Abstract
Pulmonary arterial hypertension (PAH) is a rare multifactorial disease with an unfavorable prognosis. Sildenafil therapy can improve functional capacity and pulmonary hemodynamics in PAH patients. Nowadays, it is increasingly recognized that the effects of sildenafil are pleiotropic and may also involve changes of the pro-/antioxidant balance, lipid peroxidation and autonomic control. In present study we aimed to assess the effects of sildenafil on the fatty acids (FAs) status, level of hydroxynonenal (HNE) and heart rate variability (HRV) in PAH patients. Patients with PAH were characterized by an increase in HNE and changes in the FAs composition with elevation of linoleic, oleic, docosahexanoic acids in phospholipids as well as reduced HRV with sympathetic predominance. Sildenafil therapy improved exercise capacity and pulmonary hemodynamics and reduced NT-proBNP level in PAH. Antioxidant and anti-inflammatory effects of sildenafil were noted from the significant lowering of HNE level and reduction of the phopholipid derived oleic, linoleic, docosahexanoic, docosapentanoic FAs. That was also associated with some improvement of HRV on account of the activation of the neurohumoral regulatory component. Incomplete recovery of the functional metabolic disorders in PAH patients may be assumed from the persistent increase in free FAs, reduced HRV with the sympathetic predominance in the spectral structure after treatment comparing to control group. The possibilities to improve PAH treatment efficacy through mild stimulation of free radical reactions and formation of hormetic reaction in the context of improved NO signaling are discussed.
Abbreviations: DHA, docosahexanoic acid; EPA, eicosapentanoic acid; FA, fatty acids; FC, functional class; HF, high frequency; HNE, hydroxynonenal; HRV, heart rate variability; LA, linoleic acid; LF, low frequency; 6MWT, 6 min walk test; NOS, nitric oxide synthase; NT-proBNP–N, terminal pro-brain natriuretic peptide; OS, oxidative stress; PAH, pulmonary arterial hypertension; PDE, phosphodiesterase; PKA, protein kinase A; PKG, protein kinase G; pNN50, percentage of differences between adjacent normal RR intervals exceeding 50 ms; RMSSD, the square root of the mean squared differences of successive RR interval; ROS, reactive oxygen species; SDNN, standard deviation of normal RR intervals; TP, total power; VLF, very low frequency
Keywords: Pulmonary arterial hypertension, Sildenafil, Oxidative stress, Hydroxynonenal, Fatty acid composition, Heart rate variability
Highlights
-
•
Sildenafil showed antioxidant and anti-inflammatory effects in pulmonary hypertension.
-
•
Sildenafil reduced hydroxynonenal level and improved fatty acid profile in serum.
-
•
Improvement of heart rate variability and functional capacity was noted after therapy.
-
•
Mild prooxidant activity is suggested as the mechanism to improve sildenafil efficacy.
1. Introduction
Pulmonary arterial hypertension (PAH) is a rare, progressive disease characterized by sustained pulmonary arterial vasoconstriction and vascular remodeling ultimately leading to right ventricular failure and death. The pathophysiology of PAH in complex and involves abnormal proliferation of the vascular smooth muscle and endothelial cells, infiltration by the inflammatory cells, and fibrosis of the vascular elements [20]. Multiple factors including endothelial dysfunction with reduced NO bioavailability, increased production of reactive oxygen species (ROS), impaired synthesis of prostaglandin with PGI2 deficiency and excessive production of various mediators (angiotensin II, endotelin-1, etc.) play role in the pathologic remodeling of the pulmonary vasculature [1]. Recently, the action of the inflammatory, procoagulant, antiapoptotic, and autoimmune mediators, as well as cell–cell and cell–matrix interactions have been recognized as important contributors to disease progression [66]. Despite the progress in understanding PAH and major advances in its treatment the prognosis of the disease still remains unfavorable with the mortality rate of 10–15% per year and median survival of approximately 7 years [5], [25] underscoring the need for more effective therapeutic options.
Currently approved PAH specific therapies include prostacyclins, phosphodiesterase inhibitors, endothelin receptor antagonists, and soluble guanylyl cyclase agonists [21]. Impaired synthesis of prostacyclin from arachidonic acid was the first pathobiological mechanism described in PAH, which pioneered effective drug development [12]. Reduced expression of prostacyclin synthase (PGIS) was found in pulmonary arteries in PAH patients [67], and peroxynitrite-mediated nitration of specific protein tyrosine residues was shown to be responsible for selective inactivation of PGIS and development of endothelial dysfunction [14], [81].
Increased production of ROS and oxidative stress (OS) are the major contributors to the disorders in the l-arginine-NO-signaling pathway with changes in NO synthase (NOS) activity and NO deficiency and impaired pulmonary vasodilatory responses being characteristic features of PAH [23]. The underlying mechanisms of the derangements in NO synthesis are related either to the oxidative loss of NOS cofactor tetrahydrobiopterin (BH4) or oxidative attack on the zinc tetrathiolate cluster, which is an important mediator for the NOS dimeric structure [1]. Moreover, increased nonenzymatic NO production in hypoxic and oxidative environment rather promotes formation of peroxinitrite than improves NO bioavailability.
OS is commonly recognized player at the field of PAH. Signs of OS with excessive lipid peroxidation were demonstrated in PAH patients by increased levels of isoprostanes in urine [13] and malondialdehyde in plasma [32]. The other product of lipid peroxidation is hydroxynonenal (HNE). It is regarded not only as a useful marker of oxidative inflammatory condition but also exerts a wide range of pathophysiological effects, including synthesis and release of vasoactive mediators, breakdown of the endothelial barrier function and induction of the pro-inflammatory phenotype within the vessel wall making the study of HNE role in PAH particularly important [7]. The opposing effects of HNE on the NO homeostasis have been described [56], although the data on its role in PAH is missing.
Changes in the fatty acid (FA) composition were shown to interfere with NO signaling. NO production can be stimulated by the polyunsaturated FA (PUFA) such as arachidonic, eicosapentanoic (EPA), and docosahexanoic (DHA) acids [3], [39], [43]. This relationship is bidirectional as inhibition of NO synthesis was demonstrated to increase erythrocyte membrane fluidity due to the higher content of the unsaturated FAs [16], while administration of l-arginine increased levels of linoleic (LA), γ-linolenic acid (GLA) and dihomo-GLA [45]. Increased levels of free FAs in blood have been closely linked to endothelial dysfunction and altered eNOS activity attributed to the impaired action of the phospholipase C activating receptor agonists (such as acetylcholine, ATP, bradykinin) and reduced [Ca2+]i increments [17], [64]. Thus, metabolic alterations contributing to the impaired NO bioavailability observed in PAH involve alterations in the FAs status and potentiation of the l-arginine-NO-system during treatment may be accompanied by changes in the lipid composition.
At present, targeting l-arginine-NO pathway is a cornerstone strategy in PAH treatment. The approved therapeutic options include sildenafil, tadalafil, and vardenafil – inhibitors of phosphodiesterase type 5 (PDE-5), and riociguat, which stimulates soluble guanylyl cyclase and promotes cGMP synthesis [21], [55]. Sildenafil has the highest level of evidence in PAH [21]. As the other PDE-5 inhibitors it blocks enzymatic degradation of the second messenger cGMP to GMP, increases intracellular cGMP level and leads to activation of the protein kinase G (PKG) with subsequent relaxation of smooth muscle cells, reduction of vascular remodeling and vasoconstriction. At the same time, precise molecular mechanisms of the protective effects of sildenafil are not completely understood. Recently, it was demonstrated that sildenafil suppressed multiple cytokines involved in neutrophil and mononuclear cell recruitment and reduced NF-kB and p38 MAPK activation in lungs from rats with monocrotaline induced PAH [36]. Moreover, its use caused stimulation of another PDE type 3-PKA pathway and targeted mitochondrial K+ATP channels, mitochondria and inflammation in hypertrophied right ventricle in PAH patients [48]. The inhibitory action of sildenafil on the main sources of free radicals production such as xanthine oxidase [63] and NADPH oxidase [47] points to its role as an antioxidant. Thus, the action of sildenafil is not limited to mechanistic inhibition of PDE5-PKG system but also includes modulation of the inflammatory and redox status, which are increasingly recognized as important contributors to PAH progression.
Development of PAH is characterized not only by the defective vasodilator but also exaggerated vasoconstrictor responses. The latter involve activation of the sympathetic nervous system, which was confirmed by the studies of the heart rate variability (HRV) in PAH subjects [6], [71]. Interestingly, that inhibition of NOS in healthy volunteers caused sympathetic activation [76], while administration of PDE-5 inhibitor improved NO bioavailability and prevented hypoxic pulmonary hypertension [42]. There is increasing evidence that HRV is informative not only about the autonomic balance but also reflects the severity of the inflammatory conditions and depth of the oxidative stress [74] which has not been addressed in PAH.
