Abstract
In response to injury, reparative processes are triggered to restore the damaged tissue; however, such processes are not always successful in rebuilding the original state. The formation of fibrous connective tissue is known as fibrosis, a hallmark of the reparative process. For fibrosis to be successful, delicately balanced cellular events involving cell proliferation, cell migration, and extracellular matrix (ECM) remodeling must occur in a highly orchestrated manner. While successful repair may result in a fibrous scar, this often restores structural stability and functionality to the injured tissue. However, depending on the functionality of the injured tissue, a fibrotic scar can have a devastating effect. For example, in the retina, fibrotic scarring may compromise vision and ultimately lead to blindness. In this review, we discuss some of the retinal fibrotic complications and highlight mechanisms underlying the development of retinal fibrosis in diabetic retinopathy.
Keywords: Diabetic retinopathy, retinal fibrosis, Müller cell, vascular endothelial growth factor, extracellular matrix
Introduction
Fibrosis is an intricate, reparative process that develops in response to acute or chronic injury. It requires a normally functioning vascular network, and its success in restoring the damaged tissue depends at least in part on the proper remodeling of extracellular matrix (ECM). Following injury-induced release of chemotactic agents, the vascular system facilitates timely recruitment of appropriate inflammatory cells to mediate the reparative process and facilitate synthesis and remodeling of new ECM. However, if the new ECM deposition is altered and leads to disturbed tissue architecture, it can contribute to a compromised fibrotic process. Additionally, the success of the reparative process and restoration of the functionality of the damaged tissue is, at least in part, dependent on the location of such fibrotic processes (Yang et al. 2013). In the retina, for example, the location of the damaged area can profoundly affect retinal functionality and visual acuity.
The involvement of fibroblasts in the fibrotic process is well established. Fibroblasts exist ubiquitously in connective tissues extrinsic to the central nervous system (CNS) and are distinct from epithelial cells in that they are nonpolar and are not bound to the basal lamina unilaterally, allowing migratory ability across surfaces. A primary function of fibroblasts is to respond to injury by synthesizing various ECM components including collagens, elastic fibers, glycosaminoglycans, and glycoproteins, and thereby mediating a reparative process. The glial cells, particularly the Müller cells, mediate the tissue-healing response in the retina, much like the fibroblasts in non-CNS tissue.
Cell proliferation, ECM expansion, and neovascularization are key steps in the development and progression of proliferative diabetic retinopathy (PDR) (Ban and Twigg 2008). These events are typically growth factor-driven in response to hypoxia and inflammatory insults, and they promote the formation of fibrotic tissue on the retinal surface or in the vitreous cavity (Friedlander 2007, Ban and Twigg 2008). Ultimately, the tractional forces generated by the fibrotic process during neovascularization result in the separation of the inner neurosensory retina from the outer retinal pigment epithelium (RPE) resulting in retinal detachment (Yang et al. 2008). Although it is unclear which cell types participate in generating tractional force contributing to retinal detachment in PDR, Müller cells are known to produce stress fibers that are well established in providing mechanical strength to the retinal detachment process (Guidry et al. 2003).
Two types of fibrotic tissues, fibrovascular proliferative tissue and avascular proliferative tissue, can develop in patients with PDR and contribute to retinal detachment (McMeel 1971). Fibrovascular proliferative tissue is formed when abnormal new vessels grow during PDR on the retinal surface. Avascular proliferative tissue is less common and consists of amorphous avascular membranes. Of the three types of retinal detachment, rhegmatogenous, traction, and exudative, PDR is most often associated with traction retinal detachment. During traction retinal detachment, the scar tissue on the retinal surface “pulls” at the retina, detaching it from the underlying layer, whereas during rhegmatogenous retinal detachment, retinal tears develop allowing fluid accumulation in the subretinal space (Humphrey et al. 1993). This fluid together with the tractional force of the vitreous on the retina can promote retinal detachment.
Histopathology of retinal fibrosis
Histopathologic studies of PDR have demonstrated that fibrovascular proliferative tissue and avascular proliferative tissue are closely associated with the development of PDR. Fibrovascular proliferative tissue is most common and histological studies indicate that new vessels originating and extending from the superficial plexus or capillaries of the retina often develop within the fibrous network (McMeel 1971). The extension of the newly formed vessels and the appearance and progression of contracture of connective tissue is believed to contribute to retinal fibrosis (Dobree 1964). In the early stages of retinal fibrosis, abnormal new vessels proliferate devoid of fibrotic tissue, followed by an intermediate stage in which translucent fibrotic connective tissue progressively fills the intervascular spaces, resulting in the formation of a dense, white, avascular scar in the late stage. The fibrovascular proliferative tissue expands peripherally from its origin, while avascular proliferative tissue is typically a thin membrane that extends from the edges of fibrovascular proliferation (McMeel 1971). Of note, fibrotic tissue may also develop secondary to iatrogenic intervention, typically at sites of therapeutic treatment such as photocoagulation, especially in areas of flat neovascularization (McMeel 1971).
