Abstract
Background. Some studies suggest that influenza vaccination might be protective against severe influenza outcomes in vaccinated persons who become infected. We used data from a large surveillance network to further investigate the effect of influenza vaccination on influenza severity in adults aged ≥50 years who were hospitalized with laboratory-confirmed influenza.
Methods. We analyzed influenza vaccination and influenza severity using Influenza Hospitalization Surveillance Network (FluSurv-NET) data for the 2012−2013 influenza season. Intensive care unit (ICU) admission, death, diagnosis of pneumonia, and hospital and ICU lengths of stay served as measures of disease severity. Data were analyzed by multivariable logistic regression, parametric survival models, and propensity score matching (PSM).
Results. Overall, no differences in severity were observed in the multivariable logistic regression model. Using PSM, adults aged 50−64 years (but not other age groups) who were vaccinated against influenza had a shorter length of ICU stay than those who were unvaccinated (hazard ratio for discharge, 1.84; 95% confidence interval, 1.12−3.01).
Conclusions. Our findings show a modest effect of influenza vaccination on disease severity. Analysis of data from seasons with different predominant strains and higher estimates of vaccine effectiveness are needed.
Keywords: influenza, influenza vaccine, adults, severe illness
Influenza causes >220 000 hospitalizations [1] and 3000−49 000 deaths annually in the United States [2]; most of this morbidity and mortality occurs in older adults. The 2012–2013 influenza season was characterized by predominant circulation of influenza A(H3N2) viruses [3]. Influenza A(H3N2) viruses typically cause more morbidity and mortality than either influenza A(H1N1) or B viruses [4]. Hospitalization rates during the 2012−2013 season were high for all age groups but especially for those aged ≥65 years, whose hospitalization rate was nearly 3 times greater than that seen in this age group in the previous four seasons [3].
Influenza vaccination is the best tool for the prevention of influenza and its complications. In the last 3 seasons, with a vaccine effectiveness of 54%−65% for those aged 50−64 years and 26%−52% for those aged ≥65 years, it was estimated that influenza vaccination reduced the risk for influenza-associated medical visits by 47%–61% [5–7]. Similar vaccine effectiveness estimates were found in Europe and Canada during influenza season 2012−2013 [8, 9]. Research supporting the effect of the vaccine in reducing influenza complications in hospitalized patients is limited but suggests that influenza vaccination might be protective against severe influenza outcomes in those who, despite being vaccinated against influenza, become infected [10–12]. Specifically, Ridenhour et al [12] show that influenza vaccination prevented death after pneumonia/influenza hospitalizations by 4.8% in persons aged ≥65 years. These findings are consistent with studies showing a protective effect of vaccines against severe outcomes [13–15], explained partially by a prolonged activation of the immune system [16]. We used data from our large surveillance network to further investigate the effect of influenza vaccination on influenza severity in adults aged ≥50 years hospitalized with laboratory-confirmed influenza during the 2012–2013 influenza season.
METHODS
Data Collection
Data from the 2012–2013 influenza season for adults aged ≥50 years were collected through the Influenza Hospitalization Surveillance Network (FluSurv-NET), a US population-based influenza-associated hospitalization surveillance system. As previously described, FluSurv-NET includes data on demographic characteristics, lifestyle risk factors, medical history, influenza vaccination status, and clinical outcomes from influenza virus–positive patients that were collected through medical chart review by using a standard form [17]. The FluSurv-NET catchment area includes 81 selected counties in California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Rhode Island, Tennessee, and Utah and captures approximately 8% of the US population aged ≥50 years.
FluSurv-NET Surveillance Definitions
A laboratory-confirmed influenza hospitalization case is defined as a resident of the surveillance area admitted to a hospital ≤14 days after [18] or ≤3 days before a positive influenza virus test [19, 20]. Laboratory testing for influenza virus is done through viral culture, direct or indirect fluorescent antibody staining, rapid antigen testing, or reverse transcription–polymerase chain reaction. Influenza virus testing is ordered at clinicians' discretion. FluSurv-NET staff investigate vaccination status and/or vaccination dates missing on medical charts by accessing vaccination registries, consulting with primary care providers, and interviewing patients. A case was considered vaccinated for influenza during the 2012–2013 influenza season if the case received influenza vaccination on or after 1 July 2012 and at least 12 days before hospitalization.
Inclusion and Exclusion Criteria
We included cases aged ≥50 years who were hospitalized for laboratory-confirmed influenza and lived in the community (ie, were not institutionalized) prior to hospitalization. Cases were excluded from analysis if they had an uncertain 2012–2013 vaccination history, lacked body mass index (BMI) data, or received antiviral treatment ≥4 days before hospitalization, as we could not determine whether treatment was completed.
