence in glomeruli isolated from control SD rats and treated with protamine sulfate (right) or TGF-β1 (left) are shown. B: effect of protamine sulfate (right) and TGF-β1 (left) on permeability of the glomerulus to albumin (Palb) of isolated glomeruli. All glomeruli were isolated and stored on ice in isotonic HBSS solution with 6% BSA. The glomeruli in the protamine sulfate experiments were studied at room temperature while the glomeruli in the TGF-β1 experiments were incubated with TGF-β1 or vehicle for 15 min at 37°C before study. Values are means ± SE. Numbers in parentheses indicate the number of SD rats and glomeruli studied per group. *Significant difference (P < 0.05) compared with the corresponding value in the control glomeruli treated with vehicle.
Protocol 6: Relationships Between σAlb and Protein Excretion in Hypertensive and Diabetic Rats
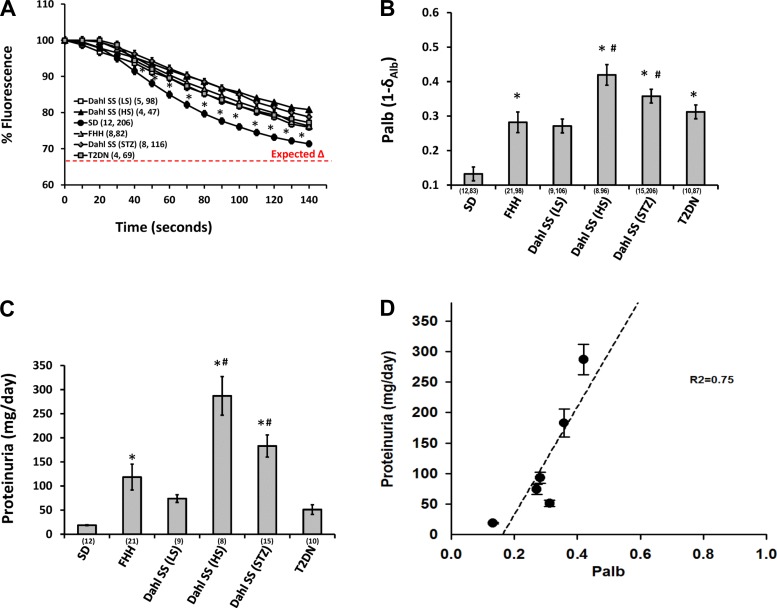
The results of these experiments are presented in Fig. 7. The fluorescence intensity in response to changes in the albumin concentration of the bath from 6 to 4% fell by 29% in glomeruli isolated from young SD rats, and was significantly greater in FHH rats (23%) and Dahl SS rats fed a HS diet (19%), but not in Dahl SS rats fed a LS diet (25%). Similarly, the fall in fluorescence intensity in response to changes in an oncotic gradient in diabetic animal models was significantly reduced in STZ-treated Dahl SS rats (21%) and 6-mo-old T2DN rats (23%) than that seen in SD rats (Fig. 7A).
Fig. 7.
Relationship between changes in albumin reflection coefficient (σAlb) and proteinuria in hypertensive and diabetic strains of rats. A: changes in the fluorescence intensity of glomeruli isolated from SD, fawn hooded hypertensive (FHH), and Dahl salt-sensitive (Dahl SS) rats fed a low-salt (LS) and high-sale (HS) diet and streptozotocin (STZ)-treated type I diabetic Dahl SS rats and type II diabetic rats with diabetic nephropathy (T2DN) in response to a fall in the protein concentration of the bath albumin concentration from 6 to 4%. B: Palb calculated from the σAlb measurement in the various strains of rats. C: comparison of proteinuria in the various strains of rats. Urinary protein excretion was compared in age-matched SD, FHH, Dahl SS rats fed a LS and HS diet, or STZ-induced type I diabetic Dahl SS rats and T2DN rats. D: relationship between protein excretion and Palb in the various hypertensive and diabetic strains of rats. Urine protein excretion is positively correlated with Palb in different strains of rats. All glomeruli were isolated and stored on ice in isotonic HBSS solution with 6% BSA and were measured within 30 min from isolation. Values are means ± SE. Numbers in parentheses indicate the number of rats and glomeruli studied per group at each time period. *Significant difference compared with SD control rats.