Müller cells mediate retinal fibrosis
Retinal Müller cells play an important role in retinal fibrosis by participating in the maintenance of retinal homeostasis (Reichenbach et al. 1993, Newman and Reichenbach 1996, Mizutani et al. 1998, Bringmann and Reichenbach 2001); however, following retinal injury, disturbed homeostatic balance activates Müller cells resulting in increased cell proliferation, cellular shape change, and vascular endothelial growth factor (VEGF) production (Humphrey et al. 1993, MacLaren 1996, Amin et al. 1997, Reichenbach et al. 1997, Dyer and Cepko 2000). Müller cells are reported to assume the role of fibroblasts, which likely do not exist in the retina (Friedlander 2007). Importantly, the Müller cells, astrocytes, and microglia together with the vascular cells participate in the fibrotic process (Bringmann and Reichenbach 2001, Guidry 2005, Friedlander 2007, Yang et al. 2013).
Apart from Müller cells, astrocytes and microglial cells contribute to retinal fibrosis as well. A study suggests that in the mouse retina, astrocytes act as a proangiogenic cell type in the retinal vascular system because they are capable of producing VEGF and fibronectin, and contribute to retinal angiogenesis in retinal fibrosis (Uemura et al. 2006). Additionally, much like Müller cells astrocytes express intermediate filament, glial fibrillary acid protein (GFAP) in response to injury (Lewis and Fisher 2003). Another study reported that microglia in human diabetic retina expresses CTGF, which promotes ECM formation, fibrosis, and angiogenesis (Kuiper et al. 2004).
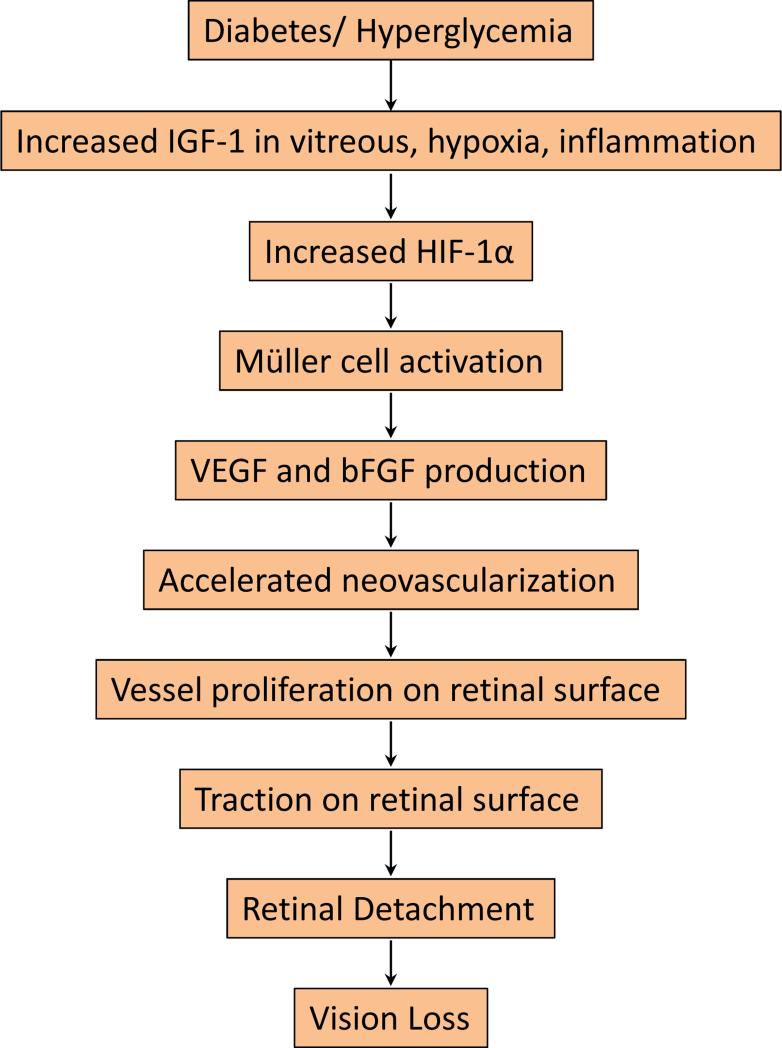
Although Müller cell-mediated fibrosis is essential for retinal repair, this process may also contribute to the progression of diabetic retinopathy (Amin et al. 1997, Lieth et al. 1998, Dyer and Cepko 2000, Friedlander 2007, Yafai et al. 2013) (Figure 1). In PDR, increased vitreal insulin-like growth factor-1 (IGF-1) upregulates hypoxia-inducible factor-1[.alpha] (HIF-1[.alpha]) (Grant et al. 1993, Meyer-Schwickerath et al. 1993, Boulton et al. 1997, Poulaki et al. 2004, Jiang et al. 2013, Tarr et al. 2013). Hypoxic Müller cells increase the stability of HIF-1[.alpha] and induce its nuclear localization (Xin et al. 2013), leading to VEGF overexpression (Rodrigues et al. 2013). HIF-2[.alpha] has also been implicated in the activation of Müller cells and their response to hypoxia (Mowat et al. 2010). As such, the HIF factors mediate Müller cell activation. Müller cells respond to hypoxia by expressing increased levels of VEGF and basic fibroblast growth factor (bFGF) (Ai et al. 2013, Rodrigues et al. 2013, Xin et al. 2013, Yafai et al. 2013), which together promote retinal neovascularization (Cheng et al. 1998). Growth of aberrant vasculature on the retinal surface promotes retinal gliosis inducing tractional forces, which significantly increases the risk of retinal detachment and subsequent vision loss (Guidry 2005, Friedlander 2007, Morello 2007).
