Abstract
Pulmonary hypertension (PH) is a progressive lung disease associated with proliferation of smooth muscle cells and constriction of lung microvasculature, leading to increased pulmonary arterial pressure, right ventricular failure, and death. We have previously shown that genistein rescues preexisting established PH by significantly improving lung and heart function. (Matori H, Umar S, Nadadur RD, Sharma S, Partow-Navid R, Afkhami M, Amjedi M, Eghbali M. Hypertension 60: 425–430, 2012). Here, we have examined the role of microRNAs (miRs) in the rescue action of genistein in monocrotaline (MCT)-induced PH in rats. Our miR microarray analysis on the lung samples from control, PH, and genistein-rescue group revealed that miR206, which was robustly upregulated to ∼11-fold by PH, was completely normalized to control levels by genistein treatment. Next, we examined whether knockdown of miR206 could reverse preexisting established PH. PH was induced in male rats by 60 mg/kg of MCT, and rats received three intratracheal doses of either miR206 antagomir (10 mg/kg body wt) or scrambled miR control at days 17, 21, and 26. Knockdown of miR206 resulted in significant improvement in the cardiopulmonary function, as right ventricular pressure was significantly reduced to 38.6 ± 3.61 mmHg from 61.2 ± 5.4 mmHg in PH, and right ventricular hypertrophy index was decreased to 0.35 ± 0.04 from 0.59 ± 0.037 in PH. Knockdown of miR206 reversed PH-induced pulmonary vascular remodeling in vivo and was associated with restoration of PH-induced loss of capillaries in the lungs and induction of vascular endothelial growth factor A expression. In conclusion, miR206 antagomir therapy improves cardiopulmonary function and structure and rescues preexisting severe PH in MCT rat model possibly by stimulating angiogenesis in the lung.
Keywords: pulmonary hypertension, right heart failure, genistein, microRNA, angiogenesis
pulmonary arterial hypertension (PAH) is a cardiopulmonary disease associated with severe pulmonary vascular remodeling, progressive increase in pulmonary artery (PA) pressure leading to right ventricular (RV) hypertrophy, and eventually right heart failure and death (14, 38). Many therapies are designed to target the vascular changes and inflammation associated with the etiology of PAH (22, 24). Our laboratory has previously shown that genistein, a natural soybean-derived phytoestrogen, with much higher affinity for estrogen receptor-β (ER-β) than -α is very effective in rescuing preexisting advanced pulmonary hypertension (PH) in rats induced by monocrotaline (MCT) (20).
microRNAs (miRs) are single-stranded noncoding RNA molecules that can regulate gene expression through binding to the 3′ untranslated regions of their target genes and thereby degrade mRNA transcripts or block protein translation (3, 4, 19). There is growing evidence for the involvement of many miRs in the progression of PH. For example, downregulation of microRNA-204 (miR204) in both human and rodent models has been associated with the pro-proliferative and antiapoptotic phenotypes of pulmonary smooth muscle cells (10). miR145 has been reported to be dysregulated in mouse models of PH and patient samples, and its downregulation is protective against the development of PAH (7). miR21 has been shown to play an important role in the pathogenesis of chronic hypoxia-induced pulmonary vascular remodeling (23). In the search for potential miR candidates that can be regulated by genistein in its rescue action of PH in the MCT rat model, we found that miR206, which is expressed at very low levels in healthy lungs, was robustly upregulated by ∼11-fold in MCT-induced PH in rats and genistein treatment was associated with restoration of miR206 expression to control levels. Many studies have shown that disordered angiogenesis may contribute to the progression of PH (36, 45). Regeneration of pulmonary vasculature and overexpression of proangiogenesis factors have been shown to ameliorate the effects of PH and may play potential therapeutic role in this disease (6, 44, 45). To explore further whether normalization of miR206 levels in the lungs of MCT rats could play a protective role, we performed the knockdown of miR206 in the lungs of PH animals and found that miR206 downregulation is sufficient to rescue advanced preexisting PH. Downregulation of miR206 was associated with an increase of vascular endothelial growth factor A (VEGFA) and angiogenesis. Thus it is likely that the rescue action by knockdown of miR206 was accompanied with an increase in angiogenesis through induction of VEGFA.
METHODS
Animals and treatments.
Male Sprague-Dawley rats (250-300 g) received a single subcutaneous injection of MCT (60 mg/kg; Sigma) at day 0 to induce PH or saline as controls (CTRL). MCT was dissolved in 1 N HCl (the pH was adjusted to 7.4) and diluted with PBS before injection. Genistein was dissolved in a mixture of DMSO (part no. 20684, Thermo Scientific) and polyethylene glycol (part no. B21992, Alfa Aesar). miR206 antagomir and scramble oligonucleotides were dissolved in ultrapure water. MCT rats were assigned to receive either genistein (1 mg·kg−1·day−1 sc) or vehicle (100 μl of a mixture containing 1.25% DMSO and 98.75% polyethylene glycol) from day 21 to day 30. For miR206 knockdown study, MCT rats were assigned to receive either miR206 inhibitor oligonucleotide [10 mg/kg body wt in 50 μl PBS (n = 5–6), mature miR206 sequence, 3′-UGGAAUGUAAGGAAGUGUGUGG-5′] or scramble miR oligonucleotide (n = 5–6) on days 17, 21, and 26. miR206 antagomir or scramble miR oligonucleotides were delivered through the oropharyngeal cavity into the trachea by intratracheal instillation. Knockdown of miR206 in the lungs was confirmed by quantitative real-time reverse transcriptase-polymerase chain reaction (QRT-PCR). Protocols received institutional review and committee approval. The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no. 85-23, Revised 1996).
Echocardiographic assessments and cardiopulmonary hemodynamic measurements.