Statistical Analysis
We evaluated the following clinical outcomes: severe influenza, diagnosis of pneumonia, length of stay in the intensive care unit (ICU), and length of stay in the hospital. For this analysis, we defined severe influenza as being admitted to an ICU or dying during hospitalization. Pneumonia was defined by abnormal chest radiography findings (including consolidation/opacity or pleural effusion) detected within the first 3 days of hospital admission and only included those who underwent chest radiography within that same time frame. We categorized age into 3 groups: 50–64, 65–74, and ≥75 years.
We examined demographic characteristics (sex, age, and race/ethnicity), clinical characteristics (BMI, asthma, chronic lung disease, cardiovascular disease, chronic metabolic disease, neurologic disease, immunosuppression, blood disorder, renal disease, and liver disease), lifestyle risk factors (alcohol abuse and smoking status), influenza virus type, and clinical course during hospitalization (receipt of antiviral treatment, admission to the ICU, death, diagnosis of pneumonia, length of stay in the ICU, and length of hospital stay) by influenza vaccination status. For descriptive analyses, we used the Pearson χ2 test and the Fisher exact test or the Wilcoxon–Mann–Whitney test, when appropriate.
Influenza Vaccination and Severity of Influenza Analysis, Using Multivariable Logistic Regression
We excluded cases who were not treated with antivirals, because most cases received antivirals, and there were substantial differences between the characteristics of the treated cases and those of the untreated cases (data not shown). Among cases treated with antivirals, we used multivariable logistic regression models for each age category to evaluate the association between influenza vaccination and (1) severe influenza and (2) diagnosis of pneumonia, after adjustment for sex, race, BMI, alcohol abuse and smoking status, virus type, and medical conditions. In addition, we used Cox proportional hazards regression for each age category to evaluate the association between influenza vaccination and (1) length of stay in the ICU (in days) and (2) length of hospital stay (in days), after adjustment for similar covariates.
Influenza Vaccination and Severity of Influenza Analysis, Using Propensity Score Matching (PSM)
To account for dissimilar distributions of baseline characteristics between vaccinated and unvaccinated groups and to reduce confounding introduced by selection bias, we predicted the probability of influenza vaccination, using propensity score matching (PSM). Because influenza vaccination rates among older adults were high and increased substantially with age, we included all unvaccinated cases and randomly sampled 300 cases among those vaccinated for each age category, to obtain an unvaccinated to vaccinated ratio of ≥1 [21, 22]. For PSM, we performed a 1:1 nearest neighbor match on propensity score of the following variables: sex, race, state of residence, BMI, underlying medical conditions, presence of any medical condition, alcohol abuse status, and smoking status for each age category (Supplementary Figure A1) [23]. The overall distribution of baseline characteristics between the 2 groups was comparable; race was the only variable that remained slightly unbalanced between the vaccinated and unvaccinated cases (Supplementary Table A1). We considered adding race as a covariate in the final model, but it was excluded for parsimony.
Finally, among propensity score–matched cases treated with antivirals, we used logistic regression models within age strata to evaluate the association between influenza vaccination and (1) severe influenza and (2) diagnosis of pneumonia, after adjustment for the Charlson comorbidity index (a weighted score of medical conditions used to predict health outcomes) [24–26]. Using the same approach, we evaluated the association between influenza vaccination and (1) length of stay (in days) in the ICU and (2) length of stay (in days) in the hospital, using Cox proportional hazards regression analyses, by age category, with adjustment for the Charlson comorbidity index. Kaplan–Meier (nonparametric) survival models were used to illustrate length of stay in the ICU, and accelerated failure time models were used to estimate the time ratio and median length of ICU stay, by vaccination status. We selected the best model on the basis of Akaike's information criterion [27]. We present odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (CIS) for logistic regression models and Cox proportional hazards regression analyses, respectively. All analyses were performed using R software (version 3.0.2).
Ethics
The institutional review board (IRB) at the Centers for Disease Control and Prevention (CDC) determined that data collected via FluSurv-NET represents public health practice, and therefore, it is not subject to IRB approval for human research protection. Participating sites submitted the FluSurv-NET surveillance project to their state and IRBs as per state and local requirements.
RESULTS
Exclusion of Data
During the 2012–2013 influenza season, FluSurv-NET collected data on 8172 adults aged ≥50 years who were hospitalized with laboratory-confirmed influenza. Of those, we excluded 2558 (32%), leaving 5614 for inclusion in our analysis. Reasons for exclusion were institutionalization in a long-term care facility prior to hospitalization (1639 [20%]), missing information about BMI (471 [6%]), unknown vaccination status (392 [5%]), <12 days between vaccination and hospitalization (39 [0.5%]), and antiviral treatment ≥4 days before hospitalization (17 [0.2%]; Figure 1).