Figure 1.
A schematic flowchart showing potential effects of diabetes or hyperglycemia on retinal fibrosis and vision loss in diabetic retinopathy. Diabetes and associated hyperglycemia can increase serum and vitreous levels of IGF-1, an angiogenic neuroprotective factor. IGF-1, hypoxia, and inflammation are all contributing factors, which can increase HIF-1α level. Upregulation of HIF-1α is known to contribute to Müller cell activation and induce angiogenic factors VEGF and bFGF that aid in the development and progression of neovascularization, which contributes to retinal fibrosis. The combined effect of new abnormal blood vessel growth and retinal fibrosis generates tractional forces, which pull on the retina, contributing to retinal detachment and subsequent vision loss, in diabetic retinopathy.
Müller cell activation represents one of the earliest steps in the pathogenesis of diabetic retinopathy (Nork et al. 1987, Robison et al. 1990, Amin et al. 1997). Targeting cyclin D and p27Kip1, modulators of Müller cell activation, could regulate the progression of fibrosis in diabetic retinopathy, since activation of Müller cells is an early step in both processes (Dyer and Cepko 2000). In cases of chronic or severe exposure to stress, Müller cells undergo “massive gliosis” (Bringmann and Reichenbach 2001, Guidry 2005), which represents significant cell proliferation and subsequent formation of “gliotic scars” both within the retina and on the subretinal and epiretinal surfaces (Guidry 2005). Since diabetic retinopathy is a long-term complication, the likelihood of massive gliosis due to chronic hyperglycemia may increase (Sueishi et al. 1996, Amin et al. 1997).
Inflammation in retinal fibrosis
Inflammation promotes fibrovascular scarring primarily by contributing to the process of angiogenesis. IL-8 and TNF-α, angiogenic inflammatory cytokines, increase endothelial cell proliferation and promote angiogenesis, which in turn, contributes to diabetes-related retinal fibrosis (Friedlander 2007). A study demonstrated that upregulation of thioredoxin-interacting protein (TXNIP), a proinflammatory and proapoptotic protein plays a significant role in promoting retinal injury in the diabetic retina. The results correlated with induction of sclerotic fibronectin, which facilitates retinal angiogenesis and fibrosis. Furthermore, blockade of TXNIP ameliorated retinal fibrosis in diabetic rats (Perrone et al. 2010). Interestingly, toll like receptors 2 and 4, which are part of the innate immune system, play a significant role in subretinal fibrosis formation (Yang et al. 2013). IL-10, an anti-inflammatory cytokine, has also been shown to play a role in reducing subretinal fibrosis, which implicates inflammation as an underlying cause of fibrosis (Yang et al. 2013).
Connective tissue growth factor (CTGF), a critical mediator in retinal fibrosis
In patients with PDR, overexpression of CTGF and subsequent increased levels in the vitreous has been reported (Tikellis et al. 2004, Kuiper et al. 2007, Abu El-Asrar et al. 2012, Hu et al. 2014). CTGF acts as a downstream mediator of transforming growth factor-ß (TGF-ß), which promotes pro-fibrotic effects. In PDR, both VEGF and CTGF participate in promoting neovascularization and subsequent fibrosis; however, the mechanisms underlying theses processes are not well understood. A negative feedback mechanism between these two factors has been identified in which VEGF upregulates CTGF, which in turn inhibits VEGF production (Kuiper et al. 2008). This negative feedback allows for a balance between the two growth factors and drives the transition from the angiogenic phase to the fibrotic phase, a process known as the “angiofibrotic switch” (Kuiper et al. 2008). The balance between these two phases is critical in understanding how the anti-VEGF agent, bevacizumab, decreases neovascularization but may also induce fibrosis, as evident from the development of fibrotic membranes. Taken together, these findings suggest that VEGF and CTGF work together in facilitating retinal fibrosis in PDR (Kuiper et al. 2008).
