Abstract
The choice of second-line chemotherapy in patients with recurrent ovarian cancer (ROC) is complex, with several factors to be considered, the most important of which is the length of the platinum-free treatment interval (PFI). Recently ROC patients have been further stratified into platinum sensitive (PS), partially platinum sensitive (PPS) and platinum resistant (PR) subgroups depending on the length of the PFI. Response to second-line therapy, progression-free survival (PFS) and overall survival (OS) are linked to the PFI, all of them improving as the PFI increases. Consequently, there is increasing interest in PFI extension strategies with platinum-free therapeutic options. Such strategies are currently being studied in patients with partially platinum-sensitive disease (PFI 6-12 months), as the treatment of these patients remains clinically challenging. A non-platinum option, trabectedin + pegylated liposomal doxorubicin (PLD) combination, has been evaluated in ROC patients in the pivotal phase III OVA-301 study. The OVA-301 study differed from previous trials in the same setting as it included only patients who were not expected to benefit from or who were ineligible for or who were unwilling to receive re-treatment with platinum-based chemotherapy, including those with PPS and PR disease. Subset analysis of patients with PPS disease in OVA-301 showed that the trabectedin + PLD combination significantly improved PFS compared with PLD alone; median PFS 7.4 versus 5.5 months, p=0.0152. Final survival data from the same subset of patients, showed that trabectedin + PLD also achieved a significant 36% decrease in the risk of death compared with PLD alone (HR=0.64; 95% CI, 0.47–0.88; p=0.0027). Median overall survival (OS) was 22.4 months in the trabectedin + PLD arm versus 16.4 months in the PLD arm. This represents a statistically significant 6-month improvement in median OS in patients treated with trabectedin + PLD compared to those treated with PLD alone.
Keywords: Ovarian Cancer, Relapse, Chemotherapy
Stratification of ROC patients according to length of PFI to tailor therapeutic strategies at relapse
The majority (approximately 80%) of patients with ovarian cancer will relapse, with a median progression-free survival (PFS) of 18 months, and require second-line therapy. Further treatment of these patients is complex with several factors to be considered, including the length of the platinum-free interval (PFI) [1].
The stratification of the ROC patient population into different subgroups by PFI (≤4 weeks and <6 months, 6-12 months and >12 months) is important for defining specific therapeutic approaches (Box 1). Clinical studies up until this point have classified relapsing patients as having either platinum-sensitive or platinum-resistant disease.
Box 1. Identifiable subgroups within the ROC patient population [2].
Classifications:
-
•
Platinum sensitive (PS) disease: PFI >12 months
-
•
Partially platinum sensitive (PPS) disease: PFI 6 – 12 months
-
•
Platinum resistant (PR) disease: PFI < 6 months
-
•
(Refractory disease: disease progression while receiving the last line of platinum therapy or within 4 weeks of the last platinum dose)
Rationale for platinum intercalation in PPS patients
Response to second-line therapy and prognosis are linked to the PFI, with both improving as the PFI increases [3, 4]. PFI is the most important predictive factor for response to platinum retreatment and the most important prognostic factor for PFS and overall survival (OS) [3, 5, 6].
As a result, there is increasing interest in PFI extension strategies, including platinum-free therapeutic options. Studies evaluating PFI extension strategies with non-platinum drug regimens have shown pegylated liposomal doxorubicin (PLD) to have equivalent efficacy and less toxicity compared with paclitaxel or topotecan, in patients relapsing after 6 months (PLD vs. topotecan: n=122; HR=1.58; p=0.021); however, until recently, relatively few studies report separate data for the PPS patient population, and the majority of these data are from unplanned retrospective subset analyses [7, 8].
The clinical management and optimal treatment of the partially platinum-sensitive subgroup of ROC patients continues to present challenges [4]. Approximately 20% of patients with ROC will relapse within the 6-12 month period. In vitro and clinical data suggest that extending the PFI in patients with relapsed PPS ovarian cancer through intercalation of a non-platinum therapy before continuing platinum-based regimens at disease progression could be clinically beneficial [9]. In this clinical setting, the potential benefit of artificially prolonging the PFI has been evaluated in several studies, with data suggesting that extending the PFI may restore platinum sensitivity and lead to clinical responses or stable disease with platinum retreatment, even in heavily pre-treated patients [4, 7]. PLD has been included in the National Institute for Health and Care Excellence (NICE) recommendations as a non-platinum treatment option for patients with partially platinum-sensitive disease [10]. A head-to-head randomised study has since proven that the trabectedin + PLD combination is superior to PLD alone. As a result, this guidance is subject to an ongoing NICE appraisal (ROC MTA222/91).
