Abstract
Oxidative stress is a hallmark of many liver diseases including viral and drug-induced hepatitis, ischemia-reperfusion injury, and non-alcoholic steatohepatitis. One of the consequences of oxidative stress in the liver is deregulation of Ca2+ homeostasis, resulting in a sustained elevation of the free cytosolic Ca2+ concentration ([Ca2+]c) in hepatocytes, which leads to irreversible cellular damage. Recently it has been shown that liver damage induced by paracetamol and subsequent oxidative stress is, in large part, mediated by Ca2+ entry through Transient Receptor Potential Melastatin 2 (TRPM2) channels. Involvement of TRPM2 channels in hepatocellular damage induced by oxidative stress makes TRPM2 a potential therapeutic target for treatment of a range of oxidative stress-related liver diseases. We report here the identification of curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), a natural plant-derived polyphenol in turmeric spice, as a novel inhibitor of TRPM2 channel. Presence of 5 µM curcumin in the incubation medium prevented the H2O2- and paracetamol-induced [Ca2+]c rise in rat hepatocytes. Furthermore, in patch clamping experiments incubation of hepatocytes with curcumin inhibited activation of TRPM2 current by intracellular ADPR with IC50 of approximately 50 nM. These findings enhance understanding of the actions of curcumin and suggest that the known hepatoprotective properties of curcumin are, at least in part, mediated through inhibition of TRPM2 channels.
Keywords: Curcumin, TRPM2 channel, Patch clamping, Oxidative stress, Hepatocytes
Graphical abstract
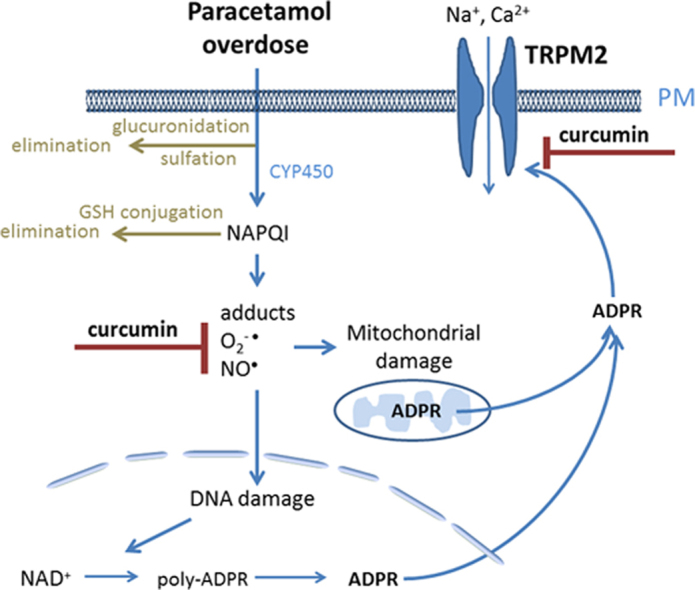
Highlights
-
•
Curcumin inhibits paracetamol- and H2O2- induced [Ca2+]c rise in rat hepatocytes.
-
•
Curcumin inhibits paracetamol- and H2O2-induced activation of TRPM2 current.
-
•
Curcumin inhibits activation of TRPM2 channels by intracellular ADP-ribose.
-
•
Liver-protective properties of curcumin are partly mediated through TRPM2 inhibition.
1. Introduction
The increased production of reactive oxygen and nitrogen species plays a central role in development of a number of liver disorders associated with hepatocellular death and impaired cell regeneration [1], [2], [3]. Liver injury induced by drug toxicity, ischemia-reperfusion, excessive alcohol consumption and viral hepatitis is mediated by oxidative stress. Curcumin, ((1E,6E)-1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione), a polyphenol found in the rhizome of Curcuma longa plant, has for centuries been used in traditional medicine in many Asian countries for treatment of liver disorders associated with oxidative stress [4]. Recent research have demonstrated that curcumin has a range of antioxidant and anti-inflammatory properties that can explain its hepatoprotective effects [5], [6], [7], [8]. Previously it has been shown that curcumin is very effective in preventing liver damage induced by paracetamol overdose, and that the level of protection afforded by curcumin against paracetamol-induced liver damage is comparable to that of N-acetyl cysteine (NAC), which is presently the main clinical treatment for paracetamol overdose in humans [9], [10]. It has been suggested that the beneficial effects of curcumin in paracetamol overdose are mainly mediated by its ability to scavenge free radicals, to induce expression of antioxidant enzymes and to inhibit NF-kB, the transcription factor involved in regulation of pro-inflammatory pathways [11]. However, our recent research into the role of Transient Receptor Potential Melastatin 2 (TRPM2) channels in oxidative stress-induced liver damage during paracetamol overdose suggests that some other mechanisms, unrelated to free radical scavenging, may also play an important role in paracetamol-induced liver toxicity [12].
