Abstract
In acute respiratory distress syndrome, both reactive oxygen species (ROS) and increased intracellular calcium ([Ca2+]i) are thought to play important roles in promoting endothelial paracellular permeability, but the mechanisms linking ROS and [Ca2+]i in microvascular endothelial cells are not known. In this study, we assessed the effect of hydrogen peroxide (H2O2) on [Ca2+]i in mouse and human lung microvascular endothelial cells (MLMVEC and HLMVEC, respectively). We found that in both MLMVECs and HLMVECs, exogenously applied H2O2 increased [Ca2+]i through Ca2+ influx and that pharmacologic inhibition of the calcium channel transient receptor potential vanilloid 4 (TRPV4) attenuated the H2O2-induced Ca2+ influx. Additionally, knockdown of TRPV4 in HLMVEC also attenuated calcium influx following H2O2 challenge. Administration of H2O2 or TRPV4 agonists decreased transmembrane electrical resistance (TER), suggesting increased barrier permeability. To explore the regulatory mechanisms underlying TRPV4 activation by ROS, we examined H2O2-induced Ca2+ influx in MLMVECs and HLMVECs with either genetic deletion, silencing, or pharmacologic inhibition of Fyn, a Src family kinase. In both MLMVECs derived from mice deficient for Fyn and HLMVECs treated with either siRNA targeted to Fyn or the Src family kinase inhibitor SU-6656 for 24 or 48 h, the H2O2-induced Ca2+ influx was attenuated. Treatment with SU-6656 decreased the levels of phosphorylated, but not total, TRPV4 protein and had no effect on TRPV4 response to the external agonist, GSK1016790A. In conclusion, our data suggest that application of exogenous H2O2 increases [Ca2+]i and decreases TER in microvascular endothelial cells via activation of TRPV4 through a mechanism that requires the Src kinase Fyn.
Keywords: ARDS, calcium, lung injury, ROS, TRPV4
acute respiratory distress syndrome (ARDS) is a common condition that affects ∼200,000 patients every year (43) with no effective pharmacologic therapies. Loss of endothelial barrier function and formation of paracellular gaps in the lung microvascular endothelium are key features in the pathobiology of ARDS (62). The lung microvascular endothelium is composed of a specific phenotype of lung endothelial cells that are present in the alveolar capillaries and are known to be distinct from the endothelial cells of the larger conduit vessels (i.e., macrovascular endothelium) with regards to various signaling pathways and expression of membrane channels (13, 21, 31, 51). Two mediators known to be involved in lung microvascular endothelial cell (LMVEC) barrier disruption in ARDS include reactive oxygen species (ROS) and increased intracellular calcium concentration ([Ca2+]i).
Increased ROS are thought to play a major role in the development of endothelial barrier dysfunction in ARDS. In particular, high levels of oxidative stress are present in ARDS, and are associated with higher mortality (41, 62). Experimentally, increasing ROS by treatment with oxidants induces edema formation in isolated perfused lungs (6, 52). Similarly, application of external oxidants or induction of intracellular ROS production worsens barrier function in vitro (5, 40, 67). Conversely, protective effects were reported with augmentation of endothelial antioxidant mechanisms (8, 49, 50). ROS, such as H2O2, serve as signaling molecules at low levels and are maintained at these levels through various intracellular and extracellular antioxidant mechanisms (9). However, ROS levels can rise quickly because of increased production from intracellular sources such as NAPDH oxidase and mitochondria. Additionally, release of ROS by adherent neutrophils can also rapidly increase endothelial cell intracellular ROS. Indeed, ROS derived from circulating inflammatory cells have been shown to induce lung injury (47), and neutrophil-derived H2O2 is thought to play a major role in endothelial injury (59). Despite these known associations between ROS and endothelial barrier dysfunction, the mechanisms by which exogenous ROS, and H2O2 in particular, induce formation of paracellular gaps in the microvascular endothelium are incompletely understood.
In addition to increased ROS, elevated [Ca2+]i via influx through membrane channels has also been shown to be a key contributor to endothelial hyperpermeability (9, 62). Intracellular Ca2+ is required for activation of the contractile apparatus in endothelial cells, leading to cell retraction and disruption of the endothelial cell-cell junctions (18). Several members of the transient receptor potential (TRP) family of Ca2+ channels, including canonical TRPs (TRPCs) and vanilloid TRPs (TRPVs), have been implicated in various models of lung injury, including ischemia-reperfusion lung injury (65), stretch-induced lung injury (22), and heart failure models of pulmonary edema (54). TRPV4 in particular has been implicated in various models of lung injury and is thought to play a key role in lung epithelial and endothelial barrier function (37). For example, Ca2+ entry via TRPV4 was recently shown to be critical in chlorine inhalation and gastric acid aspiration models of lung injury (7). Additionally, a critical role for members of the TRPC family in regulating Ca2+ influx in the setting of various injurious stimuli (such as thrombin and LPS) in both pulmonary artery and mouse lung microvascular endothelial cells (MLMVECs) has been established (2, 34, 53, 55, 56). ROS and increased [Ca2+]i may in fact be mechanistically linked; ROS increased [Ca2+]i in various tissues including the lung (40, 60), but the specific channel responsible for ROS-induced Ca2+ influx in the lung microvasculature is not known.
While TRPs clearly play a major role in endothelial cell responses to various stimuli, little is known regarding the mechanisms by which the function of these channels is regulated in LMVECs. In expression systems, TRP channels, including TRPV4, can be differentially regulated by phosphorylation (61, 63). The Src family kinase (SFK) family of tyrosine kinases contains several members, including Src, Fyn, Yes, and Lyn, that are involved in a variety of cell signaling events (42, 44). Recently, the role of SFKs in Ca2+ channel regulation has been under investigation. For instance, TRPV4 activity was modulated by SFKs in HEK cells (64, 70) while phosphorylation by a variety of kinases, including Src, has been shown to affect the activity of TRPC3 and TRPC6 (25, 27, 29). Previous work established that Fyn is expressed in LMVECs (5), but the role of Fyn, or other SFKs, in regulating lung TRPs has not been determined.
Based on these data, we sought to understand the relationship between ROS and increased [Ca2+]i in LMVECs and to determine whether inhibition of Ca2+ influx pathways could affect H2O2-induced barrier dysfunction in LMVECs. We hypothesized that H2O2 would increase [Ca2+]i by activating a membrane-bound Ca2+ channel and that inhibiting H2O2-induced Ca2+ influx would attenuate H2O2-induced endothelial permeability in vitro. Using fluorescence microscopy, measurement of endothelial resistance in monolayers, genetic deletion and silencing approaches, and pharmacological inhibitors, we determined the role of TRPV4 and the Src kinase Fyn in regulating H2O2-induced Ca2+ influx in rodent and human LMVECs.
METHODS
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of The Johns Hopkins University School of Medicine.
Isolation and culture of LMVEC.
Adult (8–10 wk), wild-type (WT) C57/B6 and Fyn-deficient (Fyn−/−; strain B6.129S7-Fyntm1Sor/J) male mice were purchased from Jackson labs. Mice were killed by cervical dislocation, and the lungs were quickly removed by dissection and immersed in DMEM (Gibco). Complete media for MLMVEC was prepared by using 400 ml DMEM with 20% FBS, 0.1% nonessential amino acids, 0.1% antibiotics/antimycotics, and endothelial cell growth supplement (Millipore). Peripheral mouse lung tissue was obtained by dissection, then minced and digested by incubation with 5% Type IA Collagenase (Sigma) for 10 min. The digested cell solution was strained through a 70-μm mesh and washed with complete media, then centrifuged at 2,000 rpm for 10 min. The pellet was resuspended in 1 ml of complete media and the cell suspension incubated for 30 min with CD31-conjugated beads (Invitrogen). The supernatant was removed, and cells adherent to the conjugated beads were washed with isolation buffer (Invitrogen), centrifuged, and the pellet resuspended and plated on a T75 flask. After reaching confluency, cells were trypsinized and incubated with beads conjugated with Griffonia simplicifolia lectin, prepared by incubating 25 μl of Biotin binder Dynabeads (Invitrogen) with 1 μg of biotin-conjugated Griffonia simplicifolia lectin (EY Labs) for 30 min at room temperature. After incubation, the beads were washed with isolation buffer, the cells centrifuged, resuspended in complete growth media, and grown to confluence. Human lung microvascular endothelial cells (HLMVECs) were purchased from Lonza; cells were grown on gelatinized cultureware with Lonza endothelial growth media-2 (EGM-2) supplemented with 1% penicillin/streptomycin and an extra 5% FBS. All experiments were performed on cells at passage 5–6. To ensure that cells did not undergo transdifferentiation in vitro, both HLMVEC and MLMVECs were stained for endothelial (Griffonia simplicifolia lectin) and smooth muscle (smooth muscle specific α-actin) markers at each passage prior to experiments. Only cells that were positive for Griffonia simplicifolia, and did not express smooth muscle specific α-actin, were used for experiments. For MLMVECs, experiments were conducted on cells isolated from at least three different mice; for HLMVEC, cells from three different donors were used.
Intracellular Ca2+ measurements.
