Abstract
Renal oxidative stress is increased in response to ureteral obstruction. In vitro, cyclooxygenase (COX)-2 activity contributes to protection against oxidants. In the present study, we tested the hypothesis that COX-2 activity counters oxidative stress and apoptosis in an in vivo model of obstructive nephropathy. Renal oxidative stress markers, antioxidant enzymes, and markers of tubular injury, tubular dilation, and apoptosis were investigated in COX-2 knockout (COX-2−/−) and wild-type (WT) mice subjected to 3 or 7 days of unilateral ureteral obstruction (UUO). In a separate series, WT sham-operated and UUO mice were treated with a selective COX-2 inhibitor, parecoxib. COX-2 increased in response to UUO; the oxidative stress markers 4-hydroxynonenal and nitrotyrosine protein residues increased in kidney tissue with no genotype difference after UUO, whereas the antioxidant enzymes heme oxygenase-1 and SOD2 displayed higher levels in COX-2−/− mice. Tubular injury was aggravated by COX-2 deletion, as measured by tubular dilatation, an increase in kidney injury molecule-1, cortical caspase-3 content, and apoptosis index. In conclusion, COX-2 is necessary to protect against tubular injury and apoptosis after UUO but not necessary to protect against oxidative stress. COX-2 is not likely to directly regulate antioxidant enzymes heme oxygenase-1 and SOD in the kidney.
Keywords: unilateral ureteral obstruction, cyclooxygenase-2, apoptosis, oxidative stress
ureteral obstruction exposes the kidney to severe oxidative stress due to mitochondrial dysfunction and increased NADPH oxidase activity. Excessive production of ROS and a decrease in antioxidant activity occur in response to hydronephrosis and contribute to the pathophysiology associated with obstructive nephropathies (18, 26). Cyclooxygenases (COXs) are responsible for the production of prostanoids. COX-1 is constitutively expressed in the murine kidney (31, 33). COX-2 is expressed primarily in renal medullary interstitial cells and macula densa in the kidney and is markedly induced in response to ureteral obstruction, leading to an increased production of proinflammatory prostaglandins (13, 19, 24). Thus, the induction of COX-2 might be associated with the inflammatory response induced by obstructive nephropathy. However, COX-2-derived prostaglandins are also involved in renal protection. For example, COX-2 is upregulated in response to hypertonicity-induced oxidative stress (12, 29, 32) and has a protective action in this condition.
Unilateral ureteral obstruction (UUO) is characterized by increased tubular diameter, increased tubular cell proliferation, and tubular atrophy (27). Intrapelvic pressure rises immediately in response to UUO. Persistent obstruction results in parenchymal thinning, proximal tubular degeneration, and tubular dilation, which leads to apoptosis and necrosis (7). Tubular apoptosis induced by UUO is most pronounced in the distal nephron and increases linearly with increased tubular dilation (3, 7). Renal macrophage infiltration in response to UUO leads to the release of TNF-α, which mediates renal tubular apoptosis (20). Concurrently, the increased production of ROS leads to the release of mitochondrial cytochrome c, resulting in the activation of caspases and apoptosis (5, 34).
He and colleagues (13) have shown that sirtuin-1-induced COX-2 protects the kidney against oxidative stress-induced apoptosis. Others have also shown that COX-2 exerts protective effects against apoptosis. The protective mechanism may involve the binding of PGE2 to EP2/EP4 receptors (9, 15). Inhibition of COX-2 induces cell death by decreasing the production of prostaglandins and increasing free arachidonic acid, which stimulates the conversion of sphingomyelin to ceramide, a known mediator of apoptosis (4, 11). Therefore, COX-2 may be crucial for maintaining cell viability during oxidative stress.
Under normal conditions, ROS, generated as a result of metabolic reactions, are scavenged by antioxidant enzymes, maintaining a healthy ratio between antioxidants and prooxidants (16). Antioxidants convert cytotoxic species, such as superoxide, to harmless end products. However, several antioxidant enzymes are downregulated in response to UUO, including SOD1, SOD2, catalase, and glutathione peroxidase. This makes the kidney more vulnerable to oxidative injury (18, 26, 35). SODs protect tissues against oxidative stress by catalyzing the conversion of superoxide radicals into less harmful ROS. Downregulation of SOD in response to UUO causes increased superoxide formation, which further leads to cytotoxic effects (17, 34). Therefore, maintaining effective antioxidant mechanisms is crucial to counteract the consequences of increased ROS induced by ureteral obstruction.
The present study was designed to investigate the role of COX-2 in the regulation of antioxidants and the progression of apoptosis in response to UUO for 3 or 7 days. We hypothesized that COX-2 activity protects against renal oxidative stress and apoptosis in consequence of ureter obstruction by stimulating antioxidant enzymes and countering apoptosis.
METHODS
Experimental animals.
All procedures conformed with Danish national guidelines for the care and handling of animals and the published guidelines from the National Institutes of Health. Animal protocols were approved by the board of the Institute of Clinical Medicine of Aarhus University, according to the licenses for use of experimental animals issued by the Danish Ministry of Justice.
Experiments were performed using adult (10–14 wk of age) wild-type (WT) or COX-2 knockout (COX-2−/−) mice backcrossed to the C57BL/6J background for 12–13 consecutive generations. COX-2−/− mice on a mixed 129/C57 genetic background were originally generated by Dinchuk et al. (6). The breeder pairs (strain name: B6;129S-Ptgs2tm1Jed/J) were obtained from Jackson Laboratories (Bar Harbor, ME), housed at the Biomedical Laboratory of the University of Southern Denmark, and genotyped as previously described (8). Furthermore, C57BL/6J mice were used for an additional experiment using parecoxib treatment to selectively inhibit COX-2. Animals had free access to a standard rodent diet (Altromin, Lage, Germany) and tap water. During the experiments, mice were housed in groups of 2–3 mice/cage on a 12:12 h light-dark cycle at a temperature of 21 ± 2°C and humidity of 55 ± 2%. Animals were allowed to acclimatize to the cages for 3–4 days before surgery.
Experimental design and surgical procedures.
Mice were anesthetized with sevoflurane and placed on a heating pad to maintain an appropriate body temperature during surgery. Through a midline abdominal incision, the left ureter was exposed and occluded with a 6-0 silk ligature. UUO was induced for 3 or 7 days in COX-2−/− mice and WT littermates. Kidneys were prepared for immunohistochemistry (for 3-day UUO, sham-operated WT: n = 4, sham-operated COX-2−/−: n = 4, 3-day UUO WT: n = 4, and 3-day UUO COX-2−/−: n = 2; for 7-day UUO, sham-operated WT: n = 5, sham-operated COX-2−/−: n = 3, 7-day UUO WT: n = 4, and 7-day UUO COX-2−/−: n = 3) or dissected into the cortex (including the cortex and outer stripe of the outer medulla) and inner medulla (including the inner medulla and inner stripe of the outer medulla). Tissues were prepared for quantitative PCR (3-day UUO: n = 5 and 7-day UUO, n = 4) or semiquantitative immunoblot analysis (n = 6). Age- and time-matched sham-operated controls were prepared and observed in parallel with each experimental group. In an additional experiment, UUO was induced for 3 and 7 days in C57BL/6J mice with concomitant treatment with parecoxib (5 mg·kg−1·day−1, Dynastat, Pfizer, New York, NY) diluted in saline (n = 5). Parecoxib was administered 2 times daily by an intraperitoneal injection and initiated on the day of surgery and throughout the study. Sham operations were performed in parallel, and control groups received saline as vehicle. The cortex was dissected free and prepared for quantitative PCR or semiquantitative immunoblot analysis (n = 5).
Tissue fixation.
Whole kidney tissues from mice subjected to either 3 or 7 days of UUO or sham operation were fixed by perfusion through the left ventricle using 4% paraformaldehyde in water. Kidneys were then immersed in fixative for 2 h and subsequently washed three times for 10 min each in PBS before dehydration and embedding in paraffin. A rotary microtome (Leica Microsystems, Ballerup, Denmark) was used to cut the embedded tissue into sections of 2 μm.