A comparison of Palb measured in these various groups is presented in Fig. 7B. Palb was significantly higher in glomeruli isolated from FHH (0.28 ± 0.03), Dahl SS rats fed a HS diet (0.42 ± 0.03), STZ-treated Dahl SS rats with type I diabetes (0.36 ± 0.02), and T2DN rats (0.31 ± 0.02) compared with SD rats (0.13 ± 0.02). The difference in Palb was not significant in SD rats vs. in Dahl SS rats fed a LS diet (0.27 ± 0.02). A comparison of the protein excretion in the various groups is presented in Fig. 7C. Protein excretion was significantly elevated in 12-wk-old Dahl SS rats fed a LS diet (74 ± 8 mg/day) and 6 mo old T2DN rats (51 ± 10 mg/day) compared with 12 wk old normal SD rats (19 ± 1 mg/day). Protein excretion was much higher in FHH rats (93 ± 9 mg/day), Dahl SS rats fed a HS diet (287 ± 40 mg/day), and STZ-treated type I diabetic Dahl SS rats (183 ± 23 mg/day). Figure 7D presents the relationship between proteinuria and Palb measured in these different strains. Urine protein excretion correlated well with the measurement of Palb of glomeruli isolated from these various strains (R2 = 0.75).
DISCUSSION
Proteinuria has long been used as markers for early detection of chronic kidney disease (2, 3, 5, 11, 14, 52). However, increases in the excretion of protein may be due to injury to the glomerular permeability barrier or defects in tubular reabsorption of filtered protein (11, 49, 52). Moreover, it is difficult to dissect the relative contributions of changes in blood pressure and renal hemodynamics vs. alterations in the barrier function of the glomerulus in vivo. Thus it is desirable to study the barrier function of glomerulus in vitro in the absence of potential differences in renal hemodynamics. In this regard, Savin et al. first described a technique to measure the relative permeability of isolated glomeruli to albumin based on the change in volume of the glomerulus in response to an imposed oncotic gradient (38). Alterations in the volume of fluid in the glomerular capillaries were assessed by measuring the increase in diameter of the glomerulus before and after imposing an oncotic gradient by lowering the protein concentration of the incubation media from 6 to 2%. This method has proven to be extremely useful in identifying factors that increase the glomerular permeability to albumin. However, it is more difficult to study the changes in permeability associated with hypertension and diabetes in which the glomerular injury is very heterogeneous because the glomeruli have to be imaged at high magnification using this method to detect the small differences in diameter. This also limits the number of glomeruli that can be studied in a given experiment. Another problem is that renal fibrosis, which reduces the compliance of the glomerulus, can lead to an overestimation of the increase in Palb.
The present study describes a high-throughput fluorescence dilution technique that is a modification of the original method (38) to measure σAlb in a population of glomeruli. The major difference is that in the present study the rats were injected with a high-molecular weight FITC-dextran in vivo to label the plasma in the glomerular capillaries and serve as a volume marker. The change in the fluorescent signal in isolated glomeruli due to water influx in response to an imposed oncotic gradient was used to determine the σAlb. The results indicate that changing the albumin concentration of the bath from 6 to 5, 4, 3, and 2% produced a linear decrease in the fluorescent signal of the glomeruli. The alterations in water and solute movement were modeled using an approach previously described by Beard et al. (1) using Kedem-Katchalsky equations, and the measured responses closely fit the predicted behavior, indicating that loss of solute by solvent drag or diffusion of albumin is negligible compared with the rapid movement of water. Changing the σAlb reduces the driving force for water movement and the magnitude of the response, whereas changes in LpA affect the shape of the response and the time to reach a new steady state. The oncotic pressure of the dextran is minimal based on the molar concentration in the plasma. This can be demonstrated in Fig. 3A from the fact that the fluorescent signal did not decrease in glomeruli bathed with 6% albumin. Water would move into the glomerulus and lower the fluorescent signal if the oncotic pressure of dextran in the plasma was significant. The generation of hydrostatic pressure in the glomerulus is most likely responsible for the deviation from the expected Palb closer to 0.1, which we could achieve when studying glomeruli isolated from SD rats (Figs. 2–7) and in mouse glomeruli (Fig. 1C) using ideal isolation and storage conditions.
