Abstract
Endothelial dysfunction has been shown to be predictive of subsequent cardiovascular events and death. Through a mechanism that is incompletely understood, increased dietary salt intake promotes endothelial dysfunction in healthy, salt-resistant humans. The present study tested the hypothesis that dietary salt-induced transforming growth factor (TGF)-β promoted endothelial dysfunction and salt-dependent changes in blood pressure (BP). Sprague-Dawley rats that received diets containing 0.3% NaCl [low salt (LS)] or 8.0% NaCl [high salt (HS)] were treated with vehicle or SB-525334, a specific inhibitor of TGF-β receptor I/activin receptor-like kinase 5, beginning on day 5. BP was monitored using radiotelemetry in four groups of rats (LS, LS + SB-525334, HS, and HS + SB-525334) for up to 14 days. By day 14 of the study, mean daytime systolic BP and mean pulse pressure of the HS group treated with vehicle was greater than those in the other three groups; mean daytime systolic BP and pulse pressure of the HS + SB-525334 group did not differ from the LS and LS + SB-525334-treated groups. Whereas mean systolic BP, mean diastolic BP, and mean arterial pressure did not differ among the groups on the seventh day of the study, endothelium-dependent vasorelaxation was impaired specifically in the HS group; treatment with the activin receptor-like kinase 5 inhibitor prevented the dietary HS intake-induced increases in phospho-Smad2 (Ser465/467) and NADPH oxidase-4 in endothelial lysates and normalized endothelial function. These findings suggest that HS-induced endothelial dysfunction and the development of salt-dependent increases in BP were related to endothelial TGF-β signaling.
Keywords: dietary salt, endothelial dysfunction, endothelium, hypertension, transforming growth factor-β
dietary NaCl (termed “salt” in the present article) content directly impacts endothelial cell function, regulating, for example, the production of nitric oxide (NO) in rodents (4, 6, 26) and humans (1). Increased salt intake induces the formation of an endothelial cell signaling complex that contains proline-rich tyrosine kinase 2, c-Src, and phosphatidylinositol 3-kinase. Phosphatidylinositol 3-kinase is an upstream activator of PKB (Akt), which phosphorylates the endothelial isoform of NO synthase (NOS3) to increase NO production. This signaling cascade also promotes the endothelial cell production of transforming growth factor (TGF)-β, which has recently been shown to serve an autocrine role in endothelial cell biology during high salt (HS) intake (35). Additional studies have demonstrated a functional interaction between TGF-β and NO (33, 35, 38). However, TGF-β also generates ROS through NADPH oxidase (NOX)4 (13, 30), which potentially reduces the bioavailability of NO (30). Thus, a dynamic balance between vascular bioavailable NO and TGF-β might exist to regulate the vascular responses to dietary salt intake.
Endothelial dysfunction (ED), defined as a reduction in arterial endothelium-dependent vasodilation, has been shown to be predictive of subsequent cardiovascular events and death (3, 21, 32). While a number of underlying pathogenetic processes may produce ED, attention has focused on the potential role that a diet high in salt content may play in this process. Recent studies have clearly demonstrated that 7 days on a HS diet impaired forearm endothelium-dependent vasodilation independently of blood pressure (BP) and serum Na+ concentration; however, endothelium-independent vasodilation did not change (7, 10). These findings highlight the need to define the underlying molecular mechanisms by which dietary salt intake affects endothelial cell biology. The hypothesis tested in the present study was that dietary salt-induced TGF-β directly promotes ED and increases salt-dependent effects on BP.
MATERIALS AND METHODS
Animal and tissue preparation.
The present study was performed in accordance with recommendations in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of the University of Alabama at Birmingham approved the project. Experiments were conducted using 1.5-mo-old male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN).
Rats were housed under standard conditions and given a 0.3% NaCl diet [low-salt (LS) diet, AIN-76A with 0.3% NaCl, Dyets, Bethlehem, PA] and water ad libitum for 4 days before initiation of the study. Rats received diets containing either 0.3% NaCl or 8.0% NaCl (HS diet; AIN-76A with 8.0% NaCl, Dyets). At day 5 of the experiment, vehicle or 6-[2-tert-butyl-5-(6-methyl-pyridin-2-yl)-1H-imidazol-4-yl]-quinoxaline (SB-525334; Selleck Chemicals, Houston, TX), which is a specific inhibitor of the kinase activity of TGF-β receptor I/activin receptor-like kinase (ALK)5, was added to the drinking water to achieve a dose of 10 mg·kg−1·day−1. SB-525334 prevented renal fibrosis in vivo in puromycin-induced nephritis (11, 17, 20) and has previously been used at this dose to demonstrate a functional role for TGF-β signaling in endothelial function (36).
