Abstract
During supine passive heating, increases in skin blood flow (SkBF) and cardiac output (Qc) are both blunted in older adults. The aim here was to determine the effect of acutely correcting the peripheral vasodilatory capacity of aged skin on the integrated cardiovascular responses to passive heating. A secondary aim was to examine the SkBF-Qc relation during hyperthermia in the presence (upright posture) and absence (dynamic exercise) of challenges to central venous pressure. We hypothesized that greater increases in SkBF would be accompanied by greater increases in Qc. Eleven healthy older adults (69 ± 3 yr) underwent supine passive heating (0.8°C rise in core temperature; water-perfused suit) after ingesting sapropterin (BH4, a nitric oxide synthase cofactor; 10 mg/kg) or placebo (randomized double-blind crossover design). Twelve young (24 ± 1 yr) subjects served as a comparison group. SkBF (laser-Doppler flowmetry) and Qc (open-circuit acetylene wash-in) were measured during supine heating, heating + upright posture, and heating + dynamic exercise. Throughout supine and upright heating, sapropterin fully restored the SkBF response of older adults to that of young adults but Qc remained blunted. During heat + upright posture, SkBF failed to decrease in untreated older subjects. There were no age- or treatment-related differences in SkBF-Qc during dynamic exercise. The principal finding of this study was that the blunted Qc response to passive heat stress is directly related to age as opposed to the blunted peripheral vasodilatory capacity of aged skin. Furthermore, peripheral impairments to SkBF in the aged may contribute to inapposite responses during challenges to central venous pressure during hyperthermia.
Keywords: heat stress, tilt, age, cutaneous vasodilation, central venous pressure, exercise
elderly adults, even in the absence of overt cardiovascular pathology, contribute disproportionately to heat-induced cardiac morbidity and mortality during environmental heat waves (9, 41, 48). Physiological responses to whole body heat exposure include precise adjustments in both peripheral cutaneous and central mechanisms designed to subserve both thermoregulation and the maintenance of blood pressure (BP). Age-related impairments in the integrated responses to increases in core temperature may directly contribute to the clinical relation between heat exposure and increased cardiovascular-related mortality with primary aging (25).
The integrated cardiovascular responses of young healthy adults to supine passive whole body heat stress involve increases in skin blood flow (SkBF) as high as 7-8 l/min (31, 38). To support this pronounced increase in cutaneous vascular conductance, cardiac output (Qc) and heart rate (HR) increases, stroke volume stays the same or increases modestly, and vascular conductance of non-cutaneous circulatory beds decreases (31, 37–39). Healthy human aging is associated with significant alterations in both the peripheral and central responses to increasing core and skin temperatures. Increases in SkBF during passive heating are substantially blunted in aged adults (16, 31, 32). Furthermore, the increase in Qc during whole body heat stress is likewise reduced in aged adults, due in part to a relative inability to maintain stroke volume (31, 32). Although the absolute chronotropic response may (13, 31, 32) or may not (31) differ with advanced age, older adults rely on a greater percentage of their heart rate reserve during whole body heat stress (31). Older adults also redistribute less blood from the renal and splanchnic vascular beds when passively heated to their thermal tolerance (31, 32).
Despite the clear attenuations in both the peripheral vascular and central hemodynamic responses to passive heating in healthy older adults, an unresolved debate centers on whether cardiac limitations (i.e., blunted increases in Qc) or peripheral vascular limitations (i.e., age-related cutaneous vascular dysfunction), or both, contribute to the relative inability of older adults to increase SkBF during heat stress. Posed another way, does the attenuated increase in Qc reflect an effect of primary aging on cardiac function when central venous pressure (CVP) falls during heat stress or is the smaller increase in Qc in accord with the smaller increase in blood flow to aged skin?
Whether or not impairments in cardiac responses contribute to the attenuated increase in SkBF during heat stress in healthy aging (31), peripheral microvascular dysfunction clearly limits cutaneous vasodilation during passive heating in older adults (16, 27, 44). The mechanisms mediating the age-related impairments in reflex cutaneous vasodilation during hyperthermia are due, in large part, to reductions in nitric oxide (NO) bioavailability (16, 18, 19, 43, 44). In the aged vasculature, reduced bioavailability of tetrahydrobiopterin (BH4), a critical enzymatic cofactor for the production of NO (33, 49), contributes to attenuated reflex cutaneous vasodilation during passive heating (44, 46). Oral administration of sapropterin (pharmaceutical grade BH4) acutely improves reflex cutaneous vasodilation during supine passive heating in older adults (44).
The influence that this restoration of peripheral cutaneous vascular function may have on cardiac hemodynamics during supine heating remains unclear but presents a unique experimental paradigm. The present study aimed to determine the effect of correcting the peripheral vascular limitation to reflex cutaneous vasodilation on the integrated cardiovascular response to passive heating in older adults. We hypothesized that normalized cutaneous vascular function (acute oral sapropterin) and subsequently greater increases in SkBF during supine passive heat stress would be accompanied by greater increases in Qc in older adults, an outcome that would be interpreted as a lack of a direct cardiac limitation to peripheral cutaneous vasodilation with aging. Furthermore, since this sapropterin-induced acute improvement of the SkBF response to passive heat stress has only been documented in the absence of a challenge to central hemodynamics (i.e., supine posture) (44), a secondary goal of this study was to determine the effect of normalized reflex cutaneous vasodilation on the integrated cardiovascular responses to heat stress in the presence (upright posture) and absence (engaging the muscle pump during dynamic exercise) of further challenges to CVP in healthy older adults.
METHODS
Subjects.
All experimental procedures and protocols were approved by The Pennsylvania State University Institutional Review Board, and verbal and written consent were obtained from all subjects prior to participation. The study conformed to the standards outlined in the Declaration of Helsinki. Twelve young (24 ± 1 yr) and 11 older (69 ± 3 yr) apparently healthy adults participated in the study. Subjects were screened for neurological, cardiovascular, and dermatological diseases and underwent a complete medical screening including a resting 12-lead electrocardiogram, physical examination, and 12-h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA). All subjects were normotensive (resting systolic BP: <140 mmHg and diastolic BP: <90 mmHg), nondiabetic, normally active, and not taking over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g., statins, anticoagulants, antidepressants, etc.). Subjects were nonobese (body mass index: <30 kg/m−2) and did not use tobacco products. Subject groups were matched for body size and resting BP. Older women taking hormone replacement therapy or who had recently taken hormone replacement therapy were excluded and young women were tested during the early follicular phase of their menstrual cycle or during the placebo phase if using oral contraceptives. All subjects were familiarized with the equipment and experimental protocol before the testing visits.
