Abstract
Chronic tobacco smoking is a major cause of preventable morbidity and mortality worldwide. In the lung, tobacco smoking increases the risk of lung cancer, and also causes chronic obstructive pulmonary disease (COPD), which encompasses both emphysema and chronic bronchitis. E-cigarettes (E-Cigs), or electronic nicotine delivery systems, were developed over a decade ago and are designed to deliver nicotine without combusting tobacco. Although tobacco smoking has declined since the 1950s, E-Cig usage has increased, attracting both former tobacco smokers and never smokers. E-Cig liquids (e-liquids) contain nicotine in a glycerol/propylene glycol vehicle with flavorings, which are vaporized and inhaled. To date, neither E-Cig devices, nor e-liquids, are regulated by the Food and Drug Administration (FDA). The FDA has proposed a deeming rule, which aims to initiate legislation to regulate E-Cigs, but the timeline to take effect is uncertain. Proponents of E-Cigs say that they are safe and should not be regulated. Opposition is varied, with some opponents proposing that E-Cig usage will introduce a new generation to nicotine addiction, reversing the decline seen with tobacco smoking, or that E-Cigs generally may not be safe and will trigger diseases like tobacco. In this review, we shall discuss what is known about the effects of E-Cigs on the mammalian lung and isolated lung cells in vitro. We hope that collating this data will help illustrate gaps in the knowledge of this burgeoning field, directing researchers toward answering whether or not E-Cigs are capable of causing disease.
Keywords: cancer, chronic obstructive pulmonary disease, cystic fibrosis, nicotine, tobacco
electronic cigarettes or e-cigarettes (E-Cigs), also known as electronic nicotine delivery systems, were designed to deliver aerosolized nicotine in a minimal liquid vehicle that was thought to be relatively safe compared with tobacco. It has been proposed by E-Cig manufacturers that, since these products do not burn tobacco, they will not expose the lung to the same toxic chemicals as regular smoked tobacco and so will not cause the lung disease that is frequently associated with chronic tobacco inhalation, including lung cancer and chronic obstructive pulmonary disease (COPD). E-Cig users are a fast-growing subset of nicotine users who are described as “vapers” rather than smokers, since E-Cigs heat and generate aerosols but do not burn e-liquids. There is considerable controversy regarding the disease risk and toxicity of E-Cigs (112, 116, 127). However, because E-Cigs do not currently fall under the auspices of the Food and Drug Administration (FDA), they have not undergone the typical toxicological evaluation, followed by human clinical trials that are required of other inhaled products (e.g., inhaled therapeutic agents), and, as such, no safety data exist from either humans or animals. Because of this, it is hard to predict whether these products will be benign when chronically inhaled, possibly over a lifetime, or whether they will induce tobacco-like disease or other types of lung disease such as bronchiolitis obliterans, a disease that has been caused by the inhalation of the buttery-tasting flavor diacetyl (83). The clinical evaluation of biomarkers of harm (e.g., inflammatory and cytotoxic markers) is required to inform the FDA and for ensuring safety and proper regulation. However, these studies are only just beginning in what can at best be described as “investigator-initiated trials” rather than formal clinical trials. A further confounder is that many E-Cig users have switched after chronically smoking tobacco products, making it difficult to differentiate between the previous effects of tobacco vs. the effects of the E-Cigs (40). To date, there are currently 1,273 E-Cig articles on Pubmed, of which 135 are reviews and only 85 include the terms “e-cigarette” and “lung.” In contrast, “tobacco” and lung yields 9,769 hits, indicating the lack of maturity of this field. In this review, we shall list and evaluate what is known about the effects of E-Cig exposure on lungs/airways in vivo and in vitro (Fig. 1).
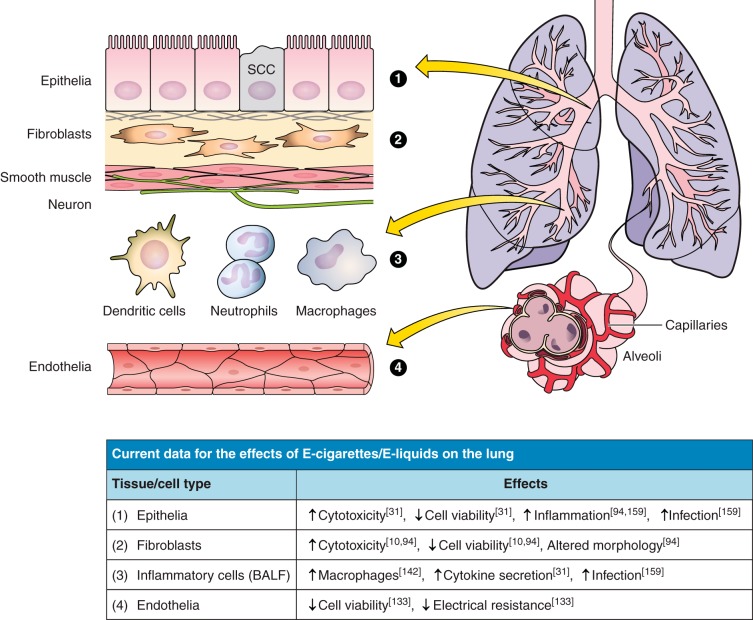
Fig. 1.
Current knowledge of the effects of E-cigarette (E-Cig) and e-liquid exposures on pulmonary cell types. Included in the table is a short list of the current in vitro and in vivo study outcomes for lung-related cell types that are depicted in the cartoon and labeled appropriately.
The problem explained.
Nicotine is a highly addictive compound that, through nicotinic acetylcholine receptors (nAChR), exerts potent effects on the brain, including the saturation and desensitization of nAChRs (α4β2-subtype) leading to significant changes in the brain's physiology such as activation of the reward/pleasure regions of the cortex and reduced anxiety (17, 50, 128). Inhaling tobacco is a relatively simple and efficient way of delivering nicotine into the bloodstream. Nicotine is a weak base that can be absorbed across the lung in its unionized form into the bloodstream (18). The effects of nicotine on the brain are complex and only just beginning to be understood. Importantly, its effects on the adolescent brain are markedly different compared with the adult brain and can affect neural development. For example, exposure to nicotine in adolescent rats led to an increased sensitivity to nicotine in these rats as adults, even if a smoking cessation period was introduced, suggesting that vaping by adolescents may have serious consequences later in life (41).
Nicotine craving causes a huge drive to continue smoking, and smokers maintain fairly constant plasma nicotine levels during waking hours, despite the significant risks: lung cancer (including both small cell and nonsmall cell) accounts for 27% of all diagnosed cancers and is the deadliest form of cancer, killing ∼150,000 people in the United States of America (USA) per year (27, 132). It is thought that ∼85% of all lung cancer is caused by smoking, and secondhand smoke exposure increases the chance of lung cancer by ∼25% (27). COPD is also caused primarily by tobacco exposure in Western countries and kills a similar amount of people as lung cancer (∼140,000/yr) (12, 60). COPD is also the third leading cause of death in the USA and worldwide (157), although tobacco exposure is a primary risk factor of COPD in first-world countries; smoke from biomass fuels is also a risk factor for COPD in second- and third-world countries (49). COPD often occurs with other comorbidities, and presentation with COPD is a major risk factor for the development of lung cancer (73, 106). The fact that tobacco exposure is a key factor in the development of both of these diseases was first denied by the tobacco industry and later accepted, following the Tobacco Master Settlement Agreement in 1998 (52, 79).
In the 1950s, ∼50% of the adult population were regular tobacco smokers in the USA (30). Following the Surgeon General's 1954 report linking tobacco smoke with disease, a series of public health campaigns led to 1) increased public awareness about the dangers of smoking, 2) bans on tobacco advertising, 3) age limits for purchasing tobacco, 4) bans on tobacco products that allegedly target minors (e.g., flavored cigarettes), 5) increased taxation on tobacco, and 6) restrictions on where tobacco products can be smoked (e.g., bans on smoking in public places) (76, 119). Due to these pressures, tobacco companies began developing “safe cigarettes.” In the 1980s, “low-tar” cigarettes were developed, which were purportedly safer than regular cigarettes because they exposed users to less of the tar phase from cigarettes. This premise was based on flawed science. In part, the reduced tar output was generated by putting holes at the base of the cigarette, which served to reduce airflow through the cigarette. However, these cigarettes were not safer than regular cigarettes. In fact, users learned to compensate by either covering up the holes with their fingers or taking larger puffs, thus negating the “low-tar effects” (67, 117). Furthermore, the gas phase of cigarette smoke is also highly toxic and was not addressed in “low-tar cigarettes” (111, 125). Additional types of safe cigarettes have been developed, including “heat not burn” types (e.g., the “Eclipse”), which heats a rod in the middle of the cigarette to give off tobacco smoke without burning it. This style of cigarette was not successful commercially (6), and there is no evidence that they are actually safer (71).