A VisualSonics Vevo 2100 echocardiogram device with a 30-MHz linear transducer was used to perform the pulmonary pulsed-wave Doppler echocardiography of PA flow. The probe was placed in a parasternal long-axis position to visualize the PA outflow tract. Pulsed flow Doppler imaging was then overlaid to observe the dynamics of blood flow through the PA valve. PA acceleration time (PAAT) was determined by calculating time taken from the start of flow to maximal velocity using echocardiogram software (Vevo 2100 version: 1.5.0). The RV systolic pressure (RVSP) was measured by direct right heart catheterization at day 30. Rats were anesthetized by ketamine (80 mg/kg) and xylazine (5 mg/kg) intraperitoneally and were placed on a controlled warming pad to keep the body temperature constant at 37°C. After tracheotomy was performed, a cannula (18G, Biovalve) was inserted, and the animals were mechanically ventilated. After a midsternal thoracotomy, rats were placed under stereomicroscope (Zeiss, Hamburg, Germany), and a pressure-conductance catheter (model 1.4F Millar SPR-671) was introduced via the apex into the RV and positioned toward the pulmonary valve. The catheter was connected to a signal processor (AD Instruments), and RV pressures were recorded digitally with a recording speed of 1 k/s. The maximum rate of rise of RV pressure (dP/dtmax), the maximum isovolumetric rate of relaxation (−dP/dtmin), and the heart rates were directly calculated from the recordings.
Gross histological evaluation.
The RV wall, the left ventricular (LV) wall and the interventricular septum were dissected, and the ratio of the RV to LV plus septum weight [RV/(LV + IVS)] was calculated as an index of RV hypertrophy, where IVS is interventricular septum.
Gene expression and miR assessment.
Total RNA was purified from whole lungs using the Trizol method. Two micrograms of total RNA were reverse transcribed to cDNA using the Omniscript RT kit (Qiagen), according to the manufacturer's instructions (Qiagen). Quantitative real-time reverse transcriptase-polymerase chain reaction (QRT-PCR) was performed using iQ SYBR Green Supermix (part no. 1708884, Bio-Rad). The VEGFA gene expression was assessed using gene-specific primers. GAPDH was used as a reference control for normalization. Levels of gene expression in each sample were determined with the comparative Ct method using Bio-Rad CFX manager 2.1 software. The primer sequences were as follows: 1) VEGFA, F: 5′-ACACGGTGGTGGAAGAAGAG-3′, R: 5′-CAAGTCTCCTGGGGACAGAA-3′; and 2) GAPDH, F: 5′-CCTGCACCACCAACTGCTTAG-3′; R: 5′-ATGACCTTGCCCACAGCCTTG-3′. For gene expression, cycling parameters were as follows: 2 min at 50°C, 20 s at 95°C, 40 cycles, 1 s at 95°C, and 20 s at 60°C. For miR studies, total RNA was isolated using the mirVana miRNA Isolation Kit (Life Technologies: part no. AM1560), according to the manufacturer's instructions. A microarray screen of miR expression in the total lung tissue of rats was performed with the use of non-Affymetrix single-channel arrays (MirBASE 17.0 MicroRNA Array, Ocean Ridge Biosciences). The miRNA microarray (miRBase17 version) contained 8,817 spots, which include mammalian miRs, spiking control, and negative controls. Each probe was represented as three spots on the microarray. Microarray data analysis was performed by Ocean Ridge Biosciences. Briefly, the data were filtered to select probes where the average signal for at least one treatment group was above the average detection threshold; the threshold was calculated from the following formula: threshold = log2[5 × SD (background) + 10% trim mean (negative controls)]. The saturated probes were then removed from the filtered list.
For miR quantification by QRT-PCR, 10 ng of total RNA were used to prepare cDNA using TaqMan MicroRNA Reverse Transcription Kit (part no. 4366596, Life Technologies) and TAQMAN specific probe for miR206 (part no. 4427975; assay ID: 000510, Life Technologies). U6 small nucleolar RNA (part no. 4427975; assay ID: 0019730, Life Technologies) was used as a reference control for normalization. QRT-PCR was performed using TaqMan Universal PCR Master Mix, No AmpErase UNG (part no. 4324018, Life Technologies). The QRT-PCR cycling parameters were 2 min at 50°C, 10 min at 95°C, 40 cycles, 15 s at 95°C, and 1 min at 60°C. Data were analyzed using Bio-Rad CFX manager 2.1 software.
Immunohistochemistry, imaging, and quantification.
Whole lungs were isolated and inflated manually using a syringe by perfusing 4% paraformaldehyde in 0.1 M Na2HPO4 and 23 mM NaH2PO4 (pH 7.4) through trachea. Isolated perfused lungs were fixed in 4% paraformaldehyde for 4 h on ice. The tissue was then immersed in ice-cold 20% sucrose overnight to cryoprotect and mounted using optimum cutting temperature compound, and transversal 4-μm sections were obtained with a cryostat (Microm HM525, Thermo Scientific). Standard hematoxylin/eosin (Sigma) stainings were performed, according to manufacturer's protocol, and images were acquired with a light microscope (Nikon). For immunohistochemistry, tissue section slides were fixed by submerging tissue in 100% acetone for 5 min and then air-drying. Tissue sections were washed three times for 10 min each in PBS and Triton X-100 (0.2%). To block background, tissue was incubated with a blocking solution containing 10% normal goat serum in PBS/0.2% Triton X-100 for 60 min at room temperature. Tissue was then incubated overnight at 4°C with primary antibody in PBS/0.2% Triton X-100/1% normal goat serum. The primary antibodies were against either α-smooth muscle actin (Sigma A2547) for the assessment of arteriole wall thickening or von Willebrand factor (Abcam ab6994) for the assessment of vessel density. The tissue was then washed three times for 10 min each in PBS/0.2% Triton X-100 and incubated with 4′,6-diamidino-2-phenylindole for 15 min at room temperature followed by secondary antibody in PBS/0.2% Triton X-100/1% normal goat serum for 1 h at room temperature. The tissue was washed three times for 10 min each in PBS/0.2% Triton X-100 and mounted on microscope slides with ProLong antifade reagent (Molecular Probes, Eugene, OR). The images were acquired using a laser-scanning confocal microscope (Nikon eclipse Ti inverted confocal microscope) and processed using Nikon NIS Elements advanced research analysis version 4.20.01.