Cells were grown to 50–60% confluence on glass coverslips and loaded with 5 μM of Fura-2 AM (Molecular Probes) for 1 h at 37°C prior to being placed in a temperature-controlled (37°C) laminar flow chamber on the stage of an inverted microscope and perfused with modified Krebs solution containing (in mM) 118 NaCl, 4.7 KCl, 0.57 MgSO4, 1.18 KH2PO4, 25 NaHCO3, 2.5 CaCl2, and 10 glucose gassed with 16% O2 and 5% CO2 at 37°C. For Ca2+-free experiments, CaCl2 was omitted and 1 mM EGTA added to the solution. Ratiometric measurement of Fura-2 fluorescence was performed by using a collimated light beam from a xenon arc lamp filtered at 340 and 380 nm and focused onto the endothelial cells via a ×20 fluorescence objective. After excitation at 340 and 380 nm (F340, F380), light emitted from the cells was returned through the objective, and detected at 510 nm by an imaging camera. An electronic shutter was used to minimize photo bleaching. The outflow rate of the flow chamber was set to 0.5 ml/min. Reservoirs containing Krebs solution as well as various drugs were connected via a manifold to a single inlet which connected to the inflow port of the chamber. At the beginning of each experiment, cells were perfused for 15 min to allow for establishment of stable baseline. Drugs (dissolved in Krebs) were added to the reservoirs prior to the experiment, thus allowing for quick switches between baseline, H2O2, and drug containing solutions. Hydrogen peroxide (Sigma) was freshly prepared in warmed Krebs prior to each experiment. For experiments involving drug pretreatment, cells were either incubated with drug in the media (1–48 h) or perfused with drug in Krebs for 10–30 min prior to exposure to H2O2. Intracellular calcium ([Ca2+]i) was estimated from F340/F380 measured in calibration solutions with Ca2+ concentrations of 0–1,350 nM (Molecular Probes, Eugene, OR).
Western blotting.
Cell lysates were prepared in T-PER (Life Biotechnologies) lysis buffer containing a protease inhibitor cocktail tablet (Boehringer) and phosphatase inhibitor cocktail solutions 2 and 3 (Sigma). Protein concentrations were determined using a BCA Protein Assay kit (Pierce). Equal amounts of protein (20 μg) were loaded into each well of an 8% gel and subjected to electrophoresis. Following separation, proteins were transferred to nitrocellulose membranes (iBlot, Life Biotechnologies), which were then blocked with blocking buffer (5% BSA in TBS-T) and incubated with primary antibody (anti-TRPV4 1:500, Alomone, or anti-phospho-tyrosine 1:3,000, Cell Signaling) at either room temperature for 1 h (phospho-tyrosine) or overnight at 4°C (TRPV4). Membranes were then washed and incubated with secondary antibodies (goat anti-mouse 1:3,000 or goat anti-rabbit 1:3,000, Bio-Rad) for 1 h. Bands were visualized by enhanced chemiluminescence. Membranes were then stripped and reprobed with anti-GAPDH horseradish peroxidase-conjugated antibody (1:25,000, Bio-rad). Densitometry was performed to quantify the amount of protein of interest, and this value was normalized to the housekeeping protein by using ImageJ software.
Electrical cell impedance sensing.
Cells were seeded on gold electrodes in 0.5-ml electrode wells (Applied BioPhysics) and grown to confluence. Following measurement of baseline transmembrane electrical resistance (TER), the media was changed to serum-free basal media (EBM, Lonza), followed by a period of equilibration (1 h). Subsequently, agonists or diluent were added directly to wells. Data were analyzed by using electrical cell impedance sensing (ECIS) ZTheta system (Applied Biophysics).
Small interference RNA transfection.
TRPV4 small interference RNA (siTRPV4) and control nontarget siRNA (siNT) were designed and synthesized by Dharmacon. HLMVEC were transfected with 100 nM of siRNA for 4 h in serum- and antibiotic-free EBM (Lonza) by using GeneSilencer (Genlantis) according to the manufacturer's instruction. After 4 h, complete media (EGM-2, Lonza) was added. After an additional 24 h, the media was changed, and the cells were harvested for protein analysis or used for Ca2+ experiments at 48 h.
H2O2 electrode.
H2O2 concentrations were measured with an electrode system (Apollo Free Radical Analyzer, World Precision Instruments). Following calibration, an H2O2-sensing electrode (ISO-HPO-2) was inserted into vials containing warmed Krebs solution as well as Krebs solution with various concentrations of H2O2 (250-1,000 μM). Measurements were taken at subsequent time points, and the solutions were maintained at 38°C with a heater during the course of the experiment.
Data analysis/statistics.
All values are expressed as means ± SE. For [Ca2+]i measurements, data were collected from up to 30 cells and the values were averaged to obtain a single value for each experiment. Change in [Ca2+]i was computed by subtracting the average basal [Ca2+]i, determined from 1 min of data collected immediately prior to challenge with agonists, from the peak [Ca2+]i measured in the first 5 min after beginning agonist challenge. For ECIS data, resistance measurements (R) from each ECIS well were normalized to the resistance value at the beginning of the experiment (R0). Data were compared by unpaired Student's t-test or by one-way ANOVA with a Holms-Sidak post hoc test to determine differences between groups. A repeated measures two-way ANOVA was used to analyze H2O2 sensor data. A P value of <0.05 was accepted as statistically significant.
RESULTS
Effect of H2O2 on [Ca2+]i in MLMVECs and HLMVECs.
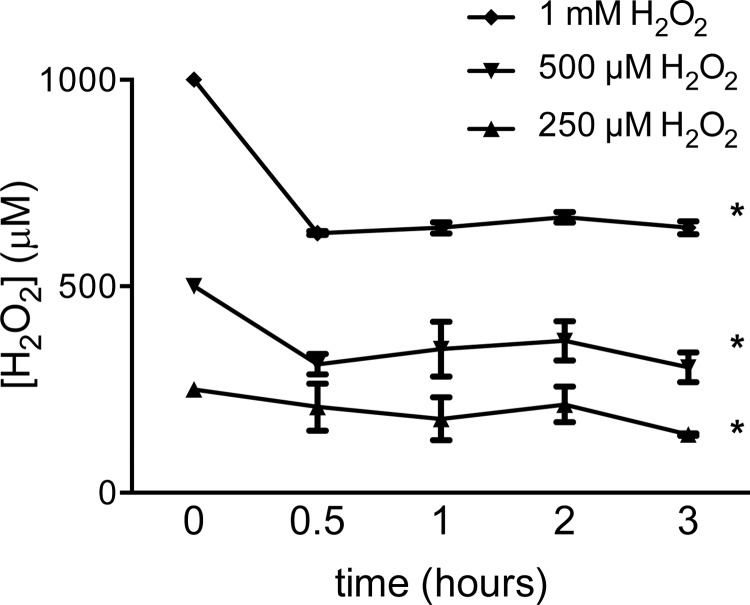
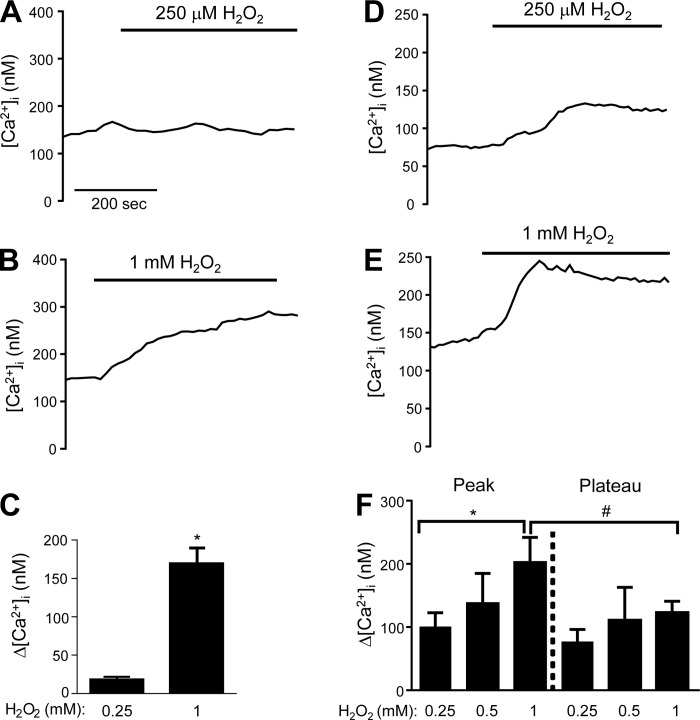
Since many of our experiments utilized H2O2 prepared in Krebs buffer solution, we measured the stability of H2O2 in this solution. Using an electrode-based ROS sensor, we measured changes in current at a fixed voltage when the electrode was placed in solutions containing 250 μM, 500 μM, and 1 mM H2O2. The concentration of [H2O2] in each of the solutions decreased within the first 30 min but subsequently remained stable up to 3 h (Fig. 1). To determine the effects of H2O2 on LMVECs, [Ca2+]i was measured in semiconfluent (50–60%) MLMVECs and HLMVECs exposed to increasing concentrations of H2O2. In MLMVEC, no significant change in [Ca2+]i was observed in response to 250 μM H2O2 (Fig. 2A). When a higher concentration of H2O2 was applied (1 mM H2O2), a large rise in [Ca2+]i was observed within 120 s that was sustained for the duration of the exposure (Fig. 2, B and C). To determine whether a change in [Ca2+]i following H2O2 exposure was a species-specific phenomenon, the effect of H2O2 on HLMVECs was also tested. Consistent with the results obtained in MLMVECs, H2O2 increased [Ca2+]i in HLMVECs, although these cells appeared much more sensitive to H2O2 in that concentrations as low as 250 μM increased [Ca2+]i (Fig. 2D). At a concentration of 1 mM, H2O2 produced a steep increase in HLMVEC [Ca2+]i that was similar in magnitude to that observed at this concentration in MLMVECs. Unlike the MLMVECs, however, the peak change in [Ca2+]i induced by H2O2 in HLMVECs was followed by a small reduction to a sustained plateau that was still significantly greater than baseline (Fig. 2, E and F).