Trabectedin + PLD provides one opportunity for prolonging the platinum-free interval
Trabectedin (Yondelis®), a marine derived anti-neoplastic agent, has been shown to have clinical benefit in patients with ROC. Trabectedin was initially isolated from the tunicate Ecteinascidia turbinata and is currently produced synthetically. It was first approved in 2007 within the European Union as monotherapy for the treatment of patients with advanced soft tissue sarcoma (STS). Trabectedin is indicated for the treatment of adult patients with advanced soft tissue sarcoma after failure of anthracyclines and ifosfamide, or who are unsuited to receive these agents [11].
In patients with relapsed, platinum-sensitive ovarian cancer trabectedin was approved in combination with PLD in 2009. Early phase II trials showed encouraging activity as a single agent in patients with relapsed ovarian cancer, particularly with platinum sensitive disease, and a large, multicentre, randomised phase III trial (OVA-301) confirmed that trabectedin + PLD improved PFS over PLD alone (Fig. 1) [12].
Fig. 1.
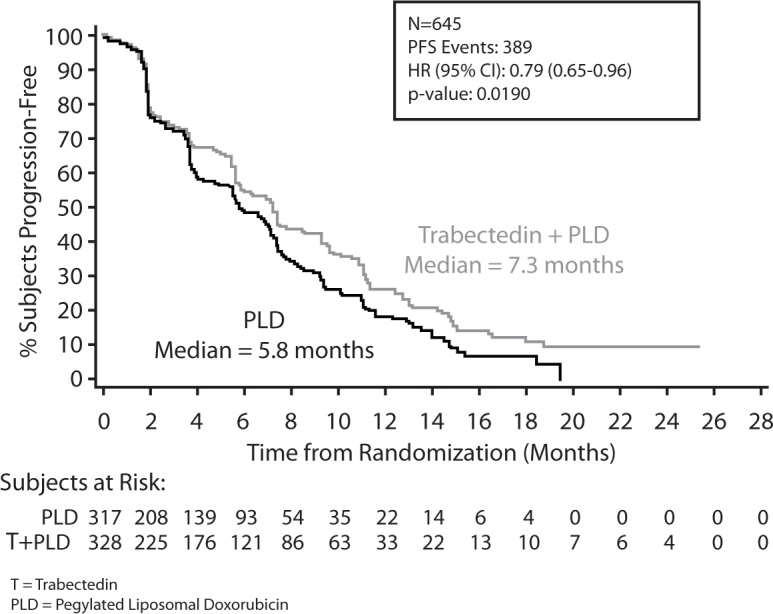
OVA-301: PFS final analysis. Final analysis of progression-free survival in OVA-301by independent radiology [12]. Reprinted with permission. © 2014 American Society of Clinical Oncology. All rights reserved.
PLD was selected as the comparator in this study due to its approval as a non-platinum alternative treatment in relapsing patients with PPS disease. The trabectedin + PLD combination also allowed a lower dose of PLD to be used in the combination. The OVA-301 study differed from previous trials in the same setting (e.g. CALYPSO) as it included only patients who were not expected to benefit from or who were ineligible for or who were unwilling to receive re-treatment with platinum-based chemotherapy. The study population comprised a total n=672 (made up of the different subgroups as follows PR n=242, PS n=216 and PPS n=214).
The results of OVA-301 in the whole study population showed that treatment with trabectedin + PLD achieved significant benefits in terms of PFS, the study's primary endpoint (median PFS: 7.3 months vs. 5.8 months; p=0.019) (Fig. 1), ORR (27.6% vs. 18.8%; p=0.008).
