Abstract
Ovarian cancer is the 5th most common cancer found in women in the UK. It is the leading cause of death from gynaecological cancer, and is the 4th most common cause of cancer death among UK women. Similar to the majority of other cancers, relative survival rates for ovarian cancer are improving, although 5-year mortality rates remain stubbornly low. The stage of the disease at diagnosis is the single most important determinant of ovarian cancer survival, as many patients first present with advanced disease. Treatment of ovarian cancer involves a combination of ‘upfront’ primary surgery followed by chemotherapy. Platinum/taxane-based chemotherapy is the recommended standard-of-care first-line chemotherapy, but the majority of patients will relapse with drug-resistant disease within 3-5 years. However, not all patients can continue with platinum combination therapies due to loss of activity or toxicity-related issues, including hypersensitivity, neurotoxicity, alopecia and ototoxicity. Therefore the choice of second-line chemotherapy must take into account factors such as platinum-free treatment interval (PFI); patient's performance status; current symptoms; history of and likely future toxicities while on chemotherapy; dosing schedule requirement; and cost of treatment. A consensus in 2010 established 4 distinct subgroups within the ROC patient population based on the PFI: (platinum sensitive <12 months, partially platinum sensitive 6-12 months, platinum resistant <6 months, and refractory disease ≤4 weeks). Within patients with platinum sensitive disease, those with partially platinum sensitive disease remain the most clinically challenging to manage effectively. Non-platinum based combination therapies, in particular trabectedin with pegylated liposomal doxorubicin (PLD), offers new options together with a significant survival advantage relative to PLD alone for these patients.
Keywords: Ovarian cancer, Relapse, Platinum-free interval, Platinum-sensitive recurrence, Partially platinum-sensitive recurrence, Tumour heterogeneity
Prevalence of ovarian cancer and recurrent ovarian cancer in the UK
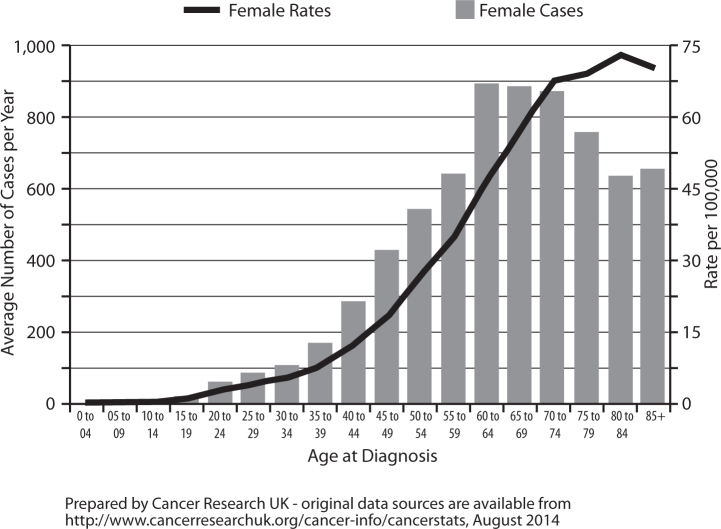
Ovarian cancer is the fifth most common cancer among women in the UK (2011), accounting for 4% of all new cases of cancer in females. Ovarian cancer incidence is strongly related to age, with the highest incidence rates being in older women; age-specific incidence rates rise sharply from around age 35-39, peak in those aged 80-84, and subsequently plateau (Fig. 1). However there has been a decrease of 11% in overall incidence over time, probably because of the contraceptive pill that is known to reduce the risk of ovarian cancer [1].
Fig. 1.
Ovarian cancer: average age of new cases per year and age-specific incidence rates per 100,000 population, females, UK (2009-2011).
Mortality rates due to ovarian cancer in the UK are significant. Ovarian cancer is the leading cause of death from gynaecological malignancies and is the fourth most common cause of cancer death among females in the UK (2011), accounting for 6% of all female deaths from cancer. This amounts to 13 ovarian cancer deaths for every 100,000 females in the UK, with the highest mortality rates being in older women (>65 years) [1, 2].