We also examined the ability of the fluorescent dilution assay to detect the effects of protamine sulfate and TGF-β1 on σAlb of isolated glomeruli. Protamine sulfate is a polyanion that has been reported to increase albumin permeability of the glomerulus (38) and cause proteinuria by reducing the effective negative charge of the slit pores. We found that σAlb decreased in glomeruli isolated from SD rats treated with protamine sulfate compared with vehicle-treated rats, consistent with previously reported results (38). Similarly, incubation of isolated glomeruli with TGF-β1 also reduced σAlb. Thus the results obtained using the fluorescent dilution assay are very consistent with previous results by Sharma et al. (45) and others (34, 38, 39, 45, 51) indicating that TGF-β1 increases Palb in isolated glomeruli.
The fluorescent dilution assay offers many advantages over the original method. First, since the glomeruli are fluorescently labeled, damage to individual glomeruli can be visualized by the loss of the label from the capillaries or a decline in the fluorescent signal with time. Second, the magnitude of the change in fluorescence or volume is much larger and easier to detect than the change in diameter, which is proportional to the cube root of the change in volume. Third, the time course of the changes in the fluorescent signal can be continuously monitored, whereas typically only steady-state changes in diameter at two time points are studied using the original technique. The most important advantage is that the glomeruli can be imaged using a low-power lens so that measurements can be made from many glomeruli simultaneously (up to 50/field) and the entire measurement can be completed in 120 s. This allows one to measure σAlb in a population of glomeruli isolated from a single kidney over a 30-min time frame, which is helpful in studying renal disease models in which the degree of glomerular hypertrophy, sclerosis, and podocyte loss is very heterogeneous. However, there are limitations to this technique. Some glomeruli in animals with severe sclerosis will not fill and therefore cannot be studied. Exclusion of these severely damaged glomeruli would lead to an underestimate of the overall increase in permeability within a strain. On the other hand, glomeruli isolated from severely diseased animals could be more susceptible to tearing and damage during the isolation procedure, leading to an overestimation of the degree of injury. These problems can be minimized by close visual inspection of each glomerulus before its selection for study.
In the present study, some findings deviated from the expected behavior. The results presented in Fig. 4 indicate that the movement of water is bidirectional, but the increase in fluorescence when the oncotic pressure was increased was much less than expected. This may be due to two factors. First, when the oncotic pressure of the bath is increased and the glomerular capillaries collapse, the transmission of both excitation and emitted light in the glomerulus would be reduced to limit the increase in fluorescence. Second, we defined the recording volume with Image 1 fluorescent imaging software to be slightly larger than the diameter of the glomerulus so that all of the fluorescent signal would remain within the recording volume when the glomeruli swell in response to a reduction in the oncotic pressure of the bath. However, when the oncotic pressure of the bath is increased, the size of the glomerulus contracts and the contribution of the background to the average signal within the recorded area increases and attenuates the increase in fluorescence. This problem can be partially mitigated by redefining a smaller recording volume after the oncotic pressure of the bath is increased, but it cannot address the issue with the decrease in transmission of light in the glomerulus.