Four groups of rats were studied. The first group of rats received the 0.3% NaCl diet and vehicle (LS group), the second group of rats received the 0.3% NaCl diet and SB-525334 (LS + SB-525334), the third group of rats received 8.0% NaCl and vehicle (HS group), and the fourth group of rats received 8.0% NaCl and SB-525334 (HS + SB-525334 group). On the seventh day of the study, rats were anesthetized with 2% isoflurane. Aortae were harvested under sterile conditions, and aortic endothelial cell lysates were obtained as previously described (34). Blood was collected for determination of serum electrolytes.
Radiotelemetry measurement of BP.
Implantable transmitters (TA11PA-C40, Data Sciences, St. Paul, MN) were used to record awake, unrestrained BP continuously. Rats were anesthetized, and right femoral arteries were isolated with a ventral incision. The catheter of the telemetry probe was inserted into the artery. Through the same ventral incision, a pocket along the right flank was formed, and the body of the transmitter slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed. In these experiments, rats were allowed 7 days on the 0.3% NaCl diet to recover before initiation of the experiments and determination of BP. Systolic BP (SBP) and diastolic BP (DBP) were monitored in 24-h periods twice weekly for 14 days. Using Dataquest ART (Data Sciences), daytime BP was recorded as the average of BP from 6 AM to 6 PM and nighttime BP was recorded as the average of BP from 6 PM to 6 AM.
Endothelial function.
On day 7 of the study, which included treatment on the last 2 days with either vehicle or SB-525334, thoracic aortae were removed and placed in cold Krebs-Ringer buffer (KRB). The aorta was carefully cut into 3-mm rings, which were subsequently mounted in a myograph chamber (DMT, Aarhus, Denmark) filled with 5 ml KRB, maintained at 37°C, and continuously aerated with 95% O2-5% CO2. Aortic ring preparations were equilibrated for 30 min and depolarized with high-K+ KRB (60 mM KCl in KRB solution). After a 30-min washout period, the experiment for a cumulative concentration-response curve to phenylephrine (Phe; 0.001–10 μM) was performed. After a second 30-min washout period, aortic rings were contracted with Phe (0.1–10 μM); the ability to maintain a sustained contraction was observed in all experimental groups. Endothelium-dependent relaxation was performed by constructing a concentration-response curve to ACh (0.001–100 μM). Endothelium-independent relaxation was tested using the NO donor sodium nitroprusside (0.001–10 μM). In separate experiments, to determine if the effects were dependent on NO, aortic rings were preincubated for 30 min with the NOS blocker Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM) before the ACh treatment.
Western blot analyses.
At the end of the experiment, the aorta was opened and endothelial cells were harvested in standard fashion (35); cell lysates were produced using modified radioimmunoprecipitation assay buffer containing 10 mmol/l Tris·HCl (pH 7.4), 100 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l EGTA, 0.5% sodium deoxycholate, 1% Triton X-100, 10% glycerol, 0.1% SDS, 20 mmol/l sodium pyrophosphate, 2 mmol/l Na3VO4, 1 mmol/l NaF, 1 mmol/l PMSF, and a protease inhibitor cocktail (Complete, EDTA-free, Roche Applied Science, Indianapolis, IN). Total protein concentration was determined using a kit (BCA Protein Assay Reagent Kit, Thermo Fisher Scientific Pierce Protein Research Products, Rockford, IL), and samples were processed for Western blot analysis. Protein extracts (10–60 μg) were boiled for 3 min in Laemmli buffer and separated by 10% SDS-PAGE (Bio-Rad Laboratories, Hercules, CA) before electrophoretic transfer onto polyvinylidene difluoride membranes. Membranes were blocked in 5% nonfat milk and then probed with antibodies (diluted 1:1,000) that recognized specifically phospho-Smad2 (Ser465/467) (Cell Signaling, Danvers, MA), total Smad2/3 (EMD Millipore, Billerica, MA), NOX4 (a kind gift from Dr. J. David Lambeth, Emory University School of Medicine) (14), and GAPDH (1:10,000 dilution, Abcam, Cambridge, MA). Density of the bands was quantified using an Odyssey CLx Imager system and Image Studio 4 software (Li-COR Biotechnology, Lincoln, NE).
Statistical analyses.
Data are expressed as means ± SE. To analyze BP responses to dietary salt intake (LS or HS) and ALK5 inhibition (treatment with vehicle or SB-525334), a repeated-measures analysis using linear mixed models was used (Proc Glimmix). Holm-Tukey's adjustment for multiple comparisons of the group by timepoint simple effects was used in post hoc analyses. For the remaining data, two-way ANOVA was performed using dietary salt intake (LS or HS) and ALK5 inhibition (treatment with vehicle or SB-525334) as independent variables. If the difference in mean values among the different levels of dietary salt intake was greater than what would be expected by chance, all pairwise multiple-comparison post hoc testing (Tukey's honestly significant difference test) was performed (Proc GLM). SAS 9.4 (Cary, NC) was used for the statistical analyses, and P values of ≤0.05 were assigned statistical significance.
RESULTS
Increased dietary salt intake elevated SBP by day 14 of the study but was prevented by ALK5 inhibition.