However, as with the majority of cancers, relative survival for ovarian cancer is improving. The latest age-standardised relative survival rates for ovarian cancer in the UK during 2005-2009 show that 72.3% of women are expected to survive their disease for at least one year; with this figure falling to 42.9% surviving five years or more. The relatively low five-year survival rates can be attributed partly to the fact that 29% of cases of ovarian cancer are emergency presentations due to the non specificity of symptoms. For example, data from the Anglia Cancer Network area for women diagnosed during 2004-08 show that five-year relative survival rates are more than 90% for early stage disease, but fall very sharply to less than 10% for late stage cases [1]. Demonstrating the importance of the stage of the disease at diagnosis as a determinant of ovarian cancer survival.
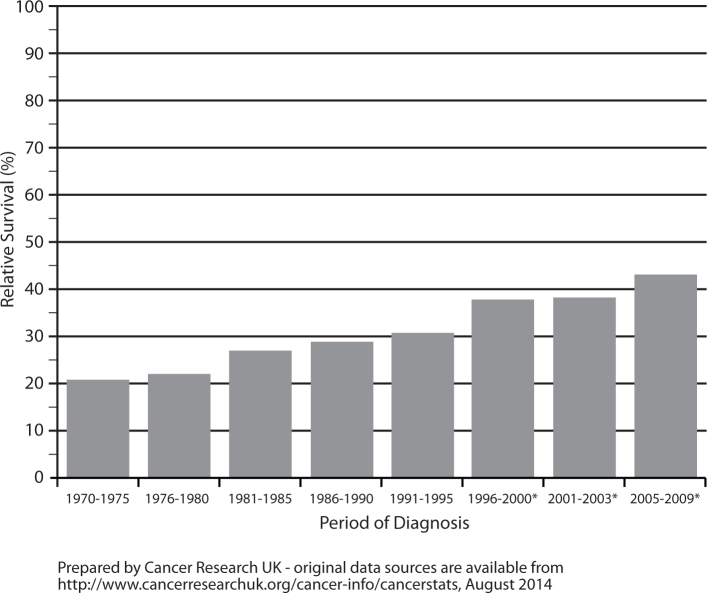
Nevertheless, five-year relative survival rates for ovarian cancer increased from 21% in England and Wales during 1971-1975 to 42.9% in England during 2005-2009 (Fig. 2). It is thought that the significant increase in one-year survival is likely to be the result of greater use of platinum-based chemotherapy regimens. And the increase in five-year survival may be due to both wider access to optimal primary treatment and a greater determination by the clinical community to treat recurrent disease [3].
Fig. 2.
Ovarian cancer, age-standardised five-year relative survival rates, England and Wales 1971-1995, England 1996-2009. *Survival rates are for England only from 1996 onwards.
When UK survival rates for ovarian cancer are compared with those of other high income countries, including in Europe, they are significantly worse. Incidence and mortality rates due to ovarian cancer in the UK are on a par with Eastern Europe and higher than those given for Germany, France and Italy. Differences in data quality and coding practices may contribute to some of the variation, but the consistently lower levels for the UK suggests real differences in survival, which demand further investigation and earlier access to specialist care and improved treatment options for UK patients [4, 5].
Management of patients with ovarian cancer
Treatment of ovarian cancer involves a combination of surgery and chemotherapy. ‘Upfront’ primary surgery for complete resection if possible or cytoreductive surgical debulking for advanced disease, followed by chemotherapy remains the international standard of care. Chemotherapy, however, has been principally responsible for the improved survival seen over the past 10 years [3, 6].
As previously described platinum combination (typically platinum-paclitaxel) chemotherapy has been established as the standard of care following surgery for ovarian cancer [7].