Quantitative PCR.
Total RNA was isolated from the kidney cortex with a NucleoSpin RNA II mini kit following the manufacturer's instructions (Macherey-Nagel, Düren, Germany). RNA was quantitated by spectrophotometry and stored at −80°C. cDNA was synthesized from 0.5 μg RNA with the AffinityScript QPCR cDNA synthesis kit (Life Technologies, Thermo Fisher Scientific, Cambridge, MA). For quantitative PCR, 100 ng cDNA served as the template for PCR amplification using the Brilliant SYBR Green QPCR Master Mix according to the manufacturer's instructions (Life Technologies). Primers used for quantitative PCR are shown in Table 1.
Table 1.
Primers used for quantitative PCR amplification
| Gene/Protein (Accession Number) | Forward Primer | Reverse Primer |
|---|---|---|
| COX-2 (NM_011198.3) | 5′-TGGGTGTGAAGGGAAATAAGG-3′ | 5′-CATCATATTTGAGCCTTGGGG-3′ |
| EP1 (NM_013641.2) | 5′-CGGCATTAGTGTGCAATACG-3′ | 5′-TGGCTGAAGTGATGGATGAG-3′ |
| EP2 (NM_008964.4) | 5′-ATGCTCCTGCTGCTTATCGT-3′ | 5′-AGGGCCTCTTAGGCTACTGC-3′ |
| EP3 (NM_011196.2) | 5′-GGATCATGTGTGTGCTGTCC-3′ | 5′-CCCATCTGTGTCTTGCATTG-3′ |
| EP4 (NM_001136079.2) | 5′-CCATCGCCACATACATGAAG-3′ | 5′-TGCATAGATGGCGAAGAGTG-3′ |
| GAPDH (NM_008084.3) | 5′-GACGGCCGCATCTTCTTGTG-3′ | 5′-GCGCCCAATACGGCCAAATC-3′ |
Immunoblot analysis.
Cortex and inner medulla tissues were homogenized separately in dissecting buffer consisting of 0.3 M sucrose, 25 mM imidazole, and 1 mM EDTA (pH 7.2) containing the following protease inhibitors: Complete Mini Protease Inhibitor Cocktail Tablets (serine, cysteine, and metalloprotease inhibitors, Roche, Hvidovre, Denmark) and Phosphatase Inhibitor Cocktail 2 and 3 (Sigma-Aldrich, St. Louis, MO). Tissues were homogenized for 4 min at 50 Hz by a TissueLyser LT (Qiagen, Hilden, Germany) and then centrifuged at 4,500 g at 4°C for 15 min. The supernatant was removed and used for gel electrophoresis in Laemmli sample buffer containing 2% SDS. Total protein concentrations of the homogenates were determined using a Pierce BCA protein assay kit (Roche), and absorbance was measured at 562 nm.
Proteins were separated on a 12% Criterion TGX Precast Gel (Bio-Rad Laboratories, København, Denmark) and transferred to a Hybond ECL nitrocellulose membrane (GE Healthcare, Hatfield, UK). The membrane was then blocked in 5% nonfat dry milk in PBS-Tween 20 (PBS-T; containing 80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, and 0.1 Tween 20; pH 7.4), washed in PBS-T, and incubated with primary antibodies overnight at 4°C. Subsequently, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 h (P448, goat anti-rabbit immunoglobulin, DAKO, Glostrup, Denmark). Antigen-antibody reactions were visualized using an enhanced chemiluminescence system (Amersham ECL Plus, GE Healthcare). All Western blots were normalized to total protein as measured by Stain-Free technology (10).
Immunofluorescence labeling.
Kidney sections were deparaffinized, rehydrated, and then boiled in target retrieval solution (sodium citrate buffer: 10 mM tri-sodium citrate and 0.05% Tween 20, pH 6.0) for 16 min to expose antigens. After being cooled, sections were placed in 50 mM NH4Cl in PBS to block nonspecific binding and preincubated in PBS containing 1% BSA, 0.2% gelatine, and 0.05% saponin. Sections were incubated overnight at 4°C with primary antibodies diluted in PBS containing 0.1% BSA and 0.3% Triton X-100. Sections were subsequently washed for 30 min in PBS containing 0.1% BSA, 0.2% gelatine, and 0.05% saponin and then incubated with Alexa fluor 488-conjugated secondary antibody at room temperature for 30 min (Life Technologies). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), washed in PBS, and mounted with SlowFade Gold Antifade Mountant (Life Technologies). Fluorescence microscopy was performed using an Olympus BX61 microscope (Olympus, Tokyo, Japan). Image processing was performed using xcellence rt software (Olympus).
Periodic acid-Schiff's reagent staining was performed on deparaffinized and rehydrated sections. Sections were immersed in 0.5% periodic acid for 5 min followed by Schiff reagent (Sigma-Aldrich) for 15 min. Sections was counterstained with Mayers hematoxylin (Ampliqon, Odense, Denmark), washed, dehydrated, and mounted with coverslips. Light microscopy was performed using a Nicon Eclipse Ci microscope (Nikon, Tokyo, Japan). Tubular dilation was measured using Fiji image-analysis software (http://www.fiji.sc, hosted at the Laboratory for Optical and Computational Instrumentation of the University of Wisconsin, Madison, WI). A grid overlay was placed on each picture, and tubules located at the points of intersections were marked. Hereafter, the lumen was quantified in percentages of the marked area. Ten pictures were captured from each specimen at ×20 magnification, with no overlapping areas.
Sections were examined by a renal pathologist to determine grade of renal injury in response to UUO. Renal injury was described from a number of injury markers: interstitial edema, interstitial inflammation, and cortical and inner medullary tubular necrosis. Each injury marker was scored as follows: 0, no injury; 1, mild injury (1–5%); 2, incipient injury (6–25%); 3, profound injury (25–50%); and 4, severe injury (50–100%). All analyses were performed blinded.
Detection of apoptosis.
Sections were deparaffinized, rehydrated, and washed in PBS. Apoptotic cells were detected using fluorescent in situ TUNEL staining. Proteinase K was added to inactivate nucleases, and sections were subsequently washed in PBS. Apoptotic cells were visualized using a commercial in situ apoptosis detection kit (product no. S7111, Millipore, Darmstadt, Germany) according to the manufacturer's protocol. Sections were counterstained with DAPI. TUNEL-positive cells were detected using a fluorescence Olympus BX61 microscope at the following wavelengths (excitation/emission): fluorescein, 488/515 nm; and DAPI, 345/455 nm. To quantify TUNEL-positive staining, 10 images were captured from each mouse using only the DAPI channel. Hereafter, TUNEL-positive cells were revealed and counted. The apoptotic index is presented as the number of positive cells per field.
Primary antibodies.
Primary antibodies were obtained from the following sources: COX-1 (product no. 160109, Cayman Chemical), SOD1 (product no. ADI-SOD-100, Enzo), SOD2 used for Western blot analysis (product no. 06-984, Merck Millipore), SOD2 used for immunofluorescence staining (product no. ADI-SOD-111, Enzo), heme oxygenase (HO)-1 (product no. ADI-SPA-896, Enzo), caspase-3 (product no. 9665, Cell Signaling Technology), and kidney injury molecule (KIM)-1 (product no. AF1817, R&D Systems).
Statistics.
All data are presented as means ± SE. Multiple comparisons between experimental groups in the study of COX-2−/− mice were performed using two-way ANOVA followed by post hoc analysis by a Tukey's multiple-comparisons test. Multiple comparisons between experimental groups in the study of pharmacological inhibition of COX-2 were performed using one-way ANOVA followed by post hoc analysis by a Tukey's multiple-comparisons test. P values of <0.05 were considered significant.
