Abstract
Pulmonary lymphangioleiomyomatosis (LAM), a rare progressive lung disease associated with mutations of the tuberous sclerosis complex 2 (Tsc2) tumor suppressor gene, manifests by neoplastic growth of LAM cells, induction of cystic lung destruction, and respiratory failure. LAM severity correlates with upregulation in serum of the prolymphangiogenic vascular endothelial growth factor D (VEGF-D) that distinguishes LAM from other cystic diseases. The goals of our study was to determine whether Tsc2 deficiency upregulates VEGF-D, and whether axitinib, the Food and Drug Administration-approved small-molecule inhibitor of VEGF receptor (VEGFR) signaling, will reduce Tsc2-null lung lesion growth in a mouse model of LAM. Our data demonstrate upregulation of VEGF-D in the serum and lung lining in mice with Tsc2-null lesions. Progressive growth of Tsc2-null lesions induces recruitment and activation of inflammatory cells and increased nitric oxide production. Recruited cells isolated from the lung lining of mice with Tsc2-null lesions demonstrate upregulated expression of provasculogenic Vegfa, prolymphangiogenic Figf, and proinflammatory Nos2, Il6, and Ccl2 genes. Importantly, axitinib is an effective inhibitor of Tsc2-null lesion growth and inflammatory cell recruitment, which correlates with reduced VEGF-D levels in serum and lung lining. Our data demonstrate that pharmacological inhibition of VEGFR signaling with axitinib inhibits Tsc2-null lesion growth, attenuates recruitment and activation of inflammatory cells, and reduces VEGF-D levels systemically and in the lung lining. Our study suggests a potential therapeutic benefit of inhibition of VEGFR signaling for treatment of LAM.
Keywords: VEGF-D, lymphangiogenesis, TCS2-null, animal models, axitinib
pulmonary lymphangioleiomyomatosis (LAM) is a rare progressive lung disease affecting predominantly women of childbearing age, which manifests by neoplastic growth of atypical smooth musclelike LAM cells in the pulmonary interstitial space, leading to cystic lung destruction and spontaneous pneumothoraces (20, 24). Mutations of the tuberous sclerosis complex 1 (Tsc1) and Tsc2 tumor suppressor genes induce abnormal growth of LAM cells in the lung (12, 20). Importantly, LAM severity correlates with upregulated serum levels of prolymphangiogenic vascular endothelial growth factor D (VEGF-D) and the degree of lymphatic vessel density within the lung (40, 46). Clinical data obtained from the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial demonstrated that VEGF-D level in serum could serve as a novel biomarker for evaluating LAM severity, response to treatment, and to prospectively distinguish LAM from other cystic lung diseases (34, 40, 46), such as pulmonary Langerhans cell histiocytosis, emphysema, Sjögren syndrome, and Birt-Hogg-Dubé syndrome (10, 47). Correlation of VEGF-D levels with the severity of LAM measured by LAM computed tomography grade, the abundance of chylous effusions, and lymphatic involvement has been considered as a prognostic tool for disease progression (46). Despite these findings, little is known about the mechanism by which growth of Tsc2-null LAM lesions induces VEGF-D upregulation and abnormal lymphangiogenesis, or whether VEGF-D signaling and lymphangiogenesis can be targeted therapeutically in LAM.
Lymphangiogenic VEGF-D promotes the formation of tumor lymphatic vessels and facilitates metastatic spread of cancer cells by regulating prostaglandin synthesis (27, 43). VEGF-D is a secreted glycoprotein that, in the form of a homodimer, binds and activates receptor protein tyrosine kinases VEGF receptor 2 (VEGFR2) and VEGFR3, which are highly expressed by lymphatic endothelium in humans, and VEGFR3 in mice (1, 43). Histopathological studies in LAM show marked VEGFR3 staining surrounding LAM lesions and abundant lymphatic channels within LAM lesions detected by lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), VEGFR3, and podoplanin expression (9, 10, 23, 31). This evidence suggests that upregulation of VEGF-D might recruit lymphatic endothelial cells forming lymphatic channels, which might induce lung remodeling in LAM disease (20).
Previously, our laboratory demonstrated upregulation of VEGF-D in a mouse model of LAM (13). Tsc2-null lung lesions develop throughout the lung and display marked upregulation of lymphatic channels similar to those observed in the human LAM (13). The goal of this study is to determine whether inhibiting VEGF-D represents a viable pharmacological approach for preventing Tsc2-null lesion growth. Several therapeutic approaches have been developed to target VEGF-D and VEGFR2/3 signaling, including small-molecule inhibitors and neutralizing antibodies for VEGF-D and VEGFR2/3 receptors, some of which successfully completed clinical trials and received Food and Drug Administration approval (43). In our study, we used axitinib, the Food and Drug Administration-approved selective small-molecule inhibitor (35), to dissect the role of VEGFR signaling in preventing Tsc2-null lesion growth using a mouse model of LAM.
MATERIALS AND METHODS
Cell lines.
Tsc2-deficient MKOC kidney epithelial cells, derived from renal tubular cystadenoma of Tsc2+/− mice created on C57BL/6 background, were generously provided by Dr. Okio Hino from the Cancer Institute, Tokyo, Japan (13, 29). To enhance the invasive characteristics of MKOC cells, 5 × 106 cells were injected subcutaneously into flanks of NCRNU female athymic nude mice (Taconic) (13). After tumors reached 1.5 cm in diameter, mice were killed and the tumors were removed, enzymatically digested, and maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) for 2 days. MKOC cells derived from these tumors were named TMKOC, as our laboratory described previously (13). Tsc2-expressing kidney tubular epithelial M-1 cells dissected from C57BL/6 mice were purchased from the American Type Culture Collection (CRL-2038) and were used as a control. MKOC, TMKOC, and M-1 cells were maintained in DMEM supplemented with 10% FBS and further characterized by immunoblot analysis to confirm Tsc2 loss and mammalian target of rapamycin complex 1 (mTORC1) activation in Tsc2-null cells.