Figure 1.
Exclusion criteria and data-cleaning algorithm. Abbreviation: BMI, body mass index.
Overall Characteristics of the Sample
A total of 5614 adults aged ≥50 years who were hospitalized with laboratory-confirmed influenza were included in this analysis, of whom 3101 (55%) received influenza vaccination at least 12 days before hospitalization but not before 1 July 2012. The distribution of age and race differed between the vaccinated and unvaccinated cases (P < .001). Those who were vaccinated against influenza were older than those who were unvaccinated; adults aged ≥75 composed 55% of vaccinated individuals, compared with 38% of unvaccinated individuals. In addition, vaccinated adults were significantly (P < .001–.01) more likely than unvaccinated adults to have chronic lung disease, cardiovascular disease, chronic metabolic disease, immunosuppression, blood disorder, renal disease, and presence of any medical condition. Furthermore, BMI, alcohol abuse status, smoking status, influenza virus type, and antiviral treatment were associated with vaccination status (P < .01; Table 1).
Table 1.
Demographic and Clinical Characteristics, Lifestyle Risk Factors, and Clinical Course of Hospitalization Among 5614 Cases, Overall and by Vaccination Status During the 2012–2013 Influenza Season
| Characteristic | Overall (n = 5614) | Vaccinated (n = 3101) | Unvaccinated (n = 2513) | P Valuea |
|---|---|---|---|---|
| Female sex | 2952 (53) | 1598 (52) | 1354 (54) | .08 |
| Age group, y | ||||
| 50–64 | 1638 (29) | 667 (22) | 971 (39) | <.001 |
| 65–74 | 1296 (23) | 721 (23) | 575 (23) | |
| ≥75 | 2680 (48) | 1713 (55) | 967 (38) | |
| Race | ||||
| Non-Hispanic white | 3845 (68) | 2288 (74) | 1557 (62) | <.001 |
| Non-Hispanic black | 860 (15) | 341 (11) | 519 (21) | |
| Hispanic | 347 (6) | 159 (5) | 188 (7) | |
| Other | 235 (4) | 139 (4) | 96 (4) | |
| Unknown | 327 (6) | 174 (6) | 153 (6) | |
| BMI | ||||
| Underweight | 239 (4) | 114 (4) | 125 (5) | <.001 |
| Normal | 1702 (30) | 1006 (32) | 696 (28) | |
| Overweight | 1716 (31) | 984 (32) | 732 (29) | |
| Obese | 1467 (26) | 763 (25) | 704 (28) | |
| Morbid obese | 490 (9) | 234 (8) | 256 (10) | |
| Medical condition | ||||
| Asthma | 945 (17) | 529 (17) | 416 (17) | .64 |
| Chronic lung disease | 1890 (34) | 1134 (37) | 756 (30) | <.001 |
| Cardiovascular disease | 2913 (52) | 1726 (56) | 1187 (47) | <.001 |
| Chronic metabolic disease | 2513 (45) | 1464 (47) | 1049 (42) | <.001 |
| Neurologic disease | 963 (17) | 555 (18) | 408 (16) | .11 |
| Immunosuppression | 921 (16) | 581 (19) | 340 (14) | <.001 |
| Blood disorder | 106 (2) | 74 (2) | 32(1) | <.01 |
| Renal disease | 1101 (20) | 708 (23) | 393 (16) | <.001 |
| Liver disease | 119 (2) | 62 (2) | 57 (2) | .55 |
| ≥1 | 5092 (91) | 2883 (93) | 2209 (88) | <.001 |
| Alcohol abuse status | ||||
| Current | 183 (3) | 70 (2) | 113 (5) | <.001 |
| Former | 185 (3) | 100 (3) | 85 (3) | |
| Never | 5246 (93) | 2931 (95) | 2315 (92) | |
| Smoking status | ||||
| Current | 1051 (19) | 454 (15) | 597 (24) | <.001 |
| Former | 1793 (32) | 1106 (36) | 687 (27) | |
| Never | 2770 (49) | 1541 (50) | 1229 (49) | |
| Influenza virus type(s) | ||||
| Influenza A | 4689 (84) | 2637 (85) | 2052 (82) | <.01b |
| Influenza B | 901 (16) | 454 (15) | 447 (18) | |
| Influenza A and B | 8 (0.1) | 4 (0.1) | 4 (0.2) | |
| Influenza A/B (not distinguished) | 16 (0.3) | 6 (0.2) | 10 (0.4) | |
| Antiviral treatment | 4631 (82) | 2607 (84) | 2024 (81) | <.001 |
| Admitted to the ICU | 803 (14) | 439 (14) | 364 (15) | .76 |
| Deceased | 118 (2) | 61 (2) | 57 (2) | .20b |
| Diagnosis of pneumoniac | 1806 (33) | 1022 (34) | 784 (32) | .19 |
| Length of ICU stay, d, median (IQR) | 3 (1–6) | 2 (1–5) | 3 (1–6) | .14d |
| Length of hospital stay, d, median (IQR) | 3 (2–6) | 3 (2–6) | 3 (2–6) | .07d |
Data are no. (%) of subjects, unless otherwise indicated, and were obtained from the Influenza Hospitalization Surveillance Network. Percentages reflect calculations involving subjects for whom data on the specified characteristic were available and might not sum to 100%, because of rounding.