Despite these failed attempts at safe cigarettes, the Institute of Medicine issued a report in 2001 that outlined the feasibility of focusing efforts on harm reduction tobacco products, which were referred to as “Potential to Reduced Exposure Products” or “PREPs,” since they wanted to avoid implying “that any product currently known is safe” (141). At that time, there were no conclusive data that the current products on the market were reducing the individual users' exposure to harmful tobacco substances. However, the committee did conclude that there was potential merit in “harm reduction” as a part of the national tobacco program that “emphasizes abstinence oriented prevention and treatment” (141). Thus, when E-Cigs were brought to market, they appeared to be likely candidate PREPs, since they neither contain tobacco nor is the vapor produced by combustion. E-Cigs were introduced into European markets in 2006 and in the USA in 2007 (126). The first generation of E-Cigs were dubbed “cigalikes” due to their resemblance to conventional cigarettes, and they came in both rechargeable/refillable and disposable formats (126, 164). Subsequently, as their popularity grew, second- and third-generation E-Cigs have been developed which, although they have ceased to resemble cigarettes, have significantly improved their ability to deliver nicotine through the lung and into the bloodstream (164). However, the health effects of long-term inhalation of E-Cig aerosols are currently unknown.
What are/what is in E-Cigs?
Cigarette smoke is a complex and highly reactive mixture that includes metals (e.g., Cr, Cd, Hg), aldehydes (e.g., 4-aminobiphenyl, acrolein, formaldehyde), carbon monoxide, free radicals, and, of course, nicotine (57, 145). Adverse effects can be caused by adduct formation, especially of aldehydes, with DNA or proteins (114), or due to excessive oxidative stress (81). Aldehydes and heavy metals have been shown to have a number of cytotoxic effects on epithelia, including adduct formation to DNA (32, 140, 154). Additionally, tobacco smoke, as well as aldehydes, cadmium, and oxidative stress, also affect plasma membrane proteins such as the cystic fibrosis transmembrane conductance regulator (CFTR)(25, 33, 70, 137), which is required for fluid secretion in the lung (35, 60). In contrast, e-liquids (the flavored liquids that are heated to form the E-Cig vapor) are thought to be much simpler and ostensibly contain nicotine (∼6–18 mg/ml) in a liquid vehicle (typically propylene glycol and/or glycerin), along with sweeteners and flavorings (65).
To date, over 400 different brands of E-Cigs have been produced (164). Unlike disposable E-Cigs, second- and third-generation E-Cigs contain a refillable tank to which the e-liquid is added, a battery-powered atomizer that generates the aerosol from the e-liquid, and a mouthpiece that collects and delivers the aerosol. This function is usually controlled by a microchip, which may activate a light-emitting diode at the tip of the E-Cig during inhalation for aesthetics. The amount of aerosol that is generated is directly proportional to the power of the battery, which has led some users to modify their E-Cig to increase battery power to get a greater nicotine “hit.” This is not without risks, however, since there is a chance of battery explosion, which can lead to injury (23). Although the number of fires and explosions from E-Cig devices has increased since inception, interestingly, many of these instances occurred while the device was being charged and are still considered rare (https://www.usfa.fema.gov/downloads/pdf/publications/electronic_cigarettes.pdf). An additional, behavioral modification that has developed among E-Cig users is “dripping,” which entails dripping e-liquid directly on the atomizer (i.e., the heating element) and inhaling the resultant vapor, which is supposed to give the largest amount of nicotine delivery possible with current E-Cig devices (146). Parameters of E-Cig emission, such as aerosol size, mass output, and chemical composition, vary by device and e-liquid types and are predicted to impact the user's exposure to the E-Cig aerosol. For example, aerosol size strongly affects how much of an aerosol is delivered to different regions of the lung and how much is retained in the oral cavity (16, 82).
There are currently over 7,000 different e-liquids that are commercially available (164). Because these e-liquids are not FDA regulated, the vendors do not have to list their e-liquid ingredients, perform any safety testing before they reach the market, nor generate these products under Good Manufacturing Practice-type conditions. For example, the reported amount of nicotine has been found to vary by up to 20% from what is reported on the e-liquid label and has even been found in purportedly nicotine-free e-liquids (38, 39, 63). It is likely that many of the compounds used in e-liquids fall within the FDA's Generally Regarded as Safe (GRAS) list [http://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS]. The typical vehicle for e-liquids contains a mix of propylene glycol and glycerol, which are both GRAS products (23). To date, most GRAS testing/toxicology has been performed following oral ingestion rather than following aerosolization to the lung. For example, diacetyl is sometimes added to foods as a buttery flavor and is on the GRAS list, based on its oral toxicology (135). Due to the potential for adverse health effects from inhaling other chemicals, the Flavor and Extract Manufacturers' Association of the United States issued a statement warning that additives on the GRAS list apply to food only and should not be characterized as safe for use in E-Cig products without further testing (https://www.femaflavor.org/safety-assessment-and-regulatory-authority-use-flavors-focus-e-cigarettes). Propylene glycol is used as a common e-liquid vehicle component in part because of its perceived low toxicity. However, both ocular and upper respiratory irritation were reported in nonasthmatic adults following a short controlled occupational exposure (158). Furthermore, diacetyl inhalation is known to cause bronchiolitis obliterans or “popcorn workers lung” (83). Bronchiolitis obliterans caused by the inhalation of diacetyl can cause a range of symptoms from mild reversible respiratory impairment to a more severe nonreversible lung obstruction from extensive scarring in the small airways (11). With no current consensus on E-Cig user topography, it is possible that even nonasthmatic users who vape frequently could experience, at the very least, respiratory irritation although to date no long-term data are available regarding chronic propylene glycol or flavorant exposure in humans.
Do e-liquids undergo thermal decomposition (pyrolysis) when vaped?
E-Cig aerosols are typically generated at temperatures of 100–250°C, which is predicted to cause pyrolysis of the e-liquid vehicle (162) and may also induce breakdown of other e-liquid constituents. Recently, formaldehyde has been detected in E-Cig emissions (77). However, these data have been disputed (55). Part of the problem lies in deciding which temperature the e-liquid is heated to during the experiment vs. what occurs during actual vaping. For example, Jensen et al. found significant amounts of formaldehyde (∼380 μg/10 puffs) in the emission from a tank-style E-Cig device when the battery voltage was set at 5.0 V, with no formaldehyde being detected when a lower voltage (3.3 V) was used (77). Because the power consumption/electrical resistance of the coil was not quoted by Jensen et al., it will be hard to see how this observation transfers to other E-Cig devices. That is, the power generated by the heating coil cannot be determined purely by the quoted voltage since it also depends on the current, and the temperature reached by the e-liquid is dependent on the power output of the heating element. Thus, for reproducibility, it may be useful for researchers to quote the power output of their E-Cig device in addition to the puff profile used. Farsalinos et al. have reported that E-Cig users do not use this higher voltage setting, and they also proposed that E-Cigs only produce formaldehyde in “dry puff” conditions (55), where a dry puff refers to the scenario where there is little liquid on the atomizer coil and temperatures get higher than would be seen with sufficient liquid, leading to the potential for increased pyrolysis. However, acrolein and other carbonyls have also been found by other investigators both in neat e-liquids and in E-Cig aerosols that were generated by unmodified E-Cig devices (133), suggesting that the occurrence/production of these compounds may be more common than originally suspected. Interestingly, neat glycerin does not pyrolyze at 900°C. However, when diluted, significant amounts of acrolein were produced following pyrolysis of glycerol (28). Similarly, these aldehydes are known to be released from vegetable oil (of which glycerol is a major component) when it is heated during cooking, even to 180°C, which is close to temperatures reported for E-Cigs (130–350°C) (146). For example, the acrid smell that occurs when oil is burned on a stove is from acrolein (13, 29). Similarly, the chemical decomposition of sugars also causes the release of aldehydes, including acrolein (144).