RESULTS
Increased COX-2 in response to 3 and 7 days of UUO.
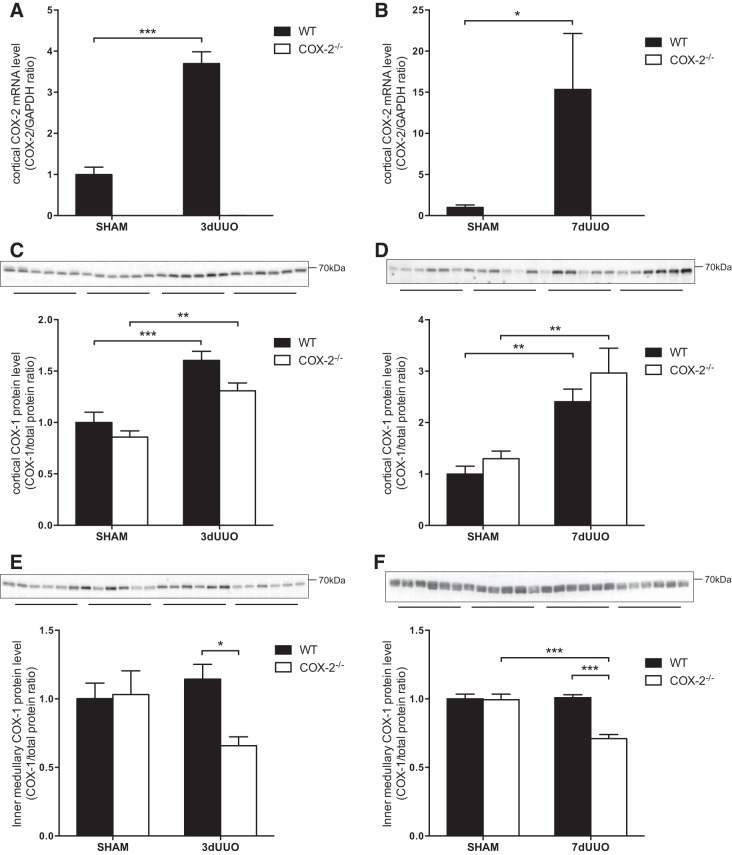
To investigate the role of COX-2 in the progression of oxidative stress and apoptosis, UUO was induced for 3 or 7 days in WT and COX-2−/− mice, and the renal changes were compared. There was a significant increase in COX-2 mRNA levels in WT mice in response to 3 or 7 days of UUO. As expected, no COX-2 mRNA was detected in COX-2−/− mice (Fig. 1, A and B). We found no difference between COX-1 protein expression in COX-2−/− and WT sham-operated mice (Fig. 1, C–F). In response to UUO, cortical COX-1 protein levels increased significantly after 3 and 7 days in both COX-2−/− and WT mice (Fig. 1, C and D). Conversely, we found no change in inner medullary COX-1 protein levels in response to UUO in WT mice. Interestingly, inner medullary COX-1 protein levels were significant lower in COX-2−/− mice subjected to UUO compared with WT mice (Fig. 1, E and F).
Fig. 1.
Cyclooxygenase (COX) expression in COX-2 knockout (COX-2−/−) and wild-type (WT) mice subjected to unilateral ureteral obstruction (UUO). A: cortical COX-2 mRNA expression in response to 3 days of UUO (3dUUO; n = 5). B: cortical COX-2 mRNA expression in response to 7 days of UUO (7dUUO). COX-2 mRNA expression was normalized to GAPDH (n = 4). C: cortical COX-1 protein abundance in response to 3 days of UUO (n = 6). D: cortical COX-1 protein abundance in response to 7 days of UUO (n = 6). E: inner medullary COX-1 protein abundance in response to 3 days of UUO (n = 6). F: inner medullary COX-1 protein abundance in response to 7 days of UUO (n = 6). COX-1 protein abundance was normalized to total protein. Sham, sham-operated animals. Data are presented as means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001.
Renal oxidative stress is increased by UUO.
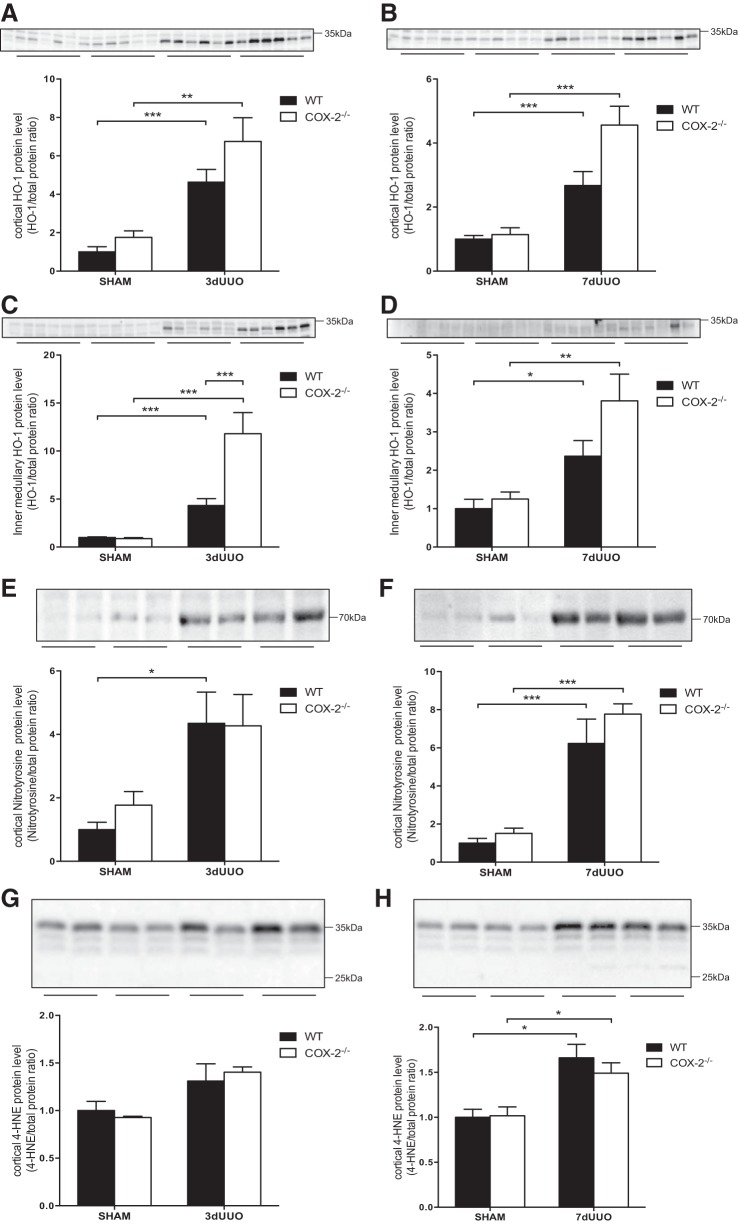
HO-1, a marker of oxidative stress, was increased in both genotypes after 3 and 7 days of UUO (Fig. 2). Although cortical HO-1 protein expression in COX-2−/− mice subjected to UUO appeared to be higher than in WT mice after both 3 and 7 days, it did not reach statistical significance (Fig. 2, A and B). COX-2−/− mice subjected to 3 days of UUO showed notably higher inner medullary HO-1 protein expression compared with WT mice (P = 0.0004; Fig. 2C). HO-1 levels were not different in COX-2−/− mice subjected to 7 days of UUO compared with WT mice (P = 0.12; Fig. 2D). Two markers of oxidative stress were investigated in response to UUO and COX-2 deficiency. Peroxinitrite induces modifications of protein tyrosine residues and forms nitrotyrosine protein adducts. The abundance of nitrotyrosine residues in the cortex was increased in response to both 3 and 7 days of UUO, and no significant change was found between COX-2−/− and WT mice (Fig. 2, E and F). 4-Hydroxynonenal (4-HNE) is produced by lipid peroxidation in cells and forms 4-HNE adducts of protein histidine residues. 4-HNE protein residues were increased in response to UUO, although they were only significant after 7 days of UUO. We found no change in the formation of 4-HNE adducts between COX-2−/− and WT mice (Fig. 2, G and H).