Abbreviations: BMI, body mass index; ICU, intensive care unit; IQR, interquartile range.
a By the χ2 test, unless otherwise indicated.
b By the Fisher test.
c Among those who underwent chest radiography within 3 days of admission (n = 5462).
d By the Wilcoxon–Mann–Whitney test.
Of the 5614 subjects hospitalized with laboratory-confirmed influenza, 803 (14%) were admitted to ICUs, and 118 (2%) died (Table 1). Of the 5462 who underwent chest radiography within 3 days of admission, 1806 (33%) received a diagnosis of pneumonia. The median length of stay at the hospital was 3 days (interquartile range [IQR], 2−6 days). The median length of stay in the ICU (n = 803) was 3 days (IQR, 1−6 days). No associations were found between vaccination status and admission to the ICU, death, diagnosis of pneumonia, or length of stay at the hospital or ICU (P > .05).
Influenza Vaccination and Severity of Influenza Analysis, Using Multivariable Logistic Regression
The odds of having severe influenza and pneumonia among the vaccinated cases were not statistically different from those of the unvaccinated cases in any of the 3 age categories, after adjustment for sex, race, BMI, medical condition, alcohol abuse and smoking status, and type of influenza virus for cases who received antiviral treatment. Likewise, among cases who received antiviral treatment, we did not find any difference in ICU and hospital length of stay between the vaccinated and unvaccinated cases, by age category, and after adjustment for sex, race, BMI, medical condition, alcohol abuse and smoking status, and type of influenza virus (Table 2).
Table 2.
Influenza Vaccination and Severity of Influenza Analysis for 4611 Cases Treated With Antivirals During the 2012–2013 Influenza Season, by Age Group, Before Propensity Score Matching
| Clinical Outcome, Measure | 50–64 y (n = 1298) |
65–74 y (n = 1054) |
≥75 y (n = 2259) |
|||
|---|---|---|---|---|---|---|
| Point Estimate (95% CI) | P Value | Point Estimate (95% CI) | P Value | Point Estimate (95% CI) | P Value | |
| Severe disease, ORa | 1.04 (.76–1.42) | .82 | 0.99 (.71–1.40) | .99 | 1.05 (.81–1.37) | .70 |
| Diagnosis of pneumonia, ORb | 0.93 (.72–1.21) | .61 | 0.80 (.61–1.06) | .12 | 1.02 (.84–1.23) | .85 |
| Length of ICU stay, HRc | 1.22 (.87–1.72) | .24 | 1.23 (.81–1.85) | .32 | 1.10 (.81–1.50) | .55 |
| Length of hospital stay, HRc | 1.02 (.91–1.15) | .70 | 1.01 (.89–1.15) | .88 | 1.05 (.96–1.15) | .28 |
Analyses were adjusted for sex, race, body mass index, medical condition (asthma, chronic lung disease, cardiovascular disease, chronic metabolic disease, neurologic disease, immunosuppression, and renal disease), alcohol abuse status, smoking status, and influenza virus type. Data exclude untreated individuals, individuals who tested positive for both influenza A and B, and those who tested positive for influenza A and/or B (not distinguished).
Abbreviations: CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio.
a Admitted to the ICU or died.
b Among those who underwent chest radiography within 3 days of admission (n = 4494).
c HR represents ICU or hospital discharge.