Fig. 1.
Traces of HPO electrode current at 450 mV poise voltage when placed in warmed Krebs solution containing 250 μM, 500 μM, and 1 mM H2O2 (n = 4–5 experiments/group). *Significant difference from control.
Fig. 2.
Representative traces (A and B) and bar graph (C) showing mean ± SE change in intracellular Ca2+ ([Ca2+]i) from baseline in mouse lung microvascular endothelial cells (MLMVEC) exposed to 250 μM and 1 mM H2O2 in Krebs buffer (n = 4–6 experiments, 25–30 cells/experiment). Representative traces (D and E) and bar graph (F) showing means ± SE in human lung microvascular endothelial cells (HLMVEC) exposed to increasing doses of H2O2 (n = 6–12 experiments, 20–40 cells/experiment for all groups). *Significant difference from 250 μM; #significant difference between peak and plateau Δ[Ca2+]i.
Identifying the source of Ca2+ in H2O2-induced responses.
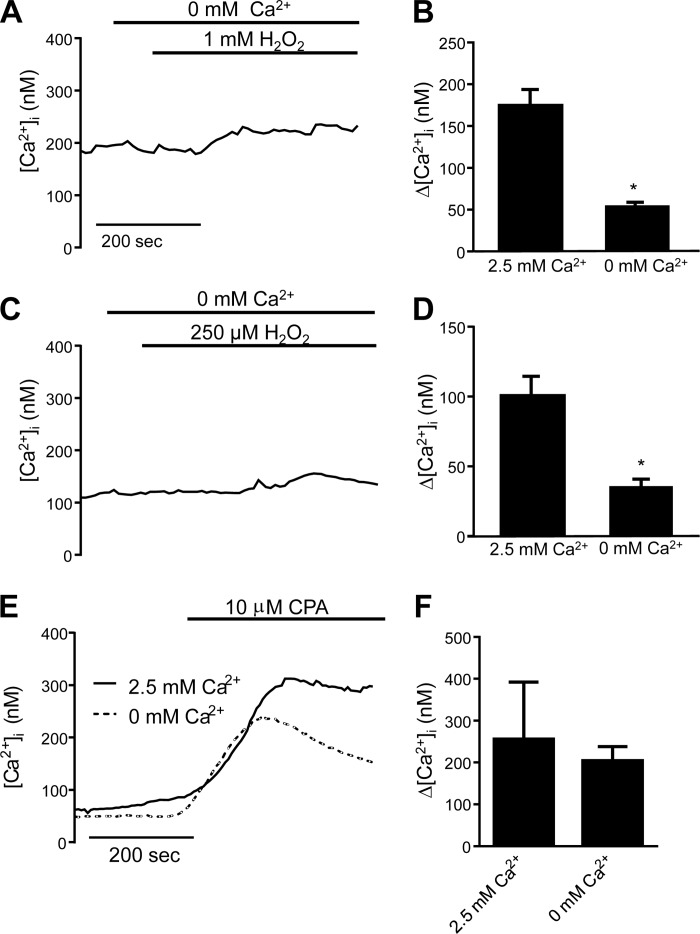
To determine whether the H2O2-induced rise in [Ca2+]i was due to Ca2+ release from the endoplasmic reticulum or influx through plasma membrane channels, MLMVECs and HLMVECs were perfused with Ca2+-free solution supplemented with EGTA (1 mM) to chelate any residual extracellular Ca2+ in the system while being challenged with H2O2. In MLMVECs, the H2O2-induced rise in [Ca2+]i was largely absent when extracellular Ca2+ was removed (Fig. 3, A and B). Similarly, in HLMVECs the change in [Ca2+]i observed in response to application of H2O2 was greatly reduced in the absence of extracellular Ca2+ (Fig. 3, C and D). To verify that perfusion with EGTA containing Ca2+-free media did not lead to depletion of Ca2+ stores, we measured [Ca2+]i in HLMVECs treated with cyclopiazonic acid (CPA; 10 μM) after being perfused with normal or Ca2+-free Krebs. The CPA-induced increase in [Ca2+]i, indicative of store release, was similar in cells perfused with normal or Ca2+-free media (Fig. 3E).
Fig. 3.
Representative trace and bar graph showing mean ± SE change in [Ca2+]i in MLMVEC (A and B) and HLMVEC (C and D) exposed to H2O2 while perfused with Krebs buffer containing 0 mM Ca2+ (n = 5–9 experiments, 20–40 cells/experiment for all groups). Mean traces (E) and bar graph (F) showing mean ± SE change in [Ca2+]i in HLMVEC exposed to cyclopiazonic acid (10 μM) in regular and Ca2+-free Krebs solutions (n = 3 experiments, 20–40 cells/experiment for all groups). *Significant difference from 2.5 mM Ca2+ group.
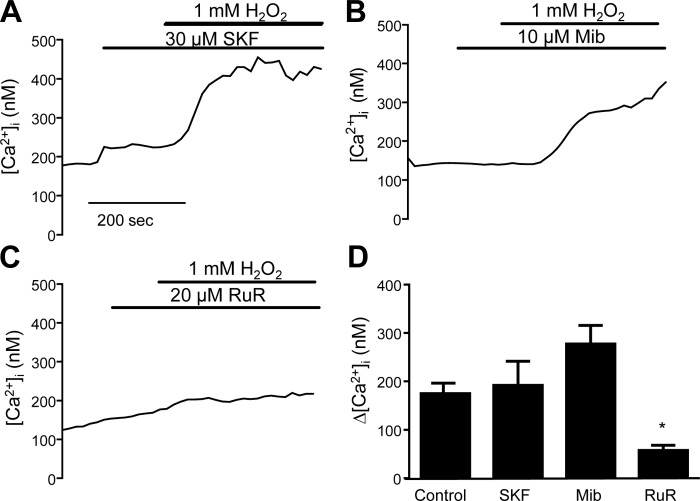
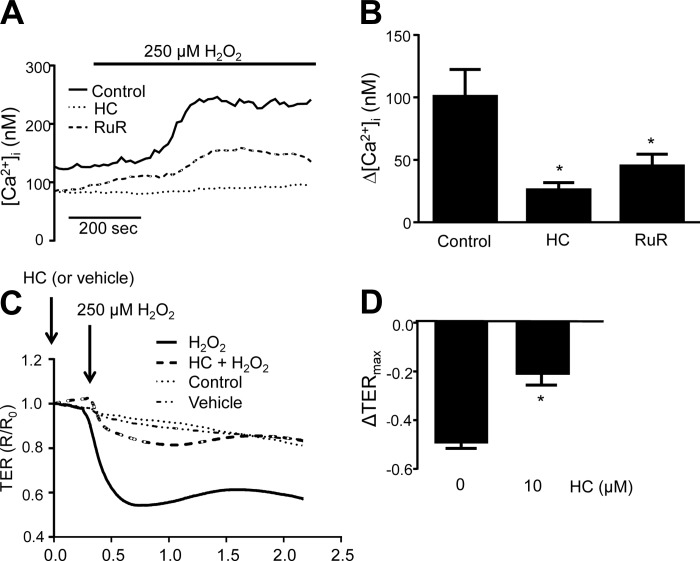
The next step was to determine the nature of the channel involved in H2O2-induced Ca2+ influx. The ability of H2O2 to increase [Ca2+]i was measured in MLMVECs pretreated with the following Ca2+ channel blockers: SKF96365 (SKF; 30 μM), a nonspecific inhibitor of nonselective cation channels (NSCC); mibefradil (Mib; 10 μm), to inhibit T-type Ca2+ channels; or ruthenium red (RuR; 20 μM), an inhibitor of TRPV4 as well as other cation channels. There was no significant change in baseline [Ca2+]i with any drug treatment (Table 1). Neither SKF nor Mib prevented the increase in [Ca2+]i after H2O2 exposure (Fig. 4, A and B). In contrast, pretreatment with RuR significantly attenuated the H2O2-induced rise in [Ca2+]i (Fig. 4, C and D).
Table 1.
Baseline [Ca2+]i
| Condition | Baseline [Ca2+]i ± SE |
|---|---|
| MLMVEC | |
| WT | 171 ± 13 |
| WT + SKF | 261 ± 40 |
| WT + Mib | 137 ± 8 |
| WT + RuR | 179 ± 33 |
| Fyn−/− | 160 ± 8 |
| HLMVEC | |
| Untreated | 90 ± 7.4 |
| HC | 84 ± 5.4 |
| RuR | 123 ± 15 |
| SU 1 h | 96 ± 19 |
| SU 24 h | 105 ± 9 |
| SU 48 h | 95 ± 5 |
| siNT | 73 ± 4 |
| siTRPV4 | 81 ± 7 |
| siFyn | 67.7 ± 10 |
MLMVEC, mouse lung microvascular endothelial cells; WT, wild-type; SKF, SKF-96365; Mib, mibefradil; RuR, ruthenium red; HLMVEC, human LMVEC; HC, HC-067047; SU, SU-6656; siNT, nontarget small interference RNA; siTRPV4, transient receptor potential vanilloid 4 siRNA; siFyn, Fyn siRNA.