One of the features of hepatocellular death mediated by oxidative stress is Ca2+ overload due the release of Ca2+ from intracellular organelles and activation of ion channels on the plasma membrane [12], [13]. We have shown that TRPM2 channels activated by intracellular ADPR are expressed in high numbers in rat and mouse hepatocytes and are responsible for H2O2- and paracetamol-induced persistent Ca2+ rise which causes hepatocellular damage [12]. Furthermore, experiments using TRPM2 KO mice have demonstrated that lack of TRPM2 channels significantly protects the liver from paracetamol-induced damage [12]. Contribution of TRPM2 channels to oxidative-stress induced cell damage makes them a potential therapeutic target for treatment of a range of oxidative stress-related diseases. There are several known inhibitors of TRPM2 channel, including anthranilic acid (ACA), clotrimazol, econazol, flufenamic acid (FF), and chlorpromazine [12], [14], [15], [16]. However, none of these agents can be used clinically to inhibit TRPM2 channels because the concentrations needed to achieve even partial block are beyond the safety margins. Here, using Ca2+ imaging and whole-cell patch clamping of primary rat hepatocytes and HEK293 cells heterologously expressing TRPM2 channels, we show that curcumin inhibits H2O2- and paracetamol-induced Ca2+ entry and the activation of TRPM2 channels by ADPR with IC50 of ~50 nM. The presented data suggests that curcumin is the most potent inhibitor of TRPM2 channels discovered so far.
2. Methods and materials
2.1. Chemicals
Paracetamol, ADPR, pleuronic acid, curcumin, BSA and NAC were purchased from Sigma-Aldrich (Rockville, Maryland, US). DMEM, penicillin/streptomycin, and trypsin-EGTA were purchased from GIBCO (Grand Island, New York, US). Fura2-AM was purchased from Invitrogen (Carlsbad, California, US). FBS was purchased from Bovogen (Melbourne, Australia). Tris and glycerin were purchased from Amresco (Solon, Ohio, US).
2.2. Animals
Hooded Wistar rats were housed and bred in the controlled environment with a 12-h light–dark cycle at least three weeks before beginning the experiments. Male rats aged 8–12 weeks were used for the experiments. All experiments were approved by the Animal Ethics Committees of the University of Adelaide and Flinders University of South Australia.
2.3. Solutions
Washing media for hepatocyte preparation (mM): 136 NaCl, 4.7 KCl, 0.85 Na2HPO4, 0.45 KH2PO4, 24 NaHCO3, 20 glucose, 1.3 CaCl2, 0.8 MgSO4, BSA (10% W/V), penicillin (100 U/ml), streptomycin (100 µg/ml) and phenol red (0.001% W/V). Krebs–Ringer–Hepes (KRH) solution (mM): 136 NaCl, 4.7 KCl, 1.3 CaCl2, 1.25 MgCl2, 10 glucose and 10 Na-HEPES with pH adjusted to 7.4 by NaOH. Control bath solution for patch clamping (mM): 140 NaCl, 4 CsCl, 2 CaCl2, 2 MgCl2 and 10 Na-HEPES, adjusted to pH 7.4 with NaOH. Pipette solution for patch clamping (mM): 130 caesium glutamate, 5 MgCl2, 5 CaCl2, 10 EGTA and 10 HEPES, adjusted to pH 7.3 with NaOH. Tris-buffered saline (TBS solution) (mM): 150 NaCl and 25 Tris with pH adjusted to 7.4 by HCl. TBST solution: TBS solution plus 0.1% Tween-20. Phosphate buffered saline (PBS) solution (mM): 137 NaCl, 2.7 KCl, 10 Na2HPO4 and 1.8 KH2PO4.
2.4. Hepatocyte isolation and culture
The isolated hepatocytes were prepared using in situ liver perfusion with collagenase as previously described [17]. The isolated hepatocytes were cultured on glass coverslips at 37 °C in 5% CO2 in air (v/v) in DMEM containing penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% FBS (v/v) for 16 to 96 hours before the experiments.
2.5. HEK 293T cell line culture and transfection
HEK 293T cells were cultured in 75 cm2 flasks at 37 °C in 5% CO2 in air (v/v) in DMEM containing penicillin (100 U/ml), streptomycin (100 µg/ml) and 10% FBS (v/v). Cells were harvested using PBS containing EGTA. The harvested cells were plated onto glass coverslips and 8 h later transfected with pCIneo expression vector containing human TRPM2 cDNA (generously provided by Professor Yasuo Mori, Japan) using TrueFect transfection reagent (United BioSystems Inc., US). Sixteen hours later, the transfected HEK 293T cells were used for the patch-clamp experiments.
2.6. Calcium imaging
Fura2-AM was dissolved in 5 µl of 20% pluronic acid in DMSO (w/v) and diluted in KRH buffer to the final concentration of 5 µM. Sixteen hours after plating on glass coverslips, the hepatocytes were loaded with Fura2-AM for 30 min, washed and incubated in KRH solution for 10 min in the CO2 incubator at 37 °C. The fluorescence of Fura-2 was recorded using a Nikon TE300 Eclipse microscope equipped with a Sutter DG-4/OF wavelength switcher, Omega XF04 filter set for Fura-2, Photonic Science ISIS-3 ICCD camera and UIC Metafluor software. Fluorescence images were obtained every 10 s using a 20×objective. Fluorescence ratio values (340/380 nm) were transformed to [Ca2+]c using the equation derived by Grynkiewicz, Poenie & Tsien, 1985 [18]; a Kd of 224 nM for binding of Fura-2 to Ca2+; and ionomycin and EGTA to determine Rmax and Rmin, respectively.