The final survival data in the overall study population confirmed the preliminary findings and showed a 14% decrease in the risk of death for patients randomised to receive the trabectedin + PLD combination (HR=0.86; p=0.0835). The median OS in the overall study population was 22.2 months for the combination, and 18.9 months for PLD alone. However, an unanticipated but significant overall imbalance in prognostic factors, including the PFI, was found between the two study arms favouring the PLD arm. A multivariate analysis based on Cox regression as specified in the study protocol was performed and found a relevant and significant improvement in OS in the overall population, with a 18% decrease in the risk of death in patients treated with the trabectedin + PLD combination compared with PLD alone (HR=0.82; 22.6 vs. 19.4 months; p=0.0285). These findings are in the range of that observed with platinum combination therapies [13].
Similar to other studies, further ad hoc analysis of the subset of patients with PPS disease (214/672 patients; 32% of OVA-301 study population) showed an improved PFS in the patients who received the combination compared with PLD alone, according to independent radiology review. A 35% risk reduction of disease progression (DP) or death was achieved, (HR=0.65, 95% confidence interval (CI), 0.45-0.92; p=0.0152; median PFS 7.4 versus 5.5 months). These data compare very favourably with the 21% achieved for the overall relapsed population [12, 14]. The risk reduction of DP or death reached 46% when these data were evaluated by independent oncology review, which included both clinical and imaging data [14].
Final survival data in the PPS subset of patients, showed that trabectedin + PLD also induced a significant 36% decrease in the risk of death compared with PLD alone (HR=0.65; 95% CI, 0.47–0.88; p=0.0027). Median OS was 22.4 months in the trabectedin + PLD arm versus 16.4 months in the PLD arm; a statistically significant 6-month improvement in median overall survival was achieved in the patients randomly assigned to receive trabectedin + PLD combination therapy (Fig. 2) [15].
Fig. 2.
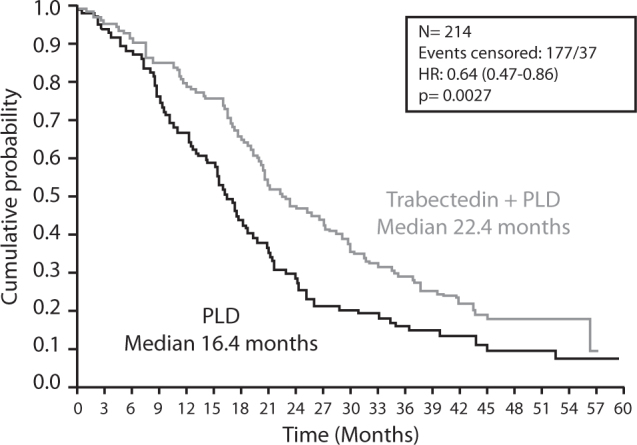
OVA-301: Final OS in PPS patients; PFI 6-12 months (Ad hoc analysis). Final overall survival in ROC patients with partially platinum-sensitive disease from OVA-301. © Elsevier [15].
Similar to the benefits in PFS seen in other unplanned ad hoc analyses of PPS patients from other studies these final survival data from OVA-301 compare very favourably with the 16–21 months survival range found in phase II trials of other combination chemotherapies, such as PLD plus carboplatin or gemcitabine plus PLD [9].
Benefits of extending the platinum-free treatment interval
Extending the PFI with a non-platinum treatment option, such as trabectedin + PLD, may benefit ROC patients by giving them some extra time to recover from the adverse effects of their prior platinum-based therapy, while preserving future treatment options. These data from OVA-301 demonstrate that the PFI can be prolonged in patients with the effective non-platinum trabectedin + PLD combination between platinum regimens. This translates both in survival advantage when reintroducing the subsequent platinum and in recovery from previous platinum-induced adverse effects [16].