It has been proposed that E-Cig users tend to avoid the bitter taste that is associated with release of aldehydes during overheating/dry puffing and that, in actual E-Cig users, aldehyde exposure never actually happens (55). However, during the aforementioned practice of dripping, where the e-liquids are placed directly on the coil, it is possible that significant pyrolysis occurs. Certainly, cigarettes can produce a harsh taste that is concomitant with the production of significant amounts of acrolein, formaldehyde, and other aldehydes, along with many other toxicants (144). However, this relatively unpleasant taste is soon overcome in new smokers due to the power of the nicotine drive (136) and due to cross-desensitization of transient receptor potential ankyrin subtype 1 (TRPA1) channels in sensory neurons (19). Therefore, it is also possible that E-Cig users will “learn” to overcome any unpleasant taste due to increased aldehyde production if the nicotine drive is great enough. It is also worth pointing out at this point that many flavors are themselves aldehydes, including anisaldehyde (sweet), cinnamaldehyde (cinnamon), and isovaleraldehyde (nutty). The effects of these flavors on pulmonary surfaces are not known. However, their potential inclusion in e-liquids may increase overall aldehyde exposure to the lung. Indeed, cinnamaldehyde is present in some e-liquids (14) and activates TRPA1 (108), suggesting that they may exert effects on the lung. Similarly, activation of this ion channel in sensory neurons in the airways of rodents by unsaturated aldehydes has previously been shown to trigger neurogenic inflammation (7) and to inhibit the CFTR ion channel (4), suggesting that a higher aldehyde burden may indeed be toxic to the lung. However, the degree of adverse effects will likely depend on dose ranging and whether aldehydes are actually generated in sufficient quantities during real vaping conditions to trigger these responses.
In addition to aldehydes, Lerner et al. also found that E-Cig aerosols generated from two separate devices produced oxidants and reactive oxygen species (OX/ROS) (94). Because the amount of OX/ROS changes with time as smoke matures, these data suggest that freshly produced E-Cig aerosols may be more potent than “aged” E-Cig aerosols, which has important implications for their study. Indeed, with regular cigarette smoke, different biological effects are seen with freshly produced vs. aged smoke, with aged smoke often being less biologically potent, which has previously been attributed to the decline in OX/ROS over time (74). Furthermore, because OX/ROS are highly reactive, they may also react with other components in the E-Cig aerosol, further changing its chemical composition. Indeed, Sussan et al. demonstrated that E-Cigs contain 1011 free radicals/puff, which is about 100 times less than is seen in regular cigarettes (142), but still likely to exert significant biological effects (45).
E-Cig topography.
When generating cigarette smoke through a smoke machine, there are several international standards. These standards are important since the rate and duration that air passes through a cigarette affects the burn temperature and the relative amount of chemicals that are subsequently produced (68, 99), and this is likely true for E-Cigs. Also, smoking tobacco in a reproducible fashion facilitates cross-laboratory data comparisons. Smoking profiles are designed to mimic the inhalation topography seen in actual smokers. For example, the Federal Trade Commission/International Standard Organization protocol calls for 2 s/35 ml puff every 60 s, and this is likely the most common puff profile used in the laboratory. However, it has been suggested that this profile underestimates how much people actually inhale, and a second profile, called “Canadian Intense,” which uses 2 s/55 ml puff every 30 s, has also been adopted, and it has recently been recommended that experiments be repeated with both profiles to study smoke generation over the range of exposures (68, 99). Similarly, for E-Cigs, knowing user's puff topography characteristics will be important for setting smoke machine parameters in the laboratory and for studying appropriate E-Cig emissions. To date, no consensus exists on how to set E-Cig parameters, and nothing comparable to the Canadian Intense profile has been developed. However, Farsalinos et al. found that E-Cig users took puffs of 4.2 s every 23 s, although they did not record the puff volume (53), whereas Lee et al. found the average puff duration to be 3.1 s (93). In contrast, Behar et al. found that the average puff duration was 2.75 s every 17 s, with an inhalation volume of 56 ml (15). These authors studied several different types of E-Cig and found that parameters varied only slightly with the type of E-Cig used. It may be that the users puff harder/more frequently on E-Cig devices that are less efficient at delivering nicotine to maintain sufficient plasma nicotine levels. Indeed, data suggested that users were able to maintain constant nicotine uptake, despite switching brands (15). Importantly, until a greater consensus is reached, these data suggest that a modified Canadian Intense profile may be a suitable parameter for studying E-Cig aerosol generation.
Will nicotine and chemical constituents in e-liquids/E-Cigs alter airway physiology?
Nicotine is a highly addictive substance that is a major component of both cigarette smoke and E-Cig aerosols that can cause physiological changes to users through nAChRs expressed throughout the body (36). Traditionally, nAChRs were primarily studied as part of the acetylcholine neurotransmitter signaling system in the central and peripheral nervous system. However, nAChR expression has been characterized in the airways as well (36, 96, 101, 165). These ligand-gated ion channels are permeable to both Na+ and divalent cations and are physiologically stimulated by acetylcholine. nAChRs contain five subunits of which different subtypes exist (e.g., α, β, γ, and δ) (3, 107). For example, the (α4)3, (β2)2 nAChR subunit configuration is the most common type in the brain while the (α7)5 or α3, α5, and β4 subunits are more common in the lung (147). Lee et al. found that inhaled nicotine from cigarette smoke caused airway irritation and a cough reflex via nAChRs expressed in pulmonary afferent neurons (89).
Interestingly, nAChRs regulate cell proliferation and inhibit apoptosis (48). For instance, Maouche et al. found that α7 nAChRs were enriched in basal lung epithelia and that, during development, α7 regulated basal cell proliferation (98), which is important for the maintenance of epithelial cell turnover and differentiation. It is well established that smoking is linked to lung cancer, and a hallmark of lung cancer is uncontrolled cell proliferation. West et al. reported that both nicotine and its metabolite (nicotine-derived nitrosamine ketone) stimulated Akt signal transduction downstream of nAChR activation, which altered cell proliferation and apoptosis in bronchial epithelia (156). Specifically, α3, α5, and β4 were identified as candidate genes for a potential role in lung cancer from genome-wide association studies (26, 75, 130, 139). Additionally, Lam et al. found different nAChR subunit gene expression profiles between nonsmokers and smokers with nonsmall cell lung cancer (86). In the same study, human bronchial epithelial cultures (HBEC) were exposed to nicotine, and expression was compared before and after removal of nicotine. Interestingly, exposing HBECs briefly upregulated nAChR α1, α5, and α7 expression at 72 h that returned to baseline levels after removal of nicotine. While all classes of nAChRs are capable of desensitization through chronic agonist exposure, there are definite immediate effects of nicotine on nAChRs in a subunit-dependent manner. Although it is currently unknown whether chronic exposure of nAChR to nicotine via E-Cigs can cause lung cancer, the role of nAChR α7 in contributing to nonsmall cell lung cancer by altering cell proliferation and apoptotic resistance has been reported (86, 113).
Many inflammatory cells contribute to COPD pathogenesis, including, but not limited to, dendritic cells, T and B lymphocytes, monocytes, macrophages, and neutrophils (12, 72). Of note, monocytes, macrophages, and neutrophils, which are impacted by inhaling cigarette smoke in the lungs, also express nAChRs. The effects of noncholinergic signaling in airway inflammatory cells have been described (64). Nicotine suppressed inflammation in human monocytes and in mouse macrophages (100, 160). Neutrophil influx occurs in COPD, and indeed neutrophils present in smokers have upregulated nAChR expression and display a reduced ability to undergo apoptosis (9, 37). Likely, these neutrophils are more sensitive to inhaled nicotine and have extended life spans, which may serve to prolong inflammation in the lungs. Taken together, these data indicate that nicotine has a proinflammatory effect on neutrophils. However, nicotine also has an anti-inflammatory effect on monocytes/macrophages, which may be negated in the case of cigarette smoke due to the inhalation of other proinflammatory products such as the tar phase. This dualism has curious implications for the chronic inhalation of nicotine from E-Cig aerosols, since many of the cigarette tobacco and tar byproducts that contribute to inflammation are not present in E-Cig aerosols. It is possible that the anti-inflammatory effects of nicotine, in the absence of proinflammatory constituents, could suppress the user's immune system. Certainly, it is reasonable to assume that high nicotine exposure from E-Cigs will be a major pharmacological player following E-Cig exposure in any organ where nAChR are expressed. Thus, E-Cig use may affect inflammation in the airways that could alter a user's susceptibility to infection and/or increase the risk of developing COPD or lung cancer.