PA medial wall thickness was quantified using ImageJ software by measuring the maximum thickness of arteriolar walls. About 50–100 arterioles/group were analyzed (n = 5–6 rats/group, 5–10 arterioles/slides, and 2 slides/animal). The number of microvessels per high power field (×60 magnification) was quantified by measuring von Willebrand factor positive microvessels with a lumen <20 μm, as our laboratory published previously (37). A total of 50 high-power magnification images from five to six animals per group were used for quantification. Sections were randomly selected, and the observers were blinded.
Statistical analysis.
Means were compared across groups using one-way ANOVA for three or more groups or t-tests for two groups for data that followed the normal distribution and met variance homogeneity. Significance was assessed using the Tukey criterion for pairwise mean comparisons under the ANOVA model. Normality was assessed using the Kolmogorov-Smirnov test. Computations were carried out using SPSS SigmaStat for Windows, version 3.0 (SPSS, Chicago, IL). P values < 0.05 were considered significant. The miR microarray statistical analysis was performed by Ocean Ridge Biosciences. The detectable probes were analyzed using NIA Array Analysis software for ANOVA. Significant probes were selected based on a criteria of P < 0.05 and false detection rate < 0.1 from a one-way ANOVA test.
RESULTS
Genistein-induced rescue of PH is associated with normalization of miR206 in the lungs of MCT-treated rats.
To identify the potential miRs involved in the rescue action of genistein, miR microarray (nonaffymetrix) was performed in the lungs of CTRL, PH, and genistein rescue groups at day 30 in the MCT model. The expression of 366 miRs was successfully detected. Multiple miRs were differentially regulated between the CTRL and the PH groups. Among these miRs, miR206 was upregulated the most by 11-fold in the PH compared with the CTRL lungs. The known regulated miR, miR21, was increased ∼3.8-fold in PH vs. CTRL lungs. Additionally, miR322 was downregulated by ∼2.6-fold; miR503 by ∼3.6-fold in PH vs. CTRL lungs, whereas miR124 remained unchanged (Fig. 1). Genistein treatment was associated with normalization of PH-induced changes of miR206, miR21, miR322, and miR503 (Fig. 1A). However, genistein was not able to prevent the induction of miR451 in PH (Fig. 1A). miR124, whose expression was not modified in PH, was, however, induced by genistein treatment (Fig. 1A). These data suggest that genistein treatment is associated with selective changes in specific miR expressions previously implicated in PH. In this study, our main focus was on miR206, because of its robust induction in the lung tissue compared with other miRs regulated by genistein in MCT-induced PH. The inhibition of miR206 by genistein treatment was further confirmed by QRT-PCR (Fig. 1B). In addition, miR206 is predominantly a skeletal muscle-specific miR and is expressed at very low levels in the lungs of healthy rats compared with miR21, miR322, and miR451 (Fig. 1C). Therefore, the aberrant expression of miR206 in the lungs of MCT-induced PH could aggravate lung injury.
Fig. 1.
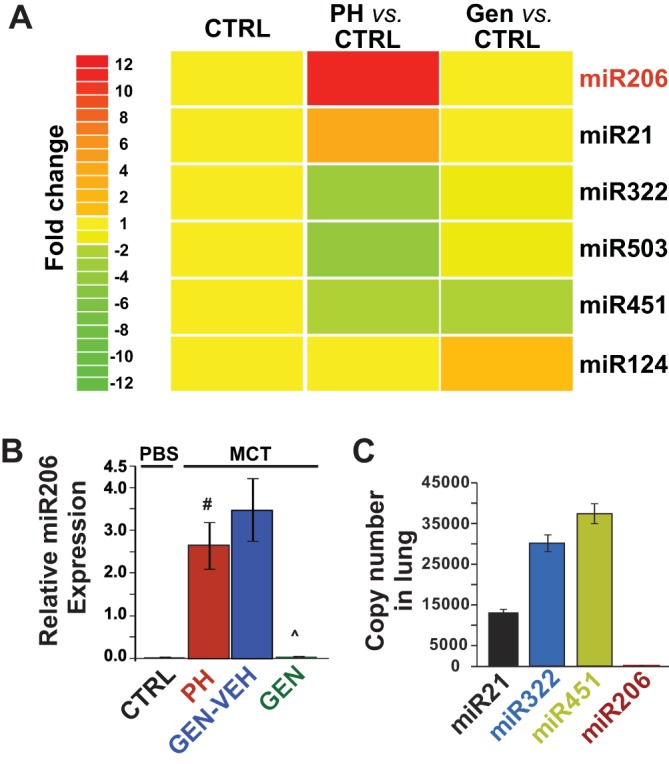
microRNA (miR) 206 is strongly upregulated in the lungs in monocrotaline (MCT)-pulmonary hypertension (PH), and genistein (GEN) therapy restores lung miR206 levels. A: heat map generated from the miR microarray analysis in the lungs of control (CTRL), PH, and GEN-rescue rats in the MCT model of PH (n = 4 rats/group). B: relative lung miR206 expression is confirmed using quantitative RT-PCR analysis in MCT model in CTRL, PH, GEN vehicle (VEH) CTRL (GEN-VEH), and GEN groups (n = 6–7 animals/group). PBS, phosphate-buffered saline. C: relative copy number of miR206 and other known miRs in PH, including miR21, miR322, and miR451, in the lungs of healthy CTRLs (n = 5 rats/group). Values are means ± SE. #P < 0.01. P̂ < 0.001.
Knockdown of miR206 rescues MCT-induced PH in rats by improving cardiac and pulmonary structure and function.