2.7. Patch-clamp recording
Membrane currents were measured at room temperature (23 °C) using standard patch clamping in a whole-cell mode, and a computer-based EPC-9 patch-clamp amplifier run by PULSE software as described previously [12]. In order to monitor the development of membrane currents, voltage ramps between −120 and +120 mV were applied every two seconds following the achievement of whole-cell configuration. The holding potential was −40 mV. The data were analyzed using PULSEFIT software. The TRPM2 current was activated by adding 0.1 mM or 1 mM ADPR to the pipette solution. Patch pipettes were pulled from borosilicate glass and fire-polished to a resistance between 1.5 and 2.5 MΩ. The series resistance did not exceed 7.5 MΩ and was 50% to 70% compensated.
2.8. Statistical analysis
Data are presented as means ± standard error of the mean (SEM). Statistical significance was determined using analysis of variance (ANOVA), followed by the Bonferroni post hoc test and Student’s t-test (two tailed).
3. Results
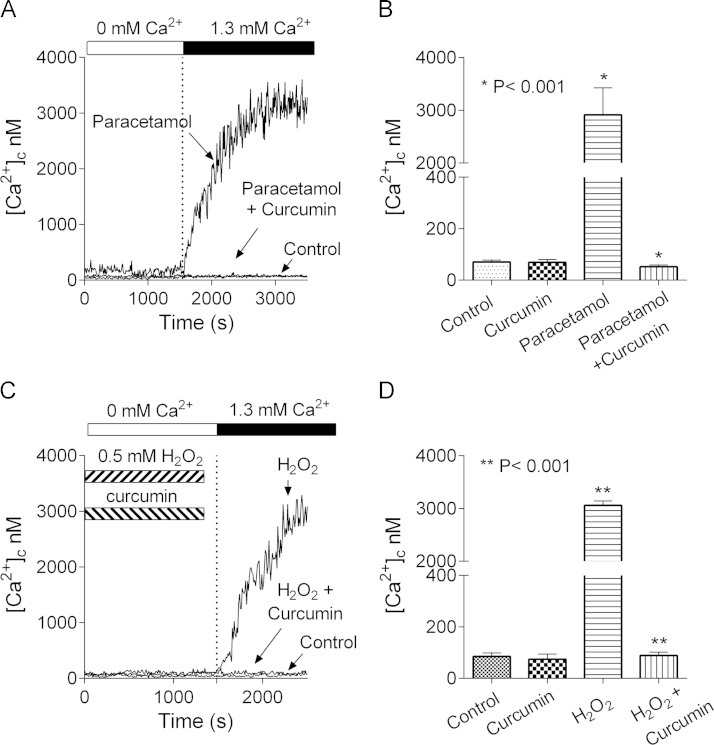
Previously, we have shown that inhibition of TRPM2 channels diminishes paracetamol- and H2O2-induced hepatocellular death in culture and that TRPM2 KO mice are significantly less susceptible to paracetamol-induced liver damage, compared to WT mice [12]. At the same, it has been demonstrated in rat and mouse that paracetamol toxicity can be ameliorated by intraperitoneal injection of curcumin prior to administration of paracetamol [8], [18]. To investigate whether liver-protective properties of curcumin are related, in any way, to TRPM2, first, we used Ca2+ imaging to ascertain the effects of curcumin on paracetamol-induced [Ca2+]c rise. Isolated rat hepatocytes plated on glass cover slips were incubated for 60 min with or without paracetamol (10 mM) in the presence or absence of 5 µM curcumin in KRH bath solution containing 1.3 mM Ca2+. Treated hepatocytes were then loaded with Fura-2AM in a nominally Ca2+ free KRH and, after washing, were transferred to the microscope stage. Introduction of 1.3 mM Ca2+ to the bath resulted in [Ca2+]c increase to levels above 3 µM in the paracetamol-treated hepatocytes incubated in the absence of curcumin (Fig. 1A and B). No such increase in [Ca2+]c was observed for cells incubated in the absence of paracetamol. These results indicate that, as shown previously [12], treatment with paracetamol activates Ca2+ entry across the plasma membrane through Ca2+ permeable channels (Fig. 1A and B). In contrast, hepatocytes treated with paracetamol in the presence of curcumin showed no change in [Ca2+]c (Fig. 1A and B).
Fig. 1.
Curcumin inhibits paracetamol- and H2O2-induced [Ca2+]c rise in rat hepatocytes. (A) Ca2+ entry activated in hepatocytes treated with paracetamol, 10 mM for 60 min, in the presence or absence of 5 µM curcumin. Control – untreated hepatocytes (B) Peak [Ca2+]c in the control, curcumin-, paracetamol-, and paracetamol- and curcumin-treated hepatocytes 15 min after introducing 1.3 mM Ca2+ to the bath. (C) Ca2+ entry activated in hepatocytes treated with 0.5 mM H2O2 in the presence or absence of 5 µM curcumin. (D) Peak [Ca2+]c in the control, curcumin-, H2O2-, and H2O2- and curcumin-treated hepatocytes 15 min after introducing 1.3 mM Ca2+ to the bath. [Ca2+]c was calculated using equation derived by Grynkiewicz, Poenie and Tsien (1985) [17].