Animals.
All animal procedures were performed according to a protocol approved by the University of Pennsylvania Animal Care and Use Committee. Briefly, 8-wk-old athymic nude female mice (NCRNU, Taconic) were injected with TMKOC cells (106/100 μl of PBS) into the tail vein, as previously described (4, 13). Starting 3 days postinjection, mice received daily intraperitoneal injections of either diluent or 25 mg of axitinib (AG-013736; Tocris Bioscience, Bristol, UK; batch no. 1A/133671) per kilogram of body weight. Each experimental group included a minimum of 10 mice per condition.
Bronchoalveolar lavage fluid analyses.
Mice were killed 3 wk post-TMKOC cell injection, and lungs were lavaged with PBS to a total volume of 5 ml. Cells isolated from bronchoalveolar lavage (BAL) were collected, counted (Beckman-Coulter, Miami, FL), and differentiated, and the remaining cells, not used for differential analysis, were frozen and further analyzed by quantitative RT-PCR (qRT-PCR), as previously described (4, 14). Aliquots of the cell-free BAL fluid were stored at −80°C and further analyzed for cytokine levels by Multiplex (Aushon, Billerica, MA), and nitric oxide (NO) metabolites by chemical reduction and chemiluminescence using the Ionics/Sievers Nitric Oxide Analyzer 280 (NOA 280; Ionics Instruments, Boulder, CO), as previously described (3, 4). Following lavage, lungs were fixed in formalin for hematoxylin and eosin staining and immunohistochemical (IHC) analysis.
Western blotting.
Total proteins from MKOC, TMKOC, and M-1 were extracted through 15-min incubation with Nonidet P-40 lysis buffer and resolved on 4–12% Bis-Tris SDS polyacrylamide gel (Life Technologies, NP0335). Phosphorylation of ribosomal protein S6 (2217), pS-6 (4856), tuberin (Tsc2, 4308), and tubulin (2125) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Equal volume of BAL fluids from axitinib-treated or untreated mice were analyzed with VEGF-D antibodies (R&D Systems, AF469) or surfactant protein (SP)-D antibodies provided by Michael F. Beers (2–4).
IHC analysis.
IHC analysis of lungs to detect lymphatic vessels was performed by immunostaining with LYVE-1 antibody (Fitzgerald Industries, Acton, MA; 70R-LR005) and with 4′,6-diamidino-2-phenylindole staining, as described (11, 13–15). Lesions from a minimum of five animals per each treatment condition were analyzed using the Nikon Eclipse TE2000-E microscope equipped with an Evolution QEi digital video camera.
qRT-PCR.
M-1, MKOC, and TMKOC cell lines and cells recovered from lung lining of treated and untreated mice were analyzed for mRNA expression levels by qRT-PCR. The reactions were carried out with SYBR Green PCR master mix on an ABI Prism 7300 sequence detection system (Applied Biosystems). Obtained Ct values were normalized to β-actin signals and further analyzed using the ΔΔCt method, as previously described(4, 14, 28).
VEGF-D ELISA.
Serum VEGF-D levels were analyzed by commercially available enzyme-linked immunoassay (ELISA) kit, according to the manufacturer's protocol (R&D Systems, catalog no. DY469) with analysis on a Molecular Devices plate reader. Data are represented as means ± SE from triplicate measurements and shown as picograms of VEGF-D per milliliter of sample.
Data analysis.
Data are shown as means ± SE. For direct comparisons between two groups, significance was assessed by Student's t-test. In experiments with multiple treatment groups, significance tests were made within the confines of a significant two-way ANOVA and using Bonferroni correction for multiple comparisons. Assessment was performed by means of GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). Values of P < 0.05 were considered significant.
RESULTS
Tsc2 deficiency upregulates VEGF-D protein and mRNA expression.
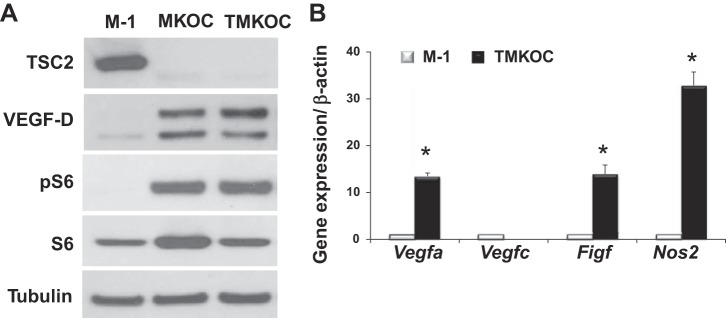
To determine whether Tsc2 deficiency upregulates VEGF-D levels, we used Tsc2-null mouse cells (MKOC and TMKOC) (13) derived from renal tubular cystadenoma of Tsc2 heterozygous mice, as described in materials and methods (29). Since these Tsc2-null cells originated from the renal tubular epithelium, we used Tsc2-expressing renal tubular epithelial M-1 cells as a control. As seen in Fig. 1A, marked upregulation of VEGF-D in Tsc2-null but not in Tsc2-expressing M-1 cells was detected by immunoblot analyses using anti-mouse VEGF-D antibody, which has been previously used to detect VEGF-D levels in mouse cells (5). Tsc2 deficiency in MKOC and TMKOC cells was confirmed by immunoblot analysis, as shown in Fig. 1A. The constitutive activation of the mTORC1 signaling pathway, a hallmark of Tsc2 loss (12, 16), in Tsc2-deficient cells, was demonstrated by phosphorylation of ribosomal protein S6 (Fig. 1A). Thus the cells, named TMKOC, utilized in the LAM mouse model were validated by demonstrating the Tsc2 loss, mTORC1 activation, and upregulation of VEGF-D.