Despite nicotine's known addictive and airway irritant properties, it is also known to be bitter tasting. Because of this, E-Cigs and their e-liquids present a novel mix of chemical constituents that not only contain nicotine but also flavors, sweeteners, and other chemicals, many of which have not been studied in the lung. Many of these chemicals are present to mask the bitter nicotine taste. Thus, while nicotine has been shown to alter many aspects of airway physiology, the potential exists for sweet- and bitter-flavored constituents from e-liquids to stimulate taste receptor signaling pathways that could alter airway physiology with chronic use. To date, however, there is no current literature on the effects of E-Cigs and chronic vaping in pulmonary physiology of nAChRs or taste receptors. Moreover, the ability to taste bitter substances may contribute to smoking behavior and nicotine addiction (24, 51, 97). Bitter taste receptors (T2Rs) are ligand-activated G protein-coupled receptors (GPCRs) that use intracellular Ca2+ as a downstream signaling molecule. There are ∼30 T2Rs expressed in humans. T2Rs are most abundant in the tongue, and T2R polymorphisms (e.g., T2R38) that impair the ability to taste bitter compounds have been correlated with populations that are more nicotine dependent and/or heavy smokers (80, 97). Furthermore, when tongue tissue was compared for T2R mRNA expression in smokers vs. nonsmokers, overall T2R gene expression was reduced in the smoking compared with the nonsmoking group (8). It is unknown whether the reduction of T2R gene expression was genetic or was suppressed by a component of cigarette smoke. Yet, a correlation between T2R expression and age was present in the nonsmoker group and absent in the smoker group, suggesting that starting smoking earlier in life could suppress T2R gene expression and contribute to nicotine addiction.
Many T2Rs have been identified in the upper and lower airway epithelia as well as airway smooth muscle cells (34, 44, 134, 150). Interestingly, T2R38 polymorphisms have also been linked to increased susceptibility of upper respiratory infections (92). Although an endogenous ligand is still unknown, known bitter agonists activate these T2Rs and increase intracellular Ca2+, stimulating ciliary beat frequency. Thus, they play a role in detecting noxious inhalants and expelling them from the airways due to increased rates of mucociliary clearance. Nasal mucosa have been reported to express both sweet receptors (T1Rs) as well as T2Rs in special nonciliated epithelial cells called solitary chemosensory cells (SCCs) (90). SCCs in the nasal epithelium harbor these receptors along with known components of the taste receptor signaling pathway and trigeminal nerve innervation. Tizzano et al. characterized the presence of SCCs with T2Rs and the T2Rs' ability to detect known bitter agonists and acyl-homoserine lactones (150), which are intercellular chemical signaling compounds secreted by Gram-negative bacteria, providing more evidence for T2R roles in innate immunity. Furthermore, Lee et al. found that T1Rs and T2Rs in nasal epithelium converge to arbitrate innate immunity (91), that is, when T1Rs are activated (e.g., hyperglycemia, chronic rhinosinusitis), they can block the antimicrobial effects of T2Rs, causing persistent airway infections. Together, these data suggest that taste reception in the airways is important to innate immunity.
Nonciliated SCCs are found throughout the lower airways, although T1Rs are not detected there (104, 105). Interestingly, Dehkordi et al. reported that intrapulmonary epithelial SCCs coexpress T2R38, its T2R signaling components, and many nAChR subunits in the same cells (42). Although it is unknown whether these two signaling pathways directly interact, it is possible that the coexpression of multiple chemosensation receptor types may increase the repertoire and sensitivity of airway cells to inhaled irritants, specifically nicotine. In this case, nicotine might be sensed by either receptor type, and an interaction might exist between downstream components of the T2R and nAChR signal transduction pathways (e.g., Ca2+ as a common second messenger) that regulate cellular responses to nicotine. For example, triggering Ca2+ influx from the activation of one nAChR subunit can attenuate the response of a second subunit through desensitization of the stimuli or prolong increases in intracellular Ca2+ (59).
Effects of E-Cig aerosols and e-liquids on cultured cells from the lung.
Tobacco smoke is highly proinflammatory and has been shown to trigger the release of inflammatory cytokines from endothelia, epithelia, and leukocytes (20, 66, 88). These cytokines can then trigger additional changes, including goblet cell metaplasia and neutrophil influx (85). Inflammation may be beneficial in the short term, especially when resolving infection. However, chronic inflammation can act as a precursor to cancer, and continued influx of neutrophils, with the subsequent increase in free elastase levels, can lead to cell damage and denudation of the epithelia (22, 143). Tobacco exposure is also associated with cellular cytotoxicity, including increased apoptosis, autophagosome formation, membrane permeability, and mitochondrial damage (46, 78, 87, 129). Furthermore, micronuclei form when chromosomes or parts of chromosomes are excluded from daughter nuclei following cell division (56). As such, micronuclei formation is associated with a high risk of cancer and is a common assay that is used to screen for genotoxic substances, and increased micronuclei formation has been observed in cigarette smokers (43, 149). Tobacco smoke has also been shown to alter gene expression and DNA methylation in both the whole lung and in airway epithelia (69, 115, 153), macrophages (47), and endothelia (161). Many of these assays have been established as outcome measures for tobacco smoke exposure, and they should be useful for probing the effects of E-Cig exposure.
To date, many cell types have been exposed to e-liquids and/or E-Cig aerosols. These cell types include lung epithelial cell lines (H292, A549), lung fibroblasts, human primary trachea-bronchial cells, and HaCaT keratinocytes (31, 94, 159). Whereas e-liquids are aerosolized, a common early approach has been to add e-liquids directly to cells at various dilutions. Although this protocol would not pick up any additional effects of pyrolysis due to heating the e-liquid, it is a useful first step to determine whether e-liquids themselves have inherent toxicity. Wu et al. exposed nondifferentiated tracheobronchial cultures to a tobacco-flavored e-liquid that contained either 18 mg/ml of nicotine (which equates to 111 mM) or was nicotine free for 24–48 h over the range (vol/vol) 0.01–0.3% (InnoVapor, Boise, ID) and found that exposures in this range did not increase lactate dehydrogenase (LDH) levels, suggesting that they were not cytotoxic (159). However, the upper levels of dosing caused significant increases in IL-6 and IL-8 levels, also increasing rhinovirus infection and rhinovirus-induced IL-6 secretion and decreasing mRNA levels of SPLUNC1, an innate defense molecule (148, 159). Whereas increases in IL-6 secretions have been detected after rhinovirus infections (122), the implication of this observation in the context of E-Cig and/or tobacco exposure is not fully understood and needs additional testing. However, increased IL-6 responses to viral infection have been detected in COPD patients, suggesting that this may be a relevant assay for E-Cig exposure (131). Lerner et al. found that e-liquids altered HFL-1 cell morphology (94). Bahl et al. tested the effects of e-liquids, directly added to murine pulmonary fibroblasts, human embryonic stem cells, and murine neural stem cells (10). Although effects of e-liquids were typically seen with ≥0.1% (vol/vol) addition, in general, the stem cells were more sensitive than the fibroblasts, suggesting that some cell types in the lung may be more vulnerable to E-Cigs than others. Furthermore, of the 36 e-liquids tested, ∼15 showed cytotoxicity, with cinnamon flavors being especially toxic. Of interest, the authors found significant variability in cytotoxicity from batch to batch, even for one flavor from one vendor, which suggests that poor quality control may exist in some cases.
In addition to directly studying the effects of e-liquids, they can be heated/aerosolized and then studied. Cervellati et al. exposed A549 (lung epithelial) and HaCaT (keratinocytes) cells to whole cigarette smoke or E-Cig vapor from three combinations of E-Cigs (nicotine; nicotine + flavor; no flavor, no nicotine) (31). After 50 min of smoke or aerosol exposure, cultures were then left for 24 h, and LDH release and cell viability were studied. No information was given regarding whether these cells were polarized or not. However, they found that, under these conditions, E-Cigs with nicotine and/or flavor induced similar cytotoxicity (increased LDH release and decreased cell viability) as standard cigarettes while nicotine and flavor-free E-Cigs did not have any effect. E-Cig aerosols (generated using a 4 s/35 ml pulse) also caused an increase in IL-6 and IL-8 secretion, which in the case of one flavor (cinnamon roll) was greater than the IL-8 secretion seen with cigarette smoke extract addition (94).