Fig. 2.
Effect of COX-2 on oxidative stress markers induced by UUO. A: cortical heme oxygenase (HO)-1 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. B: cortical HO-1 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. C: inner medullary HO-1 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. D: inner medullary HO-1 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. E and F: cortical nitrotyrosine protein expression in COX-2−/− and WT mice subjected to 3 days (E) or 7 days (F) of UUO. G and H: cortical 4-hydroxynonenal (4-HNE) protein expression in COX-2−/− and WT mice subjected to 3 days (G) or 7 days (H) of UUO. Protein abundance was normalized to total protein. Data are presented as means ± SE; n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Regulation of renal SOD in response to UUO.
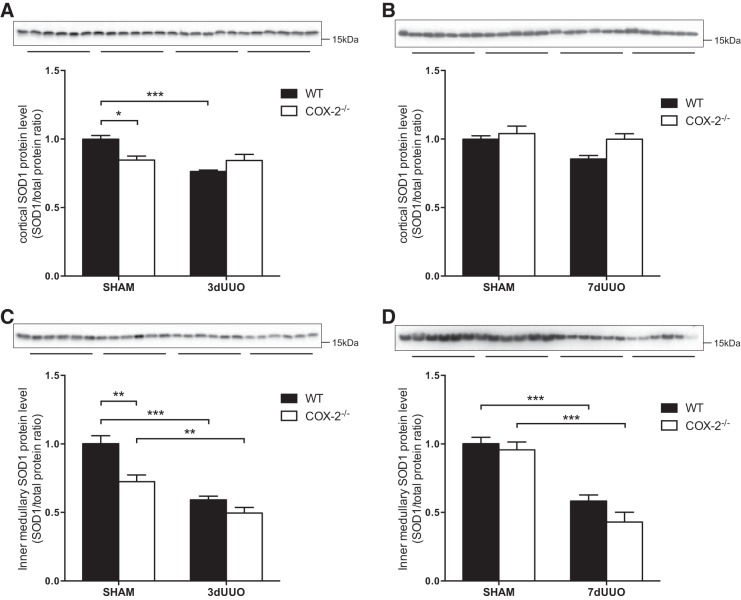
Cortical SOD1 protein levels were lower at baseline in COX-2−/− mice and were reduced after 3 days of UUO in WT mice but not in COX-2−/− mice (Fig. 3A). In inner medulla tissue, SOD1 protein levels were also lower at baseline in COX-2−/− mice and were significantly reduced in response to UUO after both 3 and 7 days of UUO in both groups (Fig. 3, C and D). No difference in SOD1 protein levels was found in this region between COX-2−/− and WT mice subjected to UUO.
Fig. 3.
Effect of COX-2 on SOD1 protein abundance after UUO. A: cortical SOD1 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. B: cortical SOD1 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. C: inner medullary SOD1 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. D: inner medullary SOD1 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. SOD1 protein abundance was normalized to total protein. Data are presented as means ± SE; n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
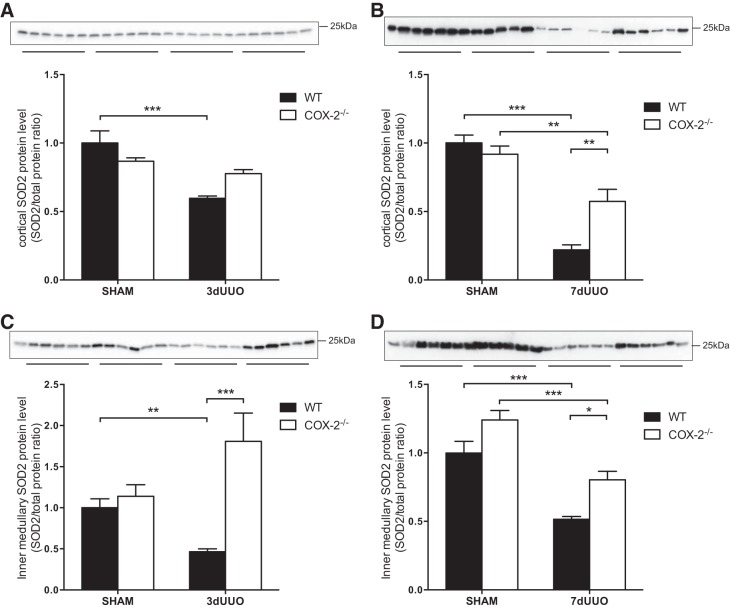
Expression of cortical SOD2 protein in WT mice was decreased in response to UUO after 3 days and even further after 7 days (Fig. 4, A and B). This downregulation was not seen in COX-2−/− mice after 3 days of UUO (P = 0.07; Fig. 4A). There was a further reduction in SOD2 after 7 days UUO in WT mice that was attenuated in COX-2−/− mice (P = 0.004; Fig. 4B). In the inner medulla, SOD2 was likewise reduced in response to 3 and 7 days of UUO in WT mice (Fig. 4, C and D). Interestingly, expression of SOD2 protein in COX-2−/− mice subjected to 3 days of UUO increased significantly compared with WT mice (P < 0.0001) and to an even higher level than sham-operated animals, although this was not significant (Fig. 4C). In addition, after 7 days of UUO, inner medullary SOD2 was significantly higher in COX-2−/− mice subjected to UUO compared with WT mice (P = 0.022; Fig. 4D).
Fig. 4.
Effect of COX-2 on SOD2 protein abundance after UUO. A: cortical SOD2 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. B: cortical SOD2 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. C: inner medullary SOD2 protein abundance in COX-2−/− and WT mice subjected to 3 days of UUO. D: inner medullary SOD2 protein abundance in COX-2−/− and WT mice subjected to 7 days of UUO. SOD2 protein abundance was normalized to total protein. Data are presented as means ± SE; n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Renal localization of SOD2 in COX-2−/− and WT mice subjected to UUO.
In the renal cortex, fluorescence microscopy showed that the SOD2 signal was associated broadly with the tubular epithelium with a predominant signal from proximal convoluted tubules in the cortex in both COX-2−/− and WT sham-operated mice (Fig. 5, A and B). WT mice subjected to 3 and 7 days of UUO showed weaker labeling of SOD2. COX-2−/− mice subjected to UUO showed stronger staining compared with WT mice, in accordance with our Western blot analyses. SOD2 was evident in the majority of tubules in the renal inner medulla in sham-operated animals (Fig. 5, C and D). After UUO, SOD2 labeling was faint and confined to a small number of tubules in WT animals. Interestingly, evaluation of SOD2 localization in COX-2−/− mice subjected to 3 or 7 days of UUO revealed intense SOD2 staining in defined structures throughout the inner medulla (Fig. 5, C and D).
Fig. 5.
Immunofluorescence staining for SOD2 in kidney sections from COX-2−/− and WT mice after 3 and 7 days of UUO. A and B: cortex after 3 days (A) and 7 days (B) of UUO. C and D: inner medulla after 3 days (C) and 7 days (D) of UUO. Green, SOD2; blue, counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). Original magnification: ×20.
Increased tubular injury in response to UUO.