Fig. 1.
Tuberous sclerosis complex 2 (Tsc2) deficiency upregulates vascular endothelial growth factor D (VEGF-D) protein and mRNA expression. A: representative micrographs of three independent immunoblot analysis of Tsc2-positive (M-1) and Tsc2-null (MKOC and TMKOC) cells with indicated antibody. Equal amounts of total protein of M-1, MKOC, and TMKOC were subjected to SDS-PAGE under reduced conditions, followed by immunoblotting with antibody against tuberin (TSC2), VEGF-D, pS6, S6, and tubulin. B: M-1 or TMKOC cells were analyzed for gene expression by quantitative RT-PCR, as described in materials and methods. Obtained Ct values were normalized to β-actin signals and further analyzed using the relative quantization (ΔΔCt) method. Values are fold change (means ± SE, n = 2 in each group, 3 independent experiments). *P < 0.05 vs. Tsc2-expressing cells (M-1) by Student's t-test.
To investigate whether Tsc2 deficiency affects VEGF-D expression, Tsc2-null and Tsc2-expressing cells were analyzed by qRT-PCR for specific VEGF family genes. As seen in Fig. 1B, the Figf gene expressing VEGF-D is markedly increased in TMKOC cells compared with M-1 cells. As additional controls, we examined mRNA levels of Vegfa, a well-known angiogenic growth factor, whose expression is regulated by Tsc2 (6, 7). Figure 1B shows marked upregulation of Vegfa in correspondence with high Figf levels in TMKOC cells (Fig. 1B). In contrast, the Vegfc gene, which is required for lymphangiogenesis during development (26), shows undetectable levels in TMKOC cells (Fig. 1B). Interestingly, TMKOC cells exhibited increased expression of the inflammatory gene Nos2.
Collectively, these data demonstrate that TMKOC cells utilized in the mouse LAM model are characterized by Tsc2 loss, mTORC1 activation, and upregulation of the VEGF-D protein level and gene expression.
Axitinib treatment inhibits Tsc2-null lung lesion growth and abnormal lymphangiogenesis.
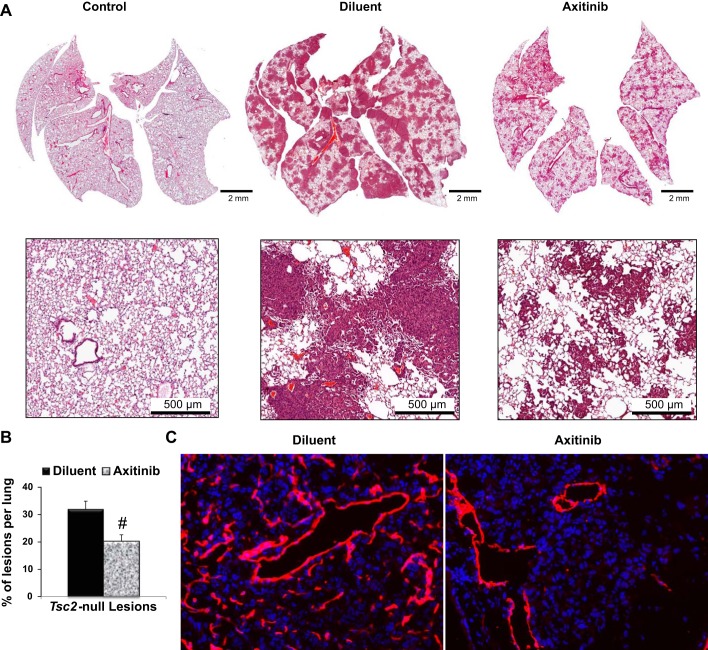
To investigate whether inhibition of the VEGF receptor signaling will affect Tsc2-null lung lesion growth, we used a mouse model of LAM (4, 13) utilizing Tsc2-null cells described above. In accordance with our laboratory's previously published data (4, 13), multiple Tsc2-null lesions of various sizes developed in the lungs 21 days postinjection of Tsc2-null TMKOC cells, with total tumor burden of ∼30% of the lung, as it was detected by morphometric analysis of hematoxylin and eosin staining of lung tissue sections (Fig. 2A). Axitinib treatment markedly suppressed tumor development and density of lesion size, and it significantly reduced total tumor burden to 20% of the lung tissue sections (32.0 ± 2.9 vs. 20.2 ± 2.4% of lesions per untreated or axitinib-treated lung, n = 7 per group, P < 0.005), (Fig. 2B).
Fig. 2.