The effects of E-Cig exposure have also been studied on the lung's microvasculature. For example, Schweitzer et al. found that E-Cigs decreased the electrical resistance of endothelial cells derived from mice, rats, and humans, and exerted significant effects on cell viability and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide production that were associated with changes in cell signaling (activation of p38 mitogen-activated protein kinase). Interestingly, these changes were similar to those observed after exposure to cigarette smoke extract (133). They also detected increased phosphorylation of myosin light chain and Rho kinase following E-Cig exposure, which may have been due to activation of sphingolipids (133). Changes in the permeability in the lung's microvasculature may induce edema and/or increase the number of leukocytes that can enter the lung, thus increasing inflammation, as described elsewhere (84).
Effects of E-Cigs on the murine lung.
Although little is known about the effects of E-Cigs on humans, some studies have been performed in mice. E-Cig exposure has been shown to elicit neuropharmacological effects, including upregulation of nAChR, in different areas of the brain and also caused signs of addiction and increased serum and cotinine levels, suggesting that comparable systemic nicotine levels can be obtained with E-Cig exposure as are seen with tobacco exposure (2, 118). Lerner et al. exposed mice to E-Cig aerosols for 5 h/day for 3 days and examined the mice 1 day later. They found that several cytokines were increased in the bronchoalveolar lavage of these mice, including IL-1α, IL-6, IL-13, and monocyte chemoattractant protein-1 (MCP-1) (94). Of note, MCP-1 recruits macrophages to the lung, IL-6 is a proinflammatory cytokine, and IL-13 induces cellular remodeling and goblet cell hyperplasia. Schweitzer et al. found that an E-Cig exposure regimen, which was equivalent in dose to exposure to smoke from two cigarettes, caused a significant increase in 8-oxo-2′-deoxyguanosine (8-oxo-dG) in both plasma and bronchoalveolar lavage (133). 8-oxo-dG is a marker of systemic oxidative stress and is indicative of DNA damage (109). They also detected increased nitrotyrosine levels in plasma. Nitrotyrosine can be formed following exposure to reactive nitrogen species such as peroxynitrite anion and nitrogen dioxide and is also a marker of cell stress/damage (124). A 2-wk exposure to E-Cigs smoked under relatively standard conditions (2-s 35-ml puff) caused a significant increase in the number of macrophages in murine lungs and actually decreased IL-6 (133). The differences in IL-6 levels observed between the two experiments are most likely due to differences in smoking (vaping) regimens since one was acute and the other was chronic (94, 133). Mouse strain differences and/or differences in E-Cig device/e-liquid may also have been factors.
After infection with Streptococcus pneumonia, E-Cig-exposed mice were less able to clear this infection, suggesting that innate defense was impaired (142). They also found that H1N1 influenza virus infection also was poorly cleared. Although these data will need to be repeated by other groups, it is the first report that E-Cig exposure leads to increased susceptibility to infection, which has important implications for the safety of E-Cig users. Interestingly, increased susceptibility to pathogens is a hallmark of tobacco exposure and is seen following both viral and bacterial infections (58, 110, 123). In addition to these aerosol exposures, a 50-fold-diluted E-Cig liquid has been shown to increase IL-4, IL-5, and IL-13 in allergen-sensitized mice when tracheally instilled (95), again suggesting that e-liquids can still have adverse effects even before they are vaporized.
The adverse effects of tobacco on both prenatal and postnatal development have been well described and include low birth weight, increased incidence of sudden infant death syndrome, and development of lung disease later in life (e.g., asthma) (1, 62). Whereas most published studies to date have focused on adult mice, it has been demonstrated that E-Cig exposure also adversely effects neonatal mice and leads to impaired development, including decreased weight gain and reduced cell proliferation in the lungs, suggesting that secondhand vaping may also potentially be a cause for concern and could adversely affect lung development (103).
What is known about the effects of E-Cig exposure in humans?
Currently, many adult E-Cig users are former smokers and have a significant history of tobacco usage before using E-Cigs and/or continue to be mixed tobacco/E-Cig users (61, 120). This will make studying the chronic effects of E-Cigs difficult since the airways/lung retain a significant memory of smoking history/exposure even after smoking cessation. For example, Rager et al. found significant evidence of DNA methylation in the nasal epithelia of ex-smokers (121). Thus, for any observed effects on E-Cig smokers, the previous and/or current tobacco smoking history must be taken into account. That said, the largest and fastest-growing population of E-Cig users who have never smoked tobacco is adolescents. For example, in North Carolina, 15% of high school students have vaped E-Cigs, and 60% thought that E-Cigs were safe. In contrast, among the same group, 24% had smoked cigarettes (5). This trend is reflected nationally (102).
It has been shown that short-term (e.g., 5 min) E-Cig inhalation leads to comparable plasma cotinine levels as regular tobacco smoking and exerts rapid physiological effects on the cardiovascular system, including elevated heart rate (151). These data indicate that modern E-Cigs are delivering significant amounts of nicotine to the bloodstream. Although to date no studies have been performed to look at the adverse effects of E-Cigs on pulmonary health (e.g., inflammation, etc.), Vardavas et al. looked at the effects of 5 min E-Cig exposure on pulmonary function using standard spirometry (152). Interestingly, they found that a 5-min E-Cig exposure caused a significant increase in peripheral airway resistance, which is indicative of changes to the small airways. The authors noted that these changes were relatively small and likely not great enough to be of immediate clinical significance. However, the changes were observed after only 5 min of exposure, and they speculated that chronic exposure may lead to greater changes in resistance. They also found that this 5-min exposure caused a significant decrease in exhaled nitric oxide (NO) levels. NO has a number of functions in the lung, and changes in NO levels can affect ciliary beating, transcription, inflammation, ion transport, and airway smooth muscle tone (21). NO is altered in many diseases, including asthma (increased), cystic fibrosis (decreased), primary ciliary dyskinesia (decreased), and COPD (may be suppressed or mildly increased) (163). Thus, it is possible that E-cig exposure could cause different lung disease to COPD.
Conclusions and future directions.
There is a long history of deceptive marketing tactics used by the tobacco industry regarding the ‘safety’ of cigarettes (52). Thus, it is interesting to speculate whether the same will hold true for the nascent E-Cig industry. Certainly, users want to believe that E-Cig products are safe, but, unfortunately, no definitive data currently exist to prove or disprove this hypothesis. Studying E-Cig exposure is very much like trying to hit a moving target, but one where researchers are not completely sure what the target looks like, since E-Cig devices, the way that they are used, and the types/flavors of e-liquids available are constantly changing. However, some facts have been established: 1) current E-Cig devices deliver nicotine at comparable levels to cigarettes, and certainly at levels high enough to evoke physiological responses in humans and rodents (54, 103, 138), 2) nicotine is highly addictive and, along with its metabolites, can cause cancer and affect neuronal development in adolescents irrespective of its source (50, 155), 3) e-liquids have been shown to contain potentially toxic aldehydes and ROS (146), and 4) some type of a biological response (e.g., change in cytokine levels) has been observed in the vast majority of murine in vivo and in vitro studies following E-Cig vapor/e-liquid exposure (94, 133, 152). Although it seems certain that E-Cig aerosols contain toxicants, it is fair to say that they likely contain less types of toxicants than cigarette smoke (i.e., E-Cig aerosols likely have hundreds of chemicals in them while tobacco smoke has thousands of chemicals). The remaining question is then one of dose ranging, that is, are the toxicants in E-Cigs present in sufficiently high concentrations to elicit lung disease over a similar time frame as tobacco smoking?