In present study we aimed to assess the effects of sildenafil on the FAs status, level of hydroxynonenal (HNE) and heart rate variability (HRV) in PAH patients. We hypothesize that improvement of NO bioavailability with sildenafil will reduce lipid peroxidation, diminish signs of OS, providing antioxidant effect in PAH patients. Correction of the metabolic disorders at the level of an organism can be reflected by increased HRV and improvement in the balance between autonomic components which influence the heart rhythm.
2. Materials and methods
2.1. Selection of patients and study design
Patients with newly diagnosed PAH (idiopathic, familial or occurring after surgical repair of congenital systemic-to-pulmonary shunts that had been performed at least five years previously) with a six-minute walking distance from 100 m to 450 m were included into the study. PAH was characterized by the presence of pre-capillary PH, i.e., mPAP≥25 mmHg, pulmonary artery wedge pressure ≤15 mmHg by right heart catheterization, in the absence of other causes of pre-capillary PH. Thermodilution method was used for cardiac output measurement. Upon confirmation of PAH sildenafil at a dose of 25 mg three times per day was initiated in addition to the conventional therapies including diuretics and anticoagulants. The duration of the study period was 12 weeks. Patients treated with other PAH specific medications such as inhaled or intravenous prostanoids, endothelin receptor blocker, calcium channel blockers and supplementation with l-arginine, and fish oil were not included into the study. Patients remained on their usual diet throughout the study. Healthy volunteers comprised a control group. They underwent standard clinical evaluation, blood sampling and HRV measurement once and did not receive any medications. The study was approved by Ethics Committee of the Danylo Halytsky Lviv National Medical University and written informed consent was obtained from all participants.
2.2. Efficacy measures
The measures of efficacy included the change in exercise capacity, as measured by the total distance walked in six minutes (6 min walk test, 6MWT), from baseline to week 12, score on the Borg scale of dyspnea (with 0 representing no dyspnea and 10 maximal dyspnea), World Health Organization (WHO) functional class (FC) of PAH. Other measures of efficacy were the changes in the peak pressure gradient of tricuspid regurgitation and right ventricle diameter by echocardiography [20]. Physical examinations and laboratory tests were performed before and 12 weeks after initiation of sildenafil therapy. Fasting blood samples were obtained by venepuncture of the cubital vein, centrifuged and serum was stored under −80 °C until the analysis.
2.3. Fatty acids determination
Phospholipid and free FAs were determined by gas chromatography according to the method of Christie [11]. Lipid components were isolated by Folch extraction using chloroform/methanol mixture (2:1, v/v) in the presence of 0.01% butylated hydroxytoluene (BHT). Using TLC, free fatty acids and total phospholipids were separated with the mobile phase heptane–diisopropyl ether–acetic acid (60:40:3, v/v/v). All lipid fractions were transmetylated to fatty acid methyl esters (FAMEs) with boron trifluoride in methanol reagent under nitrogen atmosphere without previous separation from the layer. The FAMEs were analyzed by gas chromatography with a flame ionization detector (FID) on Clarus 500 Gas Chromatograph (Perkin Elmer). Separation of FAMEs was carried out on capillary column coated with Varian CP-Sil88 stationary phase. Operating conditions were as follows: the split–splitless injector was used in split mode with a split ratio of 1:20. The injection volume of the sample was 2 µL. The injector and FID detector temperatures were kept at 260 °C. The column temperature was programmed from 150 °C (2 min) to 230 °C (10 min) at 4 °C/min. The carrier gas was helium at a flow rate of 1 mL/min. 1,2-dinonadecanoyl-sn-glycero-3-phosphocholine (19:0 PC) was used as an internal standard. The LOD for FAMEs standards was in the range of 150–250 ng/ml and the LOQ was in the range of 500–700 ng/mL, at a signal-to-noise ratio of 3 and 10, respectively. The precision of LOQ was 7.5% (CV). The linear dynamic range was 500–50000 ng/ml.
2.4. Lipid peroxidation products and NT-proBNP determination
4-hydroxynonenal (4-HNE) was assayed as a fluorimetric derivative with 1,3-cyclohexandione (CHD) [75]. Samples were mixed with methanol (1:1, V/V) and centrifuged at 850g for 10 min. CHD reagent was added to supernatant and obtained mixture was incubated for 1 h at 60 °C. The samples were purified by using Sep-pak ODS columns (Waters Associates, USA) and eluted with methanol. The eluent was evaporated to dryness under nitrogen and samples were reconstituted in 150 μl of methanol and subjected to HPLC. Chromatographic separation of CHD-adducts was carried out on RP C18 column with a linear gradient mobile phase consisted of methanol (MeOH), water and tetrahydrofuran [75]. The HPLC fluorescence detector was used with excitation at 380 nm and emission at 445 nm. The retention time was 20.07 min. The concentration of 4-HNE in the samples was calculated using a calibration curve (625–2000 nmol/l, R2=0.9997).
Evaluation of the N-terminal natriuretic peptide (NT-proBNP) level in the venous blood was performed by electro-chemiluminesence immunoassay using Roche Diagnostic reagents. The reference values of NT-proBNP for the individual aged 18–44 years are<85.8 pg/ml for male and<130 pg/ml for female.
2.5. Study of heart rate variability
During quiet wakefulness in the morning hours the short time ECG records were obtained using computer electrocardiograph “VNS-Micro” (Neurosoft®, Russia). After 20 min of rest, subjects were asked to stay supine quietly for 5 min for stationary HRV recording; afterwards they were asked to stand up rapidly and remained in the standing position for 6 min (orthostatic test). RR intervals were determined with a sampling frequency of 2 kHz and were analyzed with “Poly-Spectrum” (Neurosoft®, Russia) software designed according to HRV standards [28]. The time-domain indices – standard deviation of normal RR intervals (SDNN), the square root of the mean squared differences of successive RR interval (RMSSD), and percentage of differences between adjacent normal RR intervals exceeding 50 milliseconds (pNN50) were determined. The power spectral analysis was performed sequentially with a fast Fourier transformation. The following frequency-domain variables were studied: total power (TP, 0.01 to 0.40 Hz), high frequency power (HF, 0.15 to 0.40 Hz), low frequency power (LF, 0.04 to 0.15 Hz), and very low frequency power (VLF, 0.01 to 0.04 Hz).
2.6. Statistical analysis
Data was processed using Microsoft Excel for storage and basic calculations. Statistical calculations were performed by GraphPad Prism (version 5.00, open source). Normal distribution of the obtained data was confirmed with Shapiro–Wilk's W-test. Data are presented as means±standard deviation (M±SD), median (quartile range), means±standard error mean (M±SEM). The unpaired and paired t-test for independent variables was utilized to compare means between groups. The change in Borg dyspnea score was assessed by nonparametric Wilcoxon’s matched pairs test. Values of P<0.05 were considered significant.
3. Results
3.1. Baseline characteristics of PAH patient population
From July 2009 to June 2012 totally seven newly diagnosed treatment naive patients with PAH and seven healthy controls were enrolled. Demographic data of the patients and healthy controls is presented in Table 1. Among the PAH patients four were with familial PAH, and two with idiopathic PAH and one patient with PAH related to repaired congenital systemic-to-pulmonary shunt. Groups of patients and control were compatible by age and gender. Most subjects in PAH group were WHO functional class II (85.7%). One patient from PAH group had mild arterial hypertension. The mean 6MWD was 361.1 m in PAH group. Main parameters of pulmonary hemodynamics are presented in Table 1. The values of NT-proBNP in PAH patients were significantly increased over the reference range.
Table 1.