Another difference in the measured vs. expected results was that the change in fluorescence decreased by 25, 35, and 50% compared with the expected change of 33.3, 50, and 66.7% when the protein concentration of the bath was lowered from 6 to 4, 3, and 2%. We believe there are two factors contributing to this difference. The first is that the hydrostatic pressure in the glomerulus increases from 0 to a few millimeters mercury as water moves into the capillaries in response to a decrease in oncotic pressure of the bath. This opposes the dilution of the fluorescent label and contributes to the deviation from the expected behavior especially when large gradients are used, which cause greater distention of the glomerular capillaries. One can readily appreciate that hydrostatic pressure in the glomerulus does increase in these experiments as the volume of the capillaries expands because we can sometimes see expulsion of the fluorescent dextran from the capillaries of some glomeruli if the collapsed arterioles open up, which typically occurs if the pressure in the capillaries increases by a few millimeters mercury. For this reason, we typically use a step change of 6 to 4% in the albumin concentration of the bath instead of establishing a larger gradient to minimize the potential for a rise in hydrostatic pressure. The other complicating factor is the effect of temperature and storage time on the measurements. The results presented in Fig. 5 indicate that the change in fluorescence in response to a step change in oncotic pressure is significantly larger in glomeruli isolated and stored in ice-cold media than in those isolated and stored at room temperature. Moreover, the change in fluorescence is reduced even in glomeruli stored on ice for longer than 30 min. In this regard, we did store the glomeruli on ice for longer periods in some of our initial studies, and this may be the reason the change in fluorescence was less than expected.
Additional studies were performed to determine whether the fluorescence dilution assay could detect differences in σAlb in hypertensive and diabetic animal models with varying degrees of proteinuria. We have previously reported that Dahl SS rats develop severe hypertension, proteinuria, and glomerular injury when fed with a HS diet and diabetic nephropathy when treated with STZ to induce type I diabetes (47, 53). They have less renal injury when fed with a LS diet. We also reported that male FHH rats start to develop proteinuria and glomerular injury at ∼9 wk of age and exhibit severe renal injury by 21 wk of age (4). T2DN rats begin to exhibit a mild degree of proteinuria at 6 mo of age and develop severe diabetic nephropathy between 12 and 18 mo of age (18, 54). In the present studies, we found that the fall in fluorescence intensity in isolated glomeruli was significantly reduced following a reduction in BSA from 6 to 4% in glomeruli isolated from Dahl S rats treated with STZ or fed a HS diet. and in FHH and T2DN rats compared with the response seen in 12-wk-old SD rats. The magnitude of the rise in Palb (1 − σAlb) in these strains was correlated with the degree of proteinuria. The only strain that deviated from the regression line was the T2DN strain. Proteinuria was lower than expected given the increase in Palb. This may reflect a higher capacity of this strain to reabsorb filtered protein, at least early in the early stages of the disease process.
In summary, we have developed a high-throughput method to measure σAlb in a large number (>30 glomeruli/experiment, 3–4 experiments/30 min) of glomeruli isolated from a single animal. The measured change in σAlb fits with the predicted behavior defined in a model of water movement in isolated glomeruli using the Kedem-Katchalsky equations (16, 17) previously described by Beard et al. (1) and allows one to study differences in σAlb in populations of glomeruli derived from normal and diseased animals. The responses to different molecules that influence the glomerular permeability barrier can also be studied. Based on these results, we recommend that glomeruli should be isolated and stored at 4°C for no more than 30 min to measure Palb. They can be incubated at room temperature or at 37°C to study the effects of drugs and hormones on Palb as long as they are compared with appropriate control glomeruli studied under the same storage and incubation conditions. In conclusion, the present fluorescence dilution method provides a means to assess the development of glomerular disease in hypertensive and diabetic models and to study the effectiveness of various treatments to slow the progression of CKD.
GRANTS
This study was supported by Grants HL36279 and DK104184 (R. J. Roman) from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.F., C.C.A.C., J.Z., C.M.N.S., J.M.W., and T.H. performed experiments; F.F., C.C.A.C., J.Z., C.M.N.S., E.A.R., J.M.W., and R.J.R. analyzed data; F.F., C.C.A.C., J.Z., C.M.N.S., E.A.R., and R.J.R. interpreted results of experiments; F.F., C.C.A.C., J.Z., C.M.N.S., E.A.R., and D.A.B. prepared figures; F.F., C.C.A.C., J.Z., and C.M.N.S. drafted manuscript; F.F., C.C.A.C., M.S., V.J.S., and R.J.R. edited and revised manuscript; F.F., C.C.A.C., J.Z., C.M.N.S., E.A.R., J.M.W., T.H., M.S., D.A.B., V.J.S., and R.J.R. approved final version of manuscript; V.J.S. and R.J.R. provided conception and design of research.