Fig. 4.
Representative tracings showing change in [Ca2+]i following pretreatment with SKF96365 (SKF; 30 μM) (A), mibefradil (Mib; 10 μM) (B), and ruthenium red (RuR; 20 μM) (C). D: bar graph showing mean change ± SE in [Ca2+]i following H2O2 in cells pretreated with SKF, Mib, or RuR. *Significant difference from control; n = 6 experiments each, 25–30 cells/experiment.
Effect of TRPV4 agonism on HLMVECs.
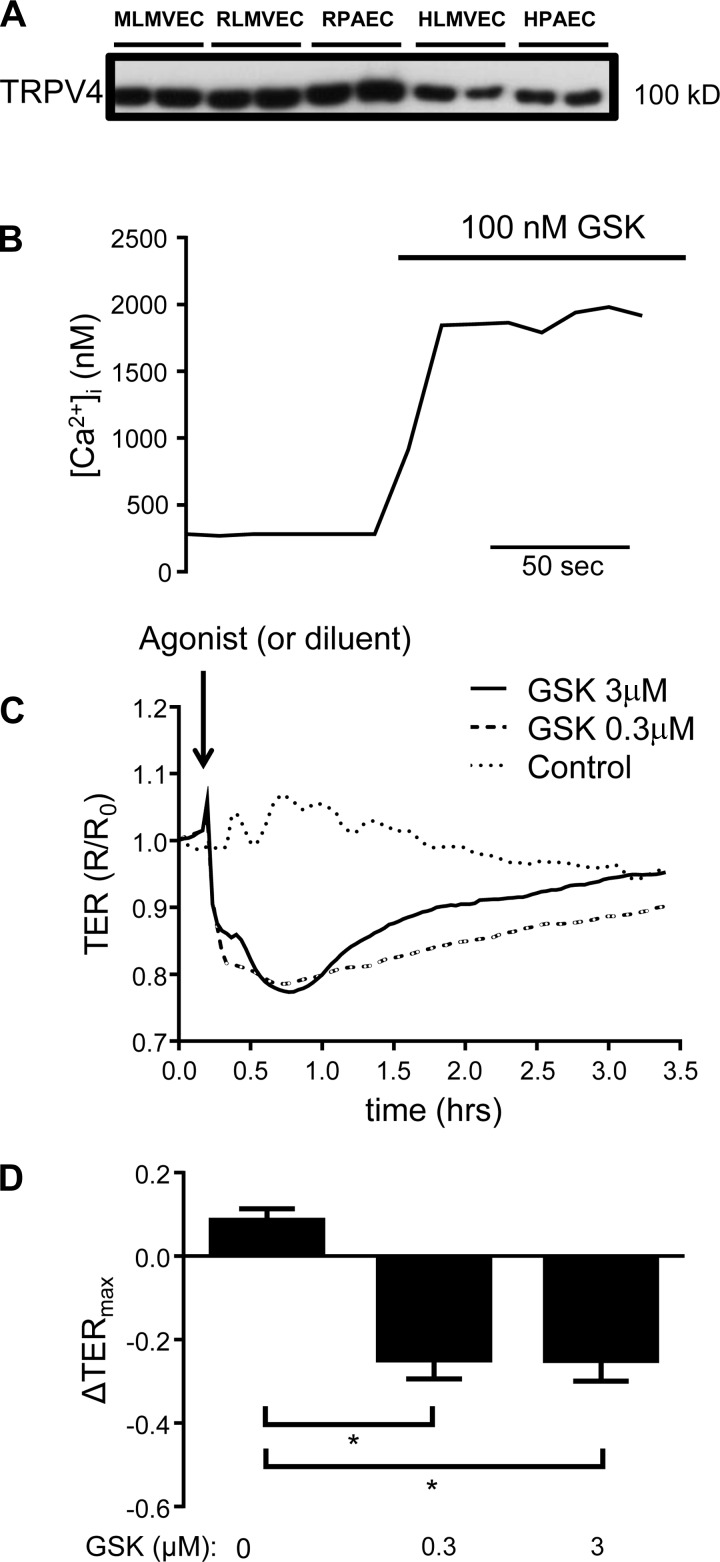
To directly evaluate the potential role of TRPV4 in the H2O2-induced increase in [Ca2+]i that we observed, we first assayed the expression of TRPV4 protein and studied the effect of TRPV4 agonism on endothelial [Ca2+]i and barrier function. Immunoblot analysis revealed that TRPV4 protein was clearly expressed in human and rat endothelial cells from conduit pulmonary arteries and in rat, mouse, and human lung microvascular endothelial cells (Fig. 5A). To determine whether TRPV4 was functional in these cells, we exposed HLMVEC to the specific TRPV4 agonist, GSK1016790A (GSK; 100 nM). Application of GSK induced a rapid, large increase in [Ca2+]i (830 ± 330 nM; n = 4; Fig. 5B). To further understand the functional consequence of TRPV4-mediated Ca2+ influx, we performed ECIS studies on confluent monolayers of HLMVECs exposed to GSK. Control cells treated with vehicle displayed no significant change in TER; however, TER transiently decreased in cells treated with GSK, followed by recovery to near baseline levels. These results suggest that Ca2+ influx via TRPV4 reduces barrier function in HLMVEC (Fig. 5, C and D).
Fig. 5.
A: expression of TRPV4 in human and rodent lung endothelial cells, including mouse and rat microvascular endothelial cells (MLMVEC, RLMVEC), rat pulmonary artery endothelial cells (RPAEC), human lung microvascular endothelial cells (HLMVEC), and human lung pulmonary artery endothelial cells (HPAEC). B: increase in [Ca2+]i in HLMVEC following exposure to GSK1016790 (GSK; 100 nM). C and D: electrical cell impedance sensing (ECIS) traces and bar graph showing mean ± SE decrease in transmembrane electrical resistance (TER) in HLMVEC following exposure to GSK. *Significant difference from control; n = 8 experiments each.
Effect of TRPV4 inhibition on H2O2-induced Ca2+ influx and barrier dysfunction.
To assess whether blockade of TRPV4 alters ROS-induced Ca2+ influx and paracellular permeability, HLMVEC were pretreated with RuR (20 μM) or the more specific TRPV4 inhibitor HC-067047 (1 μM) for 30 min and then exposed to 250 μM H2O2. Similar to MLMVEC, treatment with RuR significantly attenuated the increase in [Ca2+]i observed in response to H2O2 (Fig. 6, A and B). HC treatment inhibited this response at least as well as RuR. Indeed, small rises in [Ca2+]i, occurring several minutes after initiation of ROS exposure, were observed with RuR but not HC treatment. Baseline [Ca2+]i did not significantly change with HC or RuR treatment. With respect to barrier function, no significant change in TER was noted in control or vehicle-treated cells. Consistent with prior reports (40), we observed a decrease in TER with application of 250 μM H2O2 (Fig. 6, C and D) consisting of a large initial decrease followed by transient recovery. The H2O2-induced decrease in TER was significantly attenuated following pretreatment with HC.
Fig. 6.
Representative traces (A) and bar graph (B) showing mean ± SE change in [Ca2+]i in HLMVEC treated with the TRPV4 inhibitors RuR (20 μM) and HC-067047 (1 μM). ECIS traces (C) and bar graph (D) showing mean ± SE decrease in TER in HLMVEC following exposure to 250 μM H2O2 with and without HC pretreatment. For comparison purposes, the untreated H2O2 response shown in B is reproduced from Fig. 2. *Significant difference from control; n = 6–8 experiments/group, 20–40 cells/experiment.
Role of Fyn in H2O2-induced TRPV4 activation.