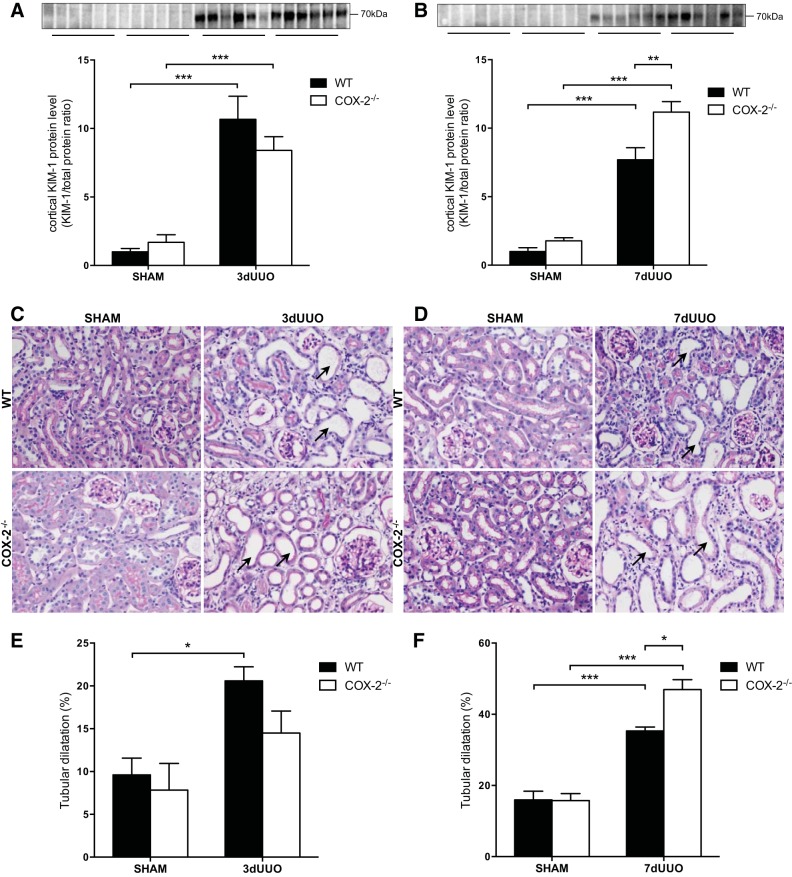
KIM-1 was used as a marker of proximal tubular injury. KIM-1 protein expression was increased in both genotypes in response to 3 and 7 days of UUO (Fig. 6, A and B). After 3 days of UUO, there was no difference between COX-2−/− and WT mice (Fig. 6A). However, after 7 days of UUO, COX-2−/− mice showed significantly higher KIM-1 protein expression compared with WT mice (P = 0.0034; Fig. 6B).
Fig. 6.
Effect of COX-2 on renal tubular injury induced by UUO. A and B: cortical kidney injury molecule (KIM)-1 protein abundance in COX-2−/− and WT mice subjected to 3 days (A) or 7 days (B) of UUO (n = 6). C and D: representative images of periodic acid-Schiff's reagent (PAS) staining of kidney sections showing cortical tubular dilation in response to 3 days (C) and 7 days (D) of UUO. Arrows denote dilated tubules. Original magnification: ×40. E and F: quantification of tubular dilation in response to 3 days (E) or 7 days (F) of UUO. Data are presented as means ± SE; for 3-day UUO: sham WT mice: n = 4, sham COX-2−/− mice: n = 4, 3-day UUO WT mice: n = 4, and 3-day UUO COX-2−/− mice: n = 2; for 7-day UUO, sham WT mice: n = 5, sham COX-2−/− mice: n = 3, 7-day UUO WT mice: n = 4, and 7-day UUO COX-2−/− mice: n = 3. *P < 0.05; **P < 0.01; ***P < 0.001.
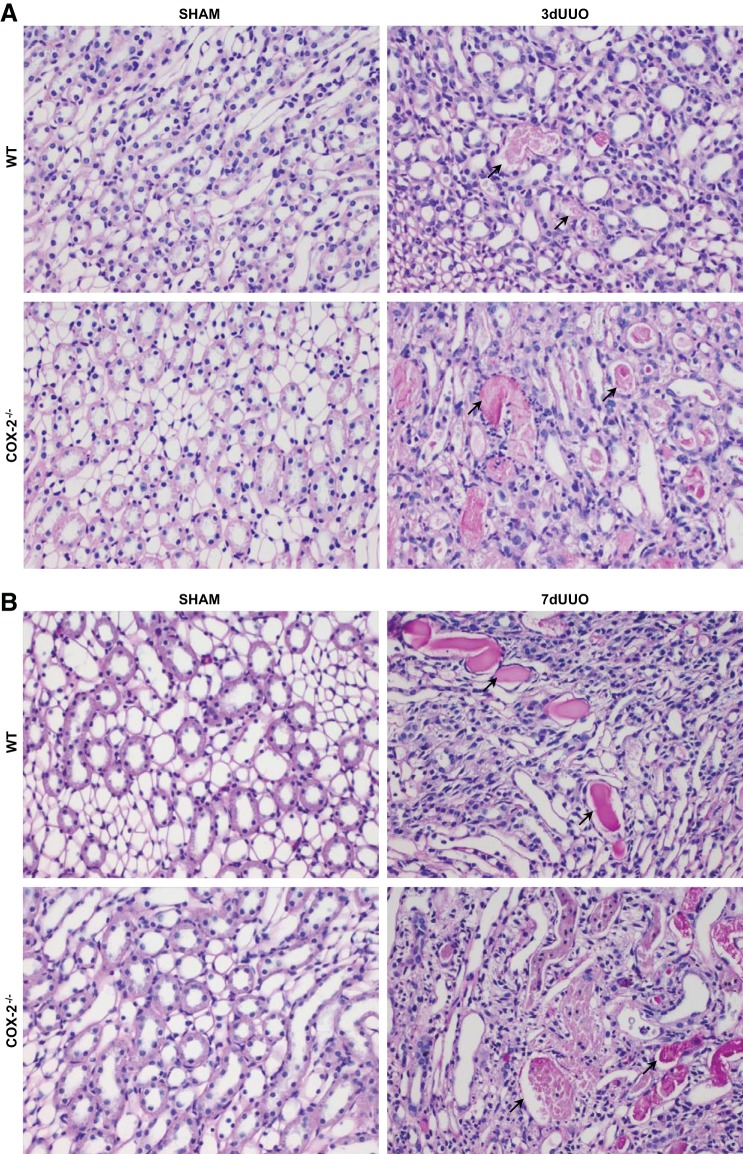
To further evaluate the effect of COX-2−/− on tubular damage, kidney sections were stained with periodic acid-Schiff's reagent to quantitate tubular dilation. Representative pictures of the cortex exhibiting dilation of the cortical tubules are shown in Fig. 6, C and D, and quantified in Fig. 6, E and F. In response to 3 and 7 days of UUO, cortical tubules were dilated compared with sham-operated animals. After 3 days of UUO, there was no difference between COX-2−/− and WT mice (Fig. 6E). However, COX-2−/− mice subjected to 7 days UUO showed more severe tubular dilation compared with WT mice (Fig. 6F). A semiquantitative histological evaluation of the renal sections demonstrated increased tubular necrosis and interstitial inflammation in response to 7 days of UUO in WT mice. Tubular necrosis was characterized by thinning of the tubular epithelium, the presence of necrotic cells in the tubule lumen, and dilation of proximal tubules with loss of brush borders. Compared with WT mice, COX-2−/− mice demonstrated more severe injuries after both 3 and 7 days UUO and additionally suffered from interstitial edema (Fig. 7 and Table 2). We found no histological difference between WT and COX-2−/− sham-operated mice.
Fig. 7.
Histological evaluation of UUO-induced kidney injury in COX-2−/− and WT mice. Representative PAS-stained kidney sections demonstrating kidney injury, characterized by thinning of tubular epithelial cells with tubular necrosis and cellular debris in tubular lumen (black arrows), are shown. A: inner medulla after 3 days of UUO. B: inner medulla after 7 days of UUO. Original magnification: ×40.
Table 2.