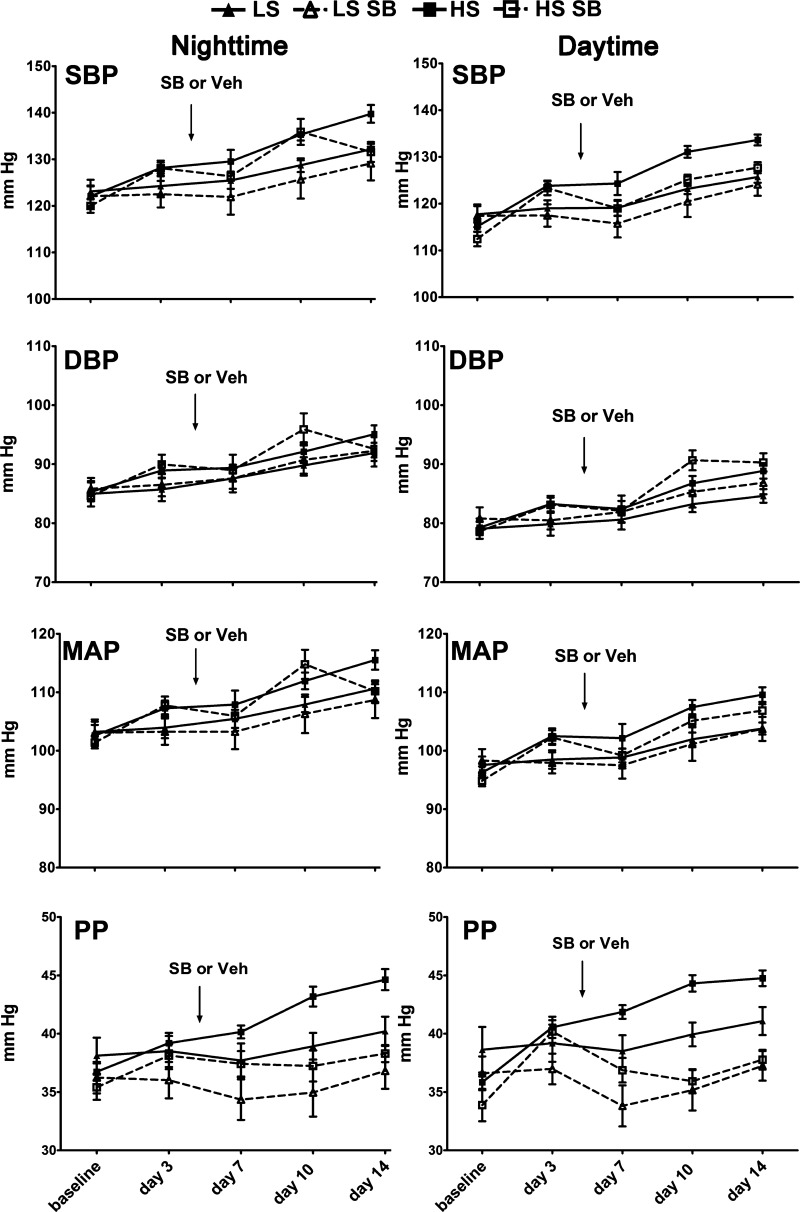
Mean body weights and mean concentrations of serum electrolytes (Na+, K+, and Cl−), which were determined on day 14 in a subset (n = 3 animals/group) of the total animals in the study, did not differ among the four groups of rats (Table 1). BP was monitored using radiotelemetry in the four groups of rats (LS, LS + SB-525334, HS, and HS + SB-525334) for 14 days. Differences in BP responses among the groups under study emerged over the experimental time period (Fig. 1). Compared with BP parameters of rats in the HS group at day 14, rats in the LS group had a lower mean daytime SBP (125.7 ± 1.2 vs. 133.6 ± 1.2 mmHg, P = 0.0038), daytime mean arterial pressure (MAP; 103.8 ± 1 vs. 109.6 ± 1.3 mmHg, P = 0.023), daytime pulse pressure (PP; 41.1 ± 1.2 vs. 44.8 ± 0.7 mmHg, P = 0.0543), and nighttime PP (40.2 ± 1.3 vs. 44.6 ± 0.9 mmHg, P = 0.0364) and higher nighttime average heart rates (439.1 ± 6.5 vs. 404 ± 5.7 beats/min, P = 0.0023).
Table 1.
Mean body weight and serum electrolytes of each of the four groups on day 7 of the experiment
| Body Weight, g | Serum Na+ Concentration, mmol/l | Serum K+ Concentration, mmol/l | Serum Cl− Concentration, mmol/l | |
|---|---|---|---|---|
| LS group | 225.0 ± 15.4 | 146.4 ± 1.8 | 5.1 ± 0.2 | 106.1 ± 1.3 |
| LS + SB-525334 group | 237.0 ± 10.9 | 143.8 ± 0.1 | 5.4 ± 0.3 | 105.1 ± 0.5 |
| HS group | 215.0 ± 15.5 | 144.8 ± 1.0 | 4.9 ± 0.2 | 103.5 ± 1.1 |
| HS + SB-525334 group | 222.8 ± 11.0 | 144.7 ± 1.4 | 5.1 ± 0.2 | 104.0 ± 0.8 |
Values are means ± SE; n = 9 rats/group.