Before the experimental session, subjects abstained from caffeinated and alcoholic beverages for 12 h and strenuous physical activity for 24 h. All protocols were performed in a thermoneutral climate-controlled chamber (22°C), and all testing occurred in the morning to reduce diurnal variation in blood flow responses (2). Studies were conducted between the months of November and April to limit any potential effects of heat acclimation. All subjects were provided a standardized breakfast meal on the morning of the experiment. For the older adults, study days were separated by at least 48 h to ensure adequate washout of the experimental drug (11). Older subjects ingested either 10 mg/kg body wt sapropterin (Kuvan; BioMarin Pharmaceutical, Novato, CA) or placebo (encapsulated ascorbate and cellulose) ∼2 h before the experiment in a double-blind, randomized crossover design. Kuvan tablets are formulated with 100 mg sapropterin and 5 mg ascorbate; therefore, the placebo treatment consisted of an equivalent dosage of ascorbate (0.5 mg ascorbate/kg−1). We have previously shown that this dosage of sapropterin is efficacious at improving reflex cutaneous vasodilation in a similar subject population (44). Young subjects completed one experimental visit (placebo treatment) and did not ingest sapropterin.
Experimental measurements.
Mean skin temperature (Tsk) was controlled using a water-perfused suit that covered the entire body except for the head, hands, feet, and left forearm. Copper-constantan thermocouples were affixed to the surface of the skin at six sites (calf, thigh, abdomen, chest, shoulder, and back) for the continuous measurement of Tsk. Esophageal temperature (Tes) was measured at the level of the left atrium from a copper-constantan thermocouple in the lumen of a sealed pediatric feeding tube. HR was monitored throughout the protocol (Polar Electro, Lake Success, NY), and arterial BP was measured by brachial auscultation every 5 min.
To obtain an index of SkBF, red blood cell flux was continuously measured on the left forearm with a laser-Doppler flowmetry probe placed in a local heating unit (Moor-Lab, Temperature Monitor, SHO2; Moor Instruments, Devon, UK); this arm was supported at heart level throughout the protocol. To ensure that changes in cutaneous blood flow were reflex in origin, the local heater was clamped at 33°C throughout the protocol. Thus this research strategy specifically isolated reflex mechanisms of cutaneous vasodilation by eliminating the influence of changes in local Tsk.
Qc was determined using the open-circuit acetylene uptake method (20, 36), which has been validated against the direct Fick technique (20). This technique provides reproducible measures of Qc during exercise in both young and older adults (3). During all experimental conditions, Qc measurements were obtained in triplicate. Paced breathing (20 breaths/min) was used during the measurement of Qc at rest in both postures, as described below. Pulmonary oxygen uptake (V̇o2) was measured using analysis of expired gases. Subjects were encouraged to breathe in a regular rhythm, avoiding coughs, swallows, and partial breaths. In a subset of older subjects (n = 2), there was acceptable test-retest reliability in Qc measured repeatedly during supine passive heating during placebo treatment (r2 = 0.82, P < 0.05).
Experimental protocol.
After instrumentation, subjects were seated in an upright posture on a customized adjustable cycle ergometer that allowed for passive, automated movement between supine rest or cycle exercise with the lower legs parallel to the floor at extension and upright rest or cycle exercise with the lower legs perpendicular to the floor at flexion. Throughout baseline, thermoneutrality was maintained by circulating ∼30°C water through the suit. After 10 min of quiet rest, baseline measurements of SkBF and Qc were obtained while subjects were in the seated upright posture. Thereafter, subjects were reclined and baseline measures of all variables were repeated in the supine posture.
Following 10 min of thermoneutral baseline, warm water (52°C) was perfused through the suit to clamp Tsk at ∼39°C and to cause a gradual rise in Tes. Whole body heating began exactly 3 h after pill ingestion, a time chosen to ensure peak plasma concentrations of sapropterin during heating and for consistency with previous studies (11, 44, 45). Qc was measured when Tes increased by 0.4 and 0.8°C. At a 0.8°C increase in Tes, mean body temperature was adjusted such that Tes plateaued at ΔTes = 0.8°C; Tes was clamped at this increase during upright posture and dynamic exercise. Thereafter, subjects were passively moved to the upright seated posture and the leg support was removed; after HR stabilized, measurements in the upright seated posture were performed.
Subjects then began cycling at a fixed power output based on body mass and body surface area (AD), as previously described in detail (5), to ensure that metabolic heat production and further changes in Tes were not different between subject groups and for a duration short enough to limit further increases in Tes. Pedaling rate was maintained by the subjects using a visual-feedback display. Subjects exercised for at least 5 min until a steady-state HR and V̇o2 were achieved, at which time Qc was again measured. After upright cycling, subjects were returned to the supine posture, cycle exercise was performed as described above, and all measurements were again obtained. During both upright and supine cycling, Qc measurements were obtained at the natural respiratory rate of the subject. Throughout all phases of the experimental protocol, red cell flux was continuously measured.
At the completion of the protocol, whole body heating was terminated, ∼30°C water was circulated through the water-perfused suit, and subjects were returned to thermoneutrality. Maximal SkBF was then obtained by increasing the local Tsk (43°C) over the site of measurement (21).
Data analysis.
SkBF, Tsk, and Tes data were recorded at 40 Hz (WinDaq; Dataq Instruments, Akron, OH). Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by mean arterial pressure (MAP; calculated as diastolic pressure plus one-third pulse pressure) and expressed as both an absolute CVC and as a percentage of maximal CVC (%CVCmax). CVC was calculated during stable periods of laser-Doppler flux during upright and supine thermoneutral baseline, at each 0.1°C increase in Tes during passive heating, during upright heat stress, and during upright and supine cycle exercise in the heat. Maximal CVC was calculated from a stable plateau in laser-Doppler flux when local Tsk was increased to 43°C at the end of the experiment. Qc and V̇o2 were analyzed immediately after each maneuver using customized and validated software (20, 36). Stroke volume was calculated as Qc·HR−1. Total peripheral resistance (TPR) was calculated as Qc·MAP−1, and systemic vascular conductance was calculated as the reciprocal of TPR. Heart rate reserve was determined as the difference between age-predicted maximal HR [208 − (0.7·age)] (47) and resting HR.
Statistical analysis.
Based on our previous data (44), 10 subjects per group would provide sufficient power (α = 0.05, P = 0.80) to detect a meaningful physiological effect of both age and sapropterin treatment. Student's t-tests were used to detect age differences in subject characteristics. Three separate two-way repeated-measures mixed-model ANOVAs were used to detect age and treatment differences in all variables at each phase of the experiment (SAS version 9.3; Cary, NC). Post hoc comparisons with Bonferroni corrections were performed for planned comparisons. The level of significance was set at α = 0.05 for main effects. Values are presented as means ± SE.