Given the paucity of information that is available regarding the effects, not only of E-Cigs, but also of many of the chemical constituents of e-liquids on the lungs, we propose that all commercially available E-Cig products be regulated in a similar fashion as any inhaled therapeutic agent, that is, thorough inhalation toxicology and safety-based clinical trials. Although this would be an undeniably expensive undertaking, the estimated value of the E-Cig market is in the billion dollar range, indicating that tobacco and E-Cig companies could likely foot this bill.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant P50-HL-120100. Research reported in this publication was in part supported by the National Institutes of Health and the Food and Drug Administration Center for Tobacco Products.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.R.R. prepared figures; T.R.R. and R.T. drafted manuscript; T.R.R. and R.T. edited and revised manuscript; T.R.R. and R.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our colleagues at the University of North Carolina at Chapel Hill and elsewhere for stimulating discussion regarding studying the effects of E-Cig exposure.
REFERENCES
- 1.Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol 42: 279–303, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology 27: 212–224, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander NS, Blount A, Zhang S, Skinner D, Hicks SB, Chestnut M, Kebbel FA, Sorscher EJ, Woodworth BA. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope 122: 1193–1197, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand V, McGinty KL, O'Brien K, Guenthner G, Hahn E, Martin CA. E-cigarette use and beliefs among urban public high school students in north carolina. J Adolesc Health 57: 46–51, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SJ, Ling PM. “And they told two friends ․ ․ ․ and so on”: RJ Reynolds' viral marketing of Eclipse and its potential to mislead the public. Tob Control 17: 222–229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, Baraldi PG, Poole DP, Bunnett NW, Geppetti P, Patacchini R. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 118: 2574–2582, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki M, Takao T, Takao K, Koike F, Suganuma N. Lower expressions of the human bitter taste receptor TAS2R in smokers: reverse transcriptase-polymerase chain reaction analysis. Tob Induc Dis 12: 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoshiba K, Nagai A, Yasui S, Konno K. Nicotine prolongs neutrophil survival by suppressing apoptosis. J Lab Clin Med 127: 186–194, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Bahl V, Lin S, Xu N, Davis B, Wang YH, Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol 34: 529–537, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med 370: 1820–1828, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med 35: 71–86, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Bastos LC, Pereira PA. Influence of heating time and metal ions on the amount of free fatty acids and formation rates of selected carbonyl compounds during the thermal oxidation of canola oil. J Agric Food Chem 58: 12777–12783, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Behar RZ, Davis B, Wang Y, Bahl V, Lin S, Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol In Vitro 28: 198–208, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One 10: e0117222, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett WD, Brown JS, Zeman KL, Hu SC, Scheuch G, Sommerer K. Targeting delivery of aerosols to different lung regions. J Aerosol Med 15: 179–188, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL. Nicotine addiction. N Engl J Med 362: 2295–2303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 29–60, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360–370, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: a mechanistic perspective. J Toxicol Environ Health B Crit Rev 12: 45–64, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic Biol Med 41: 515–527, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Bozinovski S, Vlahos R, Anthony D, McQualter J, Anderson G, Irving L, Steinfort D. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br J Pharmacol In press. [DOI] [PMC free article] [PubMed]
- 23.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control 23, Suppl 2: ii4–ii10, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon DS, Baker TB, Piper ME, Scholand MB, Lawrence DL, Drayna DT, McMahon WM, Villegas GM, Caton TC, Coon H, Leppert MF. Associations between phenylthiocarbamide gene polymorphisms and cigarette smoking. Nicotine Tob Res 7: 853–858, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 173: 1139–1144, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, Ziegler RG, Hunter DJ, Chanock S, Hankinson S, Kraft P, Bergen AW. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One 4: e4653, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramori G, Casolari P, Cavallesco GN, Giuffre S, Adcock I, Papi A. Mechanisms involved in lung cancer development in COPD. Int J Biochem Cell Biol 43: 1030–1044, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Carmines EL, Gaworski CL. Toxicological evaluation of glycerin as a cigarette ingredient. Food Chem Toxicol 43: 1521–1539, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Casella IG, Contursi M. Quantitative analysis of acrolein in heated vegetable oils by liquid chromatography with pulsed electrochemical detection. J Agric Food Chem 52: 5816–5821, 2004. [DOI] [PubMed] [Google Scholar]
- 30.CDC. Tobacco use among U.S. racial/ethnic minority groups–African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: report of the Surgeon General. Atlanta, GA: CDC, US Dept. of Health and Human Services, CDC, 1998. [PubMed] [Google Scholar]
- 31.Cervellati F, Muresan XM, Sticozzi C, Gambari R, Montagner G, Forman HJ, Torricelli C, Maioli E, Valacchi G. Comparative effects between electronic and cigarette smoke in human keratinocytes and epithelial lung cells. Toxicol In Vitro 28: 999–1005, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KM, Sacks PG, Spratt TE, Lin JM, Boyiri T, Schwartz J, Richie JP, Calcagnotto A, Das A, Bortner J, Zhao Z, Amin S, Guttenplan J, El-Bayoumy K. Modulations of benzo[a]pyrene-induced DNA adduct, cyclin D1 and PCNA in oral tissue by 1,4-phenylenebis(methylene) selenocyanate. Biochem Biophys Res Commun 383: 151–155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, Bennett WD, Riordan JR, Boucher RC, Tarran R. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 26: 533–545, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, Tarran R, Siderovski DP, Kimple AJ. Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem 287: 41706–41719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collawn JF, Matalon S. CFTR and lung homeostasis. Am J Physiol Lung Cell Mol Physiol 307: L917–L923, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol 393: 279–294, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Cormier A, Paas Y, Zini R, Tillement JP, Lagrue G, Changeux JP, Grailhe R. Long-term exposure to nicotine modulates the level and activity of acetylcholine receptors in white blood cells of smokers and model mice. Mol Pharmacol 66: 1712–1718, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res 17: 134–141, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis B, Razo A, Nothnagel E, Chen M, Talbot P. Unexpected nicotine in Do-it-Yourself electronic cigarette flavourings. Tob Control In press. [DOI] [PMC free article] [PubMed]
- 40.Dawkins L, Turner J, Roberts A, Soar K. “Vaping” profiles and preferences: an online survey of electronic cigarette users. Addiction 108: 1115–1125, 2013. [DOI] [PubMed] [Google Scholar]
- 41.de la Pena JB, Ahsan HM, Tampus R, Botanas CJ, Dela Pena IJ, Kim HJ, Sohn A, Dela Pena I, Shin CY, Ryu JH, Cheong JH. Cigarette smoke exposure during adolescence enhances sensitivity to the rewarding effects of nicotine in adulthood, even after a long period of abstinence. Neuropharmacology 99: 9–14, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Dehkordi O, Millis RM, Dennis GC, Jazini E, Williams C, Hussain D, Jayam-Trouth A. Expression of alpha-7 and alpha-4 nicotinic acetylcholine receptors by GABAergic neurons of rostral ventral medulla and caudal pons. Brain Res 1185: 95–102, 2007. [DOI] [PubMed] [Google Scholar]
- 43.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res 567: 447–474, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis 9: 1207–1224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doolittle DJ, Lee CK, Ivett JL, Mirsalis JC, Riccio E, Rudd CJ, Burger GT, Hayes AW. Comparative studies on the genotoxic activity of mainstream smoke condensate from cigarettes which burn or only heat tobacco. Environ Mol Mutagen 15: 93–105, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Doyle I, Ratcliffe M, Walding A, Vanden Bon E, Dymond M, Tomlinson W, Tilley D, Shelton P, Dougall I. Differential gene expression analysis in human monocyte-derived macrophages: impact of cigarette smoke on host defence. Mol Immunol 47: 1058–1065, 2010. [DOI] [PubMed] [Google Scholar]
- 48.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci 29: 151–158, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR, Committee on Nonsmoking Copd, and Occupational Health. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 693–718, 2010. [DOI] [PubMed] [Google Scholar]