Semiquantitative histological evaluation of renal specimens
| 3 Days of UUO |
7 Days of UUO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sham WT | Sham COX-2−/− | UUO WT | UUO COX-2−/− | Sham WT | Sham COX-2−/− | UUO WT | UUO COX-2−/− | |
| Interstitial edema | 0 (0;0) | 0 (0;0) | 0 (0;0) | 2 (2;2) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 2 (2;2) |
| Interstitial inflammation | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0.5 (0;1) | 1.3 (1;2) |
| Medullary tubular necrosis | 0 (0;0) | 0 (0;0) | 0.3 (0;1) | 3.5 (3;4) | 0 (0;0) | 0 (0;0) | 0.5 (0;2) | 3.3 (3;4) |
| Cortical tubular necrosis | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0 (0;0) | 0.5 (0;2) | 1 (1;1) |
Values are presented as means; ranges within each group are shown in parentheses. Each sample was scored as follows: 0, no injury; 1, mild injury (1–5%); 2, incipient injury (6–25%); 3, profound injury (25–50%); and 4, severe injury (50–100%). UUO, unilateral ureteral obstruction; sham, sham operation; WT, wild type; COX-2−/−, cyclooxygenase-2 knockout.
Deletion of COX-2 exacerbates UUO-induced apoptosis.
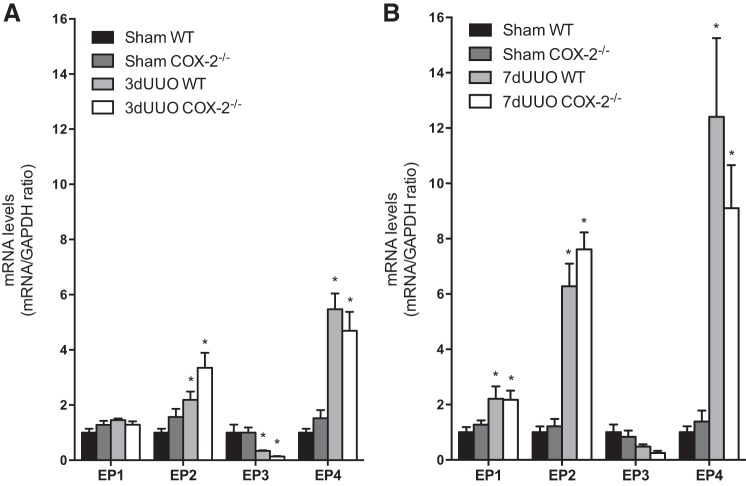
Caspase-3 levels and TUNEL staining were used to evaluate the progression of the apoptosis in response to UUO (Fig. 8). Cortical caspase-3 protein expression was increased after both 3 and 7 days of UUO (Fig. 8, A and B). COX-2−/− mice displayed higher caspase-3 protein expression than WT mice in response to 3 days of UUO (Fig. 8A). However, this difference was not observed after 7 days of UUO (Fig. 8B). Cortical, cleaved caspase-3 protein abundance was significantly increased in response to both 3 and 7 days of UUO. Cleaved caspase-3 tended to increase at both time points in COX-2−/− mice compared with WT mice (3 days: P = 0.11 and 7 days: P = 0.18; Fig. 8, C and D). The apoptotic index in the cortex and inner medulla, as determined by TUNEL staining, showed no difference between COX-2−/− and WT animals subjected to sham operation (Fig. 8, G and H). In response to both 3 and 7 days of UUO, there was a significant increase in TUNEL-positive nuclei in WT mice, consistent with increased apoptosis. Interestingly, COX-2−/− mice subjected to UUO revealed an even higher apoptotic index compared with WT mice after 3 and 7 days in the cortex (Fig. 8G) and in the inner medulla (Fig. 8H). To explore whether EP receptors might be involved in the COX-2-exerted protective effects against apoptosis, we measured the expression of EP1–EP4 in mice subjected to both 3 and 7 days of UUO. The data demonstrated increased EP2 and EP4 after 3 days of UUO and a further increase of both receptors in response to 7 days of UUO. Furthermore, EP1 was increased in response to 7 days of UUO, whereas EP3 expression was downregulated in response to 3 days of UUO. We found no difference in EP receptor expression between COX-2−/− and WT mice (Fig. 9, A and B).
Fig. 8.
Effect of COX-2 on apoptosis induced by UUO. A and B: cortical caspase-3 protein abundance in COX-2−/− and WT mice subjected to 3 days (A) or 7 days (B) of UUO (n = 6). C and D: cortical cleaved caspase-3 protein abundance in COX-2−/− and WT mice subjected to 3 days (C) or 7 days (D) of UUO (n = 6). E: TUNEL staining of the cortical region from COX-2−/− and WT mice subjected to 3 or 7 days of UUO. F: TUNEL staining of the medullary region from COX-2−/− and WT mice subjected to 3 or 7 days of UUO. Green, TUNEL-positive cells; blue, counterstaining with DAPI. Original magnification: ×20. G: quantification of the cortical apoptotic index (TUNEL-positive cells per field) in COX-2−/− and WT mice subjected to 3 or 7 days of UUO. H: quantification of the medullary apoptotic index (TUNEL-positive cells per field). Data are presented as means ± SE; for 3-day UUO, sham WT mice: n = 4, sham COX-2−/− mice: n = 4, 3-day UUO WT mice n = 4, and 3-day UUO COX-2−/− mice: n = 2; for 7-day UUO, sham WTmice: n = 5, sham COX-2−/− mice: n = 3, 7-day UUO WT mice: n = 4, and 7-day UUO COX-2−/− mice: n = 3. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 9.
Expression of PGE2 receptors in response to UUO. A: mRNA expression of EP1–EP4 receptors in the cortex was analyzed in COX-2−/− and WT mice in response to 3 days of UUO (n = 5). B: mRNA expression of EP1–EP4 receptors in the cortex in response to 7 days of UUO (n = 4). mRNA expression was normalized to GAPDH. Data are presented as means ± SE. *P < 0.05 vs. sham.
COX-2 inhibition exacerbates UUO-induced apoptosis.
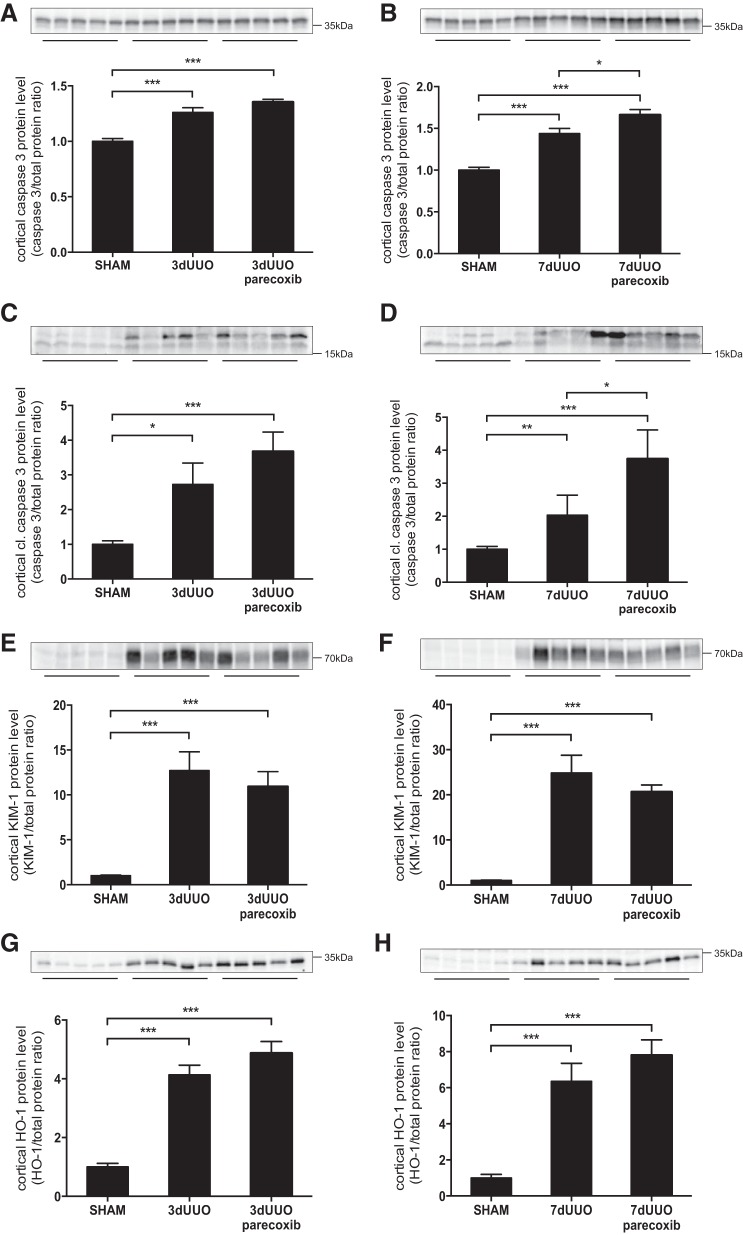
An additional experiment was performed to compare the effect of congenital COX-2 knockout with the effect of pharmacological inhibition of COX-2 on injury indexes in response to UUO. Cortical caspase-3 and cleaved caspase-3 protein abundance were increased in response to both 3 and 7 days of UUO (Fig. 10, A–D). No change in caspase-3 and cleaved caspase-3 abundance was found in response to parecoxib treatment after 3 days of UUO (Fig. 10, A and C). However, after 7 days of UUO, both caspase-3 and the cleaved form of caspase-3 were higher in mice that received parecoxib compared with vehicle-treated mice (Fig. 10, B and D). Protein expression of cortical KIM-1 was induced in response to both 3 and 7 days of UUO, and we found no change in KIM-1 expression in response to parecoxib treatment (Fig. 10, E and F). Furthermore, protein expression of HO-1 was significantly induced in response to 3 and 7 days of UUO, and no significant change was observed after parecoxib treatment (Fig. 10, G and H).