Management of patients with recurrent ovarian cancer
For relapsing patients with recurrent disease, relatively few phase III studies have been conducted and these have been primarily in patients with platinum-sensitive (PS) disease. The International Collaborative Ovarian Neoplasm 4 (ICON-4) trial was the first to show that a combination of platinum and paclitaxel was more effective than single-agent platinum compounds in patients with relapsing PS ovarian cancer. The carboplatin/paclitaxel combination increased PFS by a median of 3 months (12.0 vs. 9.0 months; p=0.0004) and OS by 5 months (29.0 vs. 24.0 months; p=0.02) when compared with carboplatin alone. [8] By comparison, the Intergroup study by Pfisterer et al. showed that a gemcitabine/carboplatin combination was associated with a median improvement in PFS of 2.8 months (8.6 vs. 5.8 months; p=0.0031) in patients with PS disease, but with greater toxicity and no improvement in OS (18.0 vs. 17.3 months) and QoL compared with carboplatin alone [9]. And, the large phase III CALYPSO trial showed that a combination of PLD and carboplatin produced a modest improvement in median PFS, and was a less toxic alternative to the standard regimen of carboplatin and paclitaxel in relapsing PS patients. However, no statistically significant difference was observed in OS (30.7 vs. 33.0 months; p=0.94) [10, 11].
Segmentation of the ROC patient population to help tailor therapeutic strategies at relapse
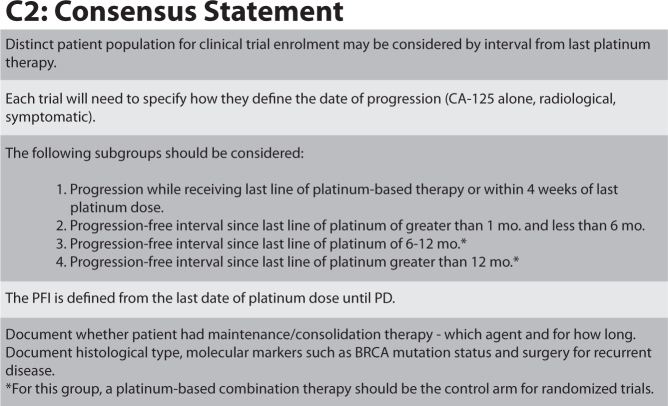
A consensus was established in 2010 at the 4th Ovarian Cancer Consensus Conference of the Gynecological Cancer InterGroup (GCIG) held in Vancouver on issues critical to conducting large randomised trials, including in the ROC patient population [12]. One of the key questions debated was “How do we define distinct patient populations in need of specific therapeutic approaches?” which can then be used across all relevant trials to help tailor therapeutic strategies for these patients.
The platinum-free interval (PFI) was defined at the meeting as the interval from the last date of platinum dose until documented progressive disease. Participants at the meeting also identified four distinct patient populations that require specific therapeutic approaches (Fig. 3) [12].
Fig. 3.
How to define distinct patient populations in need of specific therapeutic approaches? © GCIG, adapted from [12].
Optimisation of treatment strategies for patients with ROC based on platinum-free interval
It is worth bearing in mind that the ICON-4, Intergroup and CALYPSO studies were conducted before the recent stratification of the ROC patient population was fully defined. Therefore analyses of responses in the clinically challenging partially platinum-sensitive (PPS) subgroup of patients were not performed. Until 2010, patients were typically classified as being either platinum sensitive (PS) relapsing after >6 months from last platinum treatment, or platinum resistant (PR) relapsing <6 months after previous platinum, according to the platinum-free interval. The ROC patient population has now been stratified as being either PS; relapsing after 12 months or more, PPS; relapsing within 6-12 months, or PR; relapsing within <6 months, to optimise treatment approaches for the different groups. (This will be discussed in more detail in the article by Professor Nicoletta Colombo).
For patients with platinum sensitive disease, platinum/taxane-based chemotherapy is recommended in most cases, yet despite high sensitivity to platinum-based chemotherapy the majority of patients with ovarian cancer will relapse with drug-resistant disease within 3-5 years. Carboplatin-based combination regimens are the backbone of treatment for those patients who have a late relapse (>12 months), producing clinical benefit with higher rates for progression-free and overall survival. However for patients with PPS or PR disease, and also those who are unsuited to platinum, the decision for second-line treatment is far more complex with several additional factors needing to be considered (see Box 1). Patients in the PPS subgroup remains the most clinically challenging to manage effectively, however non-platinum based combination therapies, in particular trabectedin+PLD, offers new hope together with a significant survival advantage for these patients.