Similarly, ranibizumab, another anti-VEGF agent, lowers VEGF level but also elevates CTGF level (Hu et al. 2014). A dual-therapy approach was tested against VEGF and CTGF in the diabetic rat retina using ranibizumab with CTGF siRNA, which normalized both targets and markedly improved the ultrastructure of the retinal microvessels (Hu et al. 2014). Although the functions of VEGF and CTGF appear integrated, each has their own distinct actions (Kuiper et al. 2007). Studies indicate that the degree of fibrosis in vitreoretinal disorders is at least in part dependent on CTGF activity, which makes CTGF a potential therapeutic target for preventing vitreoretinal scarring and progression of fibrosis in PDR (Kuiper et al. 2006, Kuiper et al. 2007, Kuiper et al. 2008). Towards developing this type of target, a novel angiogenic inhibitor has been shown to be effective in reducing CTGF activity at the post-translational level in human endothelial cells (Kondo et al. 2006).
Retinal fibrosis secondary to treatment against diabetic retinopathy
Panretinal photocoagulation (PRP) and anti-VEGF intravitreal injections are the current mainstay of treatment modalities to reduce vascular complications of diabetic retinopathy. Numerous studies have analyzed the short- and long-term beneficial effects of these interventions in patients with diabetic macular edema (DME) or diabetic retinopathy (Friedlander 2007, Sinawat et al. 2013, Alasil and Waheed 2014, Chhablani et al. 2014, Ferenchak et al. 2014, Nitta et al. 2014, Oh et al. 2014, Parikh et al. 2014, Regnier et al. 2014, Stefanini et al. 2014). With the popularity of PRP and anti-VEGF therapies on the rise, iatrogenic subretinal fibrosis (SRF) has emerged as a significant concern (Guyer et al. 1992, Batman and Ozdamar 2010). SRF develops following laser treatment of the retina of patients with DME (Guyer et al. 1992, Han et al. 1992). Interestingly, patients with severe hard exudates from long-standing diabetic retinopathy were found to be most susceptible to developing SRF following PRP. Although side effects may arise due to PRP, the significance of these detrimental effects remains unclear.
Other types of ocular fibrosis
Fibrosis in the cornea exhibits some similarity in etiology compared to retinal fibrosis. For example, both types of fibrosis typically develop in response to chronic inflammation or disease. In addition, both the cornea and retina undergo common wound-healing processes including injured tissue removal, cell proliferation and migration, cytokine-mediated interactions, and aberrant remodeling of the underlying ECM. Importantly, the salient features of corneal and retinal fibrosis retain specific distinctions. For example, neovascularization is a major contributory factor in retinal fibrosis; however, the cornea is an avascular tissue, and corneal fibrosis therefore does not involve neovascularization. This lack of vascularity may account for slower healing in the cornea compared to the retina. However, the cornea has one layer known as the Descemet's membrane, which is able to regenerate to full functioning capacity, unlike any part of the retina. Corneal fibroblasts are known as keratocytes, which become activated in response to injury; similarly, the retina contains Müller cells, which are fibroblast-like glial cells activated in response to injury. Ultimately, if the cornea is scarred centrally, this can lead to vision loss, the common endpoint seen in retinal fibrosis as well (Wilson et al. 2012). Diabetes is risk factor for other ocular conditions such as glaucoma. Briefly, in late stage diabetic retinopathy, progressive fibrosis of the trabecular meshwork and iris (rubeosis iridis) affects the outflow of aqueous humor leading to glaucoma (Wilkinson-Berka 2004, Friedlander 2007).
Treatment modalities for retinal fibrosis
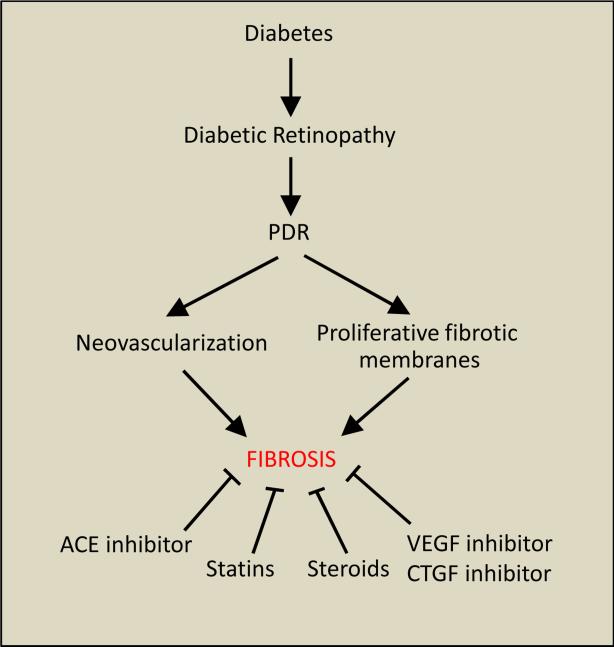
Potential inhibitory strategies for prevention of retinal fibrosis in PDR include the use of statins, steroids, and inhibitors of VEGF, CTGF, and angiotensin converting enzyme (ACE) (Figure 2). Dual suppression of pro-angiogenic VEGF and CTGF improves retinal vasculature integrity and facilitates a decrease in fibrosis in the diabetic rat retina (Hu et al. 2014). Additionally, the CTGF inhibitor SERPINA3K, reduces retinal ECM production, fibrogenic activity, and angiogenesis via the Wnt/ß-catenin pathway in the diabetic rat retina (Zhang et al. 2010). Moreover, CTGF-siRNA decreased ECM components, laminin β1 and collagen IVα3 mRNA in the diabetic rat retina (Winkler et al. 2012).