Characteristics of patients with PAH and healthy controls.
| Variable | PAH patient, n=7 | Control, n=7 | p Value |
|---|---|---|---|
| Age,years | 30.9±7.3 | 30.6±4.2 | 0.930 |
| Gender, M/F | 3/4 | 3/4 | 1.000 |
| FC, II/III | 6/1 | - | - |
| BMI, kg/m2 | 26.3±6.7 | 24.8±3.4 | 0.611 |
| Heart rate, bpm | 84.6±8.6 | 69.3±6.2 | 0.012 |
| Syst. BP, mmHg | 120.8±10.7 | 117.8±7.6 | 0.590 |
| Diast. BP, mmHg | 82.8±11.7 | 79.1±7.4 | 0.530 |
| Hypertension, n (%) | 1 (14.3) | - | - |
| 6-MWT, m | 361.1±69.4 | > 580 male* | - |
| > 500 female* | |||
| NT-proBNP, pg/ml | 3101±1952 | < 85.8 male* | - |
| < 130 female* | |||
| mPAP, mmHg | 58.9±16.3 | 7-19* | - |
| PAWP, mmHg | 9.5±3.1 | 5-13* | |
| PVR, dyn sec m2/cm5 | 1052±494 | 11-99* | - |
| CO, L/min | 3.46 (3.22, 4.16) | 4.4–8.4* | - |
| CI, L/min/m2 | 2.00 (1.88, 2.27) | 2.6–4.2* | - |
| FEV1, % | 79.0±6.5 | - | - |
| VC, % | 76.0±7.5 | - | - |
Data are presented as mean±SD or median (interquartile range).
Values were taken from [50]; PAH=pulmonary arterial hypertension; M/F=male/female; FC=functional class; BMI=body mass index; 6MWT=6-min walk test; NT-proBNP=N-terminal pro-brain natriuretic peptide; mPAP=mean pulmonary arterial pressure; PAWP – pulmonary artery wedge pressure; PVR=pulmonary vascular resistance; CO=cardiac output; CI=cardiac index; FEV1 – forced expiratory volume in 1 s; VC- vital capacity.
3.2. Comparison between PAH patients and control
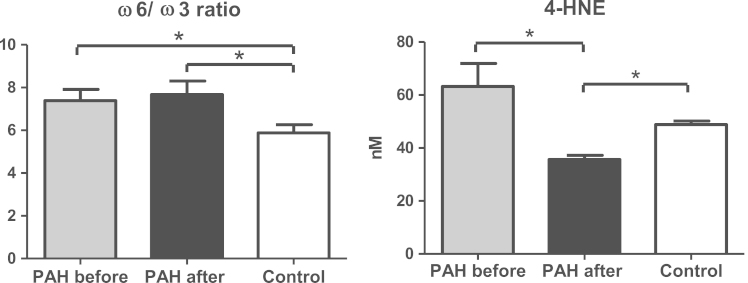
Groups of PAH patients and control were compatible by age, gender and BMI. PAH subjects had an increased heart rate but no significant difference in blood pressure comparing to healthy volunteers was noted (Table 1). Higher levels of unsaturated FA (oleic (18:1), linoleic (18:2)) in free and phospholipid derived fractions were observed in PAH patients (Table 2). The total amount and majority of the saturated FA within phospholipids were elevated in PAH comparing to control group. Interestingly, that insignificant increase of DHA (22:6) in phospholipids was observed before treatment in PAH. The ω6/ω3 ratio as well as level of the oxidative stress marker HNE (63.2±21.3 nM vs. 48.9±3.2 nM in control) were significantly elevated in PAH (Fig. 1).
Table 2.
Changes in the fatty acids composition associated with sildenafil therapy in PAH
| Fatty acids |
PAH |
Control | |
|---|---|---|---|
| Before treatment | After treatment | ||
| Free FA | |||
| Saturated | |||
| Palmitic acid, (16:0) | 1.40±0.26 | 1.64±0.18# | 1.08±0.08 |
| Stearic acid, (18:0) | 0.85±0.13 | 1.22±0.08# | 0.98±0.09 |
| Sum | 2.24±0.16 | 2.85±0.25# | 2.05±0.17 |
| Unsaturated | |||
| Palmitoleic acid, (16:1, cis-9) | 0.11±0.05 | 0.13±0.02# | 0.05±0.01 |
| Oleic acid, (18:1, cis-9) | 0.51±0.07 | 1.13±0.28# | 0.43±0.06 |
| Linoleic acid, (18:2, cis-9,12) | 0.83±0.31 | 1.14±0.24# | 0.36±0.14 |
| Sum | 1.45±0.33 | 2.40±0.52# | 0.83±0.20 |
| Phospholipids | |||
| Saturated | |||
| Palmitic acid, (16:0) | 46.30±1.68# | 44.71±2.13 | 42.18±2.06 |
| Stearic acid, (18:0) | 16.73±0.98# | 15.38±1.43 | 13.04±1.73 |
| Arachidic acid, (20:0) | 0.18±0.01# | 0.18±0.02# | 0.12±0.02 |
| Behenic acid, (22:0) | 0.19±0.04 | 0.25±0.05a | 0.12±0.08 |
| Sum | 63.40±2.70# | 60.53±3.60 | 55.47±3.41 |
| Unsaturated | |||
| Palmitoleic acid, (16:1, cis-9) | 1.57±0.07 | 1.47±0.06 | 1.41±0.08 |
| Oleic acid, (18:1, cis-9) | 12.06±1.15# | 10.62±0.64 | 9.38±0.55 |
| Linoleic acid, (18:2, cis-9,12) | 26.02±1.78# | 22.88±2.81 | 21.31±3.06 |
| Arachidonic acid, (20:4, cis-5,8,11,14,17) | 20.93±1.48 | 19.18±1.25 | 19.33±2.46 |
| Eicosapentanoic acid, | |||
| (20:5, cis-5,8,11,14,17) | 1.58±0.08 | 1.53±0.10 | 1.76±0.22 |
| Nervonic acid, (24:1, cis-15) | 0.55±0.04# | 0.60±0.06# | 0.39±0.04 |
| Docosapentanoic acid, | |||
| (22:5, cis- 7,10,13,16,19) | 0.92±0.12 | 0.82±0.11 | 0.81±0.18 |
| Docosahexanoic acid, | |||
| (22:6, cis- 4,7,10,13,16,19) | 3.90±0.17 | 3.33±0.34* | 3.32±0.86 |
| Sum | 67.52±4.0 | 60.42±3.60 | 57.71±6.15 |
Note: Data are presented as mean±SEM in ng/mL;
difference significant between PAH before or after treatment and control group, p<0.05.
difference significant before and after sildenafil, p<0.05;
Fig. 1.
Changes in the ω6/ω3 ratios and 4-HNE in PAH patients with sildenafil therapy. Note: data presented as mean±SEM; ω6/ω3 ratio was calculated as (18:2+20:4)/(22:5+22:6+20:5).
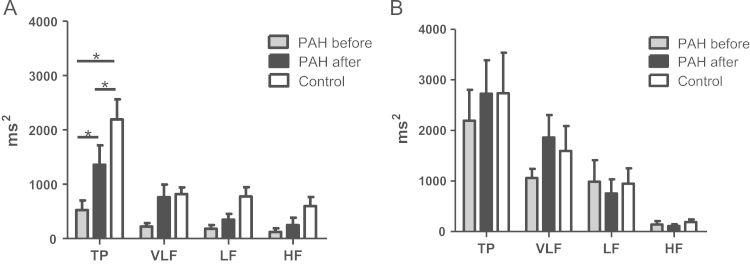
Study of HRV showed marked reduction of the time-domain indexes in supine position in PAH population. SDNN and RMSSD were reduced to 18.3±10.1 ms and 12.7±11.2 ms respectively (Table 3). Prominent lowering in total spectral power (TP 524±469 ms2 vs. 2191±975 ms2 in control group) was accompanied by marked reduction in the VLF, LF, and HF in PAH patients (Fig. 2). Sympathetic predominance with higher LF/HF ratio (3.7±2.2 vs. 2.7±4.2) in supine position and similar tendency in orthostasis were noted.
Table 3.
Changes in HRV indexes in PAH patients with sildenafil therapy
| Parameter |
PAH |
Control | ||
|---|---|---|---|---|
| Before treatment | After treatment | |||
| Supine position | SDNN, ms | 18.3±10.1a | 32.0±16.5 | 44.3±9.0 |
| RMSSD, ms | 12.9±11.2a | 21.4±23.7a | 36.5±11.7 | |
| pNN50,% | 1.66±3.62 | 6.9±14.4 | 13.6±12.0 | |
| LF/HF | 3.7±2.2 | 3.6±2.6 | 2.7±4.2 | |
| Orthostasis | SDNN, ms | 40.9±16.5 | 45.9±17.0 | 45.8±17.7 |
| RMSSD, ms | 19.4±16.2 | 14.1±7.1 | 19.6±9.8 | |
| pNN50,% | 3.92±8.05 | 1.2±1.4 | 3.0±3.6 | |
| LF/HF | 8.0±4.5 | 7.2±3.1 | 5.8±3.3 | |
Note: data presented as mean ± SD;
Difference significant comparing to control group.
Fig. 2.