- 50.England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 49: 286–293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enoch MA, Harris CR, Goldman D. Does a reduced sensitivity to bitter taste increase the risk of becoming nicotine addicted? Addict Behav 26: 399–404, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Fallin A, Glantz SA. Tobacco-control policies in tobacco-growing States: where tobacco was king. Milbank Q 93: 319–358, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Public Health 10: 2500–2514, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers). Sci Rep 5: 11269, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in “dry puff” conditions. Addiction In press. [DOI] [PubMed]
- 56.Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmond DA, Tucker JD, Thomas P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26: 125–132, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Fowles J, Dybing E. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control 12: 424–430, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garmendia J, Morey P, Bengoechea JA. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J 39: 467–477, 2012. [DOI] [PubMed] [Google Scholar]
- 59.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. alpha 5 Subunit alters desensitization, pharmacology, Ca++ permeability and Ca++ modulation of human neuronal alpha 3 nicotinic receptors. J Pharmacol Exp Ther 286: 311–320, 1998. [PubMed] [Google Scholar]
- 60.Ghosh A, Boucher RC, Tarran R. Airway hydration and COPD. Cell Mol Life Sci In press. [DOI] [PMC free article] [PubMed]
- 61.Giovenco DP, Lewis MJ, Delnevo CD. Factors associated with e-cigarette use: a national population survey of current and former smokers. Am J Prev Med 47: 476–480, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goksor E, Amark M, Alm B, Gustafsson PM, Wennergren G. The impact of pre- and post-natal smoke exposure on future asthma and bronchial hyper-responsiveness. Acta Paediatr 96: 1030–1035, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, Kosmider L, Sobczak A. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the US, Korea, and Poland. Int J Drug Policy 26: 583–588, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther 115: 208–222, 2007. [DOI] [PubMed] [Google Scholar]
- 65.Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, Kuballa T, Lachenmeier DW. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis 12: 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol 151: 1–15, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Hammond D, Collishaw NE, Callard C. Secret science: tobacco industry research on smoking behaviour and cigarette toxicity. Lancet 367: 781–787, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Hammond D, Wiebel F, Kozlowski LT, Borland R, Cummings KM, O'Connor RJ, McNeill A, Connolly GN, Arnott D, Fong GT. Revising the machine smoking regime for cigarette emissions: implications for tobacco control policy. Tob Control 16: 8–14, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harvey BG, Strulovici-Barel Y, Vincent TL, Mezey JG, Raviram R, Gordon C, Salit J, Tilley AE, Chung A, Sanders A, Crystal RG. High correlation of the response of upper and lower lobe small airway epithelium to smoking. PLoS One 8: e72669, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hassan F, Xu X, Nuovo G, Killilea DW, Tyrrell J, Da Tan C, Tarran R, Diaz P, Jee J, Knoell D, Boyaka PN, Cormet-Boyaka E. Accumulation of metals in GOLD4 COPD lungs is associated with decreased CFTR levels. Respir Res 15: 69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. Am J Prev Med 33: S368–S378, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 4: 435–459, 2009. [DOI] [PubMed] [Google Scholar]
- 73.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 13: 233–245, 2013. [DOI] [PubMed] [Google Scholar]
- 74.Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air 15: 135–140, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Chen C, Goodman G, Field JK, Liloglou T, Xinarianos G, Cassidy A, McLaughlin J, Liu G, Narod S, Krokan HE, Skorpen F, Elvestad MB, Hveem K, Vatten L, Linseisen J, Clavel-Chapelon F, Vineis P, Bueno-de-Mesquita HB, Lund E, Martinez C, Bingham S, Rasmuson T, Hainaut P, Riboli E, Ahrens W, Benhamou S, Lagiou P, Trichopoulos D, Holcatova I, Merletti F, Kjaerheim K, Agudo A, Macfarlane G, Talamini R, Simonato L, Lowry R, Conway DI, Znaor A, Healy C, Zelenika D, Boland A, Delepine M, Foglio M, Lechner D, Matsuda F, Blanche H, Gut I, Heath S, Lathrop M, Brennan P. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 452: 633–637, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Hurt RD, Murphy JG, Dunn WF. Did we finally slay the evil dragon of cigarette smoking in the late 20th century?: unfortunately, the answer is no: the dragon is still alive and well in the 21st century and living in the third world. Shame on us! Chest 146: 1438–1443, 2014. [DOI] [PubMed] [Google Scholar]
- 77.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med 372: 392–394, 2015. [DOI] [PubMed] [Google Scholar]
- 78.Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG. Evaluation of in vitro assays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomarkers Prev 18: 3263–3304, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones WJ, Silvestri GA. The Master Settlement Agreement and its impact on tobacco use 10 years later: lessons for physicians about health policy making. Chest 137: 692–700, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keller M, Liu X, Wohland T, Rohde K, Gast MT, Stumvoll M, Kovacs P, Tonjes A, Bottcher Y. TAS2R38 and its influence on smoking behavior and glucose homeostasis in the German Sorbs. PLoS One 8: e80512, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kesarwala A, Krishna M, Mitchell J. Oxidative stress in oral diseases. Oral Dis In press. [DOI] [PMC free article] [PubMed]
- 82.Kleinstreuer C, Feng Y. Lung deposition analyses of inhaled toxic aerosols in conventional and less harmful cigarette smoke: a review. Int J Environ Res Public Health 10: 4454–4485, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med 347: 330–338, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Kuebler WM. Inflammatory pathways and microvascular responses in the lung. Pharmacol Rep Suppl 57: 196–205, 2005. [PubMed] [Google Scholar]
- 85.Kuschner WG, D'Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. Eur Respir J 9: 1989–1994, 1996. [DOI] [PubMed] [Google Scholar]
- 86.Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW, Gazdar AF, Lam WK, Minna JD. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res 67: 4638–4647, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Lee CK, Doolittle DJ, Burger GT, Hayes AW. Comparative genotoxicity testing of mainstream whole smoke from cigarettes which burn or heat tobacco. Mutat Res 242: 37–45, 1990. [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Taneja V, Vassallo R. Cigarette smoking and inflammation: cellular and molecular mechanisms. J Dent Res 91: 142–149, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther 20: 355–364, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med (Berl) 92: 1235–1244, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124: 1393–1405, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias PT, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122: 4145–4159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee YH, Gawron M, Goniewicz ML. Changes in puffing behavior among smokers who switched from tobacco to electronic cigarettes. Addict Behav 48: 1–4, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 10: e0116732, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lim HB, Kim SH. Inhallation of e-cigarette cartridge solution aggravates allergen-induced airway inflammation and hyper-responsiveness in mice. Toxicol Res 30: 13–18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Macklin KD, Maus AD, Pereira EF, Albuquerque EX, Conti-Fine BM. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J Pharmacol Exp Ther 287: 435–439, 1998. [PubMed] [Google Scholar]
- 97.Mangold JE, Payne TJ, Ma JZ, Chen G, Li MD. Bitter taste receptor gene polymorphisms are an important factor in the development of nicotine dependence in African Americans. J Med Genet 45: 578–582, 2008. [DOI] [PubMed] [Google Scholar]
- 98.Maouche K, Polette M, Jolly T, Medjber K, Cloez-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, Birembaut P, Tournier JM. α7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am J Pathol 175: 1868–1882, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marian C, O'Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev 18: 3305–3320, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 167: 6518–6524, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Maus AD, Pereira EF, Karachunski PI, Horton RM, Navaneetham D, Macklin K, Cortes WS, Albuquerque EX, Conti-Fine BM. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol Pharmacol 54: 779–788, 1998. [DOI] [PubMed] [Google Scholar]
- 102.McCarthy M. “Alarming” rise in popularity of e-cigarettes is seen among US teenagers as use triples in a year. Br Med J 350: h2083, 2015. [DOI] [PubMed] [Google Scholar]
- 103.McGrath-Morrow SA, Hayashi M, Aherrera A, Lopez A, Malinina A, Collaco JM, Neptune E, Klein JD, Winickoff JP, Breysse P, Lazarus P, Chen G. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS One 10: e0118344, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merigo F, Benati D, Di Chio M, Osculati F, Sbarbati A. Secretory cells of the airway express molecules of the chemoreceptive cascade. Cell Tissue Res 327: 231–247, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. alpha-Gustducin immunoreactivity in the airways. Cell Tissue Res 319: 211–219, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Milara J, Cortijo J. Tobacco, inflammation, and respiratory tract cancer. Curr Pharm Des 18: 3901–3938, 2012. [DOI] [PubMed] [Google Scholar]