Figure 2.
Diagram illustrating the development of retinal fibrosis in diabetic retinopathy and potential inhibitory strategies for prevention of proliferative fibrotic membranes associated with PDR.
The ACE inhibitor perindopril reduces VEGF expression in the diabetic rat retina, and directly inhibits neovascularization-mediated fibrosis. Additionally, ACE inhibitors can block the renin-angiotensin system, which is known to promote proliferation, inflammation, and fibrosis (Gilbert et al. 2000, Steckelings et al. 2009). Similarly, statins or HMG Co-A reductase inhibitors have anti-VEGF and anti-inflammatory properties and can exhibit anti-fibrotic action (Weis et al. 2002). Also, simvastatin, which reduces blood retinal barrier breakdown by suppressing VEGF-induced ICAM-1 expression in the diabetic retina has anti-fibrotic property (Miyahara et al. 2004, Fernandes et al. 2014). Pre- and post-intraocular injection of triamcinolone, a steroid, reduces VEGF and thereby causes regression of pre-retinal neovascularization (Brooks et al. 2004). Taken together, these results indicate that several treatment strategies are being currently tested against retinal fibrosis and a novel drug may soon be discovered.
Conclusion
Retinal fibrosis is closely associated with the development of PDR and is characterized by the presence of newly formed blood vessels often accompanied by a fibrous tissue spreading along the inner surface of the retina, across the optic disc, or extending into the vitreous cavity (Ryan et al. 2004). Clinical studies have identified fibrotic membranes in PDR to exhibit uncontrolled growth and cause serious complications including retinal detachment, thereby contributing to vision loss. While retinal neovessels are the primary target of clinical interventions in PDR, retinal fibrosis is another confounding factor for which therapeutic strategies are limited. A variety of potential inhibitory modalities are being investigated for prevention of proliferative fibrotic membranes associated with PDR, including angiotensin converting enzyme inhibitors, VEGF inhibitors, CTGF inhibitors, statins, and steroids (Figure 2). Additionally, future strategies are needed to target abnormal ECM remodeling and BM thickening, as these factors play a critical role in the development and progression of fibrotic membranes in PDR. While further clinical and basic research is necessary to better understand the pathobiology underlying retinal fibrosis, the recent advances hold promise towards prevention of retinal fibrosis associated with diabetic retinopathy.
Highlights.
Retinal fibrosis is usually a late stage occurrence associated with PDR.
Activated Müller cells, the glial cells of the retina, facilitate retinal fibrosis.
Interactions of various growth factors promote retinal fibrosis.
The effect of PRP and anti-VEGF therapy may contribute to retinal fibrosis.
Acknowledgments
Research was supported by NIH, NEI 018218, and in part by a departmental grant from the Massachusetts Lions Eye Research Fund to SR.
Appendix
Appendix A.
Abbreviations used in the article.