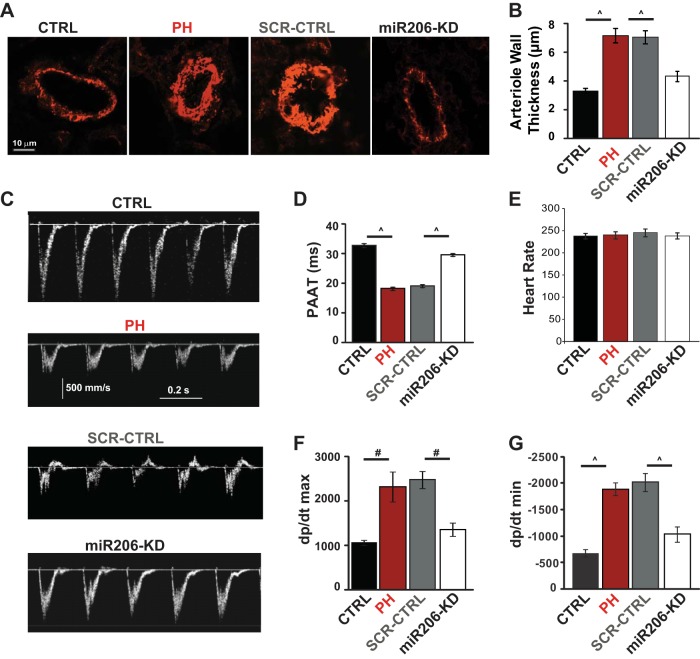
To test whether restoration of miR206 level in MCT treated rats can reverse symptoms of PH, synthetic miR206 antagomir RNA molecules were selectively delivered to the lung by intratracheal instillation at days 17, 21, and 26 in the MCT model (Fig. 2A). Synthetic miR206 antagomir delivery resulted in a significant knockdown of miR206 in the lungs of miR206-knockdown (KD) group compared with scrambled miR CTRL (SCR-CTRL) (0.07 ± 0.02 relative expression units in miR206-KD group vs. 0.7 ± 0.2 in SCR-CTRL, P < 0.05, Fig. 2B). miR206 antagomir delivery effectively rescued preexisting PH, as the RVSPs were significantly lower than the PH group (38.6 ± 3.61 mmHg in miR206-KD group vs. 61.2 ± 5.4 mmHg in PH, P < 0.01) (Fig. 2C). Also, knockdown of miR206 decreased RV hypertrophy index in MCT rats (0.35 ± 0.04 in miR206-KD vs. 0.59 ± 0.037 in PH, P < 0.001, Fig. 2D). The SCR-CTRL had no effect on RVSPs and RV hypertrophy index, as these values were not significantly different than those of the PH group (RVSP: 62.7 ± 7.0 mmHg in SCR-CTRL group vs. 61.2 ± 5.4 mmHg in PH, P < 0.32; RV hypertrophy index: 0.58 ± 0.03 mmHg in SCR-CTRL group vs. 0.59 ± 0.04 mmHg in PH, P < 0.98), suggesting the specificity of miR206 antagomir oligonucleotides.
Fig. 2.
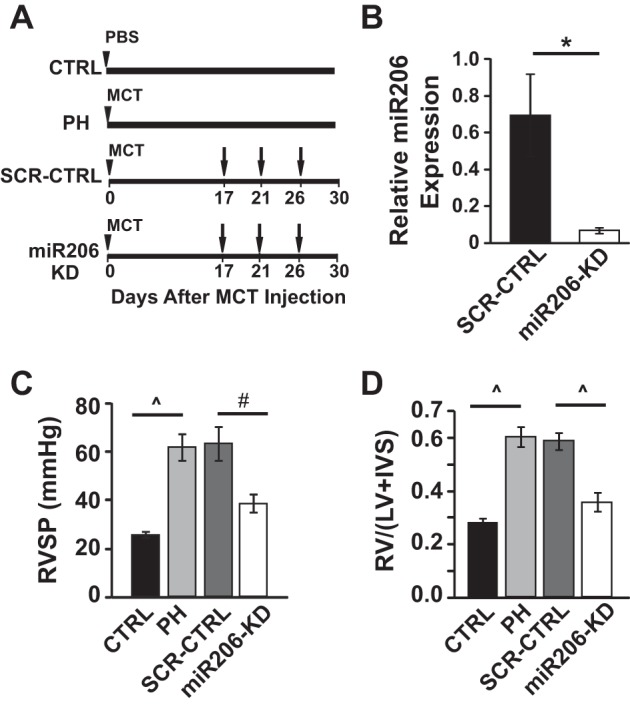
Knockdown of miR206 rescues PH by improving cardiopulmonary function and structure. A: experimental protocol for MCT model: male rats were injected with MCT or PBS at day 0 (arrowhead). The MCT-injected animals were left untreated to develop severe PH (PH group), received miR206 inhibitor [10 mg/kg body wt, miR206-knockdown (KD) group], or scrambled (SCR) miR oligonucleotide (10 mg/kg body wt, SCR-CTRL group) on days 17, 21, and 26. All of the rats were killed at day 30. B: relative miR206 expression in the lungs of SCR-CTRL vs. miR206-KD group at day 30 (n = 5–6 animals/group). C: right ventricular (RV) systolic pressure (RVSP) (n = 5–7 animals/group). D: the RV hypertrophy index [RV/(LV + IVS)], where IVS is interventricular septum, and LV is the left ventricular wall (n = 5–6 rats/group). Values are means ± SE. *P < 0.05. #P < 0.01. P̂ < 0.001.
Knockdown of miR206 also prevented the increased arteriolar muscularization in the lungs of the PH group in the MCT model (4.3 ± 0.4 μm in miR206-KD group vs. 7.2 ± 0.5 μm in PH, P < 0.001). No significant difference was observed in arteriolar muscularization between SCR-CTRL and PH groups (7.01 ± 0.46 μm in SCR-CTRL vs. 7.2 ± 0.5 μm in PH, P < 0.1) (Fig. 3, A and B). Additionally, miR206-KD considerably improved the PA flow, as evident in the Doppler echocardiography images and PAAT parameter (29.6 ± 0.5 ms in miR206-KD group vs. 18.3 ± 0.6 ms in PH, P < 0.001) (Fig. 3, C and D). SCR-CTRL had no effect on PAAT, as this parameter was not significantly different than that of the PH group (19.1 ± 0.6 ms in SCR-CTRL group vs. 18.3 ± 0.6 ms in PH, P < 0.73). The heart rates between all four groups of animals were similar (Fig. 3E). miR206-KD also significantly reduced the dP/dtmax (1,358.8 ± 153 ms in miR206-KD group vs. 2,322.6 ± 339.2 ms in PH, P < 0.05), and the maximum isovolumetric −dP/dtmin (−1,036.2 ± 148.3 in miR206-KD group vs. −1,887.3 ± 117 in PH, P < 0.001) compared with PH (Fig. 3, F and G). No significant difference was observed in dP/dtmax and −dP/dtmin between SCR-CTRL and PH groups (dP/dtmax: 2,480 ± 193.6 in SCR-CTRL group vs. 2,322.6 ± 339.2 in PH, P < 0.716; −dP/dtmin: −2,022 ± 170 in SCR-CTRL group vs. −1,887.2 ± 117 in PH, P < 0.898).