- 107.Millar NS. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans 31: 869–874, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Moon H, Kim MJ, Son HJ, Kweon HJ, Kim JT, Kim Y, Shim J, Suh BC, Rhyu MR. Five hTRPA1 Agonists Found in Indigenous Korean Mint, Agastache rugosa. PLoS One 10: e0127060, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem 387: 373–379, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Noah TL, Zhou H, Jaspers I. Alteration of the nasal responses to influenza virus by tobacco smoke. Curr Opin Allergy Clin Immunol 12: 24–31, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Noya Y, Seki K, Asano H, Mai Y, Horinouchi T, Higashi T, Terada K, Hatate C, Hoshi A, Nepal P, Horiguchi M, Kuge Y, Miwa S. Identification of stable cytotoxic factors in the gas phase extract of cigarette smoke and pharmacological characterization of their cytotoxicity. Toxicology 314: 1–10, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic cigarettes-a narrative review for clinicians. Am J Med 128: 674–681, 2015. [DOI] [PubMed] [Google Scholar]
- 113.Paleari L, Catassi A, Ciarlo M, Cavalieri Z, Bruzzo C, Servent D, Cesario A, Chessa L, Cilli M, Piccardi F, Granone P, Russo P. Role of alpha7-nicotinic acetylcholine receptor in human non-small cell lung cancer proliferation. Cell Prolif 41: 936–959, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Phillips DH, Venitt S. DNA and protein adducts in human tissues resulting from exposure to tobacco smoke. Int J Cancer 131: 2733–2753, 2012. [DOI] [PubMed] [Google Scholar]
- 115.Pickett G, Seagrave J, Boggs S, Polzin G, Richter P, Tesfaigzi Y. Effects of 10 cigarette smoke condensates on primary human airway epithelial cells by comparative gene and cytokine expression studies. Toxicol Sci 114: 79–89, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Prev Med 69: 248–260, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Pollay RW, Dewhirst T. The dark side of marketing seemingly “Light” cigarettes: successful images and failed fact. Tob Control 11, Suppl 1: I18–I31, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol In press. [DOI] [PubMed]
- 119.Proctor RN. The history of the discovery of the cigarette-lung cancer link: evidentiary traditions, corporate denial, global toll. Tob Control 21: 87–91, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Pulvers K, Hayes RB, Scheuermann TS, Romero DR, Emami AS, Resnicow K, Olendzki E, Person SD, Ahluwalia JS. Tobacco use, quitting behavior, and health characteristics among current electronic cigarette users in a national tri-ethnic adult stable smoker sample. Nicotine Tob Res 17: 1085–1095, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rager JE, Bauer RN, Muller LL, Smeester L, Carson JL, Brighton LE, Fry RC, Jaspers I. DNA methylation in nasal epithelial cells from smokers: identification of ULBP3-related effects. Am J Physiol Lung Cell Mol Physiol 305: L432–L438, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajan D, Gaston KA, McCracken CE, Erdman DD, Anderson LJ. Response to rhinovirus infection by human airway epithelial cells and peripheral blood mononuclear cells in an in vitro two-chamber tissue culture system. PLoS One 8: e66600, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rangelov K, Sethi S. Role of infections. Clin Chest Med 35: 87–100, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol 533: 240–252, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Rickert WS, Robinson JC, Young JC. Estimating the hazards of “less hazardous” cigarettes. I. Tar, nicotine, carbon monoxide, acrolein, hydrogen cyanide, and total aldehyde deliveries of Canadian cigarettes. J Toxicol Environ Health 6: 351–365, 1980. [DOI] [PubMed] [Google Scholar]
- 126.Riker CA, Lee K, Darville A, Hahn EJ. E-cigarettes: promise or peril? Nurs Clin North Am 47: 159–171, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 1340: 65–74, 2015. [DOI] [PubMed] [Google Scholar]
- 128.Rose JE. Multiple brain pathways and receptors underlying tobacco addiction. Biochem Pharmacol 74: 1263–1270, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Ryter SW, Choi AM. Autophagy in lung disease pathogenesis and therapeutics. Redox Biol 4: 215–225, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM, Bierut LJ. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res 69: 6848–6856, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL, Martinez FJ, Hershenson MB, Sajjan U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 332–340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwartz AG, Prysak GM, Bock CH, Cote ML. The molecular epidemiology of lung cancer. Carcinogenesis 28: 507–518, 2007. [DOI] [PubMed] [Google Scholar]
- 133.Schweitzer KS, Chen SX, Law S, Van Demark M, Poirier C, Justice MJ, Hubbard WC, Kim ES, Lai X, Wang M, Kranz WD, Carroll CJ, Ray BD, Bittman R, Goodpaster J, Petrache I. Endothelial disruptive proinflammatory effects of nicotine and e-cigarette vapor exposures. Am J Physiol Lung Cell Mol Physiol 309: L175–L187, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shibamoto T. Diacetyl: occurrence, analysis, toxicity. Agric Food Chem 62: 4048–4053, 2014. [DOI] [PubMed] [Google Scholar]
- 136.Slade J, Bero LA, Hanauer P, Barnes DE, Glantz SA. Nicotine and addiction. The Brown and Williamson documents. J Am Med Assoc 274: 225–233, 1995. [PubMed] [Google Scholar]
- 137.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, Byan-Parker S, Grizzle W, Sorscher EJ, Dransfield MT, Rowe SM. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 7: e39809, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res 17: 142–149, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst 100: 1552–1556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stepanov I, Muzic J, Le CT, Sebero E, Villalta P, Ma B, Jensen J, Hatsukami D, Hecht SS. Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol 26: 37–45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction–executive summary. Tob Control 10: 189–195, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim JH, Sudini K, Consolini N, Cormier SA, Lomnicki S, Hasan F, Pekosz A, Biswal S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One 10: e0116861, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J Clin Oncol 5: 660–666, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Talhout R, Opperhuizen A, van Amsterdam JG. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food Chem Toxicol 44: 1789–1798, 2006. [DOI] [PubMed] [Google Scholar]
- 145.Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8: 613–628, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Talih S, Balhas Z, Salman R, Karaoghlanian N, Shihadeh A. “Direct dripping”: a high-temperature, high-formaldehyde emission electronic cigarette use method. Nicotine Tob Res In press. [DOI] [PMC free article] [PubMed]
- 147.Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306: 1029–1032, 2004. [DOI] [PubMed] [Google Scholar]
- 148.Tarran R, Redinbo MR. Mammalian short palate lung and nasal epithelial clone 1 (SPLUNC1) in pH-dependent airway hydration. Int J Biochem Cell Biol 52: 130–135, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tice RR, Hook GG, Donner M, McRee DI, Guy AW. Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics 23: 113–126, 2002. [DOI] [PubMed] [Google Scholar]
- 150.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA 107: 3210–3215, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res 15: 267–270, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400–1406, 2012. [DOI] [PubMed] [Google Scholar]
- 153.Vucic EA, Chari R, Thu KL, Wilson IM, Cotton AM, Kennett JY, Zhang M, Lonergan KM, Steiling K, Brown CJ, McWilliams A, Ohtani K, Lenburg ME, Sin DD, Spira A, Macaulay CE, Lam S, Lam WL. DNA methylation is globally disrupted and associated with expression changes in chronic obstructive pulmonary disease small airways. Am J Respir Cell Mol Biol 50: 912–922, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Walle T, Walle UK, Sedmera D, Klausner M. Benzo[A]pyrene-induced oral carcinogenesis and chemoprevention: studies in bioengineered human tissue. Drug Metab Dispos 34: 346–350, 2006. [DOI] [PubMed] [Google Scholar]
- 155.Warren GW, Singh AK. Nicotine and lung cancer. J Carcinog 12: 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.WHO. Projections of Mortality and Causes of Death, 2015 and 2030. Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 158.Wieslander G, Norback D, Lindgren T. Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58: 649–655, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One 9: e108342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol 146: 116–123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang S, Day I, Ye S. Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis 154: 277–283, 2001. [DOI] [PubMed] [Google Scholar]
- 162.Zhou CH, Beltramini JN, Fan YX, Lu GQ. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37: 527–549, 2008. [DOI] [PubMed] [Google Scholar]
- 163.Zhou M, Liu Y, Duan Y. Breath biomarkers in diagnosis of pulmonary diseases. Clin Chim Acta 413: 1770–1780, 2012. [DOI] [PubMed] [Google Scholar]
- 164.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, Lee M. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 23, Suppl 3: iii3–iii9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zia S, Ndoye A, Nguyen VT, Grando SA. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res Commun Mol Pathol Pharmacol 97: 243–262, 1997. [PubMed] [Google Scholar]