| ABBREVIATIONS | FULL NAME |
|---|---|
| ACE | Angiotensin Converting Enzyme |
| bFGF | Basic Fibroblast Growth Factor |
| BM | Basement Membrane |
| CNS | Central Nervous System |
| CTGF | Connective Tissue Growth Factor |
| Cx43 | Connexin 43 |
| DME | Diabetic Macular Edema |
| ECM | Extracellular Matrix |
| GFAP | Glial Fibrillary Acid Protein |
| HIF | Hypoxic Inducible Factor |
| HMG Co-A | Hydroxy Methyl Glutaryl Coenzyme A |
| IGF-1 | Insulin-like Growth Factor-1 |
| PDR | Proliferative Diabetic Retinopathy |
| PRP | Panretinal Photocoagulation |
| SERPINA3K | Serine Protease Inhibitor A3K |
| SRF | Subretinal Fibrosis |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular Endothelial Growth Factor |
| TXNIP | Thioredoxin-Interacting Protein |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu El-Asrar AM, Imtiaz Nawaz M, Kangave D, Siddiquei MM, Geboes K. Osteopontin and other regulators of angiogenesis and fibrogenesis in the vitreous from patients with proliferative vitreoretinal disorders. Mediators Inflamm. 2012;2012:493043. doi: 10.1155/2012/493043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J, Liu Y, Sun JH. Advanced glycation end-products stimulate basic fibroblast growth factor expression in cultured Muller cells. Mol Med Rep. 2013;7(1):16–20. doi: 10.3892/mmr.2012.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasil T, Waheed NK. Pan retinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Curr Opin Ophthalmol. 2014;25(3):164–170. doi: 10.1097/ICU.0000000000000048. [DOI] [PubMed] [Google Scholar]
- Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38(1):36–47. [PubMed] [Google Scholar]
- Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4(3):575–596. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batman C, Ozdamar Y. The relation between bevacizumab injection and the formation of subretinal fibrosis in diabetic patients with panretinal photocoagulation. Ophthalmic Surg Lasers Imaging. 2010;41(2):190–195. doi: 10.3928/15428877-20100303-06. [DOI] [PubMed] [Google Scholar]
- Boulton M, Gregor Z, McLeod D, Charteris D, Jarvis-Evans J, Moriarty P, Khaliq A, Foreman D, Allamby D, Bardsley B. Intravitreal growth factors in proliferative diabetic retinopathy: correlation with neovascular activity and glycaemic management. Br J Ophthalmol. 1997;81(3):228–233. doi: 10.1136/bjo.81.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A, Reichenbach A. Role of Muller cells in retinal degenerations. Front Biosci. 2001;6:E72–92. doi: 10.2741/bringman. [DOI] [PubMed] [Google Scholar]
- Brooks HL, Jr., Caballero S, Jr., Newell CK, Steinmetz RL, Watson D, Segal MS, Harrison JK, Scott EW, Grant MB. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122(12):1801–1807. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39(3):581–591. [PubMed] [Google Scholar]
- Chhablani J, Mathai A, Rani P, Gupta V, Arevalo JF, Kozak I. Comparison of conventional pattern and novel navigated panretinal photocoagulation in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2014;55(6):3432–3438. doi: 10.1167/iovs.14-13936. [DOI] [PubMed] [Google Scholar]
- Dobree JH. Proliferative Diabetic Retinopathy: Evolution of the Retinal Lesions. Br J Ophthalmol. 1964;48:637–649. doi: 10.1136/bjo.48.12.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3(9):873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Ferenchak K, Duval R, Cohen JA, MacCumber MW. Intravitreal bevacizumab for postoperative recurrent vitreous hemorrhage after vitrectomy for proliferative diabetic retinopathy. Retina. 2014;34(6):1177–1181. doi: 10.1097/IAE.0000000000000058. [DOI] [PubMed] [Google Scholar]
- Fernandes R, Bento CF, Matafome P, Sena CM, Seica RM, Pereira P. Atorvastatin-mediated protection of the retina in a model of diabetes with hyperlipidemia. Can J Physiol Pharmacol. 2014;92(12):1037–1043. doi: 10.1139/cjpp-2014-0212. [DOI] [PubMed] [Google Scholar]
- Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117(3):576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RE, Kelly DJ, Cox AJ, Wilkinson-Berka JL, Rumble JR, Osicka T, Panagiotopoulos S, Lee V, Hendrich EC, Jerums G, Cooper ME. Angiotensin converting enzyme inhibition reduces retinal overexpression of vascular endothelial growth factor and hyperpermeability in experimental diabetes. Diabetologia. 2000;43(11):1360–1367. doi: 10.1007/s001250051539. [DOI] [PubMed] [Google Scholar]
- Grant MB, Mames RN, Fitzgerald C, Ellis EA, Aboufriekha M, Guy J. Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia. 1993;36(4):282–291. doi: 10.1007/BF00400229. [DOI] [PubMed] [Google Scholar]
- Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24(1):75–86. doi: 10.1016/j.preteyeres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Guidry C, Bradley KM, King JL. Tractional force generation by human muller cells: growth factor responsiveness and integrin receptor involvement. Invest Ophthalmol Vis Sci. 2003;44(3):1355–1363. doi: 10.1167/iovs.02-0046. [DOI] [PubMed] [Google Scholar]
- Guyer DR, D'Amico DJ, Smith CW. Subretinal fibrosis after laser photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113(6):652–656. doi: 10.1016/s0002-9394(14)74789-0. [DOI] [PubMed] [Google Scholar]