Fig. 10.
Effect of parecoxib on UUO-induced injury. A and B: cortical caspase-3 protein abundance in parecoxib-treated mice subjected to 3 day (A) or 7 days (B) of UUO. C and D: cortical cleaved caspase-3 protein abundance in parecoxib-treated mice subjected to 3 days (C) or 7 days (D) of UUO. E and F: cortical expression of KIM-1 protein in response to 3 days (E) or 7 days (F) of UUO and parecoxib treatment. G and H: cortical HO-1 protein abundance in response to 3 days (G) or 7 days (H) of UUO and parecoxib treatment. Protein abundance was normalized to total protein. Data are presented as means ± SE; n = 5. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
To investigate a potential protective role of COX-2-derived prostaglandins in kidney injury, we used a UUO mouse model. UUO causes an increase in renal ROS production, leading to tubulointerstitial inflammation and apoptosis (5). In vitro, COX-2 is associated with cellular oxidant defenses and may be an important protective factor in renal diseases involving oxidative stress (12, 29). In the present study, we used COX-2−/− mice to show that COX-2 is involved in protecting the kidney against ROS in vivo. An integrated measure of prevailing level of renal oxidative stress and its consequences was obtained by measuring changes in nitrotyrosine and 4-HNE residues and apoptosis, which were markedly increased in response to UUO but not further aggravated by the deletion of COX-2 activity, whereas the apoptosis pathway and renal injury were amplified by a lack of COX-2 during UUO. It appears that protection against excessive oxidative stress in COX-2−/− mice is provided by SOD2 and HO-1, which were transiently and permanently, respectively, upregulated in COX-2−/− mice during UUO. HO-1 is a very sensitive marker of oxidative stress (2), and a beneficial response to HO-1 induction has been reported in response to acute renal injury (1). Although we found an increase in HO-1 production in response to COX-2 deficiency after UUO, this did not seem to protect the kidney against UUO-induced apoptosis and general tubular injury. In the study of pharmacological inhibition of COX-2, HO-1 protein levels were slightly increased in response to parecoxib treatment. This further supports that removal of COX-2 might have implications on the regulation of HO-1 or induced oxidative stress.
Ureteral obstruction causes an increase in the intratubular hydrostatic pressure (22) and, as shown in the present study, tubular injury, which was aggravated with time by COX-2 deficiency. This might indicate that COX-2−/− mice are more susceptible to tubular injury induced by prolonged UUO. In pharmacological inhibition of COX-2, we were not able to find the same increase in KIM-1 expression during COX-2 inhibition.
Time-dependent increases in luminal diameter and tubular cell death have been observed on 3 days after UUO (14). In the present study, apoptosis was increased after both 3 and 7 days of UUO, and lack of COX-2 exacerbated apoptosis induced by UUO. In accordance with these findings, pharmacological inhibition of COX-2 increased cleaved caspase-3 in response to UUO. This observation is consistent with previous studies (9, 15) where COX-2 exerted protective effects against apoptosis through PGE2 and EP2/EP4 receptors. In accordance, our data demonstrated an increase in EP2 and EP4 expression in response to UUO, indicating that these EP receptors may be involved in the protective response. A previous study (21) has associated the EP4 receptor in a protective role against obstructive renal diseases, through suppression of inflammation and fibrosis. However, the exact mechanism(s) by which COX-2 exerts its antiapoptotic effect in response to UUO remains to be fully elucidated.
The genotype-related differences observed in HO-1 and SOD2 protein with amplified response to UUO in COX-2−/− mice would be compatible with no direct stimulatory regulation of tubular enzyme abundance by COX-2-derived metabolites but rather a secondary response to the more pronounced primary injury (dilation, apoptosis, and tubular KIM-1) observed in COX-2−/− mice. In addition, the predominant association of genotype difference with the medulla points to events related to the primary insult. Thus, e.g., ischemia and anaerobic metabolism or pressure-induced injury could be more pronounced in COX-2−/− mice and thus initiate a larger response.
The phenotype of COX-2−/− mice includes postnatal renal maldevelopment, resulting in suppressed kidney growth, immature subcapsular glomeruli, hypertrophied juxtamedullary glomeruli, abundant undeveloped mesenchymal tissue, increased plasma blood urea nitrogen, and a decreased glomerular filtration rate (6, 23, 30). Renal pathological changes in COX-2−/− mice increase with age (23). However, COX-2−/− mice on the 129/C57 genetic background show no difference from WT mice in blood pressure or urine concentrating capacity (23, 30) and are less susceptible to lipopolysaccharide-induced inflammation (6). In the present study, COX-2−/− mice were included at the age of 10–14 wk. Based on our findings in sham-operated animals, there was no obvious histological difference between COX-2−/− and WT mice at this age. Furthermore, there was no difference in baseline indexes of kidney oxidative stress status, apoptosis, or tubular injury between COX-2−/− and WT mice. However, we cannot eliminate the possibility that COX-2−/− mice are more susceptible to UUO-induced kidney injury due to renal developmental abnormalities. In fact, UUO-induced tubular injury was not aggravated by short-term COX-2 inhibition with a specific blocker, whereas the change in caspases was mimicked by a COX-2 inhibitor. On the other hand, treatment with parecoxib in vivo may also give rise to uncertainties due to the fact that parecoxib displays nonselective inhibitory actions on COX-1, whereas COX-1 may act unopposed in COX-2−/− mice (25). In a previous study (28) using a chitosan delivery system of small interfering RNA targeting COX-2, we have shown improving effects on COX-2 knockdown in response to UUO. However, in this study, only macrophages were targeted for COX-2 knockdown. It is therefore interesting to note that constitutive knockout of COX-2 is not comparable with the effect of COX-2-specific knockdown in macrophages, suggesting that renal endogenous COX-2 is relevant, not COX-2 in invading cells.
In summary, COX-2-deficient mice were used to investigate a protective role of COX-2 in response to ureteral obstruction. UUO increased COX-2, and, whereas COX-2 deletion had no effect on oxidative stress markers and increased antioxidant enzymes HO-1 and SOD2, apoptosis and tubular damage were exacerbated. In conclusion, COX-2 is necessary to protect against tubular damage and apoptosis after UUO but not necessary to protect against oxidative stress. COX-2 is not likely to directly regulate the antioxidant enzymes HO-1 and SOD2.