LS, low-salt diet; HS, high-salt diet.
Fig. 1.
In the second week of the experimental period, high salt (HS) intake elevated systolic blood pressure (SBP) and pulse pressure (PP), which were normalized by activin receptor-like kinase (ALK)5 inhibition. The arrows indicate the time of addition of vehicle (Veh) or SB-525334 (SB), and the dashed lines indicate BP responses of the animals that received this treatment. For Sprague-Dawley rats on the HS (8% NaCl) diet, SBP, diastolic blood pressure (DBP), and mean arterial pressure (MAP) during week 1 did not differ from rats on the low-salt (LS; 0.3% NaCl) diet. By the end of the second week, however, the HS diet increased daytime SBP, MAP, and both daytime and nighttime PP. ALK5 inhibition normalized these BP responses to the HS diet. Data are presented as means ± SE; n = 9 animals/group.
By day 14 of the experimental time period, compared with BP parameters of rats in the HS group, rats in the HS + SB-525334 group had lower mean daytime SBP (127.7 ± 1.2 vs. 133.6 ± 1.2 mmHg, P = 0.0477) and did not differ from rats in the LS + SB-525334 group (127.7 ± 1.2 vs. 124.1 ± 2.4 mmHg, P = 0.4049) or rats in the LS group (127.7 ± 1.2 vs. 125.7 ± 1.2 mmHg, P = 0.7978). Rats in the HS + SB-525334 group also had lower mean daytime MAP (106.9 ± 1.1 vs. 109.6 ± 1.3 mmHg, P = 0.0288), mean daytime PP (37.8 ± 0.8 vs. 44.8 ± 0.7 mmHg, P = 0.0003), and nighttime PP (38.3 ± 0.7 vs. 44.6 ± 0.9 mmHg, P = 0.003) compared with rats in the HS group. These parameters did not differ with those observed in rats in the LS group or rats in the LS + SB-525334 group.
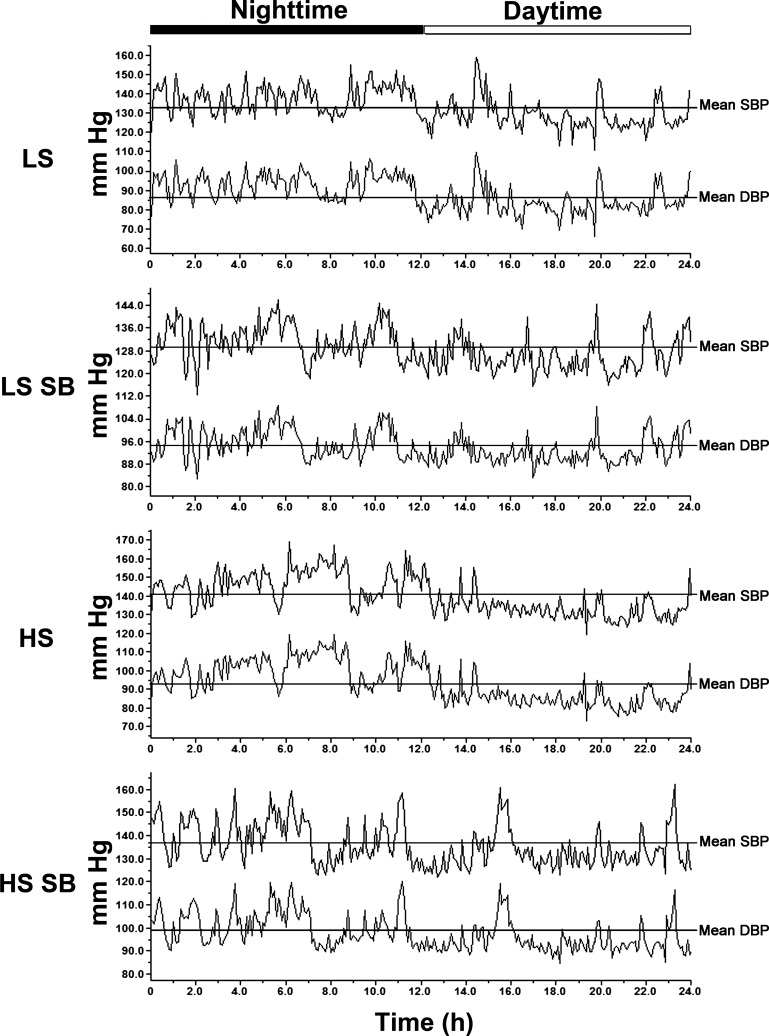
Representative tracings of SBP and DBP throughout 24 h of four rats in the LS, LS + SB-525334, HS, and HS + SB-525334 groups are shown in Fig. 2. BP increased during the nighttime period in each animal in every group and was consistent with an intact circadian rhythm of a nocturnal animal. At day 14, the differences between daytime and nighttime BP, termed ΔSBP (LS group: 6.2 ± 0.7 mmHg, LS + SB-525334 group: 6.2 ± 0.9 mmHg, HS group: 6.4 ± 1.6 mmHg, and HS + SB-525334 group: 5.4 ± 1.0 mmHg) and ΔDBP (LS group: 7.2 ± 0.5 mmHg, LS + SB-525334 group: 6.1 ± 0.4 mmHg, HS group: 6.7 ± 1.6 mmHg, and HS + SB-525334 group: 5.7 ± 2.1 mmHg), did not differ among the groups, indicating that the circadian rhythm was not affected by dietary salt intake or by treatment with the ALK5 inhibitor. In addition, the mean times at which the animals manifested nighttime peak SBP and peak DBP and daytime trough SBP and trough DBP did not differ among the four groups at day 14 of the study (data not shown).