Changes in frequency-domain HRV indexes in PAH patients with sildenafil therapy. Note: A-supine position; B-orthostatic test; data presented as mean±SD; HRV-heart rate variability; TP –total power; VLF – very low frequency; LF- low frequency; HF – high frequency.
3.3. Changes in clinical variables and hemodynamics with sildenafil therapy
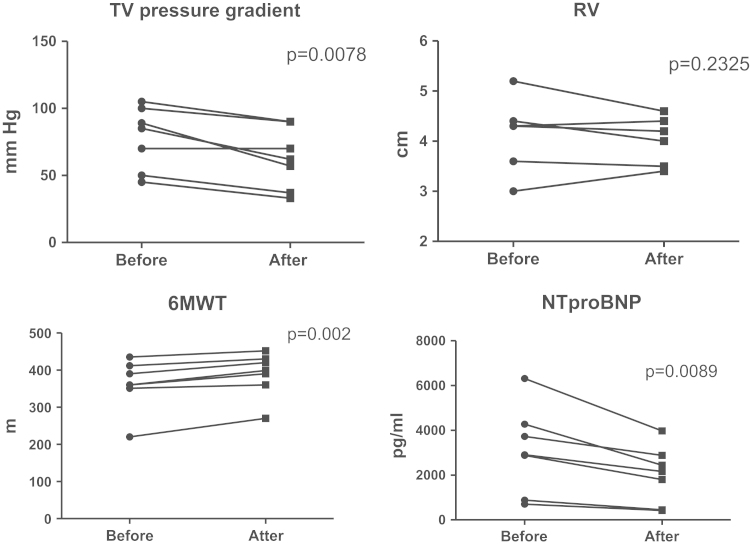
Twelve weeks of sildenafil therapy administered to newly diagnosed PAH patients caused increase in 6MWD from 361.1±69.4 m to 388.7±60.2 m (Fig. 3). At the same time, no major changes in the Borg dyspnea index were noted (4.86±1.68 before vs. 4.71±1.70 after treatment, p=0.773). Most of the patients remained within the same FC II (n=6, 85.7%), and one patient initially in FC III improved to FC II. Some decrease in the peak velocity of the tricuspid regurgitation, the parameter which correlates with the systolic pressure in the pulmonary artery from 66.9±25.3 mmHg to 61.6±25.9 mmHg without major changes in the diameter of the right ventricle were noted during echocardiographic study (Fig. 3). In general, sildenafil was well tolerated with one out of seven patients reporting headache of mild intensity during treatment.
Fig. 3.
Changes in 6MWT, echocardiographic indexes and NT-proBNP with sildenafil therapy. Note: tricuspid valve (TV) pressure gradient by echocardiography improved significantly after 12 weeks of sildenafil therapy. Also tendency to reduction of the right ventricle (RV) size was noted. Increase in the distance in 6-min walk test (6-MWT) was associated with the reduction in the NT-proBNP level. Data presented as mean±SD, *p<0.05 vs. baseline.
3.4. Changes in FAs composition and HNE with sildenafil therapy
Initially slightly elevated levels of free saturated and unsaturated FAs in PAH were further increasing reaching statistical significance with sildenafil therapy comparing to control (Table 2). Phospholipid derived saturated and unsaturated FAs tended to decrease over 12 weeks treatment period. The levels of oleic acid (18:1, cis-9) and LA (18:1, cis-9,12), which were initially elevated, lowered after treatment. Also significant reduction of DHA (22:6, cis- 4,7,10,13,16,19) and a tendency to decrease in EPA (20:5, cis-5,8,11,14,17) and DPA (22:5, cis- 7,10,13,16,19) were noted after sildenafil use (Table 2).
Initially elevated ω6/ω3 ratio further increased from 7.39±1.38 ng/mL to 7.67±1.69ng/mL with sildenafil therapy and, while high levels of 4-HNE lowered significantly after treatment, falling even below the values observed in control group (Fig. 1).
3.5. Changes in HRV with sildenafil therapy
HRV parameters in PAH improved after 12 weeks of sildenafil therapy. Time-domain indexes tended to increase but remained lower than in control group (Table 3). Significant increase in TP in supine position was noted but it was still lower comparing to control (Fig. 2). The treatment associated increase in TP in PAH patients was predominantly on account of the VLF power with its contribution to spectral structure 42% before vs. 56% after treatment. Orthostatic test also demonstrated increase in TP on account of the VLF power after sildenafil use but both autonomic components tended to decline further with LF power decreasing from 45% to 28% and HF power from 7% to 4% in spectral structure. At the same time no significant change in LF/HF ratios (3.7±2.2 before vs. 3.6±2.6 after in supine position and 8.0±4.5 before vs. 7.2±3.1 after in orthostasis) was noted.
4. Discussion
Despite major advances in our understanding of the PAH pathobiology the survival of the patients still remains unacceptably low, which substantiates further research in this area. Currently PAH is recognized as a multifactorial disease with metabolic reprogramming and inflammation and taking central stage [66]. Also OS, which is characterized by increased production of reactive oxygen and nitrogen species, uncoupling of eNOS and decreased NO bioavailability with the pathological activation of antiapoptotic and mitogenic pathways leading to cell proliferation and obliteration of the pulmonary vasculature plays an important role [62].
In this study we demonstrated signs of OS on PAH patients by increased level of HNE in blood (Fig. 1). HNE originates from the oxidation of the ω-6 polyunsaturated FAs, predominantly arachidonic acid, and is involved in the dose-dependent manner into the regulation of various cellular functions including proliferation, differentiation, apoptosis, cell cycle signaling, and modulation of the inflammatory pathways, etc. [7], [78]. The role of high levels of HNE in the pathogenesis of the vascular disease such atherosclerosis, diabetes, neurodegenerative disorders is well defined and its conjugates are frequently regarded as markers of OS [7]. The contribution of HNE to the pathogenesis of PAH may be assumed from its ability to reduce NO bioavailability via modulation of its enzymatic productiony. Elevation of peroxinitrite with reduced tetrahydrobiopterin (BH4) levels and uncoupled eNOS was shown in bovine aortic endothelial cells pretreated with HNE [72], [73]. In smooth muscle cells HNE was shown to reduce NO production through modulating gene expression and inhibiting transcriptional activation of inducible NO-synthase [27]. Deleterious effects exerted by large amount of HNE on the vasculature also include dysfunction of the endothelial barrier through modulation of the activities of proteins/enzymes by Michael adducts formation, impaired cell–cell communications, inhibition of membrane associated enzymes and development of the pro-inflammatory response [68], [7]. Thus, increased levels of HNE not only confirm presence of OS in PAH patients but also contribute to the pathologic remodeling of the pulmonary vasculature typical of the disease.
Early on, the role of FA and lipid metabolites in the development of PAH have mainly addressed changes in the eicosanoid profile [2], [61], [69], [70] and subsequently provided rationale for targeting the prostacyclin (PGI2) pathway [12]. Nowadays there is accumulating evidence on the role of FAs and their metabolism on the arginine–NOS–NO signaling [43], [56], [79]. Elevated plasma levels of free FAs were shown to modulate endothelium-dependent NO-production and to reduce eNOS activity due to an increase in the mitochondrial ROS production in cultured endothelial cells [26]. Oleic acid, which is abundantly found in human serum, was found to be elevated in PAH patients in present study (Table 2). Interestingly, it was shown to impair pulmonary arterial endothelium-dependent relaxation and endothelium-independent contraction in the canine lung injury model [34]. LA-the major ω6 acid found in western diet which is involved in the endothelial cell activation and potentiation of the inflammatory response was also increased in PAH patients. In the study of Saraswathi et al. exploring the mechanisms of proatherogenic action of LA in endothelial cells the increased Ca2+ and peroxynitrite signaling was postulated as an important pathogenetic mechanism of endothelial dysfunction [57]. Thus, contributing potential of LA to the development of obliterative pulmonary angiopathy, which is also characterized by inflammation [30], [54] may result from its proinflammatory action due to the altered NO signaling.
In present study we showed some increase in DPA and DHA in PAH patients. This can be regarded as a compensatory mechanism aimed to promote free radical production, induce antioxidant defense and diminish oxidative damage through prooxidant activity. An increase in PUFAs can also result in improved blood rheology, activation of peroxisomal oxidation and mitochondrial energy function. Supportive evidence of such speculation comes from the facts that levels of HNE and LA, both of which act as the ligands of PPARs receptors and stimulate peroxisomal function were found to be significantly increased in PAH (Fig. 1, Table 2). Recently, the major shift in the metabolic substrate preference with decreased uptake of FAs was demonstrated in the RV in rats with severe pulmonary hypertension [25]. In the model study NOS inhibition caused increase in the levels of arahidonic acid and DHA in the cell membranes due to the stimulation of Δ5 desaturases activity [16]. Whether described changes of the FAs composition contribute to the pulmonary vascular remodeling in PAH warrants further study.