RESULTS
Subject characteristics are presented in Table 1. The age groups were well matched for anthropometric characteristics, resting BP, and blood biochemistry. Although HbA1c was significantly higher in older adults, these values were within the normal recommended age-specific range. Absolute exercise intensity was not different between groups (young: 59 ± 1 W vs. older: 60 ± 1 W; P = 0.27). Absolute Tes and mean Tsk for each experimental phase are presented in Table 2. By study design, Tes increased 0.8°C during supine passive heating and remained elevated during upright posture and dynamic exercise (Table 2). There were no age- or treatment-related differences in either Tes or mean Tsk (Table 2).
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| n (male, female) | 12 (6,6) | 11 (7, 4) |
| Age, yr | 24 ± 1 | 69 ± 3* |
| Height, cm | 172 ± 3 | 173 ± 3 |
| Mass, kg | 70 ± 3 | 76 ± 5 |
| Body surface area, m2 | 1.8 ± 0.1 | 1.9 ± 0.1 |
| SBP, mmHg | 118 ± 4 | 125 ± 3 |
| DBP, mmHg | 73 ± 2 | 76 ± 2 |
| Total cholesterol, mg/dl | 175 ± 12 | 183 ± 11 |
| HDL, mg/dl | 63 ± 4 | 59 ± 6 |
| LDL, mg/dl | 94 ± 11 | 109 ± 7 |
| HbA1c,% | 5.40 ± 0.01 | 5.60 ± 0.01* |
| Fasting glucose, mg/dl | 88 ± 2 | 93 ± 2 |
Values are means ± SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
P < 0.05 vs. young.
Table 2.
Esophageal and mean skin temperature at each experimental phase
| Condition |
|||||||
|---|---|---|---|---|---|---|---|
| Thermoneutral |
ΔTes = 0.4°C |
ΔTes = 0.8°C |
|||||
| Upright | Supine | Supine | Supine | Upright | Upright exercise | Supine exercise | |
| Tes, °C | |||||||
| Young | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.6 ± 0.1 | 38.0 ± 0.1 | 38.0 ± 0.1 | 38.0 ± 0.1 | 38.1 ± 0.1 |
| Older placebo | 36.8 ± 0.1 | 36.8 ± 0.1 | 37.2 ± 0.1 | 37.6 ± 0.1 | 37.6 ± 0.1 | 37.7 ± 0.2 | 37.7 ± 0.2 |
| Older sapropterin | 36.8 ± 0.1 | 36.8 ± 0.1 | 37.2 ± 0.1 | 37.6 ± 0.1 | 37.6 ± 0.1 | 37.7 ± 0.2 | 37.7 ± 0.2 |
| Mean Tsk, °C | |||||||
| Young | 34.2 ± 0.2 | 34.1 ± 0.1 | 37.9 ± 0.2 | 38.3 ± 0.3 | 37.2 ± 0.2 | 36.7 ± 0.3 | 36.9 ± 0.2 |
| Older placebo | 34.3 ± 0.1 | 34.3 ± 0.1 | 38.1 ± 0.1 | 38.0 ± 0.1 | 37.3 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.2 |
| Older sapropterin | 34.2 ± 0.1 | 34.1 ± 0.1 | 38.0 ± 0.2 | 38.0 ± 0.2 | 37.3 ± 0.2 | 37.0 ± 0.2 | 36.8 ± 0.2 |
Values are means ± SE. Tes, esophageal temperature; Tsk, skin temperature.
Sapropterin restored cutaneous vasodilatory capacity.
At a thermoneutral baseline, there were no age- or treatment-related differences in CVC in either the upright or supine posture (Figs. 1 and 2A). Compared with young adults, reflex cutaneous vasodilation was significantly blunted throughout supine passive heating in older adults during placebo treatment, illustrated in Fig. 1 for each 0.1°C increase in Tes. By design, acute oral sapropterin fully restored the CVC response to supine passive heat stress in the older adults such that the magnitude of the response was not different from that of young adults (Figs. 1 and 2A). A sapropterin-mediated increase in the SkBF response to whole body heating was observed in all 11 older adults. Furthermore, the augmentation in SkBF with sapropterin was sustained when subjects subsequently sat upright as well as during upright dynamic exercise while hyperthermic (Fig. 2A). There were no age- or treatment-related differences in maximal CVC in response to local heating (young: 2.34 ± 0.28 flux/mmHg, older placebo: 2.68 ± 0.56 flux/mmHg, and older sapropterin: 2.01 ± 0.26 flux/mmHg; P = 0.49). Absolute CVC and flux (data not shown) yielded similar results as when representing the data as %CVCmax.
Fig. 1.
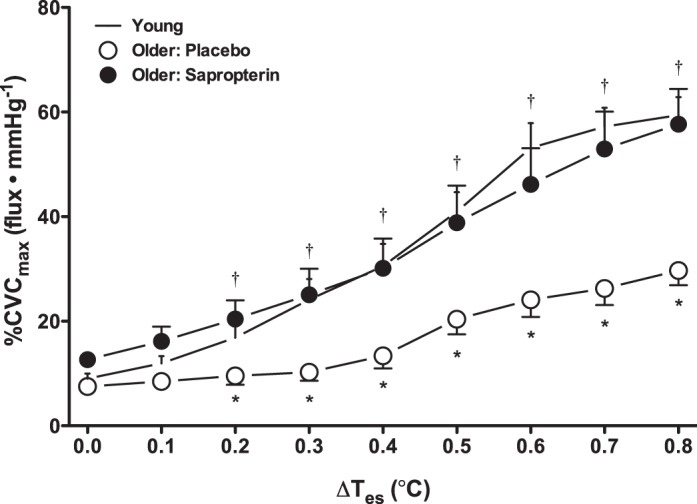
Cutaneous vascular conductance (%CVCmax) during supine passive heating in young (dash symbols) and older adults. The latter were tested after placebo (open symbols) and sapropterin administration (filled symbols). Data are shown at baseline and for each 0.1°C increase in esophageal temperature (Tes) throughout passive whole body heating. The increase in skin blood flow was blunted in older adults during placebo but was fully restored by sapropterin treatment. *P < 0.05, older placebo vs. young; †P < 0.05, older placebo vs. older sapropterin.
Fig. 2.
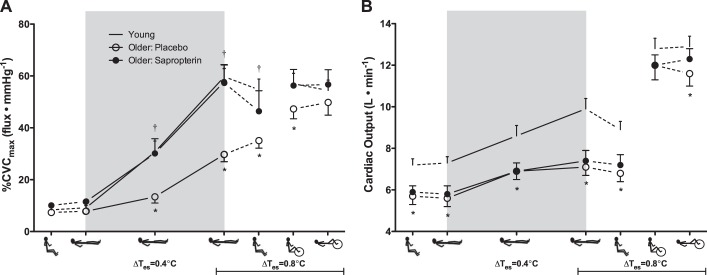
Cutaneous vascular conductance (%CVCmax; A) and cardiac output (B) in young (dash symbols) and older adults (placebo, open symbols; sapropterin, filled symbols). The experimental phases are presented in the order in which the protocol was conducted (upright thermoneutral, supine thermoneutral, supine passive heating, upright posture while heating was sustained, and upright and supine exercise while heating was sustained). Supine passive whole body heating is indicated by the gray box, with data points shown at ΔTes = 0.4 and 0.8°C. *P < 0.05, older placebo vs. young; †P < 0.05, older placebo vs. older sapropterin.