Fig. 2.
Representative tracings of SBP and DBP recordings for a 24-h period on day 14 of the study. SBP and DBP were higher during the nighttime periods in each group, indicating preservation of the circadian rhythm among the four groups.
Increased dietary salt intake impaired endothelium-dependent relaxation but was prevented by ALK5 inhibition.
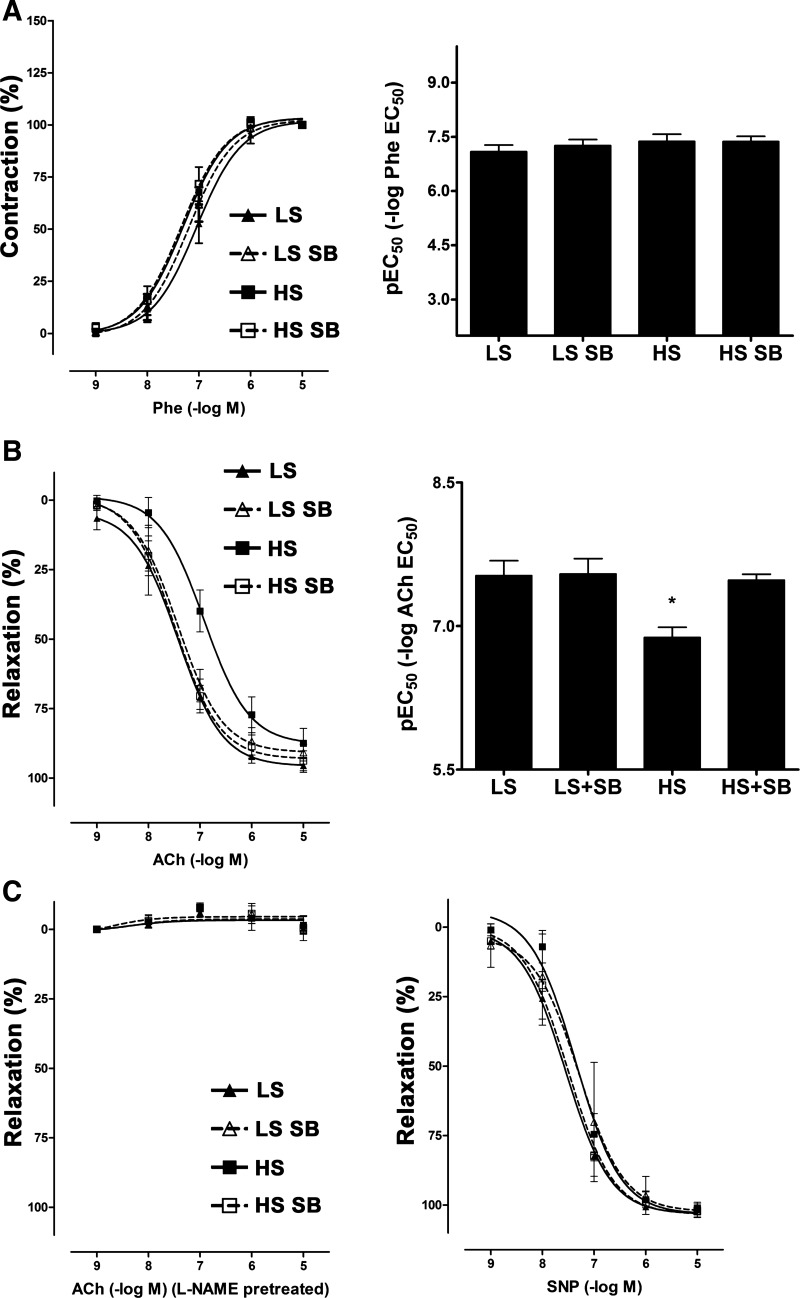
Because mean SBP, DBP, and MAP did not differ among the four groups at day 7, tests of endothelial function were performed at that time. Endothelium-dependent vasorelaxation was impaired specifically in the HS group; treatment with SB-525334 normalized this effect (Fig. 3). l-NAME pretreatment completely abolished the ACh-induced relaxation among the four groups of rats. Endothelium-independent vasorelaxation, as assessed by the addition of sodium nitroprusside, did not differ among the four groups. In addition, the contractile responses of the aortae to Phe did not differ among the four groups of rats (Fig. 3).