Clinical evidence supporting subsequent platinum rechallenge and observed OS advantage
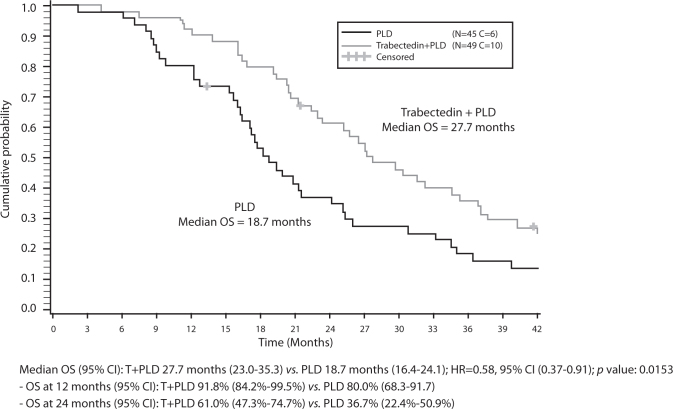
Further encouraging data in the PPS subgroup from OVA-301 showed additional benefits with the trabectedin + PLD combination upon administration of subsequent therapies. Differences were particularly pronounced in patients who were subsequently rechallenged with platinum as third-line therapy after discontinuation of OVA-301. Treatment with trabectedin + PLD resulted in a reduction of 42% in the risk of death compared with PLD alone (HR=0.58; p=0.0153). Median OS for patients receiving trabectedin + PLD was 27.7 months compared with 18.7 months for PLD; a notable improvement of 9 months in median OS (Fig. 3). Also the patients treated with trabectedin + PLD received their subsequent platinum later compared with those treated with PLD alone, with a median prolongation of the PFI by 4 months (11.5 vs. 7.5 months; HR=0.61; p=0.0203). The delay in subsequent platinum treatment has largely translated into an OS extension from previous platinum by a median of 8.9 months (18.8 vs. 9.9 months; HR=0.64; p=0.0513) compared with PLD alone [16]. This suggests that time off platinum treatment may also improve the response to subsequent reintroduction of platinum.
Fig. 3.
OVA-301: Final OS in PPS patients receiving subsequent platinum third line. Final analysis of overall survival in patients with partially platinum-sensitive disease (PFI 6-12 months) in OVA-301 receiving platinum as the first subsequent third-line chemotherapy. © Elsevier [16].
One explanation for this is that extending the PFI by an effective non-platinum intervention, such as trabectedin + PLD, may reduce cumulative platinum-induced toxicities, which in turn leads to longer survival after the reintroduction of subsequent platinum. This is now being explored in a prospective randomised trial (which will be discussed in more detail later) [16].
The impact of trabectedin plus PLD combination on PPS patients' quality of life
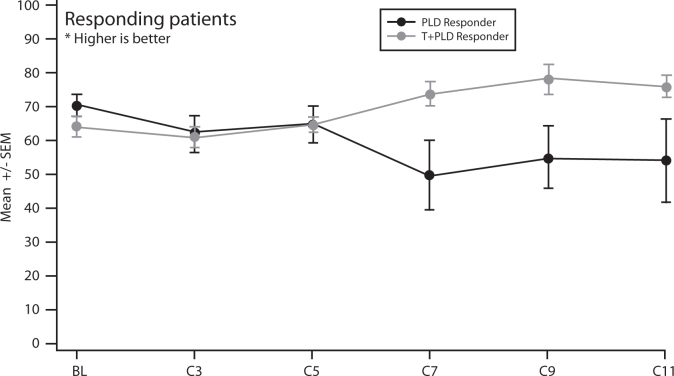
The impact of the trabectedin + PLD combination was also assessed on patients' quality of life (QoL) through patient reported outcomes via QLQ-C30, a standardised QoL instrument developed by the European Organisation for the Research and Treatment of Cancer (EORTC) to assess the quality of life of cancer patients. Results from OVA-301 showed there was a marked improvement in patient-reported functional status and symptoms in the PPS patient subgroup receiving the trabectedin + PLD combination beyond cycle 5 compared with PLD alone (Fig. 4).
Fig. 4.
OVA-301: QLQ-C30 global health status scale in the PPS population. Improved QoL for PPS patients randomised to receive trabectedin + PLD combination compared with PLD alone [17].
As a result the European Society of Medical Oncology (ESMO) has issued guidance on the treatment of ROC patients with partially platinum-sensitive disease. The trabectedin + PLD combination is recommended by ESMO in patients with relapsed partially platinum-sensitive ovarian cancer due to the observed benefit in overall survival achieved with the combination [18].
On-going clinical trials and future directions
It is likely that the introduction of novel non-platinum based chemotherapies and molecular targeted therapies, will have a major impact on the management of ROC. Some current strategies are focused on the extension of the PFI in patients with PS disease, and especially in those with PPS disease as this is the group that remains the most clinically challenging [16].