Rat hepatocytes incubated in the presence of 0.5 mM H2O2 for 30 min, showed a large rise in [Ca2+]c after the introduction of 1.3 mM Ca2+ to the bath (Fig. 1C and D) [12]. Previous results obtained using TRPM2 KO mice and inhibitors of TRPM2 and patch clamp recording have provided evidence that H2O2-initiated increase in [Ca2+]c was almost entirely due to Ca2+ entry through TRPM2 channels [12]. This H2O2-induced increase in [Ca2+]c was inhibited by the inclusion of curcumin in the 30 min incubation period. Taken together, the Fura2 Ca2+ imaging results indicate that, at a concentration of 5 µM, curcumin completely inhibits the paracetamol- and H2O2-induced increases in [Ca2+]c. in hepatocytes.
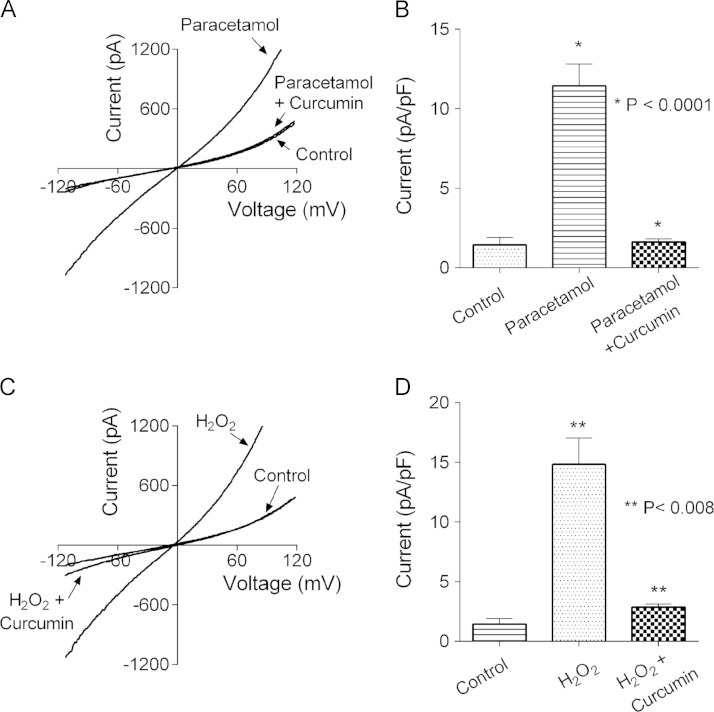
Whole-cell patch-clamp recordings confirmed the findings of Ca2+ imaging experiments (Fig. 2). As we showed previously, isolated rat hepatocytes treated with paracetamol for 60 min and then used for whole-cell patch clamping, exhibited a large non-selective cation current, mediated mostly by TRPM2 channels [12]. Addition of 5 µM curcumin into incubation medium together with paracetamol prevented development of any current above background conductance seen in untreated hepatocytes (Fig. 2A and B). Similarly, incubation with H2O2 (0.5 mM for 30 min) prior to achieving whole cell configuration resulted in a large non-selective cation current, which was absent if curcumin was added to the medium together with H2O2 (Fig. 2C and D).
Fig. 2.
Curcumin inhibits activation of a non-selective cation current in hepatocytes treated with paracetamol or H2O2. (A) Averaged current–voltage plots recorded in response to 100 ms voltage ramps between −120 and 120 mV in untreated hepatocytes (control, n=5), hepatocytes treated with 10 mM paracetamol for 60 min in control bath solution (paracetamol, n=5), and hepatocytes treated with 10 mM paracetamol and 5 µM curcumin for 60 min (paracetamol+curcumin, n=6). (B) The average amplitude of TRPM2-mediated Na+ current, obtained by replacing Na+ in the bath solution with NMDG+, in untreated hepatocytes (control) and hepatocytes treated with 10 mM paracetamol in the presence or absence of 5 µM curcumin for 60 min. (C) Averaged current–voltage plots recorded in response to 100 ms voltage ramps between −120 and 120 mV in untreated hepatocytes (control, n=5), hepatocytes treated with 0.5 mM H2O2 for 30 min (H2O2, n=5), and hepatocytes treated with 0.5 mM H2O2 in the presence of 5 µM curcumin for 30 min (H2O2+curcumin, n=5). (D) The average amplitude of TRPM2-mediated Na+ current, obtained by replacing NaCl in the bath solution with NMDGCl, in untreated hepatocytes (control) and hepatocytes treated with 0.5 mM H2O2 for 30 min, in the presence or absence of 5 µM curcumin. Standard errors in panels A and C are omitted for clarity.