GRANTS
This work was supported by the Lundbeck Foundation, the Karen Elise Jensens Foundation, the Danish Society of Nephrology, and the AP Moller Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.N., K.M., J.F., B.L.J., and R.N. conception and design of research; L.N. performed experiments; L.N. and S.K. analyzed data; L.N., J.F., and R.N. interpreted results of experiments; L.N. prepared figures; L.N. and R.N. drafted manuscript; L.N., K.M., S.K., J.F., B.L.J., and R.N. edited and revised manuscript; L.N., K.M., S.K., J.F., B.L.J., and R.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gitte Skou, Gitte Kall, and Line V. Nielsen for expert technical assistance. The authors thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 11: 965–973, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res 51: 974–978, 1991. [PubMed] [Google Scholar]
- 3.Cachat F, Lange-Sperandio B, Chang AY, Kiley SC, Thornhill BA, Forbes MS, Chevalier RL. Ureteral obstruction in neonatal mice elicits segment-specific tubular cell responses leading to nephron loss. Kidney Int 63: 564–575, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA 95: 681–686, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung SD, Lai TY, Chien CT, Yu HJ. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLos One 7: e47299, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiol Renal Physiol 303: F120–F129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friis UG, Madsen K, Svenningsen P, Hansen PB, Gulaveerasingam A, Jorgensen F, Aalkjaer C, Skott O, Jensen BL. Hypotonicity-induced renin exocytosis from juxtaglomerular cells requires aquaporin-1 and cyclooxygenase-2. J Am Soc Nephrol 20: 2154–2161, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George RJ, Sturmoski MA, Anant S, Houchen CW. EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins Other Lipid Mediat 83: 112–120, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurtler A, Kunz N, Gomolka M, Hornhardt S, Friedl AA, McDonald K, Kohn JE, Posch A. Stain-free technology as a normalization tool in Western blot analysis. Anal Biochem 433: 105–111, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Hao CM, Komhoff M, Guan Y, Redha R, Breyer MD. Selective targeting of cyclooxygenase-2 reveals its role in renal medullary interstitial cell survival. Am J Physiol Renal Physiol 277: F352–F359, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Hao CM, Yull F, Blackwell T, Komhoff M, Davis LS, Breyer MD. Dehydration activates an NF-κB-driven, COX2-dependent survival mechanism in renal medullary interstitial cells. J Clin Invest 106: 973–982, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Wang Y, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiatt MJ, Ivanova L, Trnka P, Solomon M, Matsell DG. Urinary tract obstruction in the mouse: the kinetics of distal nephron injury. Lab Invest 93: 1012–1023, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino T, Tsutsumi S, Tomisato W, Hwang HJ, Tsuchiya T, Mizushima T. Prostaglandin E2 protects gastric mucosal cells from apoptosis via EP2 and EP4 receptor activation. J Biol Chem 278: 12752–12758, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol 40: 1233–1239, 2002. [PubMed] [Google Scholar]
- 17.Liang J, Tian S, Han J, Xiong P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren Fail 36: 285–291, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Manucha W, Carrizo L, Ruete C, Molina H, Valles P. Angiotensin II type I antagonist on oxidative stress and heat shock protein 70 (HSP 70) expression in obstructive nephropathy. Cell Mol Biol (Noisy-le-grand) 51: 547–555, 2005. [PubMed] [Google Scholar]
- 19.Manucha W, Oliveros L, Carrizo L, Seltzer A, Valles P. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney Int 65: 2091–2107, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Misseri R, Meldrum DR, Dinarello CA, Dagher P, Hile KL, Rink RC, Meldrum KK. TNF-α mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am J Physiol Renal Physiol 288: F406–F411, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa N, Yuhki K, Kawabe J, Fujino T, Takahata O, Kabara M, Abe K, Kojima F, Kashiwagi H, Hasebe N, Kikuchi K, Sugimoto Y, Narumiya S, Ushikubi F. The intrinsic prostaglandin E2-EP4 system of the renal tubular epithelium limits the development of tubulointerstitial fibrosis in mice. Kidney Int 82: 158–171, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Noorafshan A, Karbalay-Doust S, Poorshahid M. Stereological survey of the ameliorative effects of sulforaphane and quercetin on renal tissue in unilateral ureteral obstruction in rats. Acta Clin Croat 51: 555–562, 2012. [PubMed] [Google Scholar]
- 23.Norwood VF, Morham SG, Smithies O. Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int 58: 2291–2300, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Ostergaard M, Christensen M, Nilsson L, Carlsen I, Frokiaer J, Norregaard R. ROS dependence of cyclooxygenase-2 induction in rats subjected to unilateral ureteral obstruction. Am J Physiol Renal Physiol 306: F259–F270, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Riendeau D, Percival MD, Brideau C, Charleson S, Dube D, Ethier D, Falgueyret JP, Friesen RW, Gordon R, Greig G, Guay J, Mancini J, Ouellet M, Wong E, Xu L, Boyce S, Visco D, Girard Y, Prasit P, Zamboni R, Rodger IW, Gresser M, Ford-Hutchinson AW, Young RN, Chan CC. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J Pharmacol Exp Ther 296: 558–566, 2001. [PubMed] [Google Scholar]
- 26.Sugiyama H, Kobayashi M, Wang DH, Sunami R, Maeshima Y, Yamasaki Y, Masuoka N, Kira S, Makino H. Telmisartan inhibits both oxidative stress and renal fibrosis after unilateral ureteral obstruction in acatalasemic mice. Nephrol Dial Transplant 20: 2670–2680, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Truong LD, Petrusevska G, Yang G, Gurpinar T, Shappell S, Lechago J, Rouse D, Suki WN. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int 50: 200–207, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Nilsson L, Cheema MU, Wang Y, Frokiaer J, Gao S, Kjems J, Norregaard R. Chitosan/siRNA nanoparticles targeting cyclooxygenase type 2 attenuate unilateral ureteral obstruction-induced kidney injury in mice. Theranostics 5: 110–123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T, Huang Y, Heasley LE, Berl T, Schnermann JB, Briggs JP. MAPK mediation of hypertonicity-stimulated cyclooxygenase-2 expression in renal medullary collecting duct cells. J Biol Chem 275: 23281–23286, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Yang T, Huang YG, Ye W, Hansen P, Schnermann JB, Briggs JP. Influence of genetic background and gender on hypertension and renal failure in COX-2-deficient mice. Am J Physiol Renal Physiol 288: F1125–F1132, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Yang T, Singh I, Pham H, Sun D, Smart A, Schnermann JB, Briggs JP. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am J Physiol Renal Physiol 274: F481–F489, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, Zhang A, Honeggar M, Kohan DE, Mizel D, Sanders K, Hoidal JR, Briggs JP, Schnermann JB. Hypertonic induction of COX-2 in collecting duct cells by reactive oxygen species of mitochondrial origin. J Biol Chem 280: 34966–34973, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Ye W, Zhang H, Hillas E, Kohan DE, Miller RL, Nelson RD, Honeggar M, Yang T. Expression and function of COX isoforms in renal medulla: evidence for regulation of salt sensitivity and blood pressure. Am J Physiol Renal Physiol 290: F542–F549, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Yeh CH, Chiang HS, Lai TY, Chien CT. Unilateral ureteral obstruction evokes renal tubular apoptosis via the enhanced oxidative stress and endoplasmic reticulum stress in the rat. Neurourol Urodyn 30: 472–479, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Zecher M, Guichard C, Velasquez MJ, Figueroa G, Rodrigo R. Implications of oxidative stress in the pathophysiology of obstructive uropathy. Urol Res 37: 19–26, 2009. [DOI] [PubMed] [Google Scholar]