OVA-301 has already demonstrated the survival advantage in prolonging the PFI through intercalation of the non-platinum combination (trabectedin + PLD) between platinum-based regimens, particularly in the PPS patient subgroup. The OS data obtained from OVA-301 demonstrate that the combination is significantly superior to PLD monotherapy in these patients.
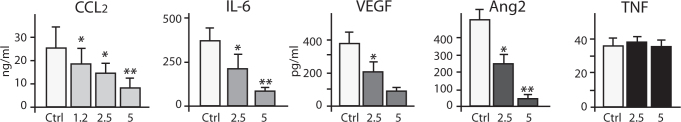
Attention is now turning to a mechanistic rationale to explain the observed clinical benefit. In vitro studies have shown platinum resistance to be an unstable, inducible and, possibly, reversible phenomenon. Platinum resistance increases after each exposure, probably due to the selection of only highly resistant cells that are less likely to respond to subsequent platinum challenge. The mechanisms for platinum resistance are complex and multifactorial. For example, cisplatin-treated ovarian cancer cells over-express IL-6, and blocking IL-6 significantly sensitises platinum-resistant ovarian cancer cells to cisplatin. Trabectedin has demonstrated inhibitory activity against the production of inflammatory cytokines, such as IL-6, in ovarian cancer cells and tumour-associated macrophages (Fig. 5) [19]. Inflammatory cytokines, such as IL-6, are thought to have tumour-promoting actions in the ovarian cancer microenvironment [20].
Fig. 5.
Trabectedin selectively suppresses inflammatory cytokines in vitro. Trabectedin selectively reduces some inflammatory cytokines in monocytes and macrophages at infra-cytotoxic levels. Adapted from [21], with permission from AACR.
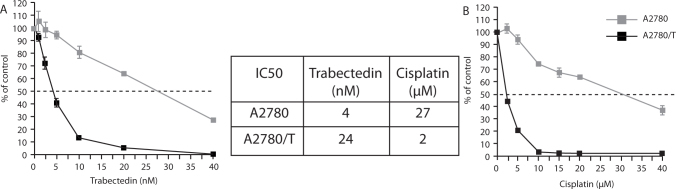
Further in vitro data with trabectedin has also demonstrated a treatment ‘sequence effect’, such that trabectedin resistance appears to improve sensitivity to platinum in ROC cells (Fig. 6) [22].
Fig. 6.
In vitro evidence of a sequence effect with trabectedin. Resistant ovarian carcinoma cell lines (A2780/T) and parental A2780 cells lines. (A) (A2780/T) cell lines were 6 fold resistant to trabectedin as compared to parental cells; (B) (A2780/T) cell lines were 13.5 fold more sensitive to cisplatin than the parental cell lines [22].
Resistance to trabectedin is associated with the loss of nucleotide excision repair (NER) function, with consequent increased sensitivity to cisplatin. This provides the rational to test the sequential combination of trabectedin + PLD and platinum in the clinic.
Conclusions
In summary, these efficacy data from the pivotal OVA-301 study suggest that the trabectedin + PLD combination is an effective second-, or any further line, non-platinum treatment option compared with PLD monotherapy for patients with ROC unable to continue the next platinum cycle. The observed efficacy and improvement in QoL particularly in patients with partially platinum-sensitive disease translates into a significant survival advantage, and therefore the combination is now recommended by ESMO in this patient subgroup. It is thought that by extending the PFI through use of the trabectedin + PLD combination allows patients time to recover from cumulative platinum-induced adverse effects, which in turn leads to longer overall survival after reintroduction of subsequent platinum. The trabectedin + PLD combination has shifted the treatment paradigm for ROC patients including the PPS subgroup or those unsuited to platinum rechallenge, by defining specific therapeutic approaches for these patients. A mechanistic rationale is currently being developed to explain the observed treatment sequencing effect, particularly in PPS patients. Looking ahead, it is anticipated that the ongoing INOVATYON study, comparing the non-platinum trabectedin + PLD with a platinum + PLD combination will confirm this hypothesis and provide guidance regarding the treatment sequence benefits in managing ROC patients, particularly in the PPS subgroup.
Conflict of Interest Statement
Professor Colombo received speaker fees from PharmaMar for her participation at the BGCS 2014 symposium. Professor Colombo is responsible for the content of this article. Patricia Howard Associates received fees from PharmaMar to provide editorial assistance.