- Han DP, Mieler WF, Burton TC. Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992;113(5):513–521. doi: 10.1016/s0002-9394(14)74722-1. [DOI] [PubMed] [Google Scholar]
- Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu M, Li X. Intravitreal injection of ranibizumab and CTGF shRNA improves retinal gene expression and microvessel ultrastructure in a rodent model of diabetes. Int J Mol Sci. 2014;15(1):1606–1624. doi: 10.3390/ijms15011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey MF, Constable IJ, Chu Y, Wiffen S. A quantitative study of the lateral spread of Muller cell responses to retinal lesions in the rabbit. J Comp Neurol. 1993;334(4):545–558. doi: 10.1002/cne.903340404. [DOI] [PubMed] [Google Scholar]
- Jiang F, Tang YT, Guo L, Jiao XY. The role of insulin-like growth factor I and hypoxia inducible factor 1alpha in vascular endothelial growth factor expression in type 2 diabetes. Ann Clin Lab Sci. 2013;43(1):37–44. [PubMed] [Google Scholar]
- Kondo S, Tanaka N, Kubota S, Mukudai Y, Yosimichi G, Sugahara T, Takigawa M. Novel angiogenic inhibitor DN-9693 that inhibits post-transcriptional induction of connective tissue growth factor (CTGF/CCN2) by vascular endothelial growth factor in human endothelial cells. Mol Cancer Ther. 2006;5(1):129–137. doi: 10.1158/1535-7163.MCT-05-0097. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, de Smet MD, van Meurs JC, Tan HS, Tanck MW, Oliver N, van Nieuwenhoven FA, Goldschmeding R, Schlingemann RO. Association of connective tissue growth factor with fibrosis in vitreoretinal disorders in the human eye. Arch Ophthalmol. 2006;124(10):1457–1462. doi: 10.1001/archopht.124.10.1457. [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Treslong-de Groot HB, Lyons KM, Agostini HJ, Rakic JM, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55(11):1139–1147. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3(7):e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol. 2004;88(8):1082–1087. doi: 10.1136/bjo.2003.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- Lieth E, Barber AJ, Xu B, Dice C, Ratz MJ, Tanase D, Strother JM. Glial reactivity and impaired glutamate metabolism in short-term experimental diabetic retinopathy. Penn State Retina Research Group. Diabetes. 1998;47(5):815–820. doi: 10.2337/diabetes.47.5.815. [DOI] [PubMed] [Google Scholar]
- MacLaren RE. Development and role of retinal glia in regeneration of ganglion cells following retinal injury. Br J Ophthalmol. 1996;80(5):458–464. doi: 10.1136/bjo.80.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeel JW. Diabetic retinopathy: fibrotic proliferation and retinal detachment. Trans Am Ophthalmol Soc. 1971;69:440–493. [PMC free article] [PubMed] [Google Scholar]
- Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Losche C, Rollmann R, Schatz H. Vitreous levels of the insulin-like growth factors I and II, and the insulin-like growth factor binding proteins 2 and 3, increase in neovascular eye disease. Studies in nondiabetic and diabetic subjects. J Clin Invest. 1993;92(6):2620–2625. doi: 10.1172/JCI116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164(5):1697–1706. doi: 10.1016/S0002-9440(10)63728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47(3):445–449. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- Morello CM. Etiology and natural history of diabetic retinopathy: an overview. Am J Health Syst Pharm. 2007;64(17 Suppl 12):S3–7. doi: 10.2146/ajhp070330. [DOI] [PubMed] [Google Scholar]
- Mowat FM, Luhmann UF, Smith AJ, Lange C, Duran Y, Harten S, Shukla D, Maxwell PH, Ali RR, Bainbridge JW. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One. 2010;5(6):e11103. doi: 10.1371/journal.pone.0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Reichenbach A. The Muller cell: a functional element of the retina. Trends Neurosci. 1996;19(8):307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Nitta F, Kunikata H, Aizawa N, Omodaka K, Shiga Y, Yasuda M, Nakazawa T. The effect of intravitreal bevacizumab on ocular blood flow in diabetic retinopathy and branch retinal vein occlusion as measured by laser speckle flowgraphy. Clin Ophthalmol. 2014;8:1119–1127. doi: 10.2147/OPTH.S62022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nork TM, Wallow IH, Sramek SJ, Anderson G. Muller's cell involvement in proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105(10):1424–1429. doi: 10.1001/archopht.1987.01060100126042. [DOI] [PubMed] [Google Scholar]
- Oh JH, Kim SW, Kwon SS, Oh J, Huh K. The change of macular thickness following single-session pattern scan laser panretinal photocoagulation for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2014 doi: 10.1007/s00417-014-2663-x. [DOI] [PubMed] [Google Scholar]
- Parikh R, Shah RJ, VanHouten JP, Cherney EF. Ocular Findings at Initial Pan Retinal Photocoagulation for Proliferative Diabetic Retinopathy Predict the Need for Future Pars Plana Vitrectomy. Retina. 2014 doi: 10.1097/IAE.0000000000000192. [DOI] [PubMed] [Google Scholar]