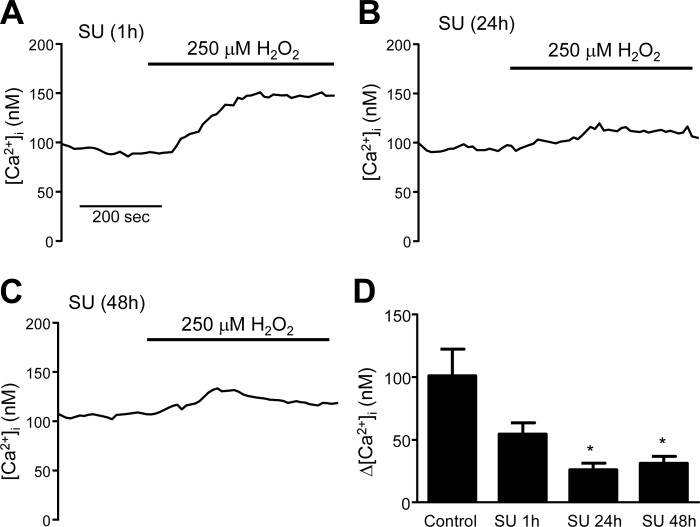
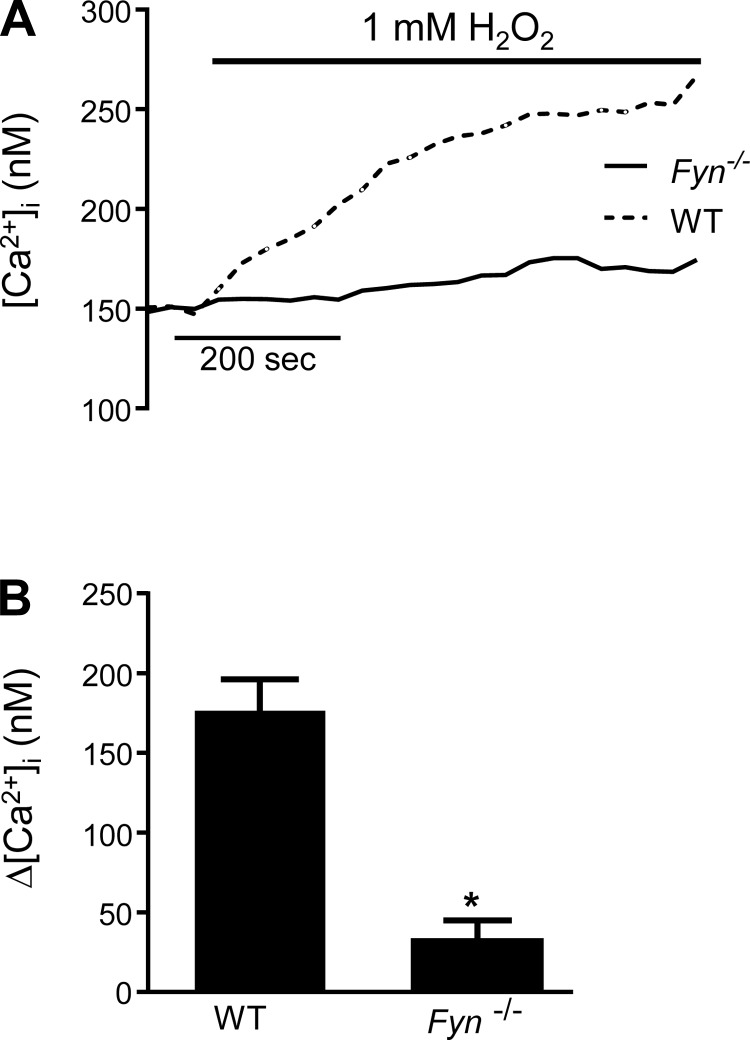
We next sought to understand the regulatory mechanisms underlying activation of TRPV4 following exposure to H2O2. The Src family of kinases (SFK) has been previously implicated in the regulation of TRP channels. We hypothesized that H2O2 might induce phosphorylation of TRPV4 via Fyn, thus activating the channel. To test this possibility, we measured H2O2-induced Ca2+ influx in HLMVEC treated with SU-6656 (SU; 5 μM), an SFK inhibitor. No statistically significant difference in H2O2-induced [Ca2+]i increase was observed in HLMVECs treated acutely (1 h) with SU (Fig. 7A). However, when HLMVECs were pretreated with SU for 24 h (Fig. 7B) or 48 h (Fig. 7C), the increase in [Ca2+]i induced by H2O2 was significantly attenuated (Fig. 7D). We also measured Ca2+ influx in MLMVECs isolated from mice deficient in Fyn (Fyn−/− mice). In these cells, no difference in basal [Ca2+]i was observed compared with MLMVEC isolated from WT mice; however, there was a complete lack of Ca2+ influx following exposure to 1 mM H2O2 (Fig. 8).
Fig. 7.
Representative traces of [Ca2+]i in HLMVEC following pretreatment with the Fyn inhibitor, SU (5 μM), for 1 (A), 24 (B), and 48 h (C). D: bar graph showing mean ± SE change in [Ca2+]i in control and SU-treated HLMVEC following exposure to 250 μM of H2O2. For comparison purposes, the 250 μM H2O2 response shown in Fig. D is reproduced from Fig. 2. *Significant difference from control; n = 6 experiments/group, 20–40 cells/experiment.
Fig. 8.
Representative traces (A) and bar graph (B) showing mean ± SE change in [Ca2+]i in MLMVEC isolated from Fyn−/− mice and exposed to 1 mM H2O2. *Significant difference from wild-type (WT); n = 3–6 experiments/group, 20–40 cells/experiment.
Effect of TRPV4 and Fyn depletion on H2O2-induced Ca2+ influx.
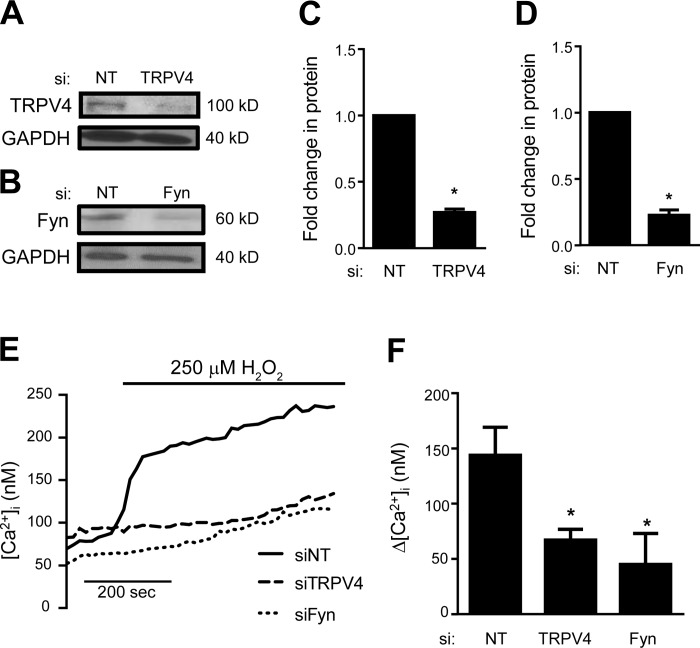
To further define the role of TRPV4 and Fyn in H2O2-induced Ca2+ influx, we measured [Ca2+]i in response to H2O2 challenge in HLMVEC following TRPV4 and Fyn depletion with siRNA. At a concentration of 100 nM, siRNAs targeted against TRPV4 and Fyn (Dharmacon “Smart Pool”) produced a >70% reduction in TRPV4 and Fyn protein levels after 48 h (Fig. 9, A–D). Interestingly, the H2O2-induced Ca2+ response in cells transfected with nontargeting siRNA (siNT) was higher than that in untransfected cells, though this increase did not reach statistical significance (P = 0.1). This effect may be related to the transfection process. Compared with siNT controls, H2O2-induced Ca2+ influx was significantly attenuated in cells transfected with siTRPV4. Similar to pharmacologic inhibition and genetic absence, silencing of Fyn (siFyn) also significantly attenuated H2O2-induced Ca2+ influx (Fig. 9, D and E).
Fig. 9.
Representative immunoblot images (A and B) and densitometry (C and D) showing TRPV4 and Fyn protein levels for HLMVEC transfected with nontargeting (siNT) and TRPV4 (siTRPV4) or Fyn (siFyn) siRNA (n = 3 independent experiments). Representative traces (E) and bar graph (F) showing mean ± SE change in [Ca2+]i in HLMVEC transfected with siNT, siTRPV4, or siFyn and exposed to 250 μM H2O2 (n = 4–7 experiments/group, 20–40 cells/experiment). *Significant difference from siNT.
Phosphorylation of TRPV4 by Fyn and Ca2+ responses.
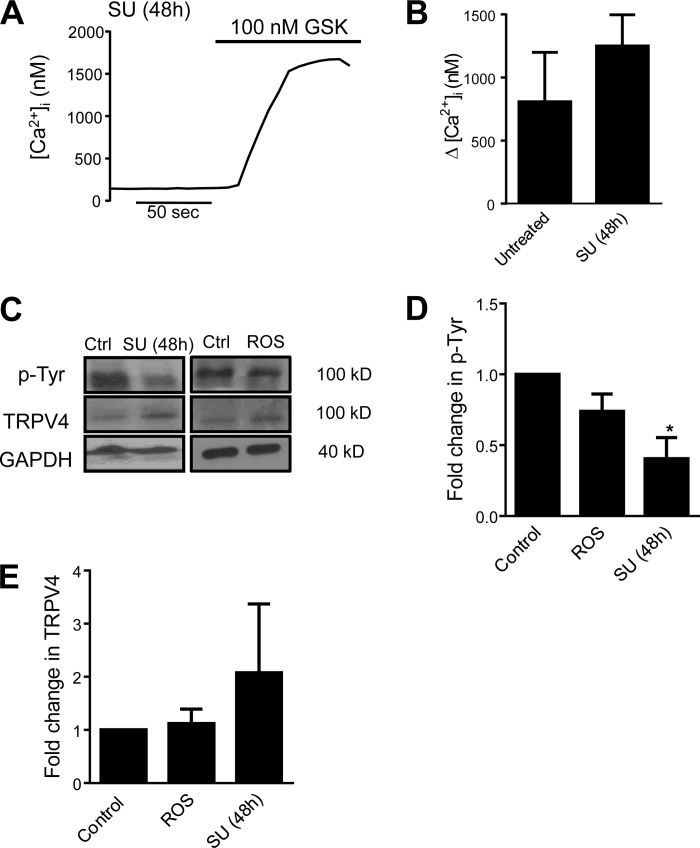
Since loss of Fyn appeared to reduce H2O2-induced Ca2+ responses, we investigated whether Fyn inhibition could alter TRPV4 activation by other stimuli or alter phosphorylation of TRPV4 protein. When HLMVECs were treated with SU for 48 h before exposure to GSK, the change in [Ca2+]i was similar in magnitude to the response observed in untreated cells (Fig. 10, A and B). When immunoblots were probed for phosphotyrosine and TRPV4 (Fig. 10, C–E), HLMVECs that had been treated with SU (5 μM for 48 h) exhibited less phosphotyrosine signal at the molecular weight of TRPV4 (∼100 kD), without any change in the total TRPV4 protein levels, suggesting that SU treatment reduced basal phosphorylation of TRPV4. We next measured whether H2O2 caused acute changes in TRPV4 phosphorylation. Treatment with 250 μM H2O2 produced no change in phosphorylated or total TRPV4, suggesting that ROS exposure does not activate TRPV4 via acute phosphorylation of the channel.