Fig. 3.
Knockdown of miR206 improves pulmonary hemodynamics and structure. A: α-smooth muscle actin staining for lung arterioles in male rats. B: quantification of arteriole wall thickness (50–100 arterioles/group were analyzed from n = 5–6 rats/group). C: echocardiographic images of pulse-wave Doppler in male rats. D: quantification of pulmonary artery acceleration time (PAAT) calculated from the pulse-wave Doppler in male rats (n = 5–6 rats/group). E: quantification of heart rate (beats/min) in male rats (n = 5–6 rats/group). Quantification of the maximum rate of rise of RV pressure (dP/dtmax; F) and the maximum isovolumetric rate of relaxation (−dP/dtmin; G) in male rats (n = 5–6 rats/group) is shown. Values are means ± SE. #P < 0.01. P̂ < 0.001.
Knockdown of miR206 is associated with induction of VEGFA and stimulation of angiogenesis in the lungs.
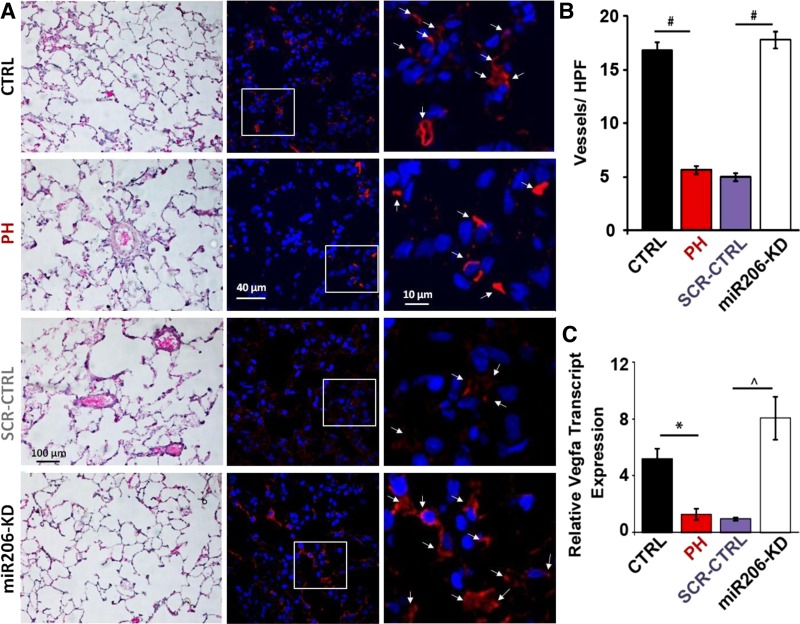
Stimulation of angiogenesis in the lungs by overexpression of angiogenic factor VEGFA has been shown to attenuate the development and progression of PAH (6). Since VEGFA is a well-studied target of miR206, we examined whether knockdown of miR206 can stimulate neoangiogenesis in the lungs (32). Figure 4A shows hematoxylin-eosin staining, as well as immnuohistochemistry of thin lung sections (4 μm) with anti-von Willebrand antibody and 4′,6-diamidino-2-phenylindole. Vessel density of microvessels up to 20 μm in the lungs was drastically reduced in PH group, and miR206-KD significantly increased the number of microvessels in MCT-treated rats (Fig. 4A). Quantification of blood vessels shows approximately threefold reduction in the microvessels in PH (5.6 ± 0.4 vessels per high-power field in PH vs. 16.7 ± 0.8 vessels in CTRL, P < 0.001). miR206-KD led to a significant approximately threefold induction of microvessels compared with PH group (17.6 ± 0.8 vessels per high-power field in miR206-KD vs. 5.6 ± 0.4 vessels in PH, P < 0.001) (Fig. 4B). SCR-CTRL had no effect on microvessel density, as this parameter was not significantly different than that of the PH group (4.9 ± 0.4 vessels per high-power field in SCR-CTRL group vs. 5.6 ± 0.4 vessels in PH, P > 0.05, Fig. 4B). Correspondingly, VEGFA transcripts were downregulated in the lungs of PH animals by approximately threefold compared with the CTRL (1.3 ± 0.4 in PH vs. 5.2 ± 0.7 in CTRL, P < 0.05). miR206-KD was associated with increase in the expression of VEGFA in the PH animals by approximately threefold above the CTRL and approximately sixfold above PH, thus reinforcing a potential anti-angiogenic role of miR206 (8.06 ± 1.5 in miR206-KD vs. 1.3 ± 0.4 in PH, P < 0.001) (Fig. 4C).
Fig. 4.
Knockdown of miR206 is associated with stimulation of angiogenesis and vascular endothelial growth factor A (VEGFA) transcripts. A: representative hematoxylin-eosin images of lung sections (left), and confocal images immunostained for von Willebrand factor (red) and 4′,6-diamidino-2-phenylindole for nuclei (blue) at lower magnification (middle) and at higher magnification (right). White arrows show representative examples of microvessels that were used for quantification. B: quantification of vessels per high-power field (HPF) in CTRL, PH, SCR miR CTRL (SCR-CTRL), and miR206-KD groups. #P < 0.001 (20 high-power magnification images from 3–4 animals/group). C: quantitative RT-PCR assessment of transcript levels of VEGFA in the lung tissues of CTRL, PH, SCR-CTRL, and miR206-KD groups in MCT rat model of PH. *P < 0.05. P̂ < 0.001. Values are means ± SE (n = 5–6 rats/group).