Cardiovascular responses to supine passive heating.
Absolute Qc (Fig. 2B) and the increase in Qc from baseline (young: Δ2.6 ± 0.3 l/min vs. older: Δ1.4 ± 0.2 l/min; P < 0.01) were both significantly lower in the older adults during placebo treatment compared with young adults throughout supine passive heating. Cardiac index (CI = Qc/AD), calculated to examine potential differences in skin-specific Qc, was likewise significantly lower in older adults during placebo treatment throughout supine heating (Table 3). Sapropterin had no effect on Qc or CI (Fig. 2B; Table 3).
Table 3.
Hemodynamic variables at each experimental phase
| Condition |
|||||||
|---|---|---|---|---|---|---|---|
| Thermoneutral |
ΔTes = 0.4°C |
ΔTes = 0.8°C |
|||||
| Upright | Supine | Supine | Supine | Upright | Upright exercise | Supine exercise | |
| SBP, mmHg | |||||||
| Young | 105 ± 3 | 103 ± 3 | 104 ± 3 | 108 ± 3 | 100 ± 2 | 128 ± 4 | 125 ± 4 |
| Older placebo | 116 ± 4* | 114 ± 4* | 111 ± 3* | 115 ± 4* | 105 ± 4 | 144 ± 7* | 142 ± 7* |
| Older sapropterin | 115 ± 3 | 112 ± 3 | 107 ± 3 | 108 ± 3 | 105 ± 5 | 143 ± 8 | 142 ± 6 |
| DBP, mmHg | |||||||
| Young | 66 ± 2 | 62 ± 2 | 59 ± 2 | 57 ± 2 | 54 ± 2 | 54 ± 2 | 55 ± 2 |
| Older placebo | 65 ± 2 | 65 ± 2 | 63 ± 2 | 61 ± 2* | 54 ± 2 | 63 ± 3* | 66 ± 2* |
| Older sapropterin | 65 ± 2 | 64 ± 2 | 61 ± 2 | 60 ± 1 | 56 ± 2 | 62 ± 3 | 65 ± 2 |
| PP, mmHg | |||||||
| Young | 39 ± 2 | 41 ± 2 | 45 ± 3 | 52 ± 4 | 47 ± 2 | 75 ± 4 | 71 ± 5 |
| Older placebo | 50 ± 4* | 50 ± 4* | 49 ± 4 | 54 ± 5 | 50 ± 4 | 81 ± 8 | 75 ± 6 |
| Older sapropterin | 50 ± 4 | 48 ± 3 | 47 ± 3 | 48 ± 4 | 50 ± 4 | 81 ± 9 | 77 ± 6 |
| %HRR, beats/min | |||||||
| Young | 8 ± 2 | — | 6 ± 6 | 18 ± 1 | 30 ± 3 | 46 ± 3 | 45 ± 3 |
| Older placebo | 8 ± 2 | — | 11 ± 2 | 16 ± 2 | 26 ± 3 | 54 ± 6 | 50 ± 5 |
| Older sapropterin | 8 ± 2 | — | 10 ± 1 | 15 ± 2 | 24 ± 3 | 53 ± 5 | 54 ± 5 |
| CI, l/m2 | |||||||
| Young | 4.0 ± 0.1 | 4.0 ± 0.1 | 4.7 ± 0.2 | 5.4 ± 0.2 | 4.9 ± 0.2 | 7.1 ± 0.2 | 7.1 ± 0.3 |
| Older placebo | 3.0 ± 0.2* | 3.0 ± 0.2* | 3.7 ± 0.2* | 3.7 ± 0.2* | 3.6 ± 0.2* | 6.5 ± 0.3* | 6.2 ± 0.3* |
| Older sapropterin | 3.1 ± 0.2 | 3.0 ± 0.1 | 3.6 ± 0.2 | 3.9 ± 0.2 | 3.8 ± 0.2 | 6.3 ± 0.2 | 6.6 ± 0.2 |
| TPR, mmHg·l−1·min | |||||||
| Young | 11 ± 0.6 | 11 ± 0.4 | 9 ± 0.4 | 8 ± 0.3 | 8 ± 0.2 | 6 ± 0.2 | 6 ± 0.2 |
| Older placebo | 15 ± 1.4 | 15 ± 1.4 | 12 ± 0.9 | 12 ± 0.9 | 11 ± 0.7 | 8 ± 0.5 | 8 ± 0.5 |
| Older sapropterin | 14 ± 1.1 | 15 ± 1.1 | 12 ± 0.9 | 11 ± 1.0 | 11 ± 0.9 | 8 ± 0.4 | 7 ± 0.4 |
| SVC, mmHg·l−1·min | |||||||
| Young | 92 ± 5 | 97 ± 5 | 115 ± 6 | 133 ± 6 | 129 ± 5 | 163 ± 5 | 165 ± 6 |
| Older placebo | 73 ± 6* | 71 ± 6* | 88 ± 6* | 91 ± 6* | 96 ± 6* | 134 ± 7* | 128 ± 7* |
| Older sapropterin | 73 ± 5 | 73 ± 5 | 91 ± 6 | 99 ± 8 | 100 ± 8 | 135 ± 6 | 138 ± 6 |
Values are means ± SE. PP, pulse pressure; HRR, heart rate reserve; CI, cardiac index; TPR, total peripheral resistance; SVC, systemic vascular conductance.
P < 0.05, older: placebo vs. young.
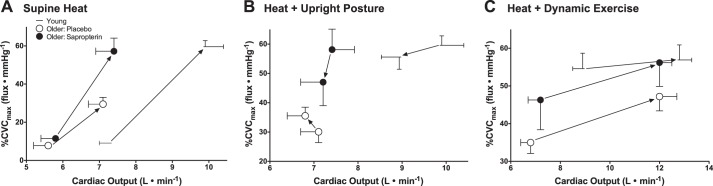
To further examine the impact of age (both with and without restoration of peripheral cutaneous vascular function) on the relation between SkBF and Qc, simultaneous changes in these variables are illustrated in Fig. 3A. Young adults exhibited robust increases in both SkBF and Qc during supine heating, but the response range of each of these variables was significantly attenuated in older adults taking placebo (Fig. 3A). Sapropterin restored the SkBF-Qc relation during heating such that it was not different from young adults without changing the age-specific response range for Qc (Fig. 3A).