More evidence which proves changes in aerobic metabolism in PAH patients comes from study of HRV, which was markedly reduced (Table 3, Fig. 2). During the past decades, data has supported the concept of a modulating effect of the aerobic metabolism on HRV [73]. Conditions that are known to be related to OS and inflammation such as ischemic heart disease, diabetes, heart failure were shown to be associated with the reduction of HRV [37], [38], [58]. Low HRV indexes in PAH patients in present study reflect not only alterations of the autonomic balance but also the depth of functional metabolic dysfunction witnessed by changes in biochemical parameters in blood. Moreover, changes in the autonomic indexes derived from assessment of HRV are believed to have independent predictive value on the long-term outcome in heart failure patients [38]. When taking into account that one of the most important clinical syndromes in PAH is right-sided heart failure study of HRV in these patients may be of particular value.
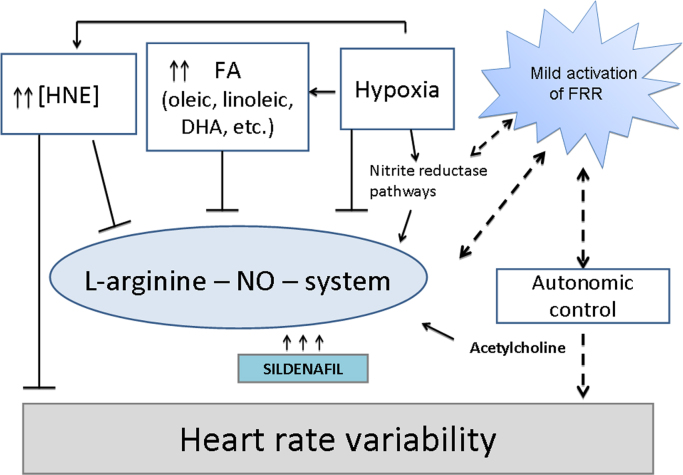
The complex interactions of the l-arginine-NO-system are presented in Fig. 4. Interestingly, that most of them are modulated by the flow of ROS. Overproduction of ROS results in reduction in NO bioavailability but their certain amounts are necessary to maintain physiological effects of the NO pathway. The level of acetylcholine, which is known to stimulate enzymatic NO production to the major extend is determined by the rate of alfa-ketoglutarate synthesis in mitochondria and, thus, their functionality [60], [77]. Therefore, optimal intensity of the redox reactions, which precludes maintenance of O2/ROS and ATP/ADP balances in mitochondria will promote functioning of the l-arginine-NO-system. These metabolic changes can be assessed in clinical practice by HRV, especially its autonomic components (HF, LF) in the spectral structure.
Fig. 4.
Metabolic interactions of the l-arginine-NO pathway during sildenafil therapy. The bioavailability of NO is influenced by a complex of factors. Oxidative stress, hypoxia, and reduced intensity of redox reactions are accompanied by increased levels of free fatty acids mostly oleic, linoleic, and docosahexanoic (DHA) with subsequent elevation of hydroxynonenal (HNE), which impairs the activity of endothelial NO synthase (eNOS) prompting NO deficiency state. Moreover, elevated levels of HNE contribute to depletion of tetrahydrobiopterin (BH4) and, thus, can mediate uncoupling and generation of superoxide [72]. Increased HNE is also associated with accumulation of the endogenous eNOS inhibitor asymmetric dimethylarginine [10]. Moreover, HNE was demonstrated to suppress iNOS and inhibit NO formation due to activation of Nrf-2 [22]. That notion is further supported by a study demonstrating HNE directly forming adducts with transcriptional inhibitor Keap-1[44]. Sildenafil improves NO signaling by blocking phosphodiesterase type 5 and increasing intracellular cGMP level leading to relaxation of smooth muscle cells, reducing vascular remodeling and vasoconstriction. At the same time, long term potentiation of l-arginine-NO-system by sildenafil may trigger hyperactivation of various cGMP-dependent signaling pathways, excessive prooxidant activity and exhaustion of antioxidant potential, which results in distorted feedback regulation. Such metabolic derangements are reflected by reduced heart rate variability and worsening of its spectral structure. The efficacy of long-term sildenafil therapy may be improved by concomitant stimulation of free radical reactions (FRR), maintenance of pO2 and development of mild prooxidant activity, which can be achieved with administration of intermittent hypoxic training, use of flavonoids or various supplements used as the sources of PUFAs (fish, plant oils, etc.).
Administration of sildenafil for 3 months resulted in significant clinical improvement and increase in the functional capacity of the PAH patients. It beneficially influenced pulmonary hymodynamics as suggested by echocardiographic evaluation and reduced the level of neurohormonal activation marker NT-proBNP. These findings are consistent with the data from the SUPER-1 clinical trial, demonstrating sildenafil to be able to increase the distance walked in six minute and reduce mean pulmonary artery pressure in PAH [19].
By blocking PDE type 5 sildenafil triggers multiple signaling pathways involved into regulation of inflammation, proliferation and apoptosis, and antioxidant defense determining the cross talk between key transcriptional pathways NFkB/Nrf2/HIF1 [29], [41], [49]. This notion is supported by the recent evidence from the model study showing upregulation of Nfr-2 after sildenafil administration [41]. Anti-inflammatory and antioxidant effects of sildenafil were confirmed in our work by decrease in HNE level which became even lower than in control after treatment. Decreased level of HNE may results from improved intensity of the redox reactions and mitochondrial function, which cause activation of the mitochondrial aldehyde dehydrogenase 2-a key enzyme in the detoxification of reactive aldehydes [[8], [24]] and, thus, promote cardioprotective effect of sildenafil.
Reduction of the OS signs was accompanied by decrease in unsaturated FAs, especially oleic, linoleic in phospholipids, which may be due to inhibition of lipid peroxidation or their better utilization for the maintenance of the redox balance. The level of DHA decreased significantly after sildenafil therapy which is suggestive about improved structural integrity of the membranes and better functionality of the membrane bound enzymes, receptors, and ion channels. Activity of mitochondrial K+ATP and BKCa channels is closely related to activation of NO/CO/H2S pathways [51], [52], [53], which was also described with sildenafil use [41]. The function of these mitochondrial membrane-bound channels is known to be coupled with the activity of the autonomic nervous system.
In present study administration of sildenafil caused increase in main time-domain index SDNN, which is informative about autonomic control in general, and both pNN50 and RMSSD, which reflect parasympathetic activity (Table 3). Significant increase in total spectral power after sildenafil use was accompanied by increase in all spectral components but predominantly VLF suggestive about neurohormonal activation without major changes in LF/HF balance. Similar tendencies were observed in orthostasis. Three month of sildenafil therapy did not restore HRV and, thus, did not provide complete recovery of the functional metabolic disorders in PAH. Increase in free FAs in blood after treatment may contribute to further inhibition of eNOS [26], [57] and confirms incomplete elimination of the oxidative damage, which was reflected by VLF predominance and persistent sympathetic activation in spectral structure of HRV.
It is worth noting that maintenance of NO flow only on account of the inhibition of PDE type 5 on the long run will result in overstimulation of PKG and associated various signaling cascades, which can lead to excessive production of NO and subsequent formation of peroxinitrite and OS persistence [65]. On the other hand, hypoxia and acidosis accompanying OS contribute to production of NO from the alternative sources, namely, nitrite reductase pathway involving a wide range of enzymes such as xanthine oxidase, cytochrome oxidase etc., which may further facilitate OS [35], [82]. In our opinion, such metabolic disturbances can be overcome through mild activation of free radical reactions, which will promote fluctuations of different ROS and oxygen [58], [74] and will help to eliminate hypoxia, signs of OS and to normalize function of the oxygen dependent enzymes, including NOS. Moreover, nonenzymetic production of NO in the NO/NO2/NO3 cycle can be activated. Such approach when applied to treatment is described as development of hormetic reaction, which can be achieved through many prooxidant influences [15], [40], [59], with the most well documented being PUFA supplementation.