DISCUSSION
Previously, our laboratory has shown that genistein therapy after the establishment of PH rescues preexisting advanced PH by restoring cardiopulmonary function and structure in rats (20). In this report, we have explored the role of downstream effector miR candidates regulated by genistein. Among the shortlisted miRs, we found that miR206 is the most robustly upregulated miR in the lungs of PH rats induced by MCT (∼11-fold) (Fig. 1A). Rescue of PH by genistein treatment was associated with normalization of miR206 in the lungs of PH, similar to levels in the CTRL group. More importantly, we found that knockdown of miR206 in the lungs of PH rats was sufficient to rescue preexisting PH (Fig. 5). Reduction of PA thickness and PA pressures (Figs. 2 and 3, A–D) by miR206-KD resulted in reduced RV afterload, as the RV was no longer required to generate high contractile forces and thus both dP/dtmax and dP/dtmin were reduced to levels similar in those in CTRL (Fig. 3, F and G). In addition, miR206-KD was associated with induction of VEGFA expression and increased angiogenesis in the lungs (Fig. 4 and 5).
Fig. 5.
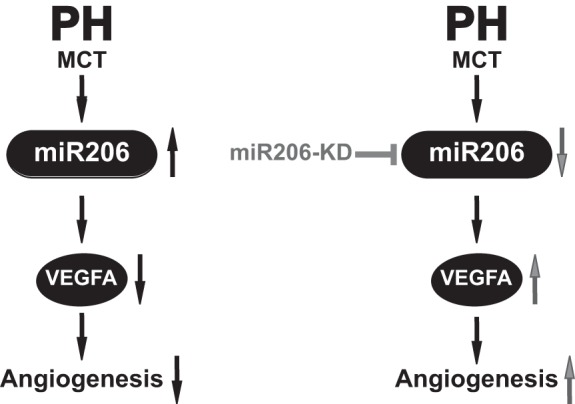
miR206-KD therapy rescues PH and induces angiogenesis in the lung. PH in MCT rat experimental model of PH results in elevated levels of miR206. Robust expression of miR206 is associated with downregulation of VEGFA and decreased angiogenesis in the lung tissue. miR206-KD therapy results in restoration of miR206 expression to healthy CTRL values and is associated with induction of angiogenesis by upregulating VEGFA expression.
Role of miR206 in muscle development and function.
miRs are noncoding RNA molecules that belong to a class of highly conserved regulatory molecules. They participate in the regulation of various physiological and pathological processes, including apoptosis, growth, differentiation, and development, as well as many cancers, cardiomyopathies, and other diseases. miR206 belongs to the miR1 family, which includes miR1/206 and miR133a/b, based on their sequence conservation. Unlike most miRs that are ubiquitous, this family of miRs is expressed in a tissue-specific manner. These miRs are muscle specific and very well characterized in terms of their function and influence on many muscle-related myopathies. Despite the similarity in their sequence and expression, these miRs have very distinct targets and promoter differences for transcriptional activation. As a result, these miRs differ in their biological roles. miR206 is primarily expressed in the skeletal muscle (21) and is regulated by many transcription factors (29, 30). In skeletal muscle, miR206 promotes myogenic differentiation by targeting several genes (28). miR206 was also shown to inhibit proliferation and induce differentiation by repressing an enzyme involved in DNA synthesis (17). These studies highlight the role of miR206 in myogenesis.
Pathological role of miR206.
Recently, the expression and role of miR206 in other tissues and diseases has also been identified. Many studies have highlighted the tumor suppressor role of miR206. Expression levels of miR206 are negligibly low in human rhabdomyosarcoma, a soft tissue sarcoma arising from skeletal muscle progenitors. Reexpression of miR206 in rhabdomyosarcoma cell lines significantly decreased proliferation, promoted apoptosis, and activated myogenic program. These studies suggest that repression of miR206 could participate in rhabdomyosarcoma development due to aberrant cell proliferation and migration (39, 43). In agreement, loss of miR206 has been shown to correlate with increased cell proliferation, migration, tumorogenesis, and apoptosis (34, 41). miR206 expression is markedly decreased in ER-α-positive human breast cancer tissues, and its overexpression in breast cancer cells results in growth inhibition (18). miR206 targets Notch3 signaling and activates apoptosis in HeLa cells (31). The role of miR206 is also highlighted in the progression of amyotrophic lateral sclerosis and in neuromuscular synaptic regeneration in mice (40). It is evident from these works that, although miR206 is highly tissue specific, it plays a regulatory role in other tissues as well via multiple targets, and its differential expression is associated with many diseases.
Controversial role of miR206 in PH.
miR206 is a skeletal muscle-specific miR, and as observed from our microarray data, the CTRL lungs have a very low copy number of miR206. However, after MCT insult, the expression of miR206 is increased by ∼11-fold in the lung tissue. Interestingly, this aberrant induction of miR206 is strikingly suppressed by genistein treatment. Even though multiple miRs, including miR206, miR21, miR322, and miR503, were dysregulated in PH, and genistein normalized their expression, knockdown of miR206 alone is sufficient to rescue preexisting PH. This could potentially be due to drastic regulation of miR206 in PH compared with other miRs.
In contrast to our observation, two recent studies have shown downregulation of miR206 in the hypoxia model of PH (16, 42). In the study by Jalali et al. (16), it was shown that pulmonary smooth muscle cells isolated from hypoxic PH mice have decreased levels of miR206 compared with CTRL mice. Gain of miR206 inhibited proliferation, migration, and contraction and increased apoptosis in these cells possibly by downregulation of Notch3 signaling (16). Although the other study by Yue et al. (42) shows that miR206 is downregulated in the lungs of hypoxic rat model of PH, contrary to their expectation gain of miR206, resulted in a significant increase in PASMC proliferation in cultured PASMCs, and the expression of its bonafide target HIF-1α remained unchanged (42). These two studies in hypoxia indicate that the exact role and function of miR206 are quite complex and are possibly influenced by multiple factors, including transcriptional regulation, effect of other miRs, and different microenvironment. In addition, the nature of insult (MCT vs. hypoxia), interaction of different cell types in lungs, and temporal regulation of miR206 in the pathogenesis of the disease could contribute to differences observed in PH from different models. Therefore, it is not surprising that miR206 expression is highly induced in the lungs of the MCT model, as shown in our study, whereas an opposite trend is observed in the hypoxia model, as shown in the studies by Yue et al. (42) and Jalali et al. (16). A recent study comparing the expression patterns of 350 miRs in MCT rat and hypoxia rat models of PH demonstrates time- and insult-dependent differences in the expression of miRs within the same species. Interestingly, only a few miRs, including let7f, miR22, and miR30c (downregulated), and miR322 and miR451 (upregulated), were consistently regulated across both MCT and hypoxia models. Notably, miR21 and let7a were significantly downregulated only in MCT-treated rats (8). These differences in the miR modulation are reflective of the distinct directions of pathogenesis in these two models that arise in part from the nature of insult.