Fig. 3.
HS intake impaired endothelium-dependent relaxation and transforming growth factor (TGF)-β receptor inhibition normalizes HS-induced endothelial dysfunction in Sprague-Dawley rats. Rats were fed with a LS or HS diet for 7 days and received either Veh or the ALK5 inhibitor SB beginning on day 5. Vascular responses were tested using aortae from the four groups of rats. A, left: contractile responses of aortic ring preparations to phenylephrine (Phe) did not differ among the four groups. Right, pEC50 values derived from the cumulative concentration-response curves did not differ among the four groups of rats in the study. B: cumulative concentration-response curves (left) were generated using ACh (0.001–10 μM) and used to calculate pEC50 values (right). HS intake significantly impaired endothelium-dependent relaxation, and ALK5 inhibition normalized this effect. Data are presented as means ± SE; n = 9 rats/group. *P < 0.05 vs. the HS SB group. C, left: pretreatment of ring preparations with Nω-nitro-l-arginine methyl ester (l-NAME) completely abolished ACh-dependent vasorelaxation. Right, responses of aortic ring preparations to sodium nitroprusside (SNP) did not differ among the four groups of rats.
Increased dietary salt intake increased endothelial phospho-Smad2 (Ser465/467) and NOX4.
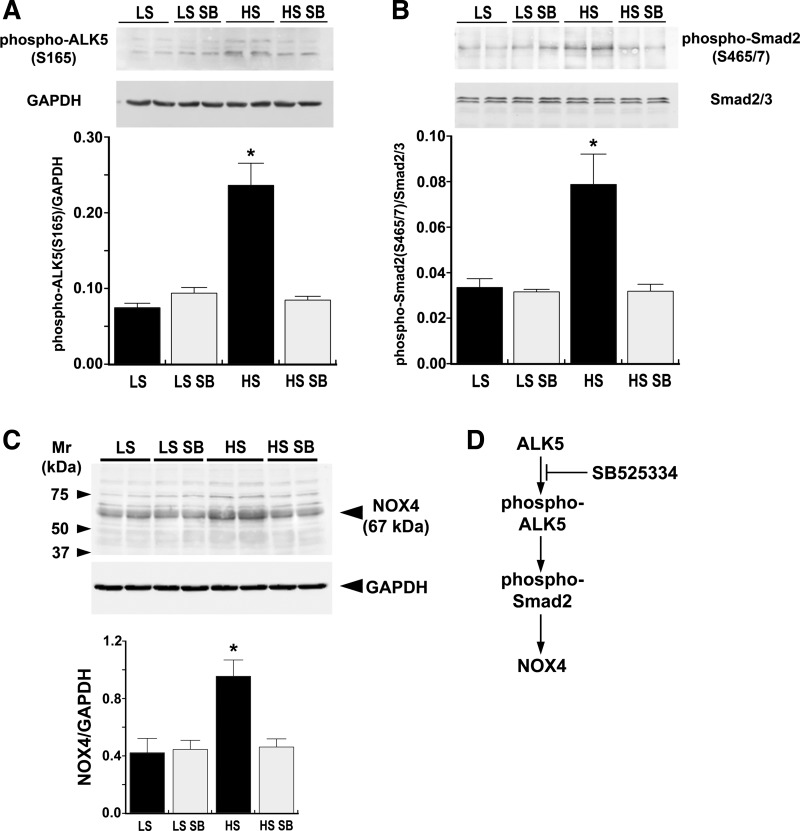
Aortic endothelial cell lysates obtained from the four groups of rats on day 7 of the experiment demonstrated activation of ALK5 by HS intake as well as the anticipated inhibition of ALK5 by SB-525334 (Fig. 4A). As a consequence, an increase in dietary salt intake increased phospho-Smad2 (Ser465/467) in endothelial lysates; this increase was also inhibited by treatment with SB-525334 (Fig. 4B). Total Smad2/3 levels did not change. The findings further showed a stimulatory effect of dietary salt intake and an inhibitory effect of treatment with SB-525334 on endothelial NOX4 levels, indicating the involvement of ALK5 in the salt-induced increase in NOX4 (Fig. 4C).
Fig. 4.
Western blot analyses of endothelial lysates performed on day 7 demonstrating the effects of ALK5 inhibition on endothelial cell function. A: increased dietary salt intake increased phospho-ALK5 (Ser165) in the endothelium. Treatment with SB reduced phospho-ALK5 (Ser165) to levels observed in LS-treated rats. The graph represents a summary of the findings. B: HS diet increased Smad2 phosphorylation by ∼2-fold compared with the LS group, and ALK5 inhibition prevented the salt-induced increases in Smad2 phosphorylation. The graph represents a summary of the findings. C: Western blots using endothelial lysates from rats on day 7 of the study showed that NADPH oxidase (NOX)4 levels increased specifically in the endothelium of rats on the HS diet. The graph represents a summary of the findings. The band at ∼80 kDa is also NOX4 (14) but was not included in the quantified results shown in the graph. ALK5 inhibition completely abolished this salt-induced response. D: the cartoon depicts a summary of the Western blot data. All data are presented as means ± SE; n = 4 rats/group. *P < 0.05 vs. LS, LS SB, and HS SB groups.