The beneficial effects of the dietary omega 3 PUFAs on the cardiovascular health are well described [18], [33], [80], while the evidence of their role in PAH is rather limited. Recently, the anti-inflammatory potential of PUFAs was shown to contribute to reverse remodeling of pulmonary vasculature in the experimental studies. Morin et al. showed that administration of monoacylglyceride residues of DPA (22:5) lowered levels of arachidonic acid in blood and tissue samples and reduced NF-kB, IL-8 and p38 MAPK activation, thus, causing a decrease in pulmonary artery pressure and an improvement of the right ventricular hypertrophy in rat [46]. In the in vitro model of human pulmonary artery overactivity induced by endothelin-1, IL-6, TNF-α a trihydroxylated DHA derivative resolvin D1 was able to reverse hyperresponsiveness caused by proinflammatory treatments [30]. EPA and DHA have been reported to prevent pressure-overload induced cardiac fibrosis [9], which may be also of particular importance in PAH.
5. Conclusions
Nowadays, although PAH patients report clinical and hemodynamic improvement with sildenafil monotherapy the prognosis of the disease remains serious and search for the ways to improve treatment efficacy continues. PAH is increasingly recognized as a metabolic disease associated with OS [1], [31], [4], which was confirmed in present study. Patients with PAH were characterized by increased HNE in blood, changes in the FAs composition with high levels of saturated and unsaturated (oleic, LA, DHA) acids in phospholipids as well as reduced HRV with sympathetic predominance. Sildenafil improved clinical status and pulmonary hemodynamics in PAH subjects, reduced signs of OS, modulated FAs composition lowering oleic, LA, DPA, DHA in phospholipids, which is suggestive about its antioxidant and anti-inflammatory action. That was accompanied by some improvement of HRV indexes with increase in total spectral power on account of the neurohumoral regulatory component. At the same time, incomplete recovery of the functional metabolic disorders in PAH patients may be assumed from the persistent increase in free FAs in serum, reduced HRV with sympathetic predominance in the spectral structure after treatment comparing to control group. The efficacy of the sildenafil therapy may be improved through mild stimulation of redox reactions and formation of the hormetic response, which facilitates not only enzymatic but also nonenzymetic NO production. Providing options to increase the efficacy of PAH specific treatment would open new possibilities for the patients once described to live in “the kingdom on near death”.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
Several authors of this review were supported by the European Cooperation in Science and Technology (COST Action BM1203/EU‐ROS).
Biographies
Dr. Khrystyna Semen, MD, PhD, associate professor, Department of Propedeutics of Internal Medicine #2, Danylo Halystky Lviv National Medical University; Postal address: 69, Pekarska street, 79010, Lviv, Ukraine . Tel: +380 676702554; e-mail: khrystyna_semen@yahoo.com
Prof. Olha Yelisyeyeva, PhD, DSc in Biochemistry Department of Histology, Cytology & Embryology, Danylo Halystky Lviv National Medical University; Postal address: 69, Pekarska street, 79010, Lviv, Ukraine. Tel: +380 939833743; fax: +380 322603277; e-mail: yelisol@gmail.com
Dr. Iwona Jarocka-Karpowicz, Department of Analytical Chemistry, Medical University of Bialystok; Postal address: 2D, Mickiewicza steet, 15222, Bialystok, Poland Tel.: +48 85 748 5486; fax: +48 85 748 5707.
Dr. Danylo Kaminskyy, PhD, associate professor, Department of Pharmaceutical, Organic, and Bioorganic Chemistry, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine Postal address: 69, Pekarska street, 79010, Lviv, Ukraine. Tel: +380 67 738 04 71; email: dankaminskyy@gmail.com
Dr. Lyubomyr Solovey, MD, department chair Intensive Care Department #2, Lviv Regional Clinical Hospital; Postal address: 7 Chernigivska str., 79010, Lviv, Ukraine. Tel: +380 976535493; e-mail: lyusol@ukr.net
Prof., dr. Elżbieta Skrzydlewska, department chair Department of Analytical Chemistry, Medical University of Bialystok; Postal address: 2D, Mickiewicza steet, 15222, Bialystok, Poland Tel.: +48 85 748 5486; fax: +48 85 748 5707.; e-mail: skrzydle@umb.edu.pl
Prof., dr. Ostap Yavorskyi, MD, DSc, department chair Department of Propedeutics of Internal Medicine #2, Danylo Halystky Lviv National Medical University; Postal address: 69, Pekarska street, 79010, Lviv, Ukraine Phone: +380 677423605 email: yavors@mail.lviv.ua
Contributor Information
Khrystyna Semen, Email: khrystyna_semen@yahoo.com.
Olha Yelisyeyeva, Email: yelisol@gmail.com.
References
- 1.Aggarwal S., Gross C.M., Sharma S., Fineman J.R., Black S.M. Reactive oxygen species in pulmonary vascular remodeling. Compr. Physiol. 2013;3:1011–1034. doi: 10.1002/cphy.c120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Husseini A., Wijesinghe D.S., Farkas L., Kraskauskas D., Drake J.I., Van Tassel B., Abbate A., Chalfant C.E., Voelkel N.F. Increased eicosanoid levels in the sugen/chronic hypoxia model of severe pulmonary hypertension. Plos One. 2015;10:e0120157. doi: 10.1371/journal.pone.0120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balakumar P., Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic. Biol. Med. 2012;53:271–279. doi: 10.1016/j.freeradbiomed.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Barnes J., Dweik R.A. Is pulmonary hypertension a metabolic disease? Am. J. Respir. Crit. Care Med. 2014;190:973–975. doi: 10.1164/rccm.201409-1702ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benza R.L., Miller Dave P., Barst Robyn J., Badesch David B., Frost Adaani E., McGoon Michael D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. CHEST J. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 6.Can M.M., Kaymaz C., Pochi N., Aktimur T. Impact of pulmonary arterial hypertension and its therapy on indices of heart rate variability. Off. Publ. Med. Assoc. Zenica -Doboj. Cant. Bosnia Herzeg. 2013;10:249–253. [PubMed] [Google Scholar]
- 7.Chapple S.J., Cheng X., Mann G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1:319–331. doi: 10.1016/j.redox.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.-H., Lihan Sun, Mochly-Rosen Daria. Mitochondrial aldehyde dehydrogenase and cardiac diseases. Cardiovasc. Res. 2010;88:51–57. doi: 10.1093/cvr/cvq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J., Shearer G.C., Chen Q., Healy C.L., Beyer A.J., Nareddy V.B., Gerdes A.M., Harris W.S., O’Connell T.D., Wang D. Omega-3 fatty acids prevent pressure overload–induced cardiac fibrosis through activation of cyclic GMP/protein kinase G signaling in cardiac fibroblasts. Circulation. 2011;123:584–593. doi: 10.1161/CIRCULATIONAHA.110.971853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.L. Chen, J.-P. Zhou, D.-B. Kuang, J. Tang, Y.-J. Li, X.-P Chen, 4-HNE increases intracellular ADMA levels in cultured HUVECs: evidence for miR-21-dependent mechanisms, 2013. [DOI] [PMC free article] [PubMed]
- 11.Christie W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv. Lipid Methodol. 1993;2:e111. [Google Scholar]
- 12.Christman B.W. Lipid mediator dysregulation in primary pulmonary hypertension. CHEST J. 1998;114:205S–207S. doi: 10.1378/chest.114.3_supplement.205s. [DOI] [PubMed] [Google Scholar]
- 13.Cracowski J.-L., Degano B., Chabot F., Labarère J., Schwedhelm E., Monneret D., Iuliano L., Schwebel C., Chaouat A., Reynaud-Gaubert M. Independent association of urinary F2-isoprostanes with survival in pulmonary arterial hypertension. CHEST J. 2012;142:869–876. doi: 10.1378/chest.11-1267. [DOI] [PubMed] [Google Scholar]