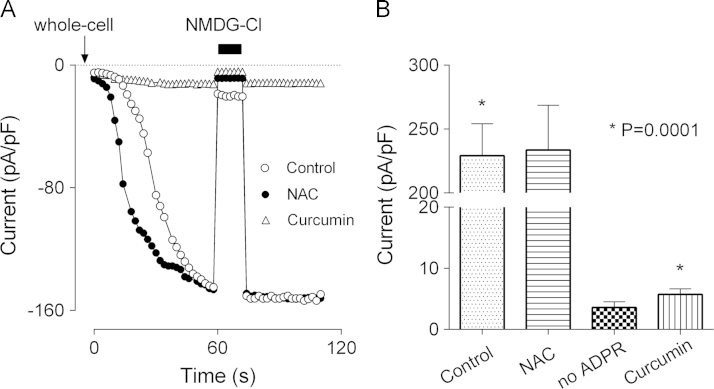
In the experiments described above curcumin was present in the medium during the treatments (H2O2 or paracetamol) that cause oxidative stress-induced cell damage. In both cases, curcumin could work as a ROS scavenger, reducing oxidative stress, and therefore, preventing the generation of ADPR and subsequent activation of TRPM2 channels. It is possible, however, that curcumin interacts with TRPM2 directly, as it does with some other channels, including TRPA1 and TRPV1 [19], [20], [21]. To investigate such a possibility, TRPM2 current was activated by intracellular application of 1 mM ADPR through a patch pipette using hepatocytes incubated with curcumin, 5 µM in the bath for 15–20 min. Whole-cell patch clamping showed that activation of TRPM2 current by ADPR was almost completely inhibited by curcumin (Fig. 3). It should be noted here that curcumin had an inhibitory effect on TRPM2 only if applied to the bath prior to achieving whole-cell configuration. If applied to the bath after the development of the current, curcumin had no effect on TRPM2, even at 50 µM concentration. For comparison, we investigated whether N-Acetyl-cysteine (NAC), which is used in therapy for paracetamol overdose, has any effect on TRPM2. In contrast to curcumin, NAC applied to the bath at 100 µM for up to 60 min before the patch clamping had no effect on activation of TRPM2 currents by ADPR (Fig. 3).
Fig. 3.
Curcumin, but not N-Acetylcysteine inhibits activation of TRPM2 current in hepatocytes by ADPR. (A) Development of TRPM2 current in response to 0.1 mM intracellular ADPR in untreated hepatocytes (control), hepatocytes treated with 5 µM curcumin for 15 min (curcumin), and hepatocytes treated with 100 µM NAC for 15 min (NAC). Current was recorded in response to 100 ms voltage ramps between −120 and 120 mV, applied every two seconds. Average current amplitude at −100 mV is plotted against time (n=6 for each condition). Standard errors are omitted for clarity. (B) The amplitude of the ADPR-activated TRPM2 current under conditions indicated on the X-axis, determined by replacing NaCl with NMDGCl in bath solution. The amplitude of Na+ current recorded in hepatocytes with no ADPR added to the pipette solution is shown for comparison (no ADPR).
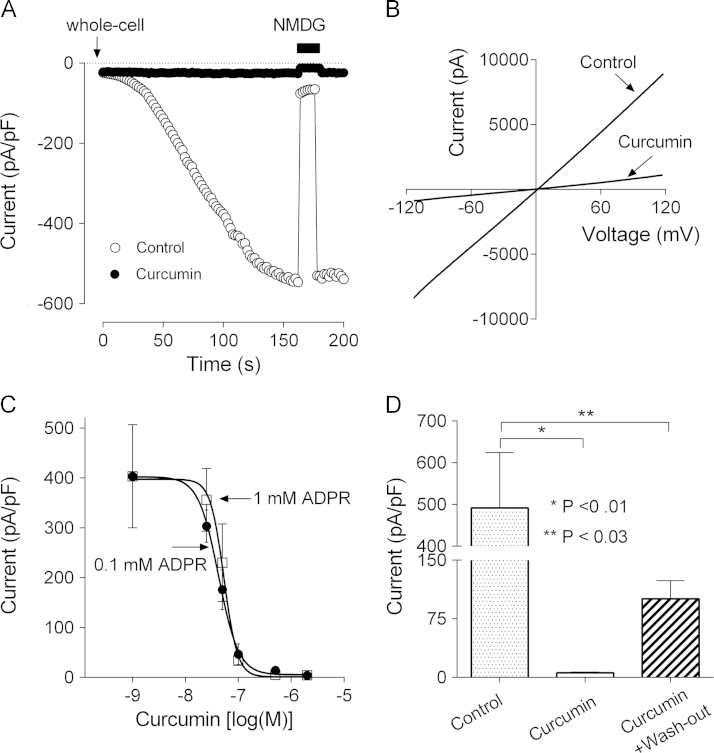
Lack of any blocking effect of curcumin on TRPM2 current after full current development suggests that curcumin does not block the TRPM2 pore, but affects the mechanism of channel activation by ADPR. One of the possibilities is that curcumin interferes with ADPR binding to the Nudix motif in the TRPM2 C-terminus. To gain some insight into this we used HEK293T cells transfected with hTRPM2 cDNA and whole cell patch clamping. Similarly to TRPM2 current in hepatocytes, current mediated by TRPM2 heterologously expressed in HEK293T cells was completely inhibited by 5 µM curcumin applied to the bath 15 min prior to patch clamping (Fig. 4A and B). To investigate whether curcumin competes with ADPR for the binding site on TRPM2, we obtained dose-response curves for curcumin at two ADPR concentrations (0.1 and 1 mM) (Fig. 4C). The IC50 values derived from the dose-response curves were not significantly different, 42±17 nM and 53±16 nM for 0.1 mM and 1 mM ADPR, respectively. This suggests that there is no competition between ADPR and curcumin.