Influenza Vaccination and Severity of Influenza Analysis, Using PSM
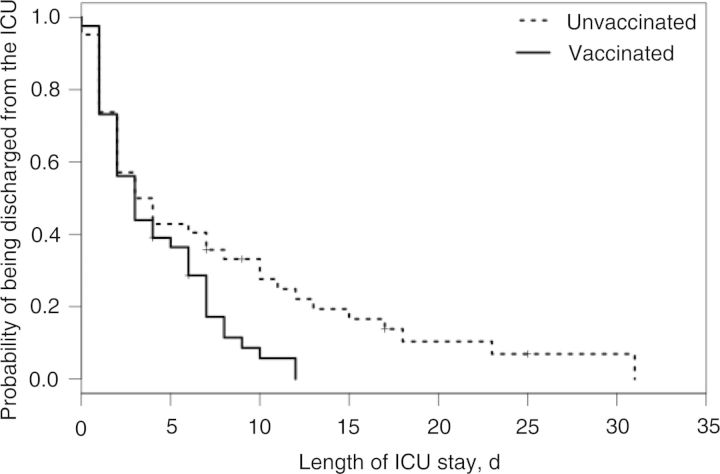
We had 300 matched pairs of vaccinated and unvaccinated patients within each age stratum, and no difference was observed regarding clinical outcomes between the 2 groups (Supplementary Table A2). After matching on the propensity score, we found that, among cases aged 50–64 years who received antiviral treatment, those who were vaccinated were almost twice as likely to be discharged earlier from the ICU than those who were unvaccinated (HR, 1.84; 95% CI, 1.12–3.01; Table 3). The accelerated failure time model estimated that the length of ICU stay for the vaccinated cases decreased by a factor of 0.6 (95% CI, .4−.8), compared with the unvaccinated cases aged 50–64 years who received antiviral treatment (P = .005), with estimated median times of 7.4 and 4.3 days, respectively (Figure 2). Although not significant, a similar trend was found for cases aged 65–74 years (HR, 1.58; 95% CI, .97–2.53; Table 3) but not for cases aged ≥75 years.
Table 3.
Influenza Vaccination and Severity of Influenza Analysis for 1509 Cases Treated With Antivirals During the 2012–2013 Influenza Season, by Age Group, After Propensity Score Matching
| Clinical Outcome | 50–64 y (n = 494) |
65–74 y (n = 495) |
≥75 y (n = 520) |
|||
|---|---|---|---|---|---|---|
| Point Estimate (95% CI) | P Value | Point Estimate (95% CI) | P Value | Point Estimate (95% CI) | P Value | |
| Severe disease, ORa | 0.97 (.62–1.52) | .89 | 1.27 (.81–1.99) | .29 | 0.99b (.59–1.66) | .97 |
| Diagnosis of pneumonia, ORc | 0.79 (.54–1.18) | .25 | 0.86b (.59–1.26) | .44 | 0.94b (.65–1.35) | .72 |
| Length of ICU stay, HRd | 1.84 (1.12–3.01) | .02 | 1.58 (.97–2.53) | .06 | 0.94 (.50–1.77) | .85 |
| Length of hospital stay, HRd | 1.08 (.90–1.29) | .39 | 0.98 (.82–1.17) | .83 | 1.03 (.87–1.23) | .72 |
Variables used for propensity score matching were sex, race, body mass index, medical condition (asthma, chronic lung disease, cardiovascular disease, chronic metabolic disease, neurologic disease, immunosuppression, hemoglobinopathy/blood disorders, renal disease, and liver disease), alcohol abuse status, and smoking status.
Abbreviations: CI, confidence interval; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio.
a Admitted to the ICU or died.
b Adjusted for the Charlson comorbidity index.
c Among those who underwent chest radiography within 3 days of admission (n = 1460).
d HR represents ICU or hospital discharge.
Figure 2.
Kaplan–Meier estimates of length of stay in the intensive care unit (ICU), by vaccination status, for cases aged 50–64 years who were treated with antivirals.
DISCUSSION
The 2012−2013 influenza season was moderately severe and, compared with previous seasons, was characterized by large increases in hospitalizations among older adults. We did not see differences in influenza severity with respect to vaccination status in hospitalized patients with laboratory-confirmed influenza during this season, when using traditional multivariate analysis. In the propensity score model, we found that individuals aged 50−64 years who were vaccinated against influenza were more likely to be discharged earlier from the ICU, compared with those who were not vaccinated (HR, 1.84; 95% CI, 1.12−3.01), with a median of a half-day reduction in time spent in the ICU. A similar trend was also seen among those aged 65−74 years, although this finding was not statistically significant. This very modest difference in outcome may suggest that, during the 2012−2013 season, influenza vaccination did not offer additional protection from severe outcomes among those who, despite vaccination, were infected, hospitalized, and treated with antivirals. Because patients who receive a diagnosis of influenza and are aged ≥50 years are at higher risk of influenza-associated complications, it is important to consider strategies to improve the effectiveness of currently available influenza vaccines [28–30].
To our knowledge, this is the first study that has evaluated the association of influenza vaccination and ICU admission, death, diagnosis of pneumonia, and hospital and ICU lengths of stay among hospitalized patients aged ≥50 years with laboratory-confirmed influenza, using traditional multivariable logistic regression and PSM. In our study, analyzing the data on the basis of the propensity for influenza vaccine receipt allowed us to balance characteristics of vaccinated and unvaccinated cases that could potentially bias results (Supplementary Table A1). For instance, older adults and those with underlying medical conditions are more likely to receive vaccination, to have influenza-associated complications, and to respond to vaccination poorly, compared with young, healthy adults. Traditional adjusted regression models measuring differences in disease severity among vaccinated and unvaccinated persons may not adequately control for these effects [31, 32].