Axitinib treatment inhibits Tsc2-null lung lesion growth and abnormal lymphangiogenesis. A: representative micrographs of hematoxylin and eosin staining of lung sections from control mice and mice with Tsc2-null lesions treated with diluent or axitinib. B: statistical analysis of the percentage of lesions per lung treated with diluent (n = 9) or axitinib (n = 6) was performed as described (13). Values are means ± SE. #P < 0.05 by Student's t-test. C: lung tissue of mice with Tsc2-null lesions treated with diluent or axitinib were analyzed for lymphatic vessels by immunohistochemical analysis with specific anti-lymphatic vessel endothelial hyaluronan receptor 1 antibodies (red). 4′,6-Diamidino-2-phenylindole staining was performed to detect nuclei (blue). Representative images were taken using a Nikon Eclipse TE-2000E microscope (n = 5 per group, magnification: ×20).
Progressive growth of Tsc2-null lesions induces lymphangiogenesis in mouse model of LAM (13). IHC analysis revealed marked upregulation of lymphatic channels within Tsc2-null lesions (Fig. 2C) detected by staining with antibody for LYVE-1, a marker of lymphatic vessels. Lymphatic endothelial cells forming new lymphatic vessels express VEGFR3 receptor, which is known to be activated by VEGF-D (5). Importantly, as seen in Fig. 2C, axitinib treatment markedly inhibited sprouting of lymphatic channels in Tsc2-null lesions.
Collectively, these data demonstrate that pharmacological inhibition of VEGFR signaling in lung with axitinib has a potential beneficial effect in decreasing Tsc2-null lesion growth and lymphatic sprouting within these lesions.
Axitinib attenuates VEGF-D upregulation in serum and lung lining fluid.
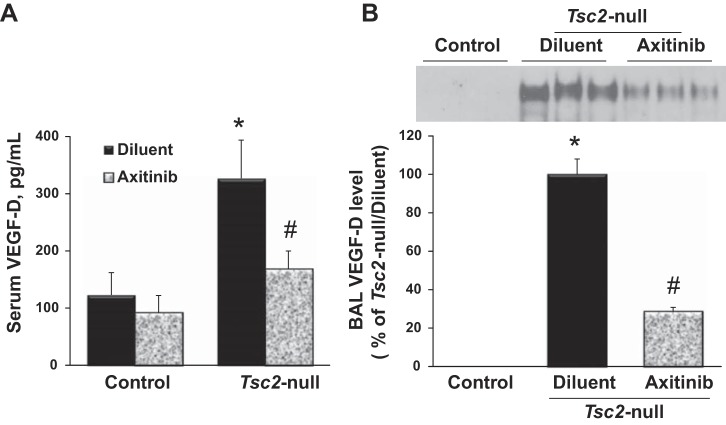
To investigate whether VEGF-D levels are increased with progressive Tsc2-null lesion growth and whether this increase is affected by axitinib, serum and BAL fluid from mice untreated or treated with axitinib were analyzed for VEGF-D level. Figure 3 demonstrates that mice with Tsc2-null lesions exhibit a significant systemic increase of VEGF-D levels in serum and BAL fluid, as determined by ELISA (Fig. 3A) and Western blot (Fig. 3B), respectively. As seen in Fig. 3, treatment with axitinib significantly reduces Tsc2-null-dependent VEGF-D levels in serum and BAL fluid. Moreover, this downregulation of VEGF-D corresponded with reduction of Tsc2-null lesions per lung (Fig. 2B) and inhibition of lymphangiogenesis (Fig. 2C).
Fig. 3.
Axitinib attenuates VEGF-D upregulation in serum and lung lining fluid. Serum and bronchoalveolar lavage (BAL) fluid was collected from control mice or mice with Tsc2-null lesions treated with diluent or axitinib and analyzed for VEGF-D level. A: serum samples were analyzed for VEGF-D level by ELISA kit. Values are means ± SE from triplicate measurements and shown as picograms of VEGF-D per milliliter of sample; n = 10 per each group. P < 0.05 vs. *control/diluent group and #Tsc2-null/diluent group by two-way ANOVA and Bonferroni correction for multiple comparisons. B, top: representative micrographs of BAL VEGF-D protein content. BAL samples containing equal volume were subjected to SDS-PAGE under reduced conditions followed by immunoblotting with anti-VEGF-D antibody. Bottom: densitometric quantification of VEGF-D content. Mean values of all of the samples from Tsc2-null lung treated with diluent or axitinib were calculated and presented as a percentage of Tsc2-null lungs treated with diluent. Values are means ± SE; n = 10 per group. P < 0.05 vs. *control/diluent group and #Tsc2-null/diluent group by t-test.
Axitinib attenuates cytokine upregulation in BAL.
Progressive growth of Tsc2-null lesions correlate with released cytokines and chemokines into the lung (4, 13). To determine whether axitinib treatment will attenuate upregulated cytokine release in the lung, BAL fluids from untreated and axitinib-treated mice were analyzed for relevant cytokines. As shown in Table 1, axitinib treatment significantly attenuates upregulated levels of VEGF-A, monocyte chemoattractant protein-1, and transforming growth factor-1β, but not interleukin-6, keratinocyte chemoattractant, or matrix metalloproteinase 9, suggesting a modulatory role of VEGFR signaling on their expression.
Table 1.