REFERENCES
- 1.Beard DA. Biosimulation: Simulation of Living Systems. New York: Cambridge University Press, 2012, p. xii, p. 307. [Google Scholar]
- 2.Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bruzzi I, Benigni A, Remuzzi G. Role of increased glomerular protein traffic in the progression of renal failure. Kidney Int Suppl 62: S29–S31, 1997. [PubMed] [Google Scholar]
- 4.Burke M, Pabbidi M, Fan F, Ge Y, Liu R, Williams JM, Sarkis A, Lazar J, Jacob HJ, Roman RJ. Genetic basis of the impaired renal myogenic response in FHH rats. Am J Physiol Renal Physiol 304: F565–F577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton C, Harris KP. The role of proteinuria in the progression of chronic renal failure. Am J Kidney Dis 27: 765–775, 1996.8651239 [Google Scholar]
- 6.Cowley AW, Stoll M, Greene AS, Kaldunski ML, Roman RJ, Tonellato PJ, Schork NJ, Dumas P, Jacob HJ. Genetically defined risk of salt sensitivity in an intercross of Brown Norway and Dahl S rats. Physiol Genomics 2: 107–115, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion. Evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med 115: 1173–1190, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature 194: 480–482, 1962. [DOI] [PubMed] [Google Scholar]
- 9.Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45: 643–648, 2005. [DOI] [PubMed] [Google Scholar]
- 10.de Keijzer MH, Provoost AP, Molenaar JC. Proteinuria is an early marker in the development of progressive renal failure in hypertensive fawn-hooded rats. J Hypertens 7: 525–528, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Erkan E. Proteinuria and progression of glomerular diseases. Pediatr Nephrol 28: 1049–1058, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One 8: e82482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis GJ, Martinez JA, Liu WQ, Xu K, Ayer A, Fine J, Tuor UI, Glazner G, Hanson LR, Frey WH 2nd, Toth C. Intranasal insulin prevents cognitive decline, cerebral atrophy and white matter changes in murine type I diabetic encephalopathy. Brain 131: 3311–3334, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Gorriz JL, Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando) 26: 3–13, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med 126: 201–205, 1967. [DOI] [PubMed] [Google Scholar]
- 16.Kedem O, Katchalsky A. A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol 45: 143–179, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta 27: 229–246, 1958. [DOI] [PubMed] [Google Scholar]
- 18.Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 345: 464–472, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreisberg JI, Karnovsky MJ. Focal glomerular sclerosis in the fawn-hooded rat. Am J Pathol 92: 637–652, 1978. [PMC free article] [PubMed] [Google Scholar]
- 20.Kuijpers MH, de Jong W. Relationship between blood pressure level, renal histopathological lesions and plasma renin activity in fawn-hooded rats. Br J Exp Pathol 68: 179–187, 1987. [PMC free article] [PubMed] [Google Scholar]
- 21.Kuijpers MH, de Jong W. Spontaneous hypertension in the fawn-hooded rat: a cardiovascular disease model. J Hypertens Suppl 4: S41–S44, 1986. [PubMed] [Google Scholar]
- 22.Kuijpers MH, Gruys E. Spontaneous hypertension and hypertensive renal disease in the fawn-hooded rat. Br J Exp Pathol 65: 181–190, 1984. [PMC free article] [PubMed] [Google Scholar]
- 23.Magro AM, Rudofsky UH. Plasma renin activity decrease precedes spontaneous focal glomerular sclerosis in aging rats. Nephron 31: 245–253, 1982. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. TNF-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol 9: 433–438, 1998. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy ET, Zhou J, Eckert R, Genochio D, Sharma R, Oni O, De A, Srivastava T, Sharma R, Savin VJ, Sharma M. Ethanol at low concentrations protects glomerular podocytes through alcohol dehydrogenase and 20-HETE. Prostaglandins Other Lipid Mediat 116–117: 88–98, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes 53: 735–742, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Provoost AP. Spontaneous glomerulosclerosis: insights from the fawn-hooded rat. Kidney Int Suppl 45: S2–S5, 1994. [PubMed] [Google Scholar]