Fig. 3.
The change (represented by the solid arrow between data points) in the relation between SkBF and cardiac output for young (dash symbols) and older adults (placebo, open symbols; sapropterin, filled symbols) depicted for each transition between experimental conditions. A: transition from supine thermoneutral to supine heating to ΔTes = 0.8°C; B: transition from supine to upright posture while maintaining ΔTes at 0.8°C; C: transition from upright rest to upright dynamic exercise while maintaining ΔTes at 0.8°C. See text for further description.
HR was significantly lower in older adults during placebo treatment compared with young adults throughout all phases of the experiment (Fig. 4A). The heart rate response to supine passive heating was attenuated in older compared with young adults (young: Δ22 ± 2 beats/min vs. older: Δ15 ± 1 beats/min; P < 0.05); however, there were no age- or treatment-related differences in percent heart rate reserve (Table 3). Stroke volume was lower in older adults during placebo treatment compared with young adults during upright and supine thermoneutrality and throughout supine passive heating (Fig. 4B). Compared with young adults, MAP and systolic BP were greater in older adults during placebo treatment during supine passive heating (Fig. 4C; Table 3). Sapropterin had no effect on HR, stroke volume, or BP during supine passive heating. Systemic vascular conductance was significantly lower in older adults during placebo treatment compared with young adults throughout all phases of the experiment, but there were no age-related differences in TPR (Table 2); sapropterin had no effect on either systemic vascular conductance or TPR (Table 3).
Fig. 4.
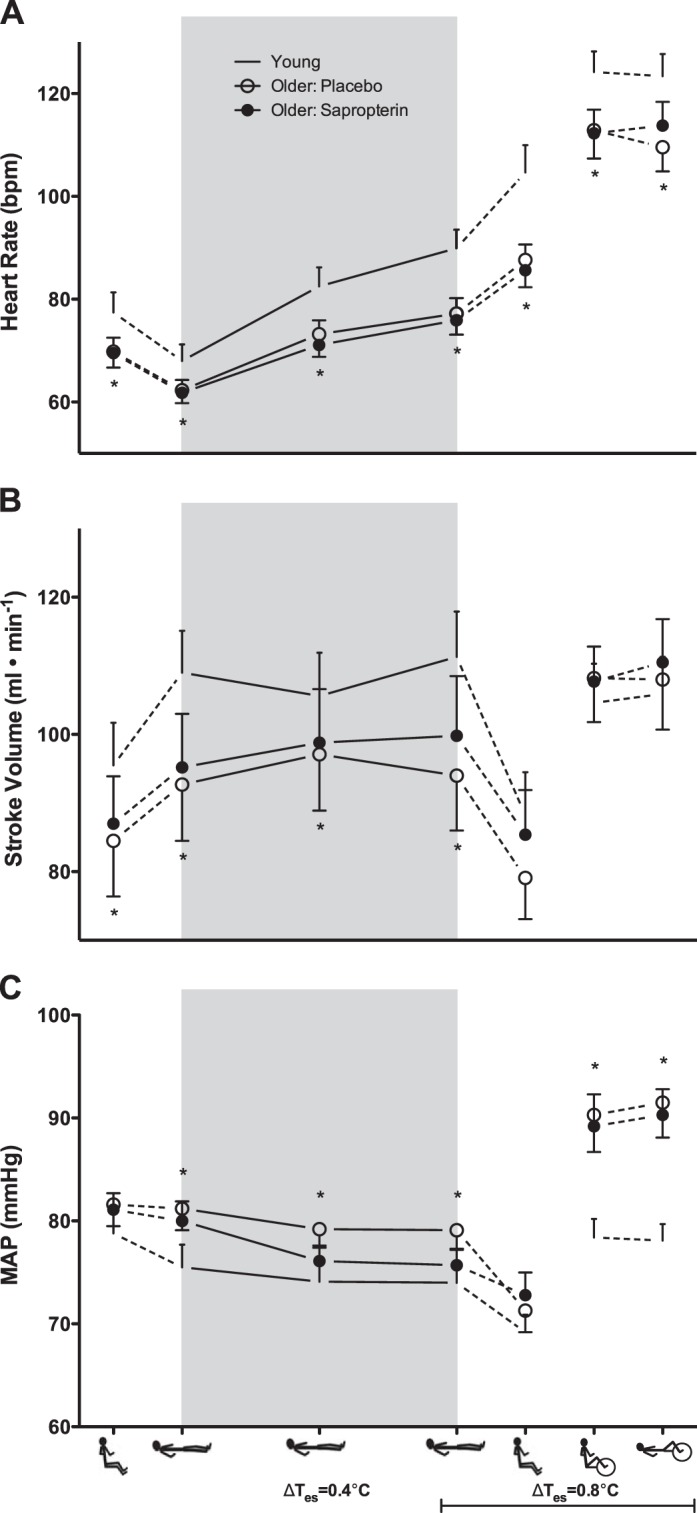
Heart rate (A), stroke volume (B), and mean arterial pressure (MAP; C) in young (dash symbols) and older adults (placebo, open symbols; sapropterin, filled symbols). The experimental conditions are presented in the order in which the protocol was conducted (upright thermoneutral, supine thermoneutral, supine heated, upright heated, upright exercise, and supine exercise). Supine passive heating is indicated by the gray box, with data points shown at ΔTes = 0.4 and 0.8°C. *P < 0.05, older placebo vs. young.
Cardiovascular responses to upright posture during hyperthermia.
During upright heating at a sustained ΔTes of 0.8°C, Qc remained lower in the older adults (Fig. 2B). There was a modest, but significant, fall in Qc upon assuming the upright posture in both the young and the older adults (placebo treatment; P < 0.05 for both), but the reduction tended to be smaller in the older adults (P = 0.08; Fig. 2B). Sapropterin had no effect on Qc during upright posture in the heat (Fig. 2B). The relation between SkBF and Qc during the transition from the supine to the upright posture (at a sustained ΔTes of 0.8°C) is illustrated in Fig. 3B. Young adults exhibited decreases in both SkBF and Qc upon assuming an upright posture as did the sapropterin-treated older group (Fig. 3B). However, in the older placebo trial, SkBF modestly increased as Qc fell, and two older subjects experienced presyncope during this transition.
The tachycardic response to assuming the upright posture while heated was significantly blunted in older adults, and the HR response was not affected by sapropterin (Fig. 4A). There were no age- or treatment-related differences in stroke volume or BP during this posture change (Fig. 4, B and C; Table 3) and sapropterin had no effect on any other cardiovascular variables (Table 3).
Cardiovascular responses to dynamic exercise during hyperthermia.