Axitinib attenuates cytokine upregulation in bronchoalveolar lavage
| IL-6 | KC | MCP-1 | VEGF-A | TGF-1β | MMP-3 | MMP-9 | |
|---|---|---|---|---|---|---|---|
| Control/diluent | 11.7 ± 1.8 | 10.7 ± 2.7 | 12.6 ± 2.3 | 0.6 ± 0.1 | 254.3 ± 94.6 | 356.9 ± 96.4 | 11.3 ± 0.8 |
| Control/axitinib | 12.6 ± 5.2 | 13.5 ± 1.4 | 11.4 ± 3.4 | 0.5 ± 0.1 | 251.5 ± 105.6 | 396.6 ± 91.0 | 13.4 ± 6.5 |
| Tsc2-null/diluent | 66.3 ± 12.5* | 29.6 ± 4.2* | 523.2 ± 56.9* | 20.5 ± 2.6* | 3,075 ± 375* | 23,982 ± 1,731* | 631.7 ± 88.2* |
| Tsc2-null/axitinib | 54.9 ± 16.6 | 21.2 ± 3.2 | 267.7 ± 48.1† | 10.3 ± 3.1† | 1,867 ± 283† | 17,391 ± 4,570 | 507.2 ± 127.2 |
Values are means ± SE in pg/ml; n = 10 mice/group. Bronchoalveolar lavage fluid was collected at day 21 post-TMKOC cell injection and analyzed for cytokine level by the Aushon Searchlight Protein Array multiplex ELISA. Tsc2, tuberous sclerosis complex 2 gene; IL-6, interleukin-6; KC, keratinocyte chemoattractant; MCP-1, monocyte chemoattractant protein-1; VEGF-A, vascular endothelial growth factor A; MMP, matrix metalloproteinase. P < 0.05 vs.
control/diluent group and
Tsc2 null/diluent group by two-way ANOVA and Bonferroni correction for multiple comparisons.
Axitinib reduces recruitment of leukocytes into the lung lining.
Previously, our laboratory has shown that Tsc2-null lesion growth promotes recruitment of immature myeloid cells to the lungs (4). To determine whether axitinib treatment affects the recruitment of leukocytes to the lung with Tsc2-null lesions, cells were isolated from the lining of the lungs with and without axitinib treatment, counted, and differentiated according to cell morphology. Table 2 shows that axitinib treatment significantly attenuated total number of cells recruited to the lung lining. Among all cell populations, there were significant reductions of multinucleated and progenitor cell populations (Table 2). Interestingly, the total number of macrophages was not significantly affected by axitinib (Table 2).
Table 2.
Axitinib reduces recruitment of leukocytes into the lung lining
| Total Cells | Macrophages | Multinucleated | Eosinophil | Progenitors | |
|---|---|---|---|---|---|
| Control/diluent | 2.35 ± 0.15 | 2.35 ± 0.15 | 0 | 0 | 0 |
| Control/axitinib | 2.2 ± 0.48 | 2.2 ± 0.48 | 0 | 0 | 0 |
| Tsc2-null/diluent | 18.86 ± 1.09* | 11.6 ± 0.74* | 4.67 ± 0.60* | 0.50 ± 0.30 | 2.34 ± 0.31* |
| Tsc2-null/axitinib | 14.13 ± 1.28† | 9.95 ± 0.78 | 2.44 ± 0.42† | 0.35 ± 0.12 | 1.44 ± 0.33† |
Values are means ± SE of cell numbers × 105; n = 10 mice/group. Total cells were collected from lung lavage of control mice or mice with Tsc2-null lesions treated with diluent or axitinib at day 21 post-TMKOC cell injection. Total count was determined by Coulter counting; differential cell counts were done using Diff-Quick staining of cytospin slides. Cells were identified as macrophages, eosinophils, multinucleated, or progenitors by standard morphology. Comparisons between groups were made by two-way ANOVA and Bonferroni correction for multiple comparisons, P < 0.05 vs.
control/diluent group and
Tsc2-null/diluent group.
Axitinib inhibits provasculogenic, prolymphangiogenic, and inflammatory gene expression in recruited BAL cells.
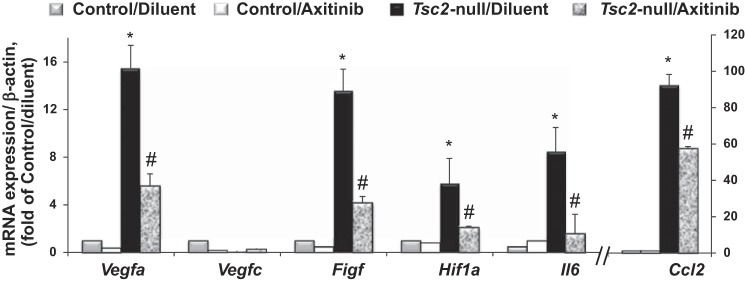
Progressive growth of Tsc2-null lesions promotes inflammatory cells recruitment (Tables 1 and 2), which correlates with increased expression of VEGF-D level in serum and lung lining (Fig. 3). To determine whether cells recruited to the lung with Tsc2-null lesions express Figf, we performed qRT-PCR analysis of BAL cell pellets. As seen in the Fig. 4, cells recruited to the lung lining expressed increased mRNA levels of Figf and Vegfa, but not Vegfc. There was also marked upregulation of proinflammatory genes Hif1a, Il6, and Ccl2. Importantly, treatment with axitinib significantly attenuates upregulation of Figf, Vegfa, and proinflammatory gene expression in BAL cells.
Fig. 4.
Recruited cells expressed increased mRNA levels that are attenuated by axitinib. RNA was extracted from BAL cells isolated from lungs of control mice and mice with Tsc2-null lesions treated with diluent or axitinib. Expression of gene markers was quantified by quantitative RT-PCR, as described in materials and methods. Ct values obtained were normalized to β-actin signals and further analyzed using the relative quantization (ΔΔCt) method. Values are fold change (means ± SE, n = 3–5 per each group). Comparisons between groups were made by two-way ANOVA and Bonferroni correction for multiple comparisons. P < 0.05 vs. *control/diluent group and #Tsc2-null/diluent group.