Box 1. Choice of second-line chemotherapy depends on the following factors:
-
•
platinum-free interval (PFI)
-
•
patient's performance status
-
•
current symptoms
-
•
history of prior toxicity on chemotherapy
-
•
anticipated future toxicities of chemotherapy
-
•
dosing schedule
-
•
cost
Incidence of patients unsuited to receive platinum second-line
Not all patients can continue with platinum at second-line or subsequent relapses due to loss of activity or toxicity-related issues including hypersensitivity, neurotoxicity, alopecia and ototoxicity. In particular, hypersensitivity reactions (HSRs) to carboplatin are a concern and have been reported in approximately 15-20% of women receiving the drug [13]. Other authors report even higher rates, with carboplatin responsible for up to approximately 27% of HSRs, and approximately 50% of initial HSRs occurring during the eighth course of treatment. HSRs usually start within 30 minutes of infusion and despite premedication with dexamethasone and antihistamines. Other anti-neoplastic drugs can also lead to HSRs with varying rates (paclitaxel/docetaxel: 10-30%; cabazitaxel: 0.3-1.8%; cetuximab: 16-19%; trastuzumab: 40%; rituximab: 77%) [14, 15, 16, 17, 18]. The retreatment interval has also been shown to be important with regard to carboplatin-related HSRs. A retreatment interval >23 months has been associated with a 36% incidence of HSRs, compared with a HSR incidence of 8% with a retreatment interval <23 months [14].
Potential solutions for managing HSRs include skin testing to identify high-risk patients, and gaining a clear understanding of the characteristics of HSRs in an individual patient in order to potentially perform rapid drug desensitization (RDD). RDD involves administering gradually increasing small doses to complete the total therapeutic dose of drug allergens. Although no molecular target has been found for specific desensitization, RDD doses induce mast cell tolerisation to the antigen. In order to reduce platinum HSRs, 12-16 step administration protocols and/or dose dilution can be undertaken, albeit with significant challenges in terms of associated burden on nursing and resource requirements, and patient preference. Successful desensitisation protocols are usually time-consuming [15, 19]. Risks and benefits of desensitisation protocols must be carefully weighed, and patients should be informed of the danger as there is still a risk of anaphylaxis or even death during the platinum rechallenge [20, 21]. Currently there is no national UK protocol on how to manage HSRs. Many centres find RDD protocols too onerous to utilise practically as management options, and other therapy options including non-platinum treatment alternatives that reduce the risk of HSRs should be considered.
As previously stated, platinum-based therapies can cause significant adverse events in patients, including hypersensitivity, neurotoxicity, alopecia and ototoxicity. (The impact of these will be discussed in more detail in the article by Professor Christina Fotopoulou.) If platinum-based regimens are not possible then alternative forms of treatment are required.
Recently, research efforts are being directed towards the development of effective non-platinum based therapies. Such therapies aim to reduce the risk of platinum-related HSRs as well as to allow patients time to recover from the well-known adverse events associated with platinum therapies, whilst at the same time effectively limiting the progression of their disease. Trabectedin + PLD has been shown to be an effective non-platinum alternative treatment option [13].
Factors to consider when deciding second-line treatment options for patients with ROC in the UK
The choice of second-line therapy is a complex decision analysis, and there are several factors to consider when deciding second-line treatment options with these patients. As highlighted in Box 1, the choice of second-line chemotherapy must take into account factors such as: the platinum-free treatment interval; the patient's performance status; their current symptoms; their history of and likely future toxicities while on chemotherapy; dosing schedule requirement; and, finally, cost of treatment [22].