Fig. 10.
A: representative trace of [Ca2+]i in HLMVEC exposed to GSK (100 nM) after treatment with SU (5 μM) for 48 h. B: bar graph showing mean ± SE change in [Ca2+]i in control and SU-treated HLMVEC following exposure to GSK (n = 5 experiments/group). C–E: representative immunoblot images and densitometry showing protein levels of phosphotyrosine and TRPV4 protein in HLMVEC under control conditions, or treated with either 250 μM H2O2 or SU (5 μM). *Significant difference from control; n = 3 independent experiments.
DISCUSSION
In this study, we demonstrated that application of H2O2 increased [Ca2+]i levels in LMVECs through a mechanism that involves the Ca2+ channel, TRPV4. Furthermore, our data suggest that the Src kinase family member Fyn participates in basal phosphorylation of TRPV4 and that this phosphorylation may be necessary for H2O2-induced Ca2+ influx. Lastly, we showed that both TRPV4 agonism and exogenous ROS worsen barrier function in vitro, and that these effects are attenuated by inhibition of TRPV4.
We have previously shown that exogenous provision of ROS using H2O2 dissolved in a solution free of serum results in rapid uptake and a sustained increase in intracellular H2O2 in MLMVEC (49). It would appear that this holds true for HLMVECs as well, as we show that both murine and human LMVECs respond to H2O2 with changes in [Ca2+]i, and that in HLMVECs the changes in [Ca2+]i are associated with changes in TER. Given the propensity of H2O2 to undergo rapid degradation in solution in the presence of trace metals and other solutes, we measured [H2O2] in the buffer solution used for our Ca2+ experiments by using an H2O2 electrode technique previously shown by us and others to provide reliable and robust measurement of [H2O2] (33, 49). Our data reveal an initial decrease in [H2O2], followed by stable [H2O2] for several hours. Since we typically allow solutions containing H2O2 to be warmed and equilibriated for 30–45 min prior to the start of experiments, these data suggest that H2O2 levels were stable in our experimental solutions over the time course (1–2 h) of our experiments, ensuring that the lack of response seen in some of our experimental conditions was not due to degradation of H2O2.
Though extracellular delivery of H2O2 was stable, it is known that large differences exist between extracellular ROS and intracellular ROS because of a variety of factors, including diffusive properties of H2O2 itself and antioxidant capacity of various cell types (15, 26). Though the exact intracellular concentration of H2O2 is not known, we do not believe that it was at a level that induced cell death. In our Ca2+ experiments, a short washout period was included at the end of H2O2 challenge, and during this time period we observed recovery of [Ca2+]i toward baseline levels (data not shown). Likewise, in our ECIS measurements of TER in HLMVEC treated with 250 μM of H2O2, some recovery (although not to baseline) was noted following H2O2 exposure. Moreover, cells tracked during Ca2+ imaging did not detach from cultureware or lose Fura-2 signal during the experiment, suggesting that cells remained viable for the duration of the experiment. Finally, our measurement of H2O2 in buffer solution revealed that the actual concentration of H2O2 delivered to cells in vitro was likely a stable lower dose than the initial concentration; thus it is unlikely that the changes observed in our experiments were due to nonspecific toxic effects of H2O2.
Our Ca2+ results are consistent with other reports where H2O2 has been shown to increase [Ca2+]i in various vascular beds (1, 36, 38, 58, 66), including bovine, human, and mouse lung endothelial cells. In bovine LMVEC (40) and human pulmonary artery endothelial cells (35), H2O2 increased [Ca2+]i and decreased TER similar to our current findings in HLMVEC. While both HLMVECs and MLMVECs responded to H2O2 with an increase in [Ca2+]i, there is a clear difference in sensitivity. Unlike HLMVECs, which exhibit substantial increases in [Ca2+]i and barrier disruption (as measured by ECIS) at concentrations of H2O2 as low as 250 μM (40), reductions in MLMVEC barrier function required very high concentrations of H2O2 (5). The difference in sensitivity to H2O2 with respect to barrier disruption in human and murine cells was reflected in the difference in [Ca2+]i responses to H2O2, with MLMVECs exhibiting little increase in [Ca2+]i at concentrations lower than 1 mM H2O2, the same concentration needed for barrier disruption. The exact reason for the varied sensitivity is unclear, but it may be explained by species-specific differences in antioxidant capacity (17). The difference in H2O2 responsiveness between mouse and human LMVECs served as a rationale for performing our inhibitor experiments in both cell types and validating our MLMVEC observations in more clinically relevant human cell cultures.
In HLMVEC, the profile of the Ca2+ response differed based on H2O2 dose. While the highest concentration of H2O2 tested produced a large peak followed by a significantly lower but still elevated plateau, exposure to H2O2 at lower doses (i.e., 250 μM) produced an increase in [Ca2+]i that was reproducibly stable around 100–150 nM with no significant differences between the peak and plateau [Ca2+]i. In MLMVEC, 1 mM of H2O2 produced sustained elevations in [Ca2+]i without a peak or plateau, similar to the sustained rise in [Ca2+]i seen in bovine lung microvascular endothelial cells (40). The reason for the differences in profile is unclear, but may reflect additional influx, efflux, or release mechanisms. In both MLMVECs and HLMVECs, the H2O2-induced increase in [Ca2+]i was significantly attenuated when extracellular Ca2+ was removed, suggesting that Ca2+ influx is the primary determinant of H2O2-induced Ca2+ influx. However, the possibility of store depletion and store-operated entry playing a role in our H2O2-induced Ca2+ influx cannot be excluded based on our data. Indeed, small, late rises in [Ca2+]i were reliably observed even in Ca2+-free experiments as well as in other experimental conditions (including TRPV4 and Fyn knockdown), perhaps corresponding to emptying of ER stores in these cells because of either prolonged absence of extracellular Ca2+ or a later H2O2-induced signaling mechanism not dependent on TRPV4. One concern when perfusing cells with Ca2+-free media containing EGTA is depletion of stores, but brisk store release was noted when HLMVEC were exposed to CPA following perfusion in Ca2+-free Krebs with EGTA, indicating that stores were still intact. Further experiments will be required to fully elucidate the source of this residual [Ca2+]i response, the possible role of store-operated Ca2+ entry, and whether these mechanisms play a role in H2O2-induced permeability.
It is clear from previous work that ROS can increase [Ca2+]i in endothelial cells (20, 23, 35, 40); however, the key channels responsible for H2O2-induced Ca2+ influx in LMVECs were unclear. Our initial inhibitor studies performed in MLMVEC served to determine the category of channels that may be responsible for H2O2-induced Ca2+ influx in LMVECs. Neither SFK96365-sensitive channels nor T-type Ca2+ channels appear to be important players in the H2O2-induced [Ca2+]i response, as pharmacologic inhibition of these channels had no significant effect. In contrast, RuR diminished H2O2-induced Ca2+ influx in MLMVEC, suggesting TRPV4 or some other RuR-sensitive channel as the channel responsible in this cell type. To our knowledge a role for TRPV4 in oxidant-induced Ca2+ influx has not been previously described. Although TRPV4 has been studied extensively in MLMVEC, less is known about TRPV4 localization and function in HLMVEC. The presence of a large Ca2+ response to GSK in HLMVEC functionally confirmed our immunoblot data, demonstrating presence of TRPV4 in these cells. Additionally, RuR also inhibited the H2O2-induced Ca2+ influx in HLMVEC. However, it should be noted that RuR also inhibits other cation channels, and has been reported to both activate and inhibit sarcoplasmic reticulum and mitochondrial channels (16, 57, 71). To more firmly establish the role of TRPV4 in H2O2-induced Ca2+ influx in HLMVECs, we inhibited TRPV4 with HC-067047, a putative specific inhibitor of TRPV4 previously shown to have minimal off-target effects (19, 69). Coupled with experiments in which TRPV4 was genetically depleted, our data suggest that TRPV4 plays a key role in mediating the acute Ca2+ response to H2O2 in the lung microvasculature.