Axitinib abrogates NO synthase 2-mediated NO production.
Our laboratory previously demonstrated that inflammatory cells recruited to the lung with Tsc2-null lesions expressed increased level of NO metabolites (4). In accordance with our previous observation, recruited leukocytes to the lung with Tsc2-null lesions exhibited marked expression of Nos2 level (Fig. 5A). Treatment with axitinib significantly attenuates upregulation of this proinflammatory gene (Fig. 5A). To investigate whether increased NO metabolite production observed in the BAL fluid of lung with Tsc2-null lesions was Nos2 dependent, BAL fluids from untreated and axitinib-treated mice with Tsc2-null lesions were examined for total NO content. Figure 5B shows that axitinib treatment also significantly decreases production of NO in the lung lining fluid.
Fig. 5.
Axitinib abrogates nitric oxide synthase 2 (NOS2)-mediated nitric oxide production. A: cells recovered from BAL fluid of control mice and mice with Tsc2-null lesions treated with diluent or axitinib were analyzed for Nos2 mRNA expression by quantitative RT-PCR and normalized to β-actin. Values are fold change (means ± SE, n = 3–5 per each group). B: BAL fluid was collected at 21 days post-TMKOC cell injection and analyzed for nitric oxide metabolites by chemical reduction, as described in materials and methods. Values are means ± SE (control/diluent, n = 5; control/axitinib, n = 5; Tcs2-null/diluent, n = 15; Tsc2/axinitib, n = 10). P < 0.05 vs. *control/diluent group and #Tsc2-null/diluent group by two-way ANOVA and Bonferroni correction for multiple comparisons.
Axitinib attenuates S-nitrosylation of surfactant protein-D.
Similar to other cystic mouse models, our mouse model of LAM is characterized by increased Nos2 function and surfactant protein-D (SP-D) modification (4). Our laboratory has recently shown that the increase in S-nitrosylated (SNO)-SP-D, a marker of pulmonary inflammation, was associated with increased Nos2 activity and NO production (4). To investigate whether axitinib treatment attenuates SNO of SP-D level, lung lining fluids from untreated and axitinib-treated mice with Tsc2-null lesions were analyzed for total SP-D and SNO-SP-D. Figure 6 shows that axitinib treatment attenuates SNO-SP-D level. The observed decreased level of SNO-SP-D was in agreement with decreased NO production within the lung lining fluid.
Fig. 6.

Axitinib attenuates S-nitrosylation (SNO) of surfactant protein-D (SP-D). BAL fluid was collected at 21 days post-TMKOC cell injection, and samples containing equal volume were subjected to SDS-PAGE under reduced conditions followed by immunoblotting with anti-SP-D antibody, as described in the materials and methods. Top: representative micrographs of total SP-D protein content. Bottom: representative micrographs of SNO-SP-D content by Biotin-Switch assay.
DISCUSSION
LAM is a devastating lung disease that primarily affects women of childbearing age. Both the genetic and spontaneous forms are caused by loss of Tsc1 or Tsc2 gene function in the smooth musclelike LAM cells in the lung. When LAM cells overgrow, they form cysts that progressively enlarge and destroy the surrounding normal lung tissue and obstruct airways and blood vessels, which eventually leads to respiratory failure. In LAM, the lymphatic system intensively expands, which results in loss of basement membrane integrity and spreading of LAM cells to other organs. While the origin of LAM cells is not well understood, one can observe that LAM manifests as a type of benign tumor (20, 30). Dr. Henske and colleges identified the same Tsc2 mutations in kidney and lung lesions of LAM patients, suggesting that kidney angiomyolipoma and lung LAM cells have common origin (8). This hypothesis is confirmed by the observation that Tsc2-null kidney epithelial cells (29) can be used to generate an animal model of LAM (4, 13). In this mouse LAM model, progressive growth of Tsc2-null lesions correlated with mTORC1 activation detected by pS6, and smooth muscle α-actin expression, abnormal lymphangiogenesis detected by LYVE-1 and upregulation of VEGF-D within lesions, similar to human LAM cells (13).
In the present study, using the same mouse LAM model, we demonstrated that mice with Tsc2-null lesions exhibit increased levels of VEGF-D in both the serum and lung lining. This parallels the human disease in which VEGF-D level in serum has been reported as a novel diagnostic biomarker for evaluating disease severity in LAM (40) and to prospectively distinguish LAM from other cystic diseases (32). From a mechanistic perspective, it is of interest that, within animal models of cancer, VEGF-D promotes tumor angiogenesis, lymphangiogenesis, and remodeling of collecting lymphatic vessels that facilitate solid tumor growth and/or metastatic spread to regional lymph nodes and distant organs (41, 42). Taken together, these observations provide a link between the metastatic nature of LAM and the lung tissue destruction, and identify VEGF-D signaling as a potential therapeutic target within this unique pathology.