References
- 1.Colombo N. Optimizing treatment of the partially platinum-sensitive ovarian cancer patient. Future Oncol. 2013;9(12s):19–23. doi: 10.2217/fon.13.206. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Rothman R, Hakes T. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991;9:389–393. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 4.Kaye S. Management of partially platinum-sensitive relapsed ovarian cancer. Eur J Cancer. 2008;6(Suppl):16–21. [Google Scholar]
- 5.Gore ME, Fryatt I, Wiltshaw E, Dawson T. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol. 1990;36:207–211. doi: 10.1016/0090-8258(90)90174-j. [DOI] [PubMed] [Google Scholar]
- 6.Pujade-Lauraine E, Paraiso D, Cure H. Predicting the effectiveness of chemotherapy (Cx) in patients with recurrent ovarian cancer (ROC): a GINECO study. Proc Am Soc Clin Oncol. 2002;21 Abstract 829. [Google Scholar]
- 7.Colombo N, Gore M. Treatment of recurrent ovarian cancer relapsing 6-12 months post platinum-based chemotherapy. Crit Rev Oncol Hematol. 2007;64:129–138. doi: 10.1016/j.critrevonc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.O'Byrne KJ, Bliss P, Graham JD. A Phase III study of doxil/caylex versus paclitaxel in platinum treated taxane naive relapsed ovarian cancer. Proc Am Soc Clin Oncol. 2002;21(203A) Abstract 808. [Google Scholar]
- 9.Sehouli J, Alfaro V, Gonzalez-Martin A. Trabectedin plus pegylated liposomal doxorubicin in the treatment of patients with partially platinum-sensitive ovarian cancer: current evidence and future perspectives. Ann Oncol. 2012;23:556–562. doi: 10.1093/annonc/mdr321. [DOI] [PubMed] [Google Scholar]
- 10.NICE Paclitaxel, pegylated liposomal doxorubicin hydrochloride and topotecan for second-line or subsequent treatment of advanced ovarian cancer: technology appraisal 91 (2005) [www.nice.org.uk/nicemedia/pdf/ta091guidance.pdf] [DOI] [PubMed]
- 11.Yondelis Summary of Product Characteristics. [http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/000773/WC500045832.pdf]
- 12.Monk BJ, Herzog TJ, Kaye SB. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–3114. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez A. Increasing the chances for platinum-sensitive ovarian cancer patients. Future Oncol. 2013;9(12 Suppl 1):29–35. doi: 10.2217/fon.13.204. [DOI] [PubMed] [Google Scholar]
- 14.Poveda A, Vergote I, Tjulandin S. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial. Ann Oncol. 2011;22:39–48. doi: 10.1093/annonc/mdq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk BJ. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. EJC. 2012;48:2361–2368. doi: 10.1016/j.ejca.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Poveda A, Ray-Coquard I, Romero I, Lopez-Guerrero JA, Colombo N. Emerging treatment strategies in recurrent platinum-sensitive ovarian cancer: Focus on trabectedin. Cancer Treat Rev. 2014;40:366–375. doi: 10.1016/j.ctrv.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Vermorken JB. Patient-reported outcomes in patients with relapsed, platinum-sensitive ovarian cancer (ROC): results from a large randomized phase III trial. Int J Gynecol Cancer. 2010;20(suppl 2) abstract 458. [Google Scholar]
- 18.Ledermann JA, on behalf of the ESMO guidelines working group Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–vi32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 19.Sehouli J, del Campo JM, Lorusso D. Discussion: session 1. What are the benefits of extending the platinum-free interval? Future Oncol. 2013;9(12 Suppl 1):25–27. doi: 10.2217/fon.13.201. [20] [DOI] [PubMed] [Google Scholar]
- 20.Coward JI. Interleukin-6 as a therapeutic target in advanced ovarian cancer. J Clin Oncol. 2010;28(Suppl):5089. [Google Scholar]
- 21.Allavena P, Signorelli M, Chieppa M. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 22.D'Incalci M, et al. AACR-NCI-EORTC Conference. International Conference on Molecular Targets and Cancer Therapeutics October 19–23, 2013. Boston, MA. C93.