Fig. 4.
Curcumin inhibits ADPR-mediated activation of TRPM2 channels heterologously expressed in HEK 293T cells. (A) Activation of TRPM2 current by 1 mM intracellular ADPR in TRPM2-transfected HEK 293T cells in normal bath solution (control), and after treatment with 5 µM curcumin for 15 min (curcumin). Current was recorded in response to 100 ms voltage ramps between −120 and 120 mV, applied every two seconds. Average current amplitude at −100 mV is plotted against time (n=6 for each condition). (B) Current–voltage plots of ADPR-activated current in TRPM2-transfected HEK 293T cells in normal bath solution (control) and cells treated with 5 µM curcumin for 15 min (curcumin). (C) The dose-response relations of curcumin inhibition of TRPM2 current in transfected HEK 293T cells at two different concentrations of ADPR in the patch pipette (0.1 and 1 mM). The amplitude of ADPR-activated current at −100 mV is plotted against curcumin concentrations (n=5 for each condition). The experimental data points are fitted with Hill equation with variable slope. The IC50 of curcumin block of ADPR-activated current were 42±17 nM and 53±16 nM for 0.1 and 1 mM ADPR, respectively. The Hill slope was 2.3 and 3.3 for 0.1 and 1 mM ADPR, respectively. (D) The wash-out the effect of curcumin on ADPR-activated current in TRPM2-expressing HEK 293T cells. The average amplitude of TRPM2 current measured at −100 mV in cells incubated in the normal bath solution (control), incubated with 2 µM curcumin for 15 min (curcumin), and incubated with 2 µM curcumin for 15-min and then washed out for 10 min (curcumin wash-out).
To determine the reversibility of inhibition of TRPM2 by curcumin, we conducted patch clamping experiments using HEK293T cells transfected with TRPM2 cDNA and attempted to remove curcumin from the bath by washing. However, we were unable to observe any change in the degree of inhibition before the patch was lost. Therefore, to determine the reversibility of inhibition of TRPM2 by curcumin, the transfected HEK293T cells were incubated with 2 µM curcumin for 15 min, then washed with normal bath media for 10 min and used for patch clamping with 1mM ADPR in the pipette solution. The amplitude of TRPM2 current in cells treated with and then washed of curcumin was 101±23 pA/pF, compared to 491±133 pA/pF in untreated cells, suggesting that curcumin had a long-lasting effect on TRPM2 activation (Fig. 4D).
4. Discussion
The results reported in this work indicate that curcumin inhibits development of TRPM2 current in response to oxidative damage or intracellular application of ADPR, but does not block active TRPM2 channels. Our previous studies have provided evidence that the mechanism by which TRPM2 is activated in hepatocytes by H2O2 or paracetamol involves the formation of ADPR in response to oxidative stress-induced DNA damage [12]. Binding of ADPR to Nudix-like domain in the C-terminus of TRPM2 leads to opening of TRPM2 channel pore and Na+ and Ca2+ entry (for review see [22]). In this study, we show for the first time that curcumin is an inhibitor of one or more steps in the pathway by which TRPM2 is activated by ADPR.
Curcumin, a component of turmeric spice, has been used in India and other countries in South-East Asia for medicinal purposes for centuries [23]. In recent years, it has been shown that curcumin is a free-radical scavenger and a powerful antioxidant, which possess anti-inflammatory, anti-carcinogenic, neuro-protective, liver-protective and many other properties, which make curcumin a potential therapeutic agent for treatment of a number of human diseases [6], [24], [25], [26], [27], [28], [29]. Measurements of free radical scavenging properties of curcumin and its analogues using cell-free assays (TEAC, FRAP, and ORAC) showed that ferulic acid, a curcumin monomer, is a more powerful free radical scavenger than curcumin itself. However, in cell culture studies, the beneficial antioxidant effects of curcumin are significantly stronger than those of ferulic acid [4]. This suggests that the medicinal properties of curcumin are mostly mediated through its interactions with the transcription factors and enzymes in the endogenous anti-oxidant and anti-inflammatory pathways, rather than just through free-radical scavenging mechanism [30].