There were no age- or treatment-related differences in Qc during upright dynamic exercise in the heat (Fig. 2B). The relation between changes in SkBF and Qc in the transition from upright rest to upright exercise is illustrated in Fig. 3C. Young and older adults exhibited increases in both variables from rest to exercise while hyperthermic (Fig. 3C). HR increased during exercise in all subject groups; however, absolute HR was lower in the older compared with young adults, and sapropterin had no effect (Fig. 4A). There were no age- or treatment-related differences in stroke volume during this phase of the protocol (Fig. 4B). The pressor response to exercise was greater in the older adults regardless of treatment (Fig. 4C; Table 3), and sapropterin had no effect on any measured variable during dynamic exercise at an elevated core temperature (Table 3).
DISCUSSION
Aging, even in the absence of overt cardiovascular disease, is characterized by impairments in the integrated thermoregulatory-cardiovascular response to passive heat stress (17). Minson et. al. (31), building on the seminal work of Rowell (38), showed discernable impairments in the extent to which older adults increase both SkBF (16, 27, 31, 32, 44) and Qc (31, 32) during supine passive heating. Peripheral limitations to cutaneous vasodilation (i.e., alterations in cutaneous vascular function) contribute at least in part to the attenuated increase in SkBF during supine passive heating in older adults because localized and systemic interventions can acutely restore the vasodilatory capacity of aged skin to that of young skin (18, 19, 43, 44, 46).
Those studies have raised the question of whether cardiac limitations (i.e., an attenuated ability to increase Qc) contribute to the relative inability of older adults to increase SkBF during heat stress or whether the smaller increase in Qc appropriately reflects the blunted increase in blood flow to aged skin. Thus, the primary aim of the present study was to examine the effect of acutely abolishing the peripheral vascular limitation to cutaneous vasodilation in healthy older adults on the central hemodynamic responses to supine passive heating. Once heated, a secondary experimental aim was to determine the SkBF and Qc responses of young and older adults to 1) passively assuming an upright posture and 2) dynamic leg exercise. Our reasoning was that the former would further decrease CVP, presenting an additional challenge to the left ventricle, while the latter would increase CVP via engagement of the muscle pump.
The primary finding of this study was that correcting the peripheral vascular limitation to reflex cutaneous vasodilation in healthy older adults had no effect on Qc during supine passive whole body heat stress. Furthermore, the sapropterin-induced increase in cutaneous vasodilation during heating did not affect Qc when central hemodynamics were further altered by superimposing upright posture and/or dynamic exercise upon the heat stress. Thus, in older adults, there was no matching of Qc to the restored SkBF response during heat stress. The inability of older adults to further increase Qc during heating when the cutaneous vasodilator response is fully restored instead suggests that their relative inability to increase Qc during passive heat stress is a consequence of primary aging.
Age group comparisons.
The aim of this study was not to reexamine age-related differences in responses to heat stress. Multiple studies have documented age-related decrements in the cardiovascular responses to supine passive heat stress, as well as during orthostatic challenges and dynamic exercise in the heat (15–17, 24, 26, 27, 30–32, 43, 44). However, a group of healthy young adults was incorporated in the present study to provide comparative values for all variables and to validate the efficacy of acute oral sapropterin in fully restoring reflex cutaneous vasodilation in apparently healthy older adults. The peripheral and central cardiovascular responses of the young adults in this study are in good agreement with those reported in multiple investigations using varied methodology (14, 31, 32, 42, 50).
As expected, profound age-related impairments in the SkBF and Qc responses to supine passive heating were evident in the current study (16, 24, 27, 31, 32, 43, 44). An inability to maintain stroke volume despite similar decreases in CVP, coupled with a reduced chronotropic response (13, 31, 32), have been suggested as primary contributing mechanisms to the attenuated increase in Qc when older adults are passively heated to the limit of their thermal tolerance (31). Although stroke volume in older adults did not fall during supine heating in the present study, our thermal stress was less severe.
When an upright posture was superimposed during heating, older adults exhibited a blunted reflex increase in HR and attenuated reductions in stroke volume such that Qc was maintained relative to supine heating but remained blunted compared with young adults, findings consistent with those reported by Minson et. al. (32). That study additionally demonstrated that older men increased vasoconstriction of the splanchnic vascular bed to a greater extent than young men during upright tilt in the heat as a means to maintain MAP. During upright dynamic exercise in the heat in the current study, HR was attenuated in older compared with young adults, yet stroke volume and Qc were relatively well maintained. Although these findings are generally in agreement with those previously reported (30), small differences likely reflect the fact that only a short bout of exercise was employed here as a means to augment CVP during hyperthermia.
Efficacy of sapropterin in restoring cutaneous vasodilation in older adults.
The significantly impaired increase in SkBF during supine passive heat stress in older adults in the present study is consistent with a wide body of literature examining this response (16, 24, 27, 43, 44). The blunted SkBF response to increasing core temperature has largely been attributed to reductions in NO-mediated cutaneous vasodilation (16). In the peripheral vasculature, the enzymatic cofactor BH4 is required for NO production; in its absence, NO synthase uncouples, decreasing functional NO synthesis and increasing superoxide production (1). BH4 bioavailability is reduced in the aged microvasculature (8, 10), likely contributing to the impaired vasodilatory response to whole body heating (44, 46). Oral administration of sapropterin (pharmaceutical grade BH4) increases plasma BH4 concentration and improves reflex cutaneous vasodilation assessed by both laser-Doppler flowmetry and venous occlusion plethysmography (44). Consistent with these previous findings, we demonstrated here that acute oral sapropterin fully restored the SkBF response to supine whole body heating in older adults to a magnitude similar to that in young adults. Thus this acute oral intervention was sufficient to temporarily eliminate the peripheral limitation to adequate cutaneous vasodilation.
The effect of normalizing SkBF on Qc during supine passive heating in older adults.
Coincident with the well-established reduction in the SkBF response to heat stress (16, 24, 27, 43, 44), older adults exhibit a substantially blunted increase in Qc during passive heating (31, 32). It has been postulated that the relative inability of older adults to increase Qc may not allow for an appropriate increase in SkBF, a scenario indicative of a cardiac limitation (in addition to the well-documented peripheral vascular limitations) (31). To test this possibility, central hemodynamics were assessed after the peripheral vascular limitation to reflex cutaneous vasodilation was removed. It is relevant to note that we previously showed that the reduction in directly measured CVP (an index of preload) during passive heating is not different between young and older adults (31). Contrary to our hypothesis that Qc would increase to match the increase in SkBF, the Qc response to supine passive heating was not different between placebo and sapropterin treatments in older adults. Moreover, during supine passive heating, stroke volume was similar during both treatments. Taken together, these data instead support the hypothesis that older adults demonstrate a relative inability to increase Qc at a given core temperature during supine passive heating and that this deficit is unrelated to a compromised SkBF.