DISCUSSION
A previous study (37) has shown that dietary salt modulated the endothelial production of TGF-β and NO through a common signaling pathway, but these mediators may produce countervailing influences on vascular function. To define the dynamic functional significance of these molecular changes during HS diet intake, the present study demonstrated that 14 days on a HS diet increased SBP and PP. Importantly, the present study clearly demonstrated that treatment with the ALK5 inhibitor prevented this elevation in SBP and PP. Furthermore, ALK5 inhibition also prevented salt-induced phosphorylation of Smad2 and increases in NOX4 and reversed the ED observed before the development of the increase in BP. The observation that dietary salt intake promoted ED in the absence of changes in BP was consistent with prior findings reported by Durand et al (8) and Raffai et al. (24) and with recent reports (7, 10) in which high dietary Na+ intake impaired endothelium-dependent vasodilation independently of BP in healthy adults. The potential limitation of using anesthetized BP recordings to show an absence of effect of salt intake in Sprague-Dawley rats (8, 37) was corrected in the present study by the use of telemetry monitoring and extending the experimental period for 2 wk. Thus, the findings supported a role for the inhibition of an endothelial TGF-β signaling pathway in the prevention of dietary salt-mediated ED and salt-dependent increases in BP observed with careful monitoring of BP.
An increase in ROS in response to HS intake has been shown to play an important role in reducing endothelium-dependent dilation in humans (10), mice (23), and rats (24). Using a model similar to that used in the present study, previous studies have demonstrated that a HS diet promoted ED in middle cerebral arteries (8) and mesenteric arteries (24). These studies further showed an increase in vascular ROS (24). Stimulation of the G protein-coupled receptor, Mas, by infusion of either ANG-(1–7) or AVE-0991, corrected salt-induced ED (8, 24). While these studies did not demonstrate the cellular mechanism of improvement in ED, Sampaio et al. (25) showed that Mas receptor activation increased endothelial NO production by activating NOS3 through posttranslational modifications (serine and threonine phosphorylation).
The present study also found that HS intake promoted an ALK5-dependent increase in endothelial NOX4. These data are consistent with Thannickal et al. (30), who found that TGF-β1 increased production of H2O2 and reduced cellular glutathione stores in endothelial cells. These investigators further demonstrated that the TGF-β1-induced increases in H2O2 were dependent on activation of ALK5 and the Smad2/3 pathway, which increased the activity of NOX4 (13). Additionally, the findings were consistent with Yan et al. (31), who also showed a TGF-β-mediated induction of NOX4 in the endothelium, and Boulden et al. (2), who demonstrated that H2O2, which is the principal product of constitutively active NOX4 (22), promoted ED by decreasing tetrahydrobiopterin and bioavailable NO. By demonstrating that ED developed during HS intake through the activation of ALK5 and the Smad2 pathway, our results suggested the pathophysiological significance of this TGF-β-dependent mechanism in the alteration of endothelial function.
Previous studies have suggested a role for TGF-β in the development of hypertension. For example, genetic disruption of elastin microfibril interfacer 1, which is involved in the vascular regulation of TGF-β, promoted the development of arterial hypertension by increasing the activity of TGF-β (39). Terrell and colleagues (29) administered recombinant human TGF-β2 at varying doses (20, 100, and 400 μg/kg) chronically to rats and rabbits. They observed that after 9 wk, TGF-β2 caused modest increases in SBP; however, renal hemodynamic end points were not significantly altered. Proteinuria and minimal tubular interstitial fibrosis developed after 6 wk of dosing at the high dose (400 μg/kg) (16). Using dietary salt intake as the stimulus for the vascular production of TGF-β, radiotelemetry monitoring of BP identified subtle but significant increases in SBP after 2 wk. Because the increase in BP was abrogated by the addition of the ALK5 inhibitor, the present findings are consistent with a role for this growth factor in the development of salt-dependent increases in BP.
Recent Framingham data have shown that large artery ED antedated the development of hypertension (15). This central pathophysiological link has been implicated in the initiation of increased arterial stiffness, hypertension, atherosclerosis, chronic kidney disease, restenosis after percutaneous coronary intervention, and stenosis of venous bypasses of coronary arteries (5, 9, 12, 18, 27, 28). Dietary salt intake promotes a dose-dependent decrease in survival in rats, related to the acceleration of cardiovascular and renal disease as salt intake exceeds 2% (19). The present study unveils the role of TGF-β in this complex interaction of the endothelium with dietary salt intake and improves understanding of the mechanism of ED in the development of hypertension.