The effect of influenza vaccination on influenza severity is uncertain. Some studies evaluating hospital admission among those with laboratory-confirmed influenza as a sign of disease severity have failed to show a protective effect of influenza vaccination [33, 34], while others that used laboratory-confirmed influenza outcomes [10, 11], as well as clinical outcomes [12, 35, 36], have found some differences. Nonetheless, the definition of influenza severity differs among studies. Nichol et al evaluated influenza vaccine effectiveness against hospitalization for influenza or pneumonia and death from any cause in community-dwelling people aged ≥65 years during 10 consecutive seasons. They found a significant decrease in hospitalizations for influenza or pneumonia (OR, 0.73; 95% CI, .68−0.77) and death (OR, 0.52; 95% CI, .50−.55) in individuals who were vaccinated, compared with those who were unvaccinated, after adjustment for demographic characteristics, medical conditions, and number of medical visits [35]; however, the study did not look at laboratory-confirmed influenza-attributable outcomes, and thus the vaccine effectiveness estimates were potentially biased [32, 37]. Another study of hospitalized cases with laboratory-confirmed influenza defined severity as being admitted in the ICU or dying during hospitalization. Their findings support the idea that influenza vaccination may protect against severe disease during hospitalization; however, the investigators did not account for antiviral treatment [10], which is associated with better outcomes, including survival [38–40]. Despite the number of studies showing the benefit of influenza vaccine in reducing disease severity among elderly individuals [10–12, 35, 36], we could not satisfactorily demonstrate this phenomenon with our data.
Influenza vaccine effectiveness during the 2012–2013 influenza season was documented to be suboptimal in older adults. The influenza A(H3N2) vaccine strain underwent minor amino acid changes, compared with the circulating strain, which had an adverse effect on the 2012–2013 vaccine efficacy [6] and likely influenced the low vaccine effectiveness in adults aged ≥65 years; the 2012−2013 vaccine effectiveness for adults aged ≥65 years was 26% (95% CI, −10% to 50%) [6]. However, other factors related to the capacity of older adults to build an immune response may have played a role [41]. Despite the low influenza vaccine effectiveness reported in older adults, a substantial number of hospitalizations were likely averted by vaccination during the 2012−2013 season [42]. The lack of substantial differences in severe outcomes between vaccinated and unvaccinated subjects in our study may reflect the modest vaccine effectiveness estimated for the 2012−2013 season. Similar analyses in other seasons may be able to confirm or expand on our findings. Nonetheless, new vaccines with improved vaccine effectiveness, such as adjuvanted influenza vaccine [30] or a high-dose vaccine [28, 29], and other strategies, such as early and aggressive antiviral treatment [17, 38, 39, 43], are needed to reduce morbidity and mortality due to influenza virus infection in vulnerable populations.
We acknowledge that the study had some limitations. Because of the high vaccination rates among hospitalized patients, we randomly selected a subset of vaccinated cases from each age group, to increase our ratio of unvaccinated subjects to vaccinated subjects, thus guaranteeing an optimal performance of the PSM algorithm [21, 22]. We detected a difference in the length of ICU stay in adults aged 50−64 years; however, reducing the sample size could have affected the ability to detect differences in disease severity and pneumonia, principally among the older age groups. For instance, older adults are reported to be less likely to be sent to the ICU [44, 45], which may have reduced the number of outcomes and, consequently, our ability to identify any significant association. Furthermore, there might be unmeasured confounders that we did not include in the PSM; however, we included a number of variables that have been reported to be associated with vaccination and severe influenza [46]. Another potential limitation is that physicians may have been more likely to test for influenza virus in patients with more-severe disease presentation, underestimating the benefit of vaccination throughout the spectrum of disease. Likewise, bias may have been introduced by not capturing all true influenza cases, owing to the low sensitivity of rapid tests [47]; however, an increased use of more-sensitive tests has been seen since the 2009 influenza pandemic [48]. In addition, antiviral treatment may have masked the effect of vaccination on the severity of influenza, as antiviral treatment has been shown to reduce influenza-related severe outcomes, including hospital length of stay [38, 39, 43, 49].