Despite the lack of an augmented increase in Qc during heating, older adults increased SkBF during oral sapropterin treatment to a magnitude similar to that of young adults. In young adults, the large increase in SkBF is supplied by both an increase in Qc and blood flow redistribution from the splanchnic and renal circulations (37); this redistribution of blood volume to the peripheral vascular beds aids in thermoregulation (7). Both of these mechanisms are attenuated during passive heating in older subjects (29). In the present study, because an augmentation in Qc and a reduction in TPR did not accompany sapropterin-induced increases in SkBF, a greater redistribution of blood from visceral circulations is the most likely explanation for the augmented SkBF response. However, Minson et. al. (32) previously noted that older adults increased vasoconstriction of the splanchnic vascular bed to a lesser extent than young men during supine passive heating. There are two plausible explanations for this contradictory finding. First, the previous study (31) heated subjects to their limit of thermal tolerance, a much more severe thermal stimulus than the heating paradigm employed in the present study. Second, BH4 bioavailability is also essential for catecholamine biosynthesis as a cofactor for tyrosine hydroxylase (22, 33, 34). Although speculative, the increase in BH4 bioavailability resulting from oral sapropterin could augment vasoconstriction in splanchnic and renal vascular beds (28, 45), thereby serving as a compensatory mechanism contributing to the increased blood flow directed to the cutaneous vasculature during passive whole body heating. This possibility remains to be mechanistically examined.
Despite the substantial increase in cutaneous vasodilation with sapropterin in the older subjects, MAP was not compromised. Consistent with previous studies (31, 32, 43, 44), MAP was fairly well maintained in older adults during both placebo and sapropterin treatment, and the small changes in arterial pressure during supine passive heating were similar to those previously reported (31, 32, 37). Furthermore, acute oral sapropterin did not affect TPR. Although oral sapropterin has been demonstrated to lower muscle sympathetic nerve activity (generally considered an index of TPR), this has only been observed in pathological conditions or with chronic (12 wks) treatment (35). Collectively, aside from functioning to fully restore peripheral cutaneous vasodilatory capacity, oral sapropterin did not directly alter cardiac function or BP.
SkBF and Qc during upright posture in hyperthermia in older adults.
The observation that sapropterin acutely restores peripheral vascular function during passive heat stress in aged skin has, to date, only been documented during supine passive heating (44). Therefore, to further examine age-related alterations in the integrated peripheral and central cardiovascular responses, hemodynamics were further manipulated by superimposing upright posture on passive heat stress to exacerbate the fall in CVP. In young adults, upright tilting or lower body negative pressure during hyperthermia results in a withdrawal of active vasodilator activity (6) and a concomitant reduction in SkBF (32). However, in older adults, the SkBF response to simulated orthostasis (upright tilt and lower body negative pressure) is impaired, and they must instead rely on alternate mechanisms, including greater increases in splanchnic vascular resistance (32, 40) to prevent a fall in MAP.
During placebo treatment in the current study, SkBF increased upon assuming the upright posture during hyperthermia, instead of decreasing (32, 40), supporting the concept that aging is associated with a decreased ability of cardiopulmonary baroreceptors to reflexively modulate SkBF, an impairment that has implications for the regulation of central blood volume and BP during orthostatic challenges in the heat (40). Notably, two older subjects taking placebo became presyncopal when transitioning to the upright posture during sustained hyperthermia. In these two subjects, a severe attenuation in the tachycardic response while assuming the upright posture was noted. In contrast to the altered SkBF responses noted during placebo treatment, the restoration of peripheral vascular function with oral sapropterin resulted in an expected decrease in SkBF in the older subjects, similar to the cutaneous vascular response observed in the young subjects. That is, sapropterin restored the relation between SkBF and Qc during upright posture in the heat such that a given small decrease in Qc was accompanied by a decrease in SkBF, albeit in a reduced range of absolute Qc. The mechanism underlying the sapropterin-induced correction of the reflex response is unknown; however, as mentioned earlier, the increase in BH4 bioavailability resulting from oral sapropterin could augment vasoconstriction in splanchnic and renal vascular beds (28, 45).
SkBF and Qc during dynamic exercise in hyperthermia.
Engaging the muscle pump during dynamic leg exercise was employed as a means to drive blood centrally, thus experimentally augmenting CVP. The subsequent increase in preload resulted in an increase in SkBF and Qc within the same response range in all three groups. During supine passive heating, the decrease in CVP is not different between young and older adults (31); however, in older adults, there is a progressive fall in stroke volume during more severe passive heating, indicative of an age-related inability to increase the contractile force of the heart. Because of decreased β-adrenergic receptor responsiveness (12), increased sympathetic drive to the heart is not sufficient to increase Qc in the face of falling CVP. Collectively, it appears that the relative inability to increase Qc during heat stress in the absence of muscle pump activation and facilitated venous return is likely due to an impaired inotropic response, thus rendering older adults more susceptible to orthostatic intolerance in the heat.
A reduced ability of older adults to increase Qc during cycle exercise in the heat has been reported (15, 26), an effect partially mediated by the an age-related reduction in cardiac β-adrenergic receptor responsiveness (12). Perhaps surprisingly, a blunted Qc response to dynamic exercise in the heat was not observed in the older adults during either treatment in the current study, likely due to the relatively greater increases in stroke volume and the short duration of the exercise bout. It is important to note that the MAP response to exercise was greater in older compared with young adults. Although all subjects exercised at the same absolute intensity, this was presumably a greater relative intensity in the older adults, accounting for the exaggerated pressor response. However, this exercise paradigm was chosen to ensure that metabolic heat production was not different between subject groups while limiting further increases in Tes (5).
Limitations.
To attempt to ascertain whether the blunted increases in SkBF during heat stress in healthy older adults results from cardiac limitations in addition to peripheral vascular dysfunction, we acutely restored cutaneous vasodilator capacity and measured Qc during experimental manipulations in CVP. An alternate approach would have been to directly augment cardiovascular function in older adults and measure cutaneous vasodilator responsiveness. Experimentally, this can be accomplished during heat stress after acute volume loading sufficient to increase ventricular filling pressure (4, 23). In young adults, rapid plasma volume expansion (23) or slow fluid administration of intravascular volume sufficient to offset fluid losses due to sweating (29) during passive heat stress counteracted simulated orthostasis-induced decreases in ventricular filling, thereby augmenting stroke volume and Qc to aid in the maintenance of MAP. To our knowledge, no studies have attempted to “restore'” the impaired Qc response to passive heating in older adults. In addition, we acknowledge that the derivation of several hemodynamic variables is a limitation of the study. Nevertheless, despite the indirect assessment, sapropterin did not affect any index of cardiac function.
Summary and conclusions.