- Perrone L, Devi TS, Hosoya KI, Terasaki T, Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulaki V, Joussen AM, Mitsiades N, Mitsiades CS, Iliaki EF, Adamis AP. Insulin-like growth factor-I plays a pathogenetic role in diabetic retinopathy. Am J Pathol. 2004;165(2):457–469. doi: 10.1016/S0002-9440(10)63311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier S, Malcolm W, Allen F, Wright J, Bezlyak V. Efficacy of Anti-VEGF and Laser Photocoagulation in the Treatment of Visual Impairment due to Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. PLoS One. 2014;9(7):e102309. doi: 10.1371/journal.pone.0102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, Faude F, Enzmann V, Bringmann A, Pannicke T, Francke M, Biedermann B, Kuhrt H, Stolzenburg JU, Skatchkov SN, Heinemann U, Wiedemann P, Reichelt W. The Muller (glial) cell in normal and diseased retina: a case for single-cell electrophysiology. Ophthalmic Res. 1997;29(5):326–340. doi: 10.1159/000268031. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Stolzenburg JU, Eberhardt W, Chao TI, Dettmer D, Hertz L. What do retinal muller (glial) cells do for their neuronal ‘small siblings’? J Chem Neuroanat. 1993;6(4):201–213. doi: 10.1016/0891-0618(93)90042-3. [DOI] [PubMed] [Google Scholar]
- Robison WG, Jr., Tillis TN, Laver N, Kinoshita JH. Diabetes-related histopathologies of the rat retina prevented with an aldose reductase inhibitor. Exp Eye Res. 1990;50(4):355–366. doi: 10.1016/0014-4835(90)90136-i. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, Solomon S, Lutty G, Semenza GL, Montaner S, Sodhi A. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62(11):3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Hinton DR, Schachat AP, Wilkinson P. Retina. Mosby Inc; St. Louis, MO: 2004. [Google Scholar]
- Sinawat S, Rattanapakorn T, Sanguansak T, Yospaiboon Y, Sinawat S. Intravitreal bevacizumab for proliferative diabetic retinopathy with new dense vitreous hemorrhage after full panretinal photocoagulation. Eye (Lond) 2013;27(12):1391–1396. doi: 10.1038/eye.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol. 2009;23(6):693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- Stefanini FR, Badaro E, Falabella P, Koss M, Farah ME, Maia M. Anti-VEGF for the management of diabetic macular edema. J Immunol Res. 2014;2014:632307. doi: 10.1155/2014/632307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueishi K, Hata Y, Murata T, Nakagawa K, Ishibashi T, Inomata H. Endothelial and glial cell interaction in diabetic retinopathy via the function of vascular endothelial growth factor (VEGF). Pol J Pharmacol. 1996;48(3):307–316. [PubMed] [Google Scholar]
- Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikellis C, Cooper ME, Twigg SM, Burns WC, Tolcos M. Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology. 2004;145(2):860–866. doi: 10.1210/en.2003-0967. [DOI] [PubMed] [Google Scholar]
- Uemura A, Kusuhara S, Wiegand SJ, Yu RT, Nishikawa S. Tlx acts as a proangiogenic switch by regulating extracellular assembly of fibronectin matrices in retinal astrocytes. J Clin Invest. 2006;116(2):369–377. doi: 10.1172/JCI25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka JL. Diabetes and retinal vascular disorders: role of the reninangiotensin system. Expert Rev Mol Med. 2004;6(15):1–18. doi: 10.1017/S1462399404008129. [DOI] [PubMed] [Google Scholar]
- Wilson SL, El Haj AJ, Yang Y. Control of scar tissue formation in the cornea: strategies in clinical and corneal tissue engineering. J Funct Biomater. 2012;3(3):642–687. doi: 10.3390/jfb3030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JL, Kedees MH, Guz Y, Teitelman G. Inhibition of connective tissue growth factor by small interfering ribonucleic acid prevents increase in extracellular matrix molecules in a rodent model of diabetic retinopathy. Mol Vis. 2012;18:874–886. [PMC free article] [PubMed] [Google Scholar]
- Xin X, Rodrigues M, Umapathi M, Kashiwabuchi F, Ma T, Babapoor-Farrokhran S, Wang S, Hu J, Bhutto I, Welsbie DS, Duh EJ, Handa JT, Eberhart CG, Lutty G, Semenza GL, Montaner S, Sodhi A. Hypoxic retinal Muller cells promote vascular permeability by HIF-1-dependent up-regulation of angiopoietin-like 4. Proc Natl Acad Sci U S A. 2013;110(36):E3425–3434. doi: 10.1073/pnas.1217091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafai Y, Iandiev I, Lange J, Yang XM, Wiedemann P, Bringmann A, Eichler W. Basic fibroblast growth factor contributes to a shift in the angioregulatory activity of retinal glial (Muller) cells. PLoS One. 2013;8(7):e68773. doi: 10.1371/journal.pone.0068773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CM, Su PY, Yeh PT, Chen MS. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: clinical manifestations and surgical outcome. Can J Ophthalmol. 2008;43(2):192–198. doi: 10.3129/i08-007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Takeda A, Yoshimura T, Oshima Y, Sonoda KH, Ishibashi T. IL-10 is significantly involved in HSP70-regulation of experimental subretinal fibrosis. PLoS One. 2013;8(12):e80288. doi: 10.1371/journal.pone.0080288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhou KK, Ma JX. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes. 2010;59(7):1809–1816. doi: 10.2337/db09-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]