In summary, we did not see substantial differences in influenza severity, by vaccination status, in older adults hospitalized with laboratory-confirmed influenza during the 2012−2013 influenza season, despite the high hospitalization rates among persons aged ≥65 years. In years in which the vaccine is a better match to circulating viruses and, consequently, has better effectiveness against influenza virus infection, we might see a stronger effect of influenza vaccination on clinical outcomes. More-immunogenic vaccines may provide higher protection from severe influenza. Annual influenza vaccination is still the best protection against influenza virus infection and its potential complications, particularly for populations at risk of developing more-severe disease. In hospitalized patients, influenza-associated antiviral treatment should be administered as soon as possible when influenza is suspected.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Pam Daily Kirley, MPH, and Brittany Martin, MPH, at the California Emerging Infections Program; Kimberly Yousey-Hindes, MPH, CPH, at the Connecticut Emerging Infections Program, Yale School of Public Health; Kyle Openo, MPH, and Monica M. Farley, MD, at the Georgia Emerging Infections Program; Maggie Ayers-Johnson, Stephanie Rothstein, Ashley Mitchell, and Malia Remackel at the Minnesota Department of Health; Patricia A. Ryan at the Maryland Emerging Infections Program, Maryland Department of Health and Mental Hygiene; Marisa Bargsten, MPH, at the New Mexico Emerging Infections Program, New Mexico Department of Health; Kathy Angeles, MPH, Lisa Butler, MPH, Sarah Khanlian, MPH, and Robert Mansmann, MPH, at the University of New Mexico; Matthew Laidler, MS, MPH, at the Oregon Public Health Division; Karen Leib, RN, and Katie Dyer from the Tennessee Emerging Infections Program; and Michelle Leon, MPH, and Alejandro Perez, MPH, at the Influenza Division, Centers for Disease Control and Prevention.
Disclaimer. The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC (cooperative agreements CDC-RFA-CK12-1202 and 5U38HM000414).
Potential conflicts of interest. W. S. is a member of the data safety monitoring board of Merck. A. E. received funding from MedImmune to conduct an unrelated clinical trial and for editorial assistance. A. E. also provides editorial assistance for Roche. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis 2012; 54:1427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson M, Shay D, Zhou H, et al. Estimates of deaths associated with seasonal influenza—United States, 1976–-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–62. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Influenza activity—United States, 2012–13 season and composition of the 2013–14 influenza vaccine. MMWR Morb Mortal Wkly Rep 2013; 62:473–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–86. [DOI] [PubMed] [Google Scholar]
- 5.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flannery B, Thaker SN, Clippard J, et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness - United States, February 2014. MMWR Morb Mortal Wkly Rep 2014; 63:137–42. [PMC free article] [PubMed] [Google Scholar]
- 8.Kissling E, Valenciano M, Buchholz U, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I-MOVE multicentre case-control study, influenza season 2012/13. Euro Surveill 2014; 19:pii:20701. [DOI] [PubMed] [Google Scholar]
- 9.Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012–13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castilla J, Godoy P, Dominguez A, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis 2013; 57:167–75. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Romero P, Aydillo TA, Perez-Ordonez A, et al. Reduced incidence of pneumonia in influenza-vaccinated solid organ transplant recipients with influenza disease. Clin Microbiol Infect 2012; 18:E533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridenhour BJ, Campitelli MA, Kwong JC, et al. Effectiveness of inactivated influenza vaccines in preventing influenza-associated deaths and hospitalizations among Ontario residents aged >/=65 years: estimates with generalized linear models accounting for healthy vaccinee effects. PLoS One 2013; 8:e76318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaby P, Bhuiya A, Nahar L, Knudsen K, de Francisco A, Strong M. The survival benefit of measles immunization may not be explained entirely by the prevention of measles disease: a community study from rural Bangladesh. Int J Epidemiol 2003; 32:106–16. [DOI] [PubMed] [Google Scholar]
- 14.Preziosi MP, Halloran ME. Effects of pertussis vaccination on disease: vaccine efficacy in reducing clinical severity. Clin Infect Dis 2003; 37:772–9. [DOI] [PubMed] [Google Scholar]
- 15.Vila-Corcoles A, Ochoa-Gondar O, Llor C, Hospital I, Rodriguez T, Gomez A. Protective effect of pneumococcal vaccine against death by pneumonia in elderly subjects. Eur Respir J 2005; 26:1086–91. [DOI] [PubMed] [Google Scholar]
- 16.Schnorr JJ, Cutts FT, Wheeler JG, et al. Immune modulation after measles vaccination of 6–9 months old Bangladeshi infants. Vaccine 2001; 19:1503–10. [DOI] [PubMed] [Google Scholar]