The fact that TRPV4 appeared to be the major channel responsible for the H2O2-induced increase in [Ca2+]i in human microvascular endothelial cells was somewhat surprising, as prior research had implicated members of the TRPM family (TRPM2 and TRPM4) as redox-sensitive Ca2+ channels that respond to increases in intracellular H2O2 (23, 46). Several explanations exist for this variation in findings. A major difference between the studies demonstrating a role for TRPM2 and our current work is the location in the lung from which cells were derived. It is now widely recognized that there is regional heterogeneity within the pulmonary endothelium, and that LMVECs are phenotypically distinct from other cell types such as pulmonary conduit or systemic endothelial cells (11, 14, 21, 51). Our results suggest that this heterogeneity may extend to the channels and/or mechanisms by which H2O2 induces barrier disruption, with TRPM2 playing a primary role in proximal, and TRPV4 a larger role, in microvascular endothelial cells. The type of Ca2+ entry (i.e., store-operated entry vs. receptor-operated Ca2+ influx) as well as the specific Ca2+ channel responsible is likely stimulus dependent; thus our findings of TRPV4 involvement are most applicable to physiologic states where ROS are delivered to the endothelium from exogenous sources, such as circulating inflammatory cells. Furthermore, it is possible that more than one Ca2+ channel is responsible for the change in [Ca2+]i seen in our experiments. For example, TRP channels can form heterotetramers, and thus it is possible that the observed Ca2+ influx occurs because of functional coupling of TRPV4 with other TRP family members (i.e., TRPM2, TRPC1, and TRPC6) as well as non-TRP channels such as Ca2+-activated K+ channels (30, 39). Lastly, Ca2+ responses in endothelial cells may be temporally heterogeneous; H2O2-induced Ca2+ influx in LMVECs may involve multiple channels depending on the dose of the stimulus and the time point studied. Thus the endothelial Ca2+ response to ROS may involve additional Ca2+ channels at later time points and/or sequential activation of multiple channels.
Our data demonstrate not only that TRPV4 is important for H2O2-mediated Ca2+ responses and permeability, but also that activation of TRPV4 is sufficient to induce barrier disruption in HLMVECs. The decrease in TER induced by TRPV4 agonists was qualitatively and quantitatively similar in profile to that induced by exogenous H2O2. Taken with our ECIS experiments showing that TRPV4 inhibition attenuated H2O2-induced decreases in TER in HLMVEC, these data suggest that TRPV4-mediated Ca2+ influx modulates the pulmonary microvascular barrier, at least in vitro. One caveat in comparing the [Ca2+]i and TER experiments is that since serum can quench H2O2 (12), we used serum-free media during our ECIS experiments. Unfortunately, HC-067047 did not dissolve easily in our media buffer, resulting in the need to use a higher dose for the ECIS compared with [Ca2+]i experiments. Thus an off-target effect of HC-067047 cannot be entirely ruled out. Additionally, since endothelial monolayers lack smooth muscle cell interactions, laminar flow, and the presence of circulating immune cells, in vivo experiments will be required to determine the role of TRPV4 in oxidant-induced increases in microvascular endothelial permeability in the intact lung.
TRPV4 is activated by multiple stimuli such as heat, osmolarity, stretch, and internal cell signaling molecules, such as the arachidonic acid metabolite epoxyeicosatrienoic acid, or EET (4, 48, 63, 68). In our study, [Ca2+]i measurements were performed in a temperature-controlled chamber with laminar flow, and measurements were obtained after a period of perfusion with Krebs buffer solution. The exact experimental conditions are relevant given TRPV4 is known to have varying stimulus sensitivity based on temperature (22) and endothelial cells are known to undergo flow-adaptive changes in cytosolic Ca2+ in response to tonic shear stress from laminar flow. In addition, site-specific regulation of TRPV4 has been previously shown in transfected cell systems (61, 64); the responsiveness of TRPV4 to certain stimuli can be experimentally abolished by site-based mutagenesis of various phosphorylation sites (61, 63). For instance, mutation of the putative SFK phosphorylation site Y110 renders TRPV4 resistant to activation by internal cell signals and shear stress (64). However, the exact SFK involved in regulating TRPV4 function in the endothelium is not known. In neuronal tissues, the SFK member, Lyn, was found to participate in Ca2+ signaling by phosphorylating TRPV4 (3). On the other hand, we have previously shown that genetic deletion of Fyn attenuated ROS-induced barrier dysfunction in MLMVEC (5). These observations, along with our current data showing significant loss of Ca2+ influx in response to H2O2 in Fyn−/− MLMVECs, suggested that Fyn inhibition would attenuate H2O2-induced Ca2+ influx in HLMVECs. Surprisingly, short-term pretreatment of HLMVECs with SFK inhibitor SU-6656 did not significantly alter H2O2-induced Ca2+ influx. Moreover, no increase in TRPV4 phosphorylation was observed upon challenge with H2O2, indicating that acute phosphorylation of TRPV4 by Fyn was not required for direct activation of TRPV4 by H2O2. However, TRPV4 protein turnover has been reported to occur slowly (24), and thus led us to speculate that the differential effect of genetic deletion and acute inhibition of Fyn might be due to baseline Fyn-dependent phosphorylation of TRPV4 being necessary, but not sufficient, to induce Ca2+ influx. In this case, we reasoned that activation of TRPV4 by H2O2 would require both basal phosphorylation and subsequent activation of TRPV4 via another mechanism. Indeed, inhibition of Fyn for a longer period of time (48 h) reduced phosphorylation, but not total membrane expression, of TRPV4 and attenuated H2O2-induced Ca2+ influx.
One limitation of the current data is that it is not possible to fully elucidate whether Fyn phosphorylates TRPV4 directly or if an intermediate kinase is involved; further investigations focused on delineating the physical interactions between TRPV4 and Fyn or the possible role of kinases downstream of Fyn will be useful in this regard. Interestingly, exogenous agonism of TRPV4 was not altered by prolonged SFK inhibition; this channel behavior is in concordance with several prior studies that have suggested that SFK-mediated phosphorylation of TRPV4 is necessary for activation by some stimuli but not exogenous agonists (3, 64, 70). While off-target effects of SU, which can target all SFK members, cannot be excluded, our hypothesis that Fyn is responsible for phosphorylation of TRPV4 is supported by the lack of H2O2-induced increases in [Ca2+]i in experiments performed in MLMVECs from Fyn−/− mice and HLMVECs in which Fyn was silenced.
The exact mechanism by which H2O2 activates TRPV4 is still under investigation. One possibility is that ROS activates Ca2+-independent phospholipase A2 (32), leading to production of arachidonic acid (AA) metabolites which are known to directly activate TRPV4 (63). Though our data suggest a role for membrane-bound Fyn and basal phosphorylation of TRPV4 in transduction of ROS signaling, prior work has shown that Fyn also functions in a protective role through effects on focal adhesion kinase phosphorylation in response to thrombin-induced endothelial injury (45). Since Fyn has been shown to translocate to various domains within the cell (10, 28, 45), we hypothesize that Fyn may function in both injurious or protective roles depending on the stimulus involved and specific cellular localization. Additionally, though our data suggest a role for basal Fyn activation, it is known that the activity of Fyn can be modulated by phosphorylation at Tyr residues 419 and 529 (10, 42). Thus it is entirely possible that stimulus-specific changes in Fyn phosphorylation may determine downstream effects of Fyn unrelated to our proposed effect on basal TRPV4 phosphorylation.
In the current study, we specifically focused on the early Ca2+ influx that occurs within 5–10 min of exposure to exogenous H2O2 in LMVECs. That blockade of TRPV4 or Fyn significantly reduced the H2O2-induced change in TER suggests that Fyn-dependent activation of TRPV4 is an initiating event and likely a major factor governing the initial barrier response to oxidant injury. However, it is well known that ROS activates many signaling pathways, and it is likely that a myriad of other kinase and Ca2+ channel pathways, as well as calcium-independent mechanisms, may also play a role during both the time interval studied as well as at later time points. Further studies utilizing time-specific blockade of various channels, as well as studies utilizing inhibitors of non-Ca2+-dependent ROS pathways, will be required to further dissect the sequence of Ca2+ channels that are likely activated by ROS in endothelial cells and to more firmly establish the role of ROS-induced Ca2+ influx in the overall ROS response.
In conclusion, our data suggest that TRPV4 plays a key role in the early rise in [Ca2+]i in mouse and human LMVECs following exposure to H2O2, and that inhibiting TRPV4-mediated Ca2+ influx attenuates barrier dysfunction in response to H2O2 in vitro. Furthermore, our data suggest a novel role for Fyn in regulating the function of TRPV4 through phosphorylation. Although these results provide insight into some of the mechanisms that regulate Ca2+ signaling in vitro, further work is needed to determine whether other TRP channels also contribute to H2O2-induced Ca2+ influx and the role of TRPV4 in oxidant injury and barrier function in vivo.
GRANTS
Support for this study was provided by National Heart, Lung, and Blood Institute Grants F32HL-124930-02, T32HL-007534, R01HL-73859, and R01HL-075078.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.S., D.B.P., and L.A.S. conception and design of research; K.S., L.E.S., J.R., S.B., and C.U. performed experiments; K.S., L.E.S., J.R., and S.B. analyzed data; K.S., J.R., M.J.C., and L.A.S. interpreted results of experiments; K.S. prepared figures; K.S. drafted manuscript; K.S., M.J.C., D.B.P., and L.A.S. edited and revised manuscript; M.J.C., D.B.P., and L.A.S. approved final version of manuscript.