Experiments using a number of animal models indicate that curcumin attenuates liver damage caused by ethanol, thioacetamide, CCl4, iron overdose, cholestasis, and paracetamol overdose [6], [8], [9], [31]. Curcumin can even reverse, to some extent, liver cirrhosis caused by chronic administration of CCl4 [31]. The exact molecular mechanisms of liver protective properties of curcumin remain to be established, however, its antioxidant activity and inhibition of NF-κB transcription factors and dependent pro-inflammatory pathways are likely to play a major role [7], [30]. The data presented in this study add inhibition of TRPM2 channels to the list of possible mechanisms of liver protection by curcumin. Our recent investigation of the mechanisms of paracetamol liver toxicity indicate that oxidative stress-induced activation of TRPM2 channels that mediate Ca2+ overload and loss of intracellular K+ constitutes a major pathway contributing to hepatocellular death [12]. In paracetamol overdose in vivo, curcumin may work as an antioxidant and free-radical scavenger, reducing damage to mitochondria and DNA, thus inhibiting production of ADPR, the main agonist of TRPM2. Such a mechanism is proposed for the protective effect of NAC, a sulfhydryl donor, which contributes to regeneration of GSH, against oxidative stress-induced cellular damage [32]. Pre-incubation of DRG neurons with NAC has been shown to inhibit activation of TRPM2 current by H2O2, most likely due to increased levels of GSH in the cells [32]. The patch clamping data presented in this study, however, indicate that NAC has no effect on activation of TRPM2 by ADPR. This suggests that a possible increase in GSH levels in hepatocytes in response to curcumin plays no role in the mechanism of inhibition of TRPM2 activation by ADPR. The mechanism of TRPM2 inhibition by curcumin is not yet clear; however, it does not involve a direct block of the pore, or competition of curcumin with ADPR for the binding site. A very slow wash-out of curcumin could be the evidence of strong binding, with slow off rate, of curcumin to TRPM2, or evidence of partitioning of curcumin into lipid membrane where it can interact with hydrophobic regions of TRPM2 protein. Alternatively, curcumin may cause TRPM2 modification through yet unknown pathway, which affects its function or localization.
Previously curcumin has been shown to inhibit a number of ion channels, including Kv1.3, Kv1.4, Kv11.1, TREK-1, and Ca2+ release activated Ca2+ (CRAC) channel [33], [34], [35]. However, the IC50 of curcumin for all these channels was 10–100 times higher than the IC50 of curcumin for TRPM2 found in this study (~50 nM). The mechanisms of inhibition of different types of channels by curcumin are also likely to be different, ranging from fast and easily reversible block of Kv1.4 channels [33] to slow and virtually irreversible block of TRPM2 activation found in this study.
5. Conclusions
In conclusion, the IC50 for curcumin (~50 nM) and even the concentration required for a complete block of TRPM2 (~1 µM) are well within the safety margins for its human consumption [27], [36], [37]. This makes curcumin a potential therapy for diseases where deleterious effects of oxidative stress, at least in part, are due to aberrant Ca2+ entry mediated by TRPM2 channels. Curcumin has already been suggested as a treatment for some neurodegenerative conditions, and TRPM2 channels are expressed in very high numbers in the brain and have been implicated in neurodegeneration [24], [26], [28]. It is possible that inhibition of TRPM2 channels, in addition to free radical scavenging, is the mechanism by which curcumin protects cells from oxidative stress-induced damage.
Acknowledgments
We thank Prof Yasuo Mori for providing TRPM2 cDNA. This work was supported by NHMRC, Australia (APP1086817).
References
- 1.Zhu R., Wang Y., Zhang L., Guo Q. Oxidative stress and liver disease. Hepatol. Res. 2012;42:741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 2.Roberts R.A., Laskin D.L., Smith C.V., Robertson F.M., Allen E.M.G., Doorn J.A. Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii H., Kurose I., Kato S. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J. Gastroenterol .Hepatol. 1997;12:S272–S282. doi: 10.1111/j.1440-1746.1997.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 4.Esatbeyoglu T., Huebbe P., Ernst I.M. a, Chin D., Wagner A.E., Rimbach G. Curcumin – from molecule to biological function. Angew. Chem. 2012;51:5308–5332. doi: 10.1002/anie.201107724. [DOI] [PubMed] [Google Scholar]
- 5.Bruck R., Ashkenazi M., Weiss S., Goldiner I., Shapiro H., Aeed H. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007;27:373–383. doi: 10.1111/j.1478-3231.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanji A.A., Jokelainen K., Tipoe G.L., Rahemtulla A., Thomas P., Dannenberg A.J. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G321–G327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 7.Manjunatha H., Srinivasan K. Hypolipidemic and antioxidant effects of dietary curcumin and capsaicin in induced hypercholesterolemic rats. Lipids. 2007;42:1133–1142. doi: 10.1007/s11745-007-3120-y. [DOI] [PubMed] [Google Scholar]