Secreted VEGF-D exerts its signaling function via the activation of the cell surface receptor VEGFR-3 (1, 25). This receptor is a tyrosine kinase, whose activation within the lymphatic endothelium leads to growth of lymphatic vessels (42, 44). Within cancer models, disruption of VEGF-D binding to VEGFR-3, using either a soluble form of VEGFR-3 (19, 33), neutralizing antibodies to VEGF-D (42) or VEGFR-3 (21, 37), has been shown to reduce tumor lymphangiogenesis and lymphatic metastasis. Previously, direct inhibition of VEGFR-3 signaling was not considered as an approach to limit LAM. Here, we have used axitinib, also known as AG013736, a small-molecule tyrosine kinase inhibitor to target VEGFR-3 signaling. Axitinib targets angiogenesis and shows activity against all three VEGF receptors, including VEGFR-1, -2, and -3 (22). It has been shown to inhibit the growth of breast cancer in xenograft models (45) and has been used successfully in trials with various tumor types, including renal cell carcinoma (38).
The present study demonstrates that pharmacological targeting of VEGFR signaling with axitinib attenuates Tsc2-null lung lesion growth. In addition, it reduces the increased levels of VEGF-D seen in the serum and BAL fluid in this mouse model of LAM. Interestingly, the leukocytes recruited to the lung lining are a significant source of VEGF-D (Fig. 4). Axitinib only partially reduced the increased cellularity in the lining of lungs with Tsc2-null lesions, whereas upregulation of Vegfa and Figf gene expression in BAL cells was markedly reduced by axitinib. Leukocytes recruited to the lung lining produced are in an activated state, as there is upregulation of proinflammatory genes such as Nos2, Ccl2, and Il6 in BAL cells from lung with Tsc2-null lesions. As with Figf, the increased expression of these genes in BAL cells was reduced by axitinib, suggesting that the activated state may be critical to VEGF-D expression.
Importantly, VEGF-D expression is increased in Tsc2-deficient cells used in LAM mouse model (Fig. 1). These data suggest that Tsc2-null lung lesions express VEGF-D, which might contribute to inflammatory cell recruitment, which further increases VEGF-D levels in serum. Further study determining the specific contribution of Tsc2-null lesions and inflammatory cells in abnormal lymphangiogenesis in LAM are warranted.
It has been shown that axitinib dose-dependently reduced the phosphorylation of Akt, endothelial NO synthase (NOS), and extracellular signal-regulated kinase-1/2, key downstream signaling molecules of VEGF (22). This suggests that the reduction in Akt, endothelial NOS, and extracellular signal-regulated kinase 1/2 phosphorylation may be due to antagonism of upstream VEGFRs by axitinib. Axitinib-dependent inhibition of NOS2 expression demonstrates a role of VEGF signaling in modulating its expression. Because axitinib affects activity of not only VEGFR2 and VEGFR3 but also PDGF receptor, cKit, and BRC-ABL (36), further study will determine specific role of these receptors in upregulation (18) of NOS2, VEGF-A, and VEGF-D.
The data presented here demonstrate that, in a mouse model of LAM, axitinib is an effective inhibitor of Tsc2-null lesion growth. However, several clinical trial reports have demonstrated that tyrosine kinase inhibitors, such as axitinib, have been shown to cause frequent cardiovascular adverse events during treatment of renal cell carcinoma (17, 39), and thus caution in axitinib use for the treatment of LAM is warranted. In the mouse model of LAM, growth of Tsc2-null lesions involves recruitment and activation of inflammatory cells; these processes are also inhibited by axitinib. Reduction in lesion growth and cell recruitment by axitinib leads to reduced VEGF-D expression. Collectively, our study demonstrates proof-of-concept of the pharmacological inhibition of VEGF signaling to induce beneficial effects on lung inflammation and Tsc2-null lung lesions seen in LAM.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-114085 (V. P. Krymskaya) and HL-086621 (A. J. Gow) and by the LAM Foundation (V. P. Krymskaya).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.N.A.-V. and V.P.K. conception and design of research; E.N.A.-V., E.A., M.L.J., R.R., and C.-J.G. performed experiments; E.N.A.-V., E.A., A.Y.L., N.-T.E., and C.-J.G. analyzed data; E.N.A.-V., C.-J.G., A.J.G., and V.P.K. interpreted results of experiments; E.N.A.-V. prepared figures; E.N.A.-V. and V.P.K. drafted manuscript; E.N.A.-V., A.J.G., and V.P.K. edited and revised manuscript; E.N.A.-V., E.A., M.L.J., R.R., A.Y.L., N.-T.E., C.-J.G., A.J.G., and V.P.K. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Irine Khavin and Dmitry A. Goncharov for technical assistance with in vivo experiments and tissue collection, and Dr. Michael Beers for providing antibody against SP-D.