Conclusions
In conclusion, despite a relatively poor long-term prognosis for patients diagnosed with ovarian cancer in the UK, the tide is begining to turn towards optimising treatment options for those with advanced recurrent disease. In contrast with first-line therapy where there is an excellent correlation between PFS and OS, this correlation is less clear for recurrent disease. As the majority of patients will relapse within 3-5 years, the decision analysis for retreatment is complex. Consideration also has to be given to those patients with recurrent disease unsuited or unwilling to continue treatment with platinum-based therapies. Recent stratification of ROC patients according to the PFI is helping to optimise different therapeutic approaches for the different subgroups, and in particular the difficult to manage PPS subgroup. In addition, there is growing evidence to suggest a survival benefit with utilising non-platinum treatment regimens, such as trabectedin + PLD combination, in patients with PPS disease when these patients are subsequently rechallenged with platinum; the rationale for which will be discussed in more detail in the next articles.
Conflict of Interest Statement
Professor Gabra received speaker fees from PharmaMar for his participation at the BGCS 2014 symposium. Professor Gabra is responsible for the content of this article. Patricia Howard Associates received fees from PharmaMar to provide editorial assistance.
References
- 1.http://www.cancerresearchuk.org/cancer-info/cancerstats/types/ovary/incidence/uk-ovarian-cancer-incidence-statistics. Accessed August 2014.
- 2.Ovarian cancer - the recognition and initial management of ovarian cancer; NICE Clinical Guideline (April 2011).
- 3.Kitchener HC. Survival from cancer of the Ovary in England & Wales up to 2001. Br J Cancer. 2008;99(Suppl 1):S73–S74. doi: 10.1038/sj.bjc.6604594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters S. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer. 2013;132:676–685. doi: 10.1002/ijc.27651. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Ervik M. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2013. [http://globocan.iarc.fr] accessed December 2013. [Google Scholar]
- 6.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized Phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 7.Kaye S. Management of partially platinum-sensitive relapsed ovarian cancer. Eur J Cancer. 2008;6(Suppl):16–21. [Google Scholar]
- 8.Parmar MK, Ledermann JA, Colombo N. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–2106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 9.Pfisterer J, Plante M, Vergote I. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28:3323–3329. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 11.Wagner U, Marth C, Largillier R. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs. paclitaxel and carboplatin in platinum-sensitive ovarian cancer patients. Br J Cancer. 2012;107:588–591. doi: 10.1038/bjc.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedlander Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez A. Increasing the chances for platinum-sensitive ovarian cancer patients. Future Oncol. 2013;9(12s):29–35. doi: 10.2217/fon.13.204. [DOI] [PubMed] [Google Scholar]
- 14.del Campo JM, Sehouli J, Lorusso D. Discussion: session 2. Future Oncol. 2013;9(12s):37. doi: 10.2217/fon.13.202. [DOI] [PubMed] [Google Scholar]
- 15.Markman M, Kennedy A, Webster K. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol. 1999;17:1141–1145. doi: 10.1200/JCO.1999.17.4.1141. [DOI] [PubMed] [Google Scholar]
- 16.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 17.Castells M, Sancho-Serra M del C, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61:1575–1584. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanai T, Iwasa S, Hashimoto H. Successful rechallenge for oxaliplatin hypersensitivity reactions in patients with metastatic colorectal cancer. Anticancer Res. 2012;32:5521–5526. [PubMed] [Google Scholar]
- 19.Rose PG, Fusco N, Smrekar M, Mossbruger K, Rodriguez M. Successful administration of carboplatin in patients with clinically documented carboplatin hypersensitivity. Gynecol Oncol. 2003;89:429–433. doi: 10.1016/s0090-8258(03)00178-1. [DOI] [PubMed] [Google Scholar]
- 20.Dizon DS, Sabbatini PJ, Aghajanian C, Hensley ML, Spriggs DR. Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol. 2002;84:378–382. doi: 10.1006/gyno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 21.Zweizig S, Roman LD, Muderspach LI. Death from anaphylaxis to cisplatin: a case report. Gynecol Oncol. 1994;53:121. doi: 10.1006/gyno.1994.1098. 121. [DOI] [PubMed] [Google Scholar]
- 22.Rakowski JA. Use of pegylated liposomal doxorubicin in the management of platinum-sensitive recurrent ovarian cancer: current concepts. Expert Rev Anticancer Ther. 2012;12:31–40. doi: 10.1586/era.11.187. [DOI] [PubMed] [Google Scholar]