- 8.Messner D.J., Sivam G., Kowdley K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009;29:63–72. doi: 10.1111/j.1478-3231.2008.01793.x. doi:LIV1793 [pii]/r10.1111/j.1478-3231.2008.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kheradpezhouh E., Panjehshahin M.R., Miri R., Javidnia K., Noorafshan A., Monabati A. Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur. J. Pharmacol. 2010;628:274–281. doi: 10.1016/j.ejphar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Lauterburg B.H., Corcoran G.B., Mitchell J.R. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J. Clin. Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yousef M.I., Omar S.A.M., El-Guendi M.I., Abdelmegid L.A. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem. Toxicol. 2010;48:3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Kheradpezhouh E., Ma L., Morphett A., Barritt G.J., Rychkov G.Y. TRPM2 channels mediate acetaminophen-induced liver damage. Proc. Natl. Acad. Sci. USA. 2014;111:3176–3181. doi: 10.1073/pnas.1322657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egnatchik R.A., Leamy A.K., Jacobson D.A., Shiota M., Young J.D. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol. Metab. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill K., Benham C.D., McNulty S., Randall A.D. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Hill K., McNulty S., Randall A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn. Schmiedebergs. Arch. Pharmacol. 2004;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- 16.Bari M.R., Akbar S., Eweida M., Kühn F.J.P., Gustafsson A.J., Lückhoff A. H2O2-induced Ca2+ influx and its inhibition by N-(p-amylcinnamoyl) anthranilic acid in the β-cells: Involvement of TRPM2 channels. J. Cell. Mol. Med. 2009;13:3260–3267. doi: 10.1111/j.1582-4934.2009.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker J.C., Barritt G.J. Evidence that lanthanum ions stimulate calcium inflow to isolated hepatocytes. Biochem. J. 1981;200:109–114. doi: 10.1042/bj2000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. doi:3838314. [PubMed] [Google Scholar]
- 19.Leamy A.W., Shukla P., McAlexander M.A., Carr M.J., Ghatta S. Curcumin ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) activates and desensitizes the nociceptor ion channel TRPA1. Neurosci. Lett. 2011;503:157–162. doi: 10.1016/j.neulet.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 20.Martelli L., Ragazzi E., Di Mario F., Martelli M., Castagliuolo I., Maschio M. Dal. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol. Motil. 2007;19:668–674. doi: 10.1111/j.1365-2982.2007.00928.x. [DOI] [PubMed] [Google Scholar]
- 21.Yeon K.Y., Kim S.A., Kim Y.H., Lee M.K., Ahn D.K., Kim H.J. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J. Dent. Res. 2010;89:170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 22.Sumoza-Toledo A., Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J. Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal B.B., Sundaram C., Malani N., Ichikawa H. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y., Chen X., Chen Z., Zeng Y., Shi G., Su Y. Curcumin protects mitochondria from oxidative damage and attenuates apoptosis in cortical neurons. Acta Pharmacol. Sin. 2004;25:1606–1612. [PubMed] [Google Scholar]
- 25.Tomita M., Matsuda T., Kawakami H., Uchihara J.N., Okudaira T., Masuda M. Curcumin targets Akt cell survival signaling pathway in HTLV-I-infected T-cell lines. Cancer Sci. 2006;97:322–327. doi: 10.1111/j.1349-7006.2006.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhang L., Fiala M., Cashman J., Sayre J., Espinosa A., Mahanian M. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2006;10:1–7. doi: 10.3233/jad-2006-10101. [DOI] [PubMed] [Google Scholar]
- 27.Zuccotti G.V., Trabattoni D., Morelli M., Borgonovo S., Schneider L., Clerici M. Immune modulation by lactoferrin and curcumin in children with recurrent respiratory infections. J. Biol. Regul. Homeost. Agents. 2009;23:119–123. doi:9 [pii] [PubMed] [Google Scholar]
- 28.Yanagisawa D., Amatsubo T., Morikawa S., Taguchi H., Urushitani M., Shirai N. In vivo detection of amyloid β deposition using 19F magnetic resonance imaging with a 19F-containing curcumin derivative in a mouse model of Alzheimer’s disease. Neuroscience. 2011;184:120–127. doi: 10.1016/j.neuroscience.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 29.Zong H., Wang F., Fan Q., Wang L. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol. Biol. Rep. 2012;39:4803–4808. doi: 10.1007/s11033-011-1273-5. [DOI] [PubMed] [Google Scholar]
- 30.Shishodia S., Singh T., Chaturvedi M.M. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007;595:127–148. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-Gordillo K., Segoviab J., Shibayama M., Tsutsumic V., Vergara P., Moreno M.G. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: Role of TGF-β modulation and oxidative stress. Fundam. Clin. Pharmacol. 2008;22:417–427. doi: 10.1111/j.1472-8206.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 32.Özgül C., Naziroǧlu M. TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion. Physiol. Behav. 2012;106:122–128. doi: 10.1016/j.physbeh.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Liu H., Danthi S.J., Enyeart J.J. Curcumin potently blocks Kv1.4 potassium channels. Biochem. Biophys. Res. Commun. 2006;344:1161–1165. doi: 10.1016/j.bbrc.2006.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin D.H., Seo E.Y., Pang B., Nam J.H., Kim H.S., Kim W.K. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. J. Pharmacol. Sci. 2011;115:144–154. doi: 10.1254/jphs.10209FP. [DOI] [PubMed] [Google Scholar]
- 35.Choi S.W., Kim K.S., Shin D.H., Yoo H.Y., Choe H., Ko T.H. Class 3 inhibition of hERG K+ channel by caffeic acid phenethyl ester (CAPE) and curcumin. Pflugers Arch. Eur. J. Physiol. 2013;465:1121–1134. doi: 10.1007/s00424-013-1239-7. [DOI] [PubMed] [Google Scholar]
- 36.Sanmukhani J., Satodia V., Trivedi J., Patel T., Tiwari D., Panchal B. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother. Res. 2014;28:579–585. doi: 10.1002/ptr.5025. [DOI] [PubMed] [Google Scholar]
- 37.Kanai M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014;20:9384–9391. doi: 10.3748/wjg.v20.i28.9384. [DOI] [PMC free article] [PubMed] [Google Scholar]