- 14.Daiber A., Steffen Daub, Markus Bachschmid, Stefan Schildknecht, Matthias Oelze, Sebastian Steven, Patrick Schmidt, Alexandra Megner, Masayuki Wada, Tadashi Tanabe. Protein tyrosine nitration and thiol oxidation by peroxynitrite—Strategies to prevent these oxidative modifications. Int. J. Mol. Sci. 2013;14:7542–7570. doi: 10.3390/ijms14047542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrzyńska I., Szachowicz-Petelska B., Skrzydlewska E., Figaszewski Z. Effect of sweet grass (Hierochloe odorata) on the physico-chemical properties of liver cell membranes from rats intoxicated with ethanol. Environ. Toxicol. Pharmacol. 2013;35:247–253. doi: 10.1016/j.etap.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Dorrance A.M., Graham D., Dominiczak A., Fraser R. Inhibition of nitric oxide synthesis increases erythrocyte membrane fluidity and unsaturated fatty acid content. Am. J. Hypertens. 2000;13:1194–1202. doi: 10.1016/s0895-7061(00)01184-5. [DOI] [PubMed] [Google Scholar]
- 17.Esenabhalu V.E., Schaeffer G., Graier W.F. Free fatty acid overload attenuates Ca2+ signaling and NO production in endothelial cells. Antioxid. Redox Signal. 2003;5:147–153. doi: 10.1089/152308603764816505. [DOI] [PubMed] [Google Scholar]
- 18.Ferreri C., Chatgilialoglu C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert. Rev. Mol. Diagn. 2012;12:767–780. doi: 10.1586/erm.12.73. [DOI] [PubMed] [Google Scholar]
- 19.Galiè N., Ghofrani H.A., Torbicki A., Barst R.J., Rubin L.J., Badesch D., Fleming T., Parpia T., Burgess G., Branzi A. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 20.Galiè N., Hoeper M.M., Humbert M., Torbicki A., Vachiery J.-L., Barbera J.A., Beghetti M., Corris P., Gaine S., Gibbs J.S. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 21.Galiè N., Humbert Marc, Vachiery, Jean-Luc Gibbs, Simon, Lang Irene, Torbicki, Adam Simonneau, Gérald, Peacock Andrew, Noordegraaf Anton Vonk, Beghetti Maurice. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 22.Gatbonton-Schwager T.N., Sadhukhan Sushabhan, Zhang Guo-Fang, Letterio John J., Tochtrop Gregory P. Identification of a negative feedback loop in biological oxidant formation fegulated by 4-hydroxy-2-(E)-nonenal. Redox Biol. 2014;2:755–763. doi: 10.1016/j.redox.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghofrani H.A., Pepke-Zaba J., Barbera J.A., Channick R., Keogh A.M., Gomez-Sanchez M.A., Kneussl M., Grimminger F. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2004;43:S68–S72. doi: 10.1016/j.jacc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Gong D., Zhang Hao, Hu Shengshou. Mitochondrial aldehyde dehydrogenase 2 activation and cardioprotection. J. Mol. Cell. Cardiol. 2013;55:58–63. doi: 10.1016/j.yjmcc.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Graham B.B., Kumar R., Mickael C., Sanders L., Gebreab L., Huber K.M., Perez M., Smith-Jones P., Serkova N.J., Tuder R.M. Severe pulmonary hypertension is associated with altered right ventricle metabolic substrate uptake. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015;309:L435–L440. doi: 10.1152/ajplung.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gremmels H., Bevers L.M., Fledderus J.O., Braam B., van Zonneveld A.J., Verhaar M.C., Joles J.A. Oleic acid increases mitochondrial reactive oxygen species production and decreases endothelial nitric oxide synthase activity in cultured endothelial cells. Eur. J. Pharmacol. 2015;751:67–72. doi: 10.1016/j.ejphar.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hattori Y., Hattori S., Kasai K. 4-Hydroxynonenal prevents no production in vascular smooth muscle cells by inhibiting nuclear factor-κB–dependent transcriptional activation of inducible NO synthase. Arterioscler. Thromb. Vasc. Biol. 2001;21:1179–1183. doi: 10.1161/hq0701.092135. [DOI] [PubMed] [Google Scholar]
- 28.Heart R. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 29.Hemnes A.R., Zaiman A., Champion H.C. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008;294:L24–L33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 30.Hiram R., Rizcallah E., Sirois C., Sirois M., Morin C., Fortin S., Rousseau E. Resolvin D1 reverses reactivity and Ca2+ sensitivity induced by ET-1, TNF-α, and IL-6 in the human pulmonary artery. Am. J. Physiol.-Heart Circ. Physiol. 2014;307:H1547–H1558. doi: 10.1152/ajpheart.00452.2014. [DOI] [PubMed] [Google Scholar]
- 31.M. Humbert, H.-A. Ghofrani, The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax, 2015, thoraxjnl-2015-207170. [DOI] [PMC free article] [PubMed]
- 32.Irodova N., Lankin V.Z., Konovalova G.K., Kochetov A.G., Chazova I.E. Oxidative stress in patients with primary pulmonary hypertension. Bull. Exp. Biol. Med. 2002;133:580–582. doi: 10.1023/a:1020238026534. [DOI] [PubMed] [Google Scholar]
- 33.Jung U.J., Torrejon C., Tighe A.P., Deckelbaum R.J. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am. J. Clin. Nutr. 2008;87(Suppl.):2003S–2009S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 34.Kafi S.A., Scillia P., Mélot C., Gevenois P.A., Pagnamenta A., Naeije R. Abnormal pulmonary vascular tone in canine oleic acid lung injury. Crit. Care Med. 2002;30:1565–1569. doi: 10.1097/00003246-200207000-00028. [DOI] [PubMed] [Google Scholar]
- 35.S. Khambata Rayomand, M., Ahluwalia Amrita, “Repurposing” of xanthine oxidoreductase as a nitrite reductase: a new paradigm for therapeutic targeting in hypertension. ANTIOXIDANTS & REDOX SIGNALING, 2015. [DOI] [PubMed]
- 36.Kiss T., Kovacs K., Komocsi A., Tornyos A., Zalan P., Sumegi B., Gallyas F., Jr Novel mechanisms of sildenafil in pulmonary hypertension involving cytokines/chemokines, MAP kinases and Akt. Plos One. 2014;9:e104890. doi: 10.1371/journal.pone.0104890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Rovere M.T., Christensen J.H. The autonomic nervous system and cardiovascular disease: role of n-3 PUFAs. Vasc. Pharmacol. 2015;71:1–10. doi: 10.1016/j.vph.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 38.La Rovere M.T., Pinna G.D., Maestri R., Barlera S., Bernardinangeli M., Veniani M., Nicolosi G.L., Marchioli R., Tavazzi L. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI‐HF trial. Eur. J. Heart Fail. 2012;14:1410–1419. doi: 10.1093/eurjhf/hfs126. [DOI] [PubMed] [Google Scholar]
- 39.Lee C.-H., Lee S.-D., Ou H.-C., Lai S.-C., Cheng Y.-J. Eicosapentaenoic acid protects against palmitic acid-induced endothelial dysfunction via activation of the AMPK/eNOS pathway. Int. J. Mol. Sci. 2014;15:10334–10349. doi: 10.3390/ijms150610334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemmens K.J., Sthijns M.M., van der Vijgh W.J., Bast A., Haenen G.R. The antioxidant flavonoid monoHER provides efficient protection and induces the innate Nrf2 mediated adaptation in endothelial cells subjected to oxidative stress. PharmaNutrition. 2014;2:69–74. [Google Scholar]
- 41.Liu X-m Peyton K.J., Wang X., Durante W. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem. Pharmacol. 2012;84:1045–1054. doi: 10.1016/j.bcp.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maggiorini M. Prevention and treatment of high-altitude pulmonary edema. Prog. Cardiovasc. Dis. 2010;52:500–506. doi: 10.1016/j.pcad.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Matesanz N., Park G., McAllister H., Leahey W., Devine A., McVeigh G.E., Gardiner T.A., McDonald D.M. Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2010;51:6815. doi: 10.1167/iovs.10-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohan I.K., Das U. Effect of l-arginine-nitric oxide system on the metabolism of essential fatty acids in chemical-induced diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids. 2000;62:35–46. doi: 10.1054/plef.1999.0122. [DOI] [PubMed] [Google Scholar]
- 46.Morin C., Hiram R., Rousseau E., Blier P.U., Fortin S. Docosapentaenoic acid monoacylglyceride reduces inflammation and vascular remodeling in experimental pulmonary hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2014;307:H574–H586. doi: 10.1152/ajpheart.00814.2013. [DOI] [PubMed] [Google Scholar]
- 47.Musicki B., Bivalacqua T.J., Champion H.C., Burnett A.L. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J. Sex. Med. 2014;11:424–430. doi: 10.1111/jsm.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagendran J., Archer Stephen L., Soliman Daniel, Gurtu Vikram, Moudgil Rohit, Haromy Alois, Aubin Chantal St, Webster Linda, Rebeyka Ivan M., Ross David B. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 49.Park H.-S., Park J.-W., Kim H.-J., Choi C.W., Lee H.-J., Kim B.I., Chun Y.-S. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am. J. Respir. Cell. Mol. Biol. 2013;48:105–113. doi: 10.1165/rcmb.2012-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peacock A.J., Naeije R., Rubin L.J., editors. Pulmonary Circulation: Diseases and Their Treatment. CRC Press, Taylor & Francis; Boca Raton, FL, USA: 2011. [Google Scholar]