REFERENCES
- 1.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A 95: 548–553, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atochina-Vasserman EN, Bates SR, Zhang P, Abramova H, Zhang Z, Gonzales L, Tao JQ, Gochuico BR, Gahl W, Guo CJ, Gow AJ, Beers MF, Guttentag S. Early alveolar epithelial dysfunction promotes lung inflammation in a mouse model of Hermansky-Pudlak syndrome. Am J Respir Crit Care Med 184: 449–458, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atochina-Vasserman EN, Gow AJ, Abramova H, Guo CJ, Tomer Y, Preston AM, Beck JM, Beers MF. Immune reconstitution during Pneumocystis lung infection: disruption of surfactant component expression and function by S-nitrosylation. J Immunol 182: 2277–2287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atochina-Vasserman EN, Guo CJ, Abramova E, Golden TN, Sims M, James ML, Beers MF, Gow AJ, Krymskaya VP. Surfactant dysfunction and lung inflammation in the female mouse model of lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 53: 96–104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin ME, Roufail S, Halford MM, Alitalo K, Stacker SA, Achen MG. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J Biol Chem 276: 44307–44314, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brugarolas J, Kaelin WG Jr. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 6: 7–10, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4: 147–158, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Choi YB, Chen HSV, Lipton SA. Three pairs of cysteine residues mediate both redox and Zn2+ modulation of the NMDA receptor. J Neurosci 21: 392–400, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JM, Hyjek E, Husain AN, Shen L, Jones J, Schuger LA. Lymphatic endothelial differentiation in pulmonary lymphangioleiomyomatosis cells. J Histochem Cytochem 61: 580–590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasgow CG, Avila NA, Lin JP, Stylianou MP, Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest 135: 1293–1300, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. TSC2 modulates actin cytoskeleton and focal adhesion through TSC1-binding domain and the Rac1 GTPase. J Cell Biol 167: 1171–1182, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, Panettieri RA Jr, Krymskaya VP. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem 277: 30958–30967, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Goncharova EA, Goncharov DA, Fehrenbach M, Khavin I, Ducka B, Hino O, Colby TV, Merrilees MJ, Haczku A, Albelda SM, Krymskaya VP. Prevention of alveolar destruction and airspace enlargement in a mouse model of pulmonary lymphangioleiomyomatosis (LAM). Sci Transl Med 4: 154ra134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncharova EA, Goncharov DA, James ML, Atochina-Vasserman EN, Stepanova V, Hong SB, Li H, Gonzales L, Baba M, Linehan WM, Gow AJ, Margulies S, Guttentag S, Schmidt LS, Krymskaya VP. Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Rep 7: 412–423, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, Krymskaya VP. mTORC2 is required for proliferation and survival of TSC2-null cells. Mol Cell Biol 31: 2484–2498, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 34: 473–480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail 1: 72–78, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Hartshorn KL, White MR, Tecle T, Sorensen G, Holmskov U, Crouch EC. Viral aggregating and opsonizing activity in collectin trimers. Am J Physiol Lung Cell Mol Physiol 298: L79–L88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 94: 819–825, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Henske EP, McCormack FX. Lymphangioleiomyomatosis–a wolf in sheep's clothing. J Clin Invest 122: 3807–3816, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 66: 8065–8075, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O'Connor P, Shalinsky DR, Bender SL. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14: 7272–7283, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Issaka RB, Oommen S, Gupta SK, Liu G, Myers JL, Ryu JH, Vlahakis NE. Vascular endothelial growth factors C and D induces proliferation of lymphangioleiomyomatosis cells through autocrine crosstalk with endothelium. Am J Pathol 175: 1410–1420, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, Bonetti F, Kingswood C, Review Panel of the ERSLAMTF. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J 35: 14–26, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 15: 290–298, 1996. [PMC free article] [PubMed] [Google Scholar]
- 26.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Karnezis T, Shayan R, Caesar C, Roufail S, Harris NC, Ardipradja K, Zhang YF, Williams SP, Farnsworth RH, Chai MG, Rupasinghe TW, Tull DL, Baldwin ME, Sloan EK, Fox SB, Achen MG, Stacker SA. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21: 181–195, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen L, Atochina-Vasserman EN, Guo CJ, Scott PA, Haenni B, Beers MF, Ochs M, Gow AJ. NOS2 is critical to the development of emphysema in Sftpd deficient mice but does not affect surfactant homeostasis. PloS One 9: e85722, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res 59: 1206–1211, 1999. [PubMed] [Google Scholar]
- 30.Krymskaya VP. Smooth muscle-like cells in pulmonary lymphangioleiomyomatosis. Proc Am Thorac Soc 5: 119–126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, Sato T, Kubo H, Gomi K, Shibuya K, Fukuchi Y, Suda K. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol 29: 1356–1366, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Lee IK, Kang KA, Zhang R, Kim BJ, Kang SS, Hyun JW. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environ Toxicol Pharmacol 31: 233–241, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res 65: 6901–6909, 2005. [DOI] [PubMed] [Google Scholar]
- 34.McCormack FX, Travis WD, Colby TV, Henske EP, Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am J Respir Crit Care Med 186: 1210–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McTigue M, Murray BW, Chen JH, Deng YL, Solowiej J, Kania RS. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A 109: 18281–18289, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pemovska T, Johnson E, Kontro M, Repasky GA, Chen J, Wells P, Cronin CN, McTigue M, Kallioniemi O, Porkka K, Murray BW, Wennerberg K. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature 519: 102–105, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res 66: 2650–2657, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, Pithavala YK, Reich SD, Freddo JL, Wilding G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 23: 5474–5483, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Schmidinger M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl 11: 172–191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, Tominaga S, Fukuchi Y. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol 4: 143–152, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis 27: 1729–1738, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 7: 186–191, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14: 159–172, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, Kubo H, Thurston G, McDonald DM, Achen MG, Stacker SA, Alitalo K. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 20: 1223–1231, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilmes LJ, Pallavicini MG, Fleming LM, Gibbs J, Wang D, Li KL, Partridge SC, Henry RG, Shalinsky DR, Hu-Lowe D, Park JW, McShane TM, Lu Y, Brasch RC, Hylton NM. AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 25: 319–327, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Young L, Lee HS, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, Brown KK, Lynch JP 3rd Goldberg HJ, Downey GP, Swigris JJ, Taveira-DaSilva AM, Krischer JP, Trapnell BC, McCormack FX. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet 1: 445–452, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, Bissler JJ, Franz DN, McCormack FX. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest 138: 674–681, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]