In summary, the findings demonstrated the dynamic function of the endothelium and subsequent changes in BP that occurred during HS intake. Our results showed that salt-induced ED preceded changes in BP and further identified TGF-β as the upstream mechanism of salt-induced ED. While the cellular events that modulate bioavailable NO are complicated, the present study supports the involvement of endothelial ALK5 activation and Smad2 signaling in salt-induced ED.
GRANTS
This work was supported by Office of Research and Development, Medical Research Service, Department of Veterans Affairs, Grant 1 IP1 BX001595, the National Institute of Diabetes and Digestive and Kidney Diseases George M. O'Brien Kidney and Urological Research Centers Program Grant P30-DK-079337, and American Heart Association Grant 15SDG25760063, and an Anderson Innovation Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.F., W.-Z.Y., and K.J.A. performed experiments; W.F., K.J.A., and P.W.S. analyzed data; W.F., W.-Z.Y., K.J.A., and P.W.S. interpreted results of experiments; W.F., W.-Z.Y., and P.W.S. prepared figures; W.F. drafted manuscript; W.F., W.-Z.Y., K.J.A., and P.W.S. edited and revised manuscript; W.F., W.-Z.Y., K.J.A., and P.W.S. approved final version of manuscript; P.W.S. conception and design of research.
REFERENCES
- 1.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol Renal Physiol 274: F914–F923, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC Jr. Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med 41: 810–817, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound 12: 34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 22: 812–818, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng X, Welch WJ, Wilcox CS. Renal vasoconstriction during inhibition of NO synthase: effects of dietary salt. Kidney Int 46: 639–646, 1994. [DOI] [PubMed] [Google Scholar]
- 7.DuPont JJ, Greaney JL, Wenner MM, Lennon-Edwards SL, Sanders PW, Farquhar WB, Edwards DG. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens 31: 530–536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1–7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 299: H1024–H1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garin G, Berk BC. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium 13: 375–384, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Greaney JL, DuPont JJ, Lennon-Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol 590: 5519–5528, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, Laping NJ. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-β type I receptor kinase in puromycin-induced nephritis. J Pharmacol Exp Ther 313: 943–951, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24: 677–683, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly FJ, Anderson S, Thompson MM, Oyama TT, Kennefick TM, Corless CL, Roman RJ, Kurtzberg L, Pratt BM, Ledbetter SR. Acute and chronic renal effects of recombinant human TGF-β2 in the rat. J Am Soc Nephrol 10: 1264–1273, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Kim DK, Jang Y, Lee HS, Park HJ, Yoo J. Synthesis and biological evaluation of 4(5)-(6-alkylpyridin-2-yl)imidazoles as transforming growth factor-β type 1 receptor kinase inhibitors. J Med Chem 50: 3143–3147, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the Chronic Renal Impairment in Birmingham (CRIB) study. Am J Kidney Dis 43: 244–253, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Meneely GR, Ball CO. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med 25: 713–725, 1958. [DOI] [PubMed] [Google Scholar]
- 20.Moon JA, Kim HT, Cho IS, Sheen YY, Kim DK. IN-1130, a novel transforming growth factor-β type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int 70: 1234–1243, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Poisa P, Porteri E, Agabiti-Rosei C, Paderno V, Belotti E, Rizzoni D, Castellano M, Agabiti-Rosei E. Prognostic role of flow-mediated dilatation of the brachial artery in hypertensive patients. J Hypertens 26: 1612–1618, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1–7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. Am J Physiol Heart Circ Physiol 301: H1341–H1352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49: 185–192, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat: role of endogenous nitric oxide. J Clin Invest 91: 642–650, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant 18: 892–898, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 101: 948–954, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Terrell TG, Working PK, Chow CP, Green JD. Pathology of recombinant human transforming growth factor-β1 in rats and rabbits. Int Rev Exp Pathol 34: 43–67, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Thannickal VJ, Hassoun PM, White AC, Fanburg BL. Enhanced rate of H2O2 release from bovine pulmonary artery endothelial cells induced by TGF-β1. Am J Physiol Lung Cell Mol Physiol 265: L622–L626, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, Jiang F. Nox4 and redox signaling mediate TGF-β-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis 5: e1010, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ying WZ, Aaron K, Sanders PW. Dietary salt activates an endothelial proline-rich tyrosine kinase 2/c-Src/phosphatidylinositol 3-kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension 52: 1134–1141, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-β1. Am J Physiol Renal Physiol 295: F406–F414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-β regulates endothelial function during high salt intake in rats. Hypertension 62: 951–956, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying WZ, Aaron KJ, Sanders PW. Transforming growth factor-β regulates endothelial function during high salt intake in rats. Hypertension 62: 951–956, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-β1 in rat aortic endothelium. Am J Physiol Heart Circ Physiol 277: H1293–H1298, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Ying WZ, Sanders PW. The interrelationship between TGF-β1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-β maturation to blood pressure homeostasis. Cell 124: 929–942, 2006. [DOI] [PubMed] [Google Scholar]