Our study is an attempt to understand the complex role of miR206 in PH. However, it is limited in its scope, as it employs only the MCT rat model of PH. Similar to most of the animal models of PH studied to date, the MCT rat model also has limitations in mimicking human PAH. Nonetheless, many aspects of this model are pertinent to human disease, especially in the context of inflammation (13), and it is an extensively used model in PH studies. Our findings highlight the role of miR206 in the MCT model of PH; however, the role of miR206 in human PAH remains to be explored.
Role of angiogenesis and VEGFA in PH.
PH is associated with complex vascular remodeling, including pulmonary vasoconstriction, excessive proliferation of endothelia cells, and dysregulated angiogenic signaling. Angiogenesis factors, including VEGF and its receptor VEGF receptor 2 are important factors for the maintenance, function and survival of endothelial cells (12, 15). More importantly, VEGF-driven angiogenesis has been shown to be critical in the normal lung alveolar development with both gain and loss of function studies (35). The development of rodent models in which PH is induced by multiple insults through combination of VEGFA receptor inhibition by specific antagonist (SU5416) and chronic hypoxic exposure support the view that VEGF/VEGF receptor 2 signaling contributes to pulmonary vascular remodeling in the pathogenesis of PH (9, 33). Cell-based gene transfer of VEGFA has been shown to minimize the development and progression of PAH by preventing the loss of existing vessels or by inducing the development of new blood vessels within the lung (6). Several studies have highlighted the fact that vascular remodeling in PAH patients not only is restricted to lungs and surrounding vasculature, but also affects the peripheral tissues (2, 26). This systemic angiogenic impairment of vasculature was shown to be associated with dysregulation of miR126 in the skeletal muscle in PAH through VEGF/ERK pathway, further confirming the role of VEGFA signaling in PH (27). miR206 has been shown to directly target VEGFA in muscle and, therefore, negatively regulates angiogenesis (32). Lung angiogenesis in the MCT model of PH has been shown to be drastically attenuated (1, 25, 37). In agreement with previous studies, Dutly et al. (11), using fluorescent microangiography, a highly quantitative tool to study lung microvascular changes in animal models of PH, demonstrated a marked decrease in the number of vessels in MCT-treated animals. In addition to miR206, other miRs have also been shown to regulate pulmonary vascular remodeling in PH. For example, miR-130/301 family has been shown to regulate vasomotor tone in PH by controlling the expression of the master protein peroxisome proliferator-activated receptor-γ. Peroxisome proliferator-activated receptor-γ, in turn, facilitates cross talk between pulmonary vascular endothelial and smooth muscle cells via regulating many downstream vasoactive factors, including endothelin-1 (5). Therefore, multiple miRs can regulate pulmonary vascular signaling in PH via different vasoactive factors.
In this study, we observe that miR206-KD in the lungs of PH rats induced by MCT is associated with a significant induction and restoration of VEGFA expression to the levels observed in CTRL. Also, high levels of miR206 were associated with a decrease in angiogenesis. However, it is possible that our quantification of microvessels may have been affected, if the lung volumes were different after the lung injury. Such an injury could affect lung volume and thus the number and thickness of vessels. A bigger lung would have thinner walls and fewer proximal vessels. Although we cannot distinguish between bronchial or pulmonary microvessels with von Willebrand factor staining, we do observe a significant global increase in angiogenesis in the lung with miR206-KD. It is quite possible that, among many factors, angiogenesis could be a contributing factor for overall improvement in cardiopulmonary function with miR206-KD. miR206-KD in MCT-treated animals resulted in the rescue of preexisting PH. This was evident from various functional parameters, including reduction of thickening of lung arterioles and increase in angiogenesis. The pressure changes in the RV were also substantially normalized to control levels in these animals, suggesting an overall improvement in the RV function. It is possible, but not exclusive, that many of the improved functional parameters were contributed by an increase in angiogenesis.
Possible regulatory mechanisms of miR206 by genistein.
Our previous studies have shown that both estrogen and genistein rescue PH, and they most likely act through ER-β receptor pathway (20). In this report, we have explored miRs as one of the potential downstream mechanisms for the rescue action of genistein in PH. Although our in vivo data clearly suggest that genistein treatment is associated with inhibition of miR206 in the MCT-induced model of PH in rats, whether this inhibition is through ER-β pathway or through genistein's tyrosine kinase inhibitory effect is not clear. It is possible that both of these pathways culminate in the net restoration of miR206 levels by genistein. The contribution of other potential mechanisms of genistein action, for example, increases in endothelial nitric oxide synthase levels and nitric oxide-mediated PA relaxation could not be ruled out as the indirect factors for modulation of miR206 levels. In addition, we cannot rule out the possibility that downregulation of miR206 expression upon genistein treatment could be a result of improvement in the cardiopulmonary function of the animals and not a direct consequence of genistein action. Therefore, genistein rescue and miR206 downregulation could ultimately converge to rescue preexisting PH in the MCT model. Exploring the direct or indirect action of both MCT and genistein on the transcriptional regulation of miR206, as well as its downstream targets, warrants further investigation to understand the model-specific transcriptional regulation of miR206 and potential link between genistein and miR206 regulation.
Conclusion.
In conclusion, as depicted in Fig. 5 this study shows that, in the lungs of MCT-induced PH rats, genistein treatment is associated with the differential regulation of several miRs. Among these, miR206 expression shows steep upregulation in PH, and its expression is completely restored by genistein treatment. Knockdown of miR206 by specific antagomir is sufficient to rescue advanced PH in MCT rat model concomitant with an increase in angiogenesis and decrease in arteriolar thickening in the lungs.