The principal finding of this study was that fully restoring the peripheral SkBF response to supine passive whole body heating did not affect Qc in healthy older adults. In addition, the sapropterin-induced increase in cutaneous vasodilation during heating did not alter Qc when CVP was further challenged by superimposing upright posture and/or dynamic exercise upon the heat stress. Taken together, the lack of a further increase in Qc during heating when cutaneous vasodilator capacity is fully restored in older adults instead suggests that their relative inability to increase Qc during passive heat stress is a consequence of primary aging.
GRANTS
This research was supported by National Institutes of Health Grants HL-120471-01 (to J. L. Greaney), HL-093238-04 (to L. M. Alexander), and AG-007004-23 (to W. L. Kenney) and an American College of Sports Medicine Research Endowment award (to J. L Greaney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.G., A.E.S., L.M.A., and W.L.K. conception and design of research; J.L.G. and A.E.S. performed experiments; J.L.G. and A.E.S. analyzed data; J.L.G., A.E.S., D.N.P., L.M.A., and W.L.K. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., A.E.S., D.N.P., L.M.A., and W.L.K. edited and revised manuscript; J.L.G., A.E.S., D.N.P., L.M.A., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all the volunteer subjects are greatly appreciated. We are grateful for the assistance of Luke A. McMahon, Susan Slimak, R.N., Jane Pierzga, M.S., Randall A. McCullough, Timothy R. McConnell, Ph.D., and Kenneth C. Beck, Ph.D. This study was conducted in the Department of Kinesiology at The Pennsylvania State University.
REFERENCES
- 1.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Aoki K, Kondo N, Shibasaki M, Takano S, Katsuura T. Circadian variation in skin blood flow responses to passive heat stress. Physiol Behav 63: 1–5, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Bell C, Monahan KD, Donato AJ, Hunt BE, Seals DR, Beck KC. Use of acetylene breathing to determine cardiac output in young and older adults. Med Sci Sports Exerc 35: 58–64, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Brothers RM, Pecini R, Dalsgaard M, Bundgaard-Nielsen M, Wilson TE, Secher NH, Crandall CG. Beneficial effects of elevating cardiac preload on left-ventricular diastolic function and volume during heat stress: implications toward tolerance during a hemorrhagic insult. Am J Physiol Regul Integr Comp Physiol 307: R1036–R1041, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer MN, Jay O. Selecting the correct exercise intensity for unbiased comparisons of thermoregulatory responses between groups of different mass and surface area. J Appl Physiol (1985) 116: 1123–1132, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Crandall CG, Johnson JM, Kosiba WA, Kellogg DL Jr. Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol (1985) 81: 2192–2198, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Crandall CG, Wilson TE, Marving J, Vogelsang TW, Kjaer A, Hesse B, Secher NH. Effects of passive heating on central blood volume and ventricular dimensions in humans. J Physiol 586: 293–301, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis FP, Nelson F, Pincus L. Mortality during heat waves in New York City July, 1972 and August and September, 1973. Environ Res 10: 1–13, 1975. [DOI] [PubMed] [Google Scholar]
- 10.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feillet F, Clarke L, Meli C, Lipson M, Morris AA, Harmatz P, Mould DR, Green B, Dorenbaum A, Giovannini M, Foehr E. Pharmacokinetics of sapropterin in patients with phenylketonuria. Clin Pharmacokinet 47: 817–825, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, Rengo C, Femminella GD, Leosco D, Bonaduce D. Beta-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol 4: 396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 593: 2225–2235, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganio MS, Overgaard M, Seifert T, Secher NH, Johansson PI, Meyer MA, Crandall CG. Effect of heat stress on cardiac output and systemic vascular conductance during simulated hemorrhage to presyncope in young men. Am J Physiol Heart Circ Physiol 302: H1756–H1761, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol (1985) 82: 1126–1135, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol 284: H1662–H1667, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev 35: 119–125, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol 291: H2965–H2970, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Holowatz LA, Thompson CS, Kenney WL. l-Arginine supplementation or arginase inhibition augments reflex cutaneous vasodilatation in aged human skin. J Physiol 574: 573–581, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol (1985) 88: 1650–1658, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman S. Establishment of tetrahydrobiopterin as the hydroxylase cofactor and a review of some recent studies in man. Psychopharmacol Bull 14: 38–40, 1978. [PubMed] [Google Scholar]
- 23.Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol 587: 1131–1139, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol 57: 120–125, 1988. [DOI] [PubMed] [Google Scholar]
- 25.Kenney WL, Craighead DH, Alexander LM. Heat waves, aging, and human cardiovascular health. Med Sci Sports Exerc 46: 1891–1899, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenney WL, Ho CW. Age alters regional distribution of blood flow during moderate-intensity exercise. J Appl Physiol (1985) 79: 1112–1119, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol 272: H1609–H1614, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem 86: 774–783, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Lucas RA, Ganio MS, Pearson J, Crandall CG. Sweat loss during heat stress contributes to subsequent reductions in lower-body negative pressure tolerance. Exp Physiol 98: 473–480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minson CT, Kenney WL. Age and cardiac output during cycle exercise in thermoneutral and warm environments. Med Sci Sports Exerc 29: 75–81, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985) 84: 1323–1332, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol 276: R203–R212, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26: 2439–2444, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol 50: 238–246, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 308: R208–R218, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridout SJ, Parker BA, Smithmyer SL, Gonzales JU, Beck KC, Proctor DN. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J Appl Physiol (1985) 108: 483–489, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. [DOI] [PubMed] [Google Scholar]
- 38.Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford Univ. Press, 1986. [Google Scholar]
- 39.Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man–role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. [DOI] [PubMed] [Google Scholar]
- 40.Scremin G, Kenney WL. Aging and the skin blood flow response to the unloading of baroreceptors during heat and cold stress. J Appl Physiol (1985) 96: 1019–1025, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Shibasaki M, Wilson TE, Bundgaard-Nielsen M, Seifert T, Secher NH, Crandall CG. Modelflow underestimates cardiac output in heat-stressed individuals. Am J Physiol Regul Integr Comp Physiol 300: R486–R491, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond) 129: 159–167, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol (1985) 115: 972–978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985) 115: 1025–1031, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanhewicz AE, Bruning RS, Smith CJ, Kenney WL, Holowatz LA. Local tetrahydrobiopterin administration augments reflex cutaneous vasodilation through nitric oxide-dependent mechanisms in aged human skin. J Appl Physiol (1985) 112: 791–797, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37: 153–156, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Vandentorren S, Bretin P, Zeghnoun A, Mandereau-Bruno L, Croisier A, Cochet C, Riberon J, Siberan I, Declercq B, Ledrans M. August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur J Public Health 16: 583–591, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 95: 9220–9225, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank-Starling relations in humans. J Physiol 587: 3383–3392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]