- 51.Peers C., Bauer C.C., Boyle J.P., Scragg J.L., Dallas M.L. Modulation of ion channels by hydrogen sulfide. Antioxid. Redox Signal. 2012;17:95–105. doi: 10.1089/ars.2011.4359. [DOI] [PubMed] [Google Scholar]
- 52.Prabhakar N.R., Semenza G.L. Gaseous messengers in oxygen sensing. J. Mol. Med. 2012;90:265–272. doi: 10.1007/s00109-012-0876-1. [DOI] [PubMed] [Google Scholar]
- 53.Pryor W.A., Houk K.N., Foote C.S., Fukuto J.M., Ignarro L.J., Squadrito G.L., Davies K.J.A. Free radical biology and medicine: it's a gas, man! Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R491–R511. doi: 10.1152/ajpregu.00614.2005. [DOI] [PubMed] [Google Scholar]
- 54.Rabinovitch M., Guignabert C., Humbert M., Nicolls M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin L.J., Badesch D.B., Fleming T.R., Galiè N., Simonneau G., Ghofrani H.A., Oakes M., Layton G., Serdarevic-Pehar M., McLaughlin V.V. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. CHEST J. 2011;140:1274–1283. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- 56.Rudolph V., Freeman B.A. Cardiovascular consequences when nitric oxide and lipid signaling converge. Circ. Res. 2009;105:511–522. doi: 10.1161/CIRCRESAHA.109.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saraswathi V., Wu G., Toborek M., Hennig B. Linoleic acid-induced endothelial activation role of calcium and peroxynitrite signaling. J. Lipid Res. 2004;45:794–804. doi: 10.1194/jlr.M300497-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Semen K., den Hartog G., Kaminskyy D., Sirota T., Maij N. Redox modulation by Amaranth oil in human lung fibroblasts. Nat. Prod. Chem. Res. 2013;2:2. [Google Scholar]
- 59.Skrzydlewska E., Sulkowski S., Koda M., Zalewski B., Kanczuga-Koda L., Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J. Gastroenterol.: WJG. 2005;11:403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starkov A.A. An update on the role of mitochondrial α-ketoglutarate dehydrogenase in oxidative stress. Mol. Cell. Neurosci. 2013;55:13–16. doi: 10.1016/j.mcn.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stenmark K.R., Morganroth M.L., Remigio L.K., Voelkel N.F., Murphy R.C., Henson P.M., Mathias M.M., Reeves J.T. Alveolar inflammation and arachidonate metabolism in monocrotaline-induced pulmonary hypertension. Am. J. Physiol.-Heart Circ. Physiol. 1985;248:H859–H866. doi: 10.1152/ajpheart.1985.248.6.H859. [DOI] [PubMed] [Google Scholar]
- 62.Tabima D.M., Frizzell Sheila, Gladwin Mark T. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radic. Biol. Med. 2012;52:1970–1986. doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taibi G., Carruba Giuseppe, Miceli Vitale, Cocciadiferro Letizia, Cucchiara Angela, Nicotra Concetta M.A. Sildenafil protects epithelial cell through the inhibition of xanthine oxidase and the impairment of ROS production. Free. Radic. Res. 2010;44:232–239. doi: 10.3109/10715760903431426. [DOI] [PubMed] [Google Scholar]
- 64.Tang Y., Li G. Chronic exposure to high fatty acids impedes receptor agonist-induced nitric oxide production and increments of cytosolic Ca2+ levels in endothelial cells. J. Mol. Endocrinol. 2011;47:315–326. doi: 10.1530/JME-11-0082. [DOI] [PubMed] [Google Scholar]
- 65.Thomas S.R., Witting P.K., Drummond G.R. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 66.R.M. Tuder, S.L. Archer, P. Dorfmüller, S.C.Erzurum, C. Guignabert, E. Michelakis, M. Rabinovitch, R. Schermuly, K.R.Stenmark and N.W. Morrell, Relevant issues in the pathology andpathobiology of pulmonary hypertension, J. Am. Coll. Cardiol. 62 (25), Suppl. D, D4-D12 [DOI] [PMC free article] [PubMed]
- 67.Tuder R.M., Cool Carlyne D., Geraci Mark W., Wang Jun, Abman Steven H., Wright Laurel, Badesch David, Voelkel Norbert F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 68.Usatyuk P.V., Natarajan V. Hydroxyalkenals and oxidized phospholipids modulation of endothelial cytoskeleton, focal adhesion and adherens junction proteins in regulating endothelial barrier function. Microvasc. Res. 2012;83:45–55. doi: 10.1016/j.mvr.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voelkel N.F., Chang S.W., McDonnell T.J., Westcott J.Y., Haynes J. Role of membrane lipids in the control of normal vascular tone. Am. Rev. Respir. Dis. 1987;136:214–217. doi: 10.1164/ajrccm/136.1.214. [DOI] [PubMed] [Google Scholar]
- 70.Walker B.R., Voelkel N.F., Reeves J.T. Pulmonary pressor response after prostaglandin synthesis inhibition in conscious dogs. J. Appl. Physiol. 1982;52:705–709. doi: 10.1152/jappl.1982.52.3.705. [DOI] [PubMed] [Google Scholar]
- 71.Wensel R., Jilek C., Dörr M., Francis D., Stadler H., Lange T., Blumberg F., Opitz C., Pfeifer M., Ewert R. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur. Respir. J. 2009;34:895–901. doi: 10.1183/09031936.00145708. [DOI] [PubMed] [Google Scholar]
- 72.Whitsett J., Picklo M.J., Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler. Thromb., Vasc. Biol. 2007;27:2340–2347. doi: 10.1161/ATVBAHA.107.153742. [DOI] [PubMed] [Google Scholar]
- 73.Yelisyeyeva O., Semen K., Ostrovska G., Kaminskyy D., Sirota T., Zarkovic N., Mazur D., Lutsyk O., Rybalchenko K., Bast A. The effect of Amaranth oil on monolayers of artificial lipids and hepatocyte plasma membranes with adrenalin-induced stress. Food Chem. 2014;147:152–159. doi: 10.1016/j.foodchem.2013.09.119. [DOI] [PubMed] [Google Scholar]
- 74.Yelisyeyeva O., Semen K., Zarkovic N., Kaminskyy D., Lutsyk O., Rybalchenko V. Activation of aerobic metabolism by Amaranth oil improves heart rate variability both in athletes and patients with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2012;118:47–57. doi: 10.3109/13813455.2012.659259. [DOI] [PubMed] [Google Scholar]
- 75.Yoshino K., Sano M., Fujita M., Tomita I. Formation of aliphatic aldehydes in rat plasma and liver due to vitamin E deficiency. Chem. Pharm. Bull. 1986;34:5184–5187. [PubMed] [Google Scholar]
- 76.Young C.N., Fisher J.P., Gallagher K.M., Whaley‐Connell A., Chaudhary K., Victor R.G., Thomas G.D., Fadel P.J. Inhibition of nitric oxide synthase evokes central sympatho-excitation in healthy humans. J. Physiol. 2009;587:4977–4986. doi: 10.1113/jphysiol.2009.177204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zakharchenko M.V., Zakharchenko A., Khunderyakova N., Tutukina M., Simonova M., Vasilieva A., Romanova O., Fedotcheva N., Litvinova E., Maevsky E. Burst of succinate dehydrogenase and α-ketoglutarate dehydrogenase activity in concert with the expression of genes coding for respiratory chain proteins underlies short-term beneficial physiological stress in mitochondria. Int. J. Biochem. Cell. Biol. 2013;45:190–200. doi: 10.1016/j.biocel.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Zarkovic N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003;24:281–291. doi: 10.1016/s0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S., Yang T., Xu X., Wang M., Zhong L., Yang Y., Zhai Z., Xiao F., Wang C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: a case control study. BMC Pulm. Med. 2015;15:50. doi: 10.1186/s12890-015-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng J., Huang T., Yu Y., Hu X., Yang B., Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012;15:725–737. doi: 10.1017/S1368980011002254. [DOI] [PubMed] [Google Scholar]
- 81.Zou M., Martin Christian, Ullrich Volker. Tyrosine nitration as a mechanism of selective inactivation of prostacyclin synthase by peroxynitrite. Biol. Chem. 1997;378:707–714. doi: 10.1515/bchm.1997.378.7.707. [DOI] [PubMed] [Google Scholar]
- 82.Zuckerbraun B.S., George P., Gladwin M.T. Nitrite in pulmonary arterial hypertension: therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc. Res. 2011;89:542–552. doi: 10.1093/cvr/cvq370. [DOI] [PMC free article] [PubMed] [Google Scholar]