GRANTS
This work was supported in part by American Heart Association Grant AHA17240020 (S. Sharma); National Heart, Lung, and Blood Institute Grants HL-089876 and HL-119886 (M. Eghbali); and Clinical and Translational Science Institute Grant UL1TR000124 (M. Eghbali).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S., S.U., A.C., and M.E. conception and design of research; S.S., S.U., and A.C. performed experiments; S.S., S.U., A.C., and M.E. analyzed data; S.S., S.U., and M.E. interpreted results of experiments; S.S., S.U., and M.E. prepared figures; S.S. and M.E. drafted manuscript; S.S., S.U., and M.E. edited and revised manuscript; S.S., S.U., A.C., and M.E. approved final version of manuscript.
REFERENCES
- 1.Arcot SS, Lipke DW, Gillespie MN, Olson JW. Alterations of growth factor transcripts in rat lungs during development of monocrotaline-induced pulmonary hypertension. Biochem Pharmacol 46: 1086–1091, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 54: S55–S66, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, Chan SY. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem 290: 2069–2085, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell AI, Zhao Y, Sandhu R, Stewart DJ. Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 104: 2242–2248, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, Upton P, Xin M, van Rooij E, Olson EN, Morrell NW, MacLean MR, Baker AH. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res 111: 290–300, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J, Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 184: 1171–1182, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutly AE, Kugathasan L, Trogadis JE, Keshavjee SH, Stewart DJ, Courtman DW. Fluorescent microangiography (FMA): an improved tool to visualize the pulmonary microvasculature. Lab Invest 86: 409–416, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol 302: L363–L369, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Jakubczak LF. Calorie and water intakes as a function of strain, age, and caloric-density of diet. Physiol Behav 20: 273–278, 1978. [DOI] [PubMed] [Google Scholar]
- 16.Jalali S, Ramanathan GK, Parthasarathy PT, Aljubran S, Galam L, Yunus A, Garcia S, Cox RR Jr, Lockey RF, Kolliputi N. Mir-206 regulates pulmonary artery smooth muscle cell proliferation and differentiation. PLoS One 7: e46808, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174: 677–687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo N, Toyama T, Sugiura H, Fujii Y, Yamashita H. miR-206 expression is down-regulated in estrogen receptor alpha-positive human breast cancer. Cancer Res 68: 5004–5008, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 294: 853–858, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Matori H, Umar S, Nadadur RD, Sharma S, Partow-Navid R, Afkhami M, Amjedi M, Eghbali M. Genistein, a soy phytoestrogen, reverses severe pulmonary hypertension and prevents right heart failure in rats. Hypertension 60: 425–430, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy JJ. MicroRNA-206: the skeletal muscle-specific myomiR. Biochim Biophys Acta 1779: 682–691, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mucke H. The role of imatinib in the treatment of pulmonary hypertension. Drugs Today (Barc) 49: 203–211, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabasi AL, Loscalzo J, Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 125: 1520–1532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, Lemarchand P, Eddahibi S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 23: 762–771, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Partovian C, Ladoux A, Eddahibi S, Teiger E, Raffestin B, Frelin C, Adnot S. Cardiac and lung VEGF mRNA expression in chronically hypoxic and monocrotaline-treated rats. Chest 114: 45S–46S, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Potus F, Graydon C, Provencher S, Bonnet S. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series). Pulm Circ 4: 175–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay E, Breuils-Bonnet S, Ribeiro F, Porlier A, Maltais F, Bonnet S, Provencher S. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 318–328, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Prugh JD, Hartman GD, Mallorga PJ, McKeever BM, Michelson SR, Murcko MA, Schwam H, Smith RL, Sondey JM, Springer JP, et al. . New isomeric classes of topically active ocular hypotensive carbonic anhydrase inhibitors: 5-substituted thieno[2,3-b]thiophene-2-sulfonamides and 5-substituted thieno[3,2-b]thiophene-2-sulfonamides. J Med Chem 34: 1805–1818, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A 103: 8721–8726, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg MI, Georges SA, Asawachaicharn A, Analau E, Tapscott SJ. MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol 175: 77–85, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem 284: 31921–31927, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stahlhut C, Suarez Y, Lu J, Mishima Y, Giraldez AJ. miR-1 and miR-206 regulate angiogenesis by modulating VegfA expression in zebrafish. Development 139: 4356–4364, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Taulli R, Bersani F, Foglizzo V, Linari A, Vigna E, Ladanyi M, Tuschl T, Ponzetto C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J Clin Invest 119: 2366–2378, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 112: 2477–2486, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension: a unique process in a unique disease. Antioxid Redox Signal 4: 833–843, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M. Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184: 715–723, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voelkel NF, Tuder RM. Cellular and molecular biology of vascular smooth muscle cells in pulmonary hypertension. Pulm Pharmacol Ther 10: 231–241, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Ling C, Bai Y, Zhao J. MicroRNA-206 is associated with invasion and metastasis of lung cancer. Anat Rec (Hoboken) 294: 88–92, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326: 1549–1554, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan D, Dong XE, Chen X, Wang L, Lu C, Wang J, Qu J, Tu L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem 284: 29596–29604, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yue J, Guan J, Wang X, Zhang L, Yang Z, Ao Q, Deng Y, Zhu P, Wang G. MicroRNA-206 is involved in hypoxia-induced pulmonary hypertension through targeting of the HIF-1alpha/Fhl-1 pathway. Lab Invest 93: 748–759, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, Liu M, Wang C, Lin C, Sun Y, Jin D. Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression. Anticancer Res 31: 3859–3863, 2011. [PubMed] [Google Scholar]
- 44.Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ. Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res 92: 984–991, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YD, Courtman DW, Ng DS, Robb MJ, Deng YP, Trogadis J, Han RN, Stewart DJ. Microvascular regeneration in established pulmonary hypertension by angiogenic gene transfer. Am J Respir Cell Mol Biol 35: 182–189, 2006. [DOI] [PubMed] [Google Scholar]