- 17.Chaves SS, Aragon D, Bennett N, et al. Patients hospitalized with laboratory-confirmed influenza during the 2010–2011 influenza season: exploring disease severity by virus type and subtype. J Infect Dis 2013; 208:1305–14. [DOI] [PubMed] [Google Scholar]
- 18.Treanor J. Influenza viruses, including avian influenza and swine influenza. In: MandellBennett GLJE, Dolin R, eds. Principles and practice of infectious diseases. Vol 2 Philadelphia: Churchill Livingstone, 2010:2265–93. [Google Scholar]
- 19.Enstone JE, Myles PR, Openshaw PJ, et al. Nosocomial pandemic (H1N1) 2009, United Kingdom, 2009–2010. Emerg Infect Dis 2011; 17:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jhung MA, D'Mello T, Perez A, et al. Hospital-onset influenza hospitalizations--United States, 2010–2011. Am J Infect Control 2014; 42:7–11. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics 1985; 41:103–16. [PubMed] [Google Scholar]
- 22.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996; 52:249–64. [PubMed] [Google Scholar]
- 23.Williamson EJ, Forbes A. Introduction to propensity scores. Respirology 2014; 19:625–35. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 25.McGregor JC, Kim PW, Perencevich EN, et al. Utility of the chronic disease score and charlson comorbidity index as comorbidity measures for use in epidemiologic studies of antibiotic-resistant organisms. Am J Epidemiol 2005; 161:483–93. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 27.Burnham KP, Anderson DR. Model selection and multimodel inference. 2nd ed New York: Springer, 1998. [Google Scholar]
- 28.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–45. [DOI] [PubMed] [Google Scholar]
- 29.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–80. [DOI] [PubMed] [Google Scholar]
- 30.van Essen GA, Beran J, Devaster JM, et al. Influenza symptoms and their impact on elderly adults: randomised trial of AS03-adjuvanted or non-adjuvanted inactivated trivalent seasonal influenza vaccines. Influenza Other Respir Viruses 2014; 8:452–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006; 35:337–44. [DOI] [PubMed] [Google Scholar]
- 32.Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine 2009; 27:6300–4. [DOI] [PubMed] [Google Scholar]
- 33.Gilca R, De Serres G, Boulianne N, et al. Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. Influenza Other Respir Viruses 2011; 5:247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean HQ, Meece JK, Belongia EA. Influenza vaccination and risk of hospitalization among adults with laboratory confirmed influenza illness. Vaccine 2014; 32:453–7. [DOI] [PubMed] [Google Scholar]
- 35.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007; 357:1373–81. [DOI] [PubMed] [Google Scholar]
- 36.Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol 2009; 170:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 2007; 7:658–66. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother 2011; 66:959–63. [DOI] [PubMed] [Google Scholar]
- 39.McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis 2007; 45:1568–75. [DOI] [PubMed] [Google Scholar]
- 40.Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014; 2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis 2008; 197:490–502. [DOI] [PubMed] [Google Scholar]
- 42.Fry AM, Kim IK, Reed C, et al. Modeling the effect of different vaccine effectiveness estimates on the number of vaccine-prevented influenza-associated hospitalizations in older adults. Clin Infect Dis 2014; 59:406–9. [DOI] [PubMed] [Google Scholar]
- 43.Hiba V, Chowers M, Levi-Vinograd I, Rubinovitch B, Leibovici L, Paul M. Benefit of early treatment with oseltamivir in hospitalized patients with documented 2009 influenza A (H1N1): retrospective cohort study. J Antimicrob Chemother 2011; 66:1150–5. [DOI] [PubMed] [Google Scholar]
- 44.Chan MC, Lee N, Ngai KL, Leung TF, Chan PK. Clinical and virologic factors associated with reduced sensitivity of rapid influenza diagnostic tests in hospitalized elderly patients and young children. J Clin Microbiol 2014; 52:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu W, Ash AS, Levinsky NG, Moskowitz MA. Intensive care unit use and mortality in the elderly. J Gen Intern Med 2000; 15:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenciano M, Kissling E, Ciancio BC, Moren A. Study designs for timely estimation of influenza vaccine effectiveness using European sentinel practitioner networks. Vaccine 2010; 28:7381–8. [DOI] [PubMed] [Google Scholar]
- 47.Chartrand C, Leeflang MM, Minion J, Brewer T, Pai M. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med 2012; 156:500–11. [DOI] [PubMed] [Google Scholar]
- 48.Millman A, Reed C, Kirby P, et al. Impact of changes in diagnostic testing on estimated influenza-associated hospitalization rates in the influenza hospitalization surveillance network (FluSurv-NET): United States, 2003–2013. In: Program and abstracts of the 63rd Annual Epidemic Intelligence Service Conference Atlanta, GA: Centers for Disease Control and Prevention, 2014:104. [Google Scholar]
- 49.Viasus D, Pano-Pardo JR, Pachon J, et al. Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A(H1N1) virus infection. Chest 2011; 140:1025–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.