REFERENCES
- 1.Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486: 252–256, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ahmmed GU, Mehta D, Vogel S, Holinstat M, Paria BC, Tiruppathi C, Malik AB. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J Biol Chem 279: 20941–20949, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci 28: 1046–1057, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anidi IU, Servinsky LE, Rentsendorj O, Stephens RS, Scott AL, Pearse DB. CD36 and Fyn kinase mediate malaria-induced lung endothelial barrier dysfunction in mice infected with Plasmodium berghei. PloS One 8: e71010, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baird BR, Cheronis JC, Sandhaus RA, Berger EM, White CW, Repine JE. O2 metabolites and neutrophil elastase synergistically cause edematous injury in isolated rat lungs. J Appl Physiol (1985) 61: 2224–2229, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L158–L172, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brigham KL, Meyrick B, Berry LC Jr, Repine JE. Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol (1985) 63: 840–850, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Chabot F, Mitchell JA, Gutteridge JM, Evans TW. Reactive oxygen species in acute lung injury. Eur Respir J 11: 745–757, 1998. [PubMed] [Google Scholar]
- 10.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol 5: 155–160, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 276: L41–L50, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Chi AY, Waypa GB, Mungai PT, Schumacker PT. Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid Redox Signal 12: 603–610, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium: calcium channels that regulate barrier function. Antioxid Redox Signal 11: 765–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark J, Alvarez DF, Alexeyev M, King JA, Huang L, Yoder MC, Stevens T. Regulatory role for nucleosome assembly protein-1 in the proliferative and vasculogenic phenotype of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 294: L431–L439, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Colton C, Wilt S, Gilbert D, Chernyshev O, Snell J, Dubois-Dalcq M. Species differences in the generation of reactive oxygen species by microglia. Mol Chem Neuropathol 28: 15–20, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Corbalan-Garcia S, Teruel JA, Gomez-Fernandez JC. Characterization of ruthenium red-binding sites of the Ca(2+)-ATPase from sarcoplasmic reticulum and their interaction with Ca(2+)-binding sites. Biochem J 287: 767–774, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cutler RG. Oxidative Stress and Aging. Basel, Switzerland: Birkhauser Verlag, 1995. [Google Scholar]
- 18.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, Hayward NJ, McNamara CR, Xue F, Moran MM, Strassmaier T, Uykal E, Owsianik G, Vennekens R, De Ridder D, Nilius B, Fanger CM, Voets T. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A 107: 19084–19089, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123: 887–902, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res 68: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hamanaka K, Jian MY, Weber DS, Alvarez DF, Townsley MI, Al-Mehdi AB, King JA, Liedtke W, Parker JC. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol 293: L923–L932, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Hecquet CM, Ahmmed GU, Vogel SM, Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res 102: 347–355, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Hills CE, Bland R, Squires PE. Functional expression of TRPV4 channels in human collecting duct cells: implications for secondary hypertension in diabetic nephropathy. Exp Diabetes Res 2012: 936518, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hisatsune C, Kuroda Y, Nakamura K, Inoue T, Nakamura T, Michikawa T, Mizutani A, Mikoshiba K. Regulation of TRPC6 channel activity by tyrosine phosphorylation. J Biol Chem 279: 18887–18894, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Huang BK, Sikes HD. Quantifying intracellular hydrogen peroxide perturbations in terms of concentration. Redox Biol 2C: 955–962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda S, Harita Y, Shibagaki Y, Sekine T, Igarashi T, Inoue T, Hattori S. Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-gamma1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell 22: 1824–1835, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaspar JW, Jaiswal AK. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J 25: 1076–1087, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawasaki BT, Liao Y, Birnbaumer L. Role of Src in C3 transient receptor potential channel function and evidence for a heterogeneous makeup of receptor- and store-operated Ca2+ entry channels. Proc Natl Acad Sci U S A 103: 335–340, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin MT, Jian MY, Taylor MS, Cioffi DL, Yap FC, Liedtke W, Townsley MI. Functional coupling of TRPV4, IK, and SK channels contributes to Ca(2+)-dependent endothelial injury in rodent lung. Pulm Circ 5: 279–290, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. J Surg Res 143: 70–77, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez J, Moreno JJ. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Arch Biochem Biophys 392: 257–262, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Mastore M, Kohler L, Nappi AJ. Production and utilization of hydrogen peroxide associated with melanogenesis and tyrosinase-mediated oxidations of DOPA and dopamine. Febs J 272: 2407–2415, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L919–L930, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Moldovan L, Mythreye K, Goldschmidt-Clermont PJ, Satterwhite LL. Reactive oxygen species in vascular endothelial cell motility. Roles of NAD(P)H oxidase and Rac1. Cardiovasc Res 71: 236–246, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Morty RE, Kuebler WM. TRPV4: an exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol 307: L817–L821, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31: 3531–3545, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh J, Kapela A, Tsoukias NM. Stochastic model of endothelial TRPV4 calcium sparklets: effect of bursting and cooperativity on EDH. Biophys J 108: 1566–1576, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearse DB, Shimoda LA, Verin AD, Bogatcheva N, Moon C, Ronnett GV, Welsh LE, Becker PM. Effect of cGMP on lung microvascular endothelial barrier dysfunction following hydrogen peroxide. Endothelium 10: 309–317, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med 155: 479–484, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Resh MD. Fyn, a Src family tyrosine kinase. Int J Biochem Cell Biol 30: 1159–1162, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: a novel molecular target in cancer. Cancer 116: 1629–1637, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saksena S, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Role of Fyn and PI3K in H2O2-induced inhibition of apical Cl-/OH- exchange activity in human intestinal epithelial cells. Biochem J 416: 99–108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarmiento D, Montorfano I, Cerda O, Cáceres M, Becerra A, Cabello-Verrugio C, Elorza AA, Riedel C, Tapia P, Velásquez LA, Varela D, Simon F. Increases in reactive oxygen species enhance vascular endothelial cell migration through a mechanism dependent on the transient receptor potential melastatin 4 ion channel. Microvasc Res 98: 187–196, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Shasby DM, Vanbenthuysen KM, Tate RM, Shasby SS, McMurtry I, Repine JE. Granulocytes mediate acute edematous lung injury in rabbits and in isolated rabbit lungs perfused with phorbol myristate acetate: role of oxygen radicals. Am Rev Respir Dis 125: 443–447, 1982. [DOI] [PubMed] [Google Scholar]
- 48.Sidhaye VK, Guler AD, Schweitzer KS, D'Alessio F, Caterina MJ, King LS. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc Natl Acad Sci U S A 103: 4747–4752, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stephens RS, Rentsendorj O, Servinsky LE, Moldobaeva A, Damico R, Pearse DB. cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 299: L323–L333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens RS, Servinsky LE, Rentsendorj O, Kolb TM, Pfeifer A, Pearse DB. Protein kinase G increases antioxidant function in lung microvascular endothelial cells by inhibiting the c-Abl tyrosine kinase. Am J Physiol Cell Physiol 306: C559–C569, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc 8: 453–457, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Tate RM, Vanbenthuysen KM, Shasby DM, McMurtry IF, Repine JE. Oxygen-radical-mediated permeability edema and vasoconstriction in isolated perfused rabbit lungs. Am Rev Respir Dis 126: 802–806, 1982. [DOI] [PubMed] [Google Scholar]
- 53.Tauseef M, Knezevic N, Chava KR, Smith M, Sukriti S, Gianaris N, Obukhov AG, Vogel SM, Schraufnagel DE, Dietrich A, Birnbaumer L, Malik AB, Mehta D. TLR4 activation of TRPC6-dependent calcium signaling mediates endotoxin-induced lung vascular permeability and inflammation. J Exp Med 209: 1953–1968, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4: 159ra148, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 13: 693–708, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol 39: 173–185, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J 79: 39–50, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71: 226–235, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Varani J, Ward PA. Mechanisms of neutrophil-dependent and neutrophil-independent endothelial cell injury. Biol Signals 3: 1–14, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Veit F, Pak O, Brandes RP, Weissmann N. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: focus on ion channels. Antioxid Redox Signal 22: 537–552, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A 101: 396–401, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 27: 337–349, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Wegierski T, Lewandrowski U, Muller B, Sickmann A, Walz G. Tyrosine phosphorylation modulates the activity of TRPV4 in response to defined stimuli. J Biol Chem 284: 2923–2933, 2009. [DOI] [PubMed] [Google Scholar]
- 65.Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M, Brandes RP, Grimminger F, Meissner M, Freichel M, Offermanns S, Veit F, Pak O, Krause KH, Schermuly RT, Brewer AC, Schmidt HH, Seeger W, Shah AM, Gudermann T, Ghofrani HA, Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun 3: 649, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolfram Kuhlmann CR, Wiebke Lüdders D, Schaefer CA, Kerstin Most A, Backenköhler U, Neumann T, Tillmanns H, Erdogan A. Lysophosphatidylcholine-induced modulation of Ca(2+)-activated K(+)channels contributes to ROS-dependent proliferation of cultured human endothelial cells. J Mol Cell Cardiol 36: 675–682, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Wu F, Szczepaniak WS, Shiva S, Liu H, Wang Y, Wang L, Kelley EE, Chen AF, Gladwin MT, McVerry BJ. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L987–L997, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu S, Jian MY, Xu YC, Zhou C, Al-Mehdi AB, Liedtke W, Shin HS, Townsley MI. Ca2+ entry via α1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol 297: L650–L657, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol 305: C704–C715, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a transient receptor potential (TRP) channel by tyrosine phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem 278: 11520–11527, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Xu L, Tripathy A, Pasek DA, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem 274: 32680–32691, 1999. [DOI] [PubMed] [Google Scholar]