- 29.Provoost AP, De Keijzer MH. The fawn-hooded rat: a model for chronic renal failure. In: Experimental and Genetic Models of Chronic Renal Failure, edited by Gretz N and Strauch M. Basel: Karger, 1993, p. 100–104. [Google Scholar]
- 30.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984. [DOI] [PubMed] [Google Scholar]
- 31.Rakieten N, Rakieten ML, Nadkarni MR. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep 29: 91–98, 1963. [PubMed] [Google Scholar]
- 32.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753–763, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131–185, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Roman RJ, Cowley A. Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 248: F190–F198, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Rudofsky UH, Magro AM. Spontaneous hypertension in fawn-hooded rats. Lab Anim Sci 32: 389–391, 1982. [PubMed] [Google Scholar]
- 36.Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54: 979–988, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savin VJ, McCarthy ET, Sharma R, Charba D, Sharma M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl Res 151: 288–292, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol 3: 1260–1269, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Savin VJ, Terreros DA. Filtration in single isolated mammalian glomeruli. Kidney Int 20: 188–197, 1981. [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, McCarthy ET, Reddy DS, Patel PK, Savin VJ, Medhora M, Falck JR. 8,9-Epoxyeicosatrienoic acid protects the glomerular filtration barrier. Prostaglandins Other Lipid Mediat 89: 43–51, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma M, McCarthy ET, Savin VJ, Lianos EA. Nitric oxide preserves the glomerular protein permeability barrier by antagonizing superoxide. Kidney Int 68: 2735–2744, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Sharma M, Sharma R, Reddy SR, McCarthy ET, Savin VJ. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation 73: 366–372, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Sharma M, Zhou J, Gauchat JF, Sharma R, McCarthy ET, Srivastava T, Savin VJ. Janus kinase 2/signal transducer and activator of transcription 3 inhibitors attenuate the effect of cardiotrophin-like cytokine factor 1 and human focal segmental glomerulosclerosis serum on glomerular filtration barrier. Transl Res 166: 384–398, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma M, Zhou Z, Miura H, Papapetropoulos A, McCarthy ET, Sharma R, Savin VJ, Lianos EA. ADMA injures the glomerular filtration barrier: role of nitric oxide and superoxide. Am J Physiol Renal Physiol 296: F1386–F1395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma R, Khanna A, Sharma M, Savin VJ. Transforming growth factor-beta1 increases albumin permeability of isolated rat glomeruli via hydroxyl radicals. Kidney Int 58: 131–136, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Sharma R, Sharma M, Li JZ, McCarthy ET, Savin VJ. Direct effects of platelet-activating factor on glomerular capillary permeability. Kidney Blood Press Res 20: 25–30, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Slaughter TN, Paige A, Spires D, Kojima N, Kyle PB, Garrett MR, Roman RJ, Williams JM. Characterization of the development of renal injury in Type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 305: R727–R734, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988. [DOI] [PubMed] [Google Scholar]
- 49.Toblli JE, Bevione P, Di Gennaro F, Madalena L, Cao G, Angerosa M. Understanding the mechanisms of proteinuria: therapeutic implications. Int J Nephrol 2012: 546039, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomlinson KC, Gardiner SM, Hebden RA, Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev 44: 103–150, 1992. [PubMed] [Google Scholar]
- 51.Vehaskari VM, Root ER, Germuth FG Jr, Robson AM. Glomerular charge and urinary protein excretion: effects of systemic and intrarenal polycation infusion in the rat. Kidney Int 22: 127–135, 1982. [DOI] [PubMed] [Google Scholar]
- 52.Waller KV, Ward KM, Mahan JD, Wismatt DK. Current concepts in proteinuria. Clin Chem 35: 755–765, 1989. [PubMed] [Google Scholar]
- 53.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams JM, Zhang J, North P, Lacy S, Yakes M, Dahly-Vernon A, Roman RJ. Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol 300: F983–F998, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]