SUMMARY
With the capacity to fine-tune protein expression via sequence-specific interactions, microRNAs (miRNAs) help regulate cell maintenance and differentiation. While some studies have also implicated miRNAs as regulators of the antiviral response, others have found that the RISC complex that facilitates miRNA-mediated silencing is rendered non-functional during cellular stress, including virus infection. To determine the global role of miRNAs in the cellular response to virus infection, we generated a vector that rapidly eliminates total cellular miRNA populations in terminally differentiated primary cultures. Loss of miRNAs has a negligible impact on both innate sensing of and immediate response to acute viral infection. In contrast, miRNA depletion specifically enhances cytokine expression, providing a post-translational mechanism for immune cell activation during cellular stress. This work highlights the physiological role of miRNAs during the antiviral response and suggests their contribution is limited to chronic infections and the acute activation of the adaptive immune response.
INTRODUCTION
The response to virus infection in chordates is largely mediated by a family of Type I interferon cytokines (IFN-I) that work to inhibit virus replication (tenOever, 2013). This stratified defense system is composed of core cellular effector proteins (intrinsic), a population of specialized hematopoietic cells that react non-specifically to pathogens (innate), and a multifaceted and highly specific antibody and cell-mediated response (adaptive). This is in contrast to many other defense strategies that solely rely on pathogen-derived small RNAs to combat infection (tenOever, 2013).
Despite our predominant usage of the protein-based stratified defense system, chordates do encode for small RNAs in the form of microRNAs (miRNAs). In an effort to comprehensively study the interplay of these miRNAs and virus infection, we developed a transformative tool that gives us the capacity to rapidly eliminate them in a wide range of terminally differentiated primary cells within hours of treatment. This tool utilizes a replication incompetent Adenovirus vector and the Vaccinia virus protein VP55, which, as we have previously reported, has the capacity to tail and degrade Ago-associated small RNAs (Backes et al., 2012). To address the role of post-transcriptional silencing in the host response to virus infection, we employed this vector to assess how loss of miRNAs impacted the intrinsic response to virus infection. Comprehensive transcriptome datasets clearly demonstrate that small RNAs do not, in any significant manner, contribute to the acute cellular response to virus or IFN signaling. In contrast, prolonged depletion of endogenous miRNAs induces a dramatic increase in pro-inflammatory cytokine production in both primary fibroblasts and in vivo. In all, we have defined stimulus-specific miRNA targetomes relating to the cellular response to virus infection and conclusively demonstrate that the role of small RNAs in this context is limited to linking the intrinsic and innate/adaptive immune responses through de-repression of diverse cytokines.
RESULTS
VP55-mediated degradation of endogenous miRNAs
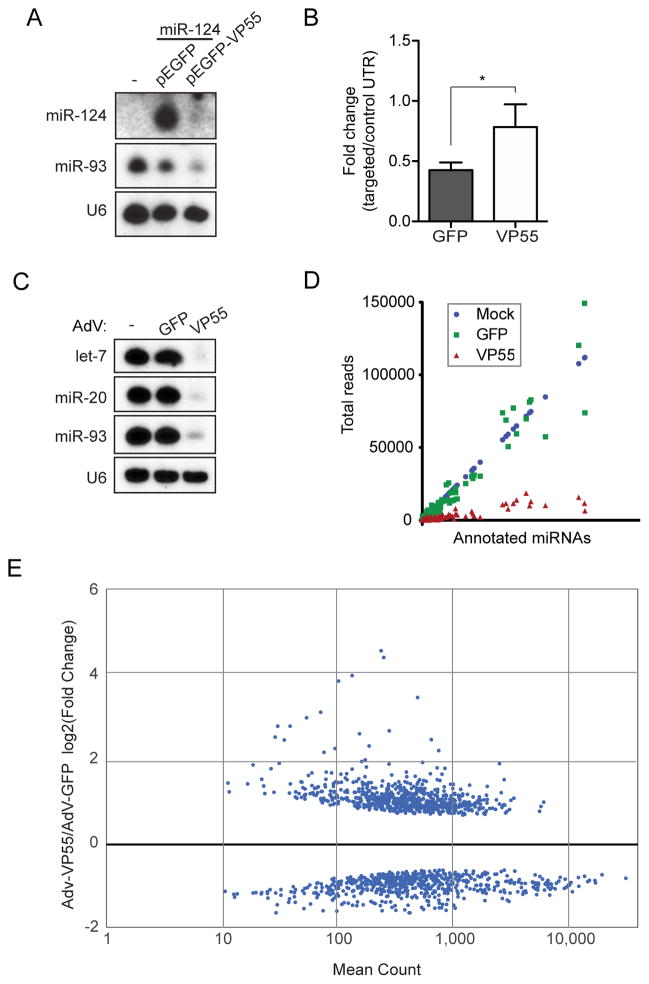
Small RNA profiling of poxvirus infected cells demonstrated that this virus family rapidly tailed and degraded miRNAs and this activity could be recapitulated with the expression of a single Vaccinia protein, VP55 (Backes et al., 2012). Further, the addition of VP55 to an unrelated RNA virus conferred the capacity to rapidly degrade both miRNAs and exogenous virus-derived siRNA (vsiRNA) mimetics (Backes, et al., 2014). Given the potential utility of rapid miRNA depletion, we chose to develop an inert expression system for VP55. First, we human codon optimized wild type VP55 and expressed it as an enhanced green fluorescent protein (EGFP) fusion (pEGFP-VP55). Transfection of pEGFP-VP55 demonstrated that ubiquitous, highly expressed miRNAs (ex. miR-93) as well as exogenous, overexpressed miRNAs (ex. miR-124) could both be degraded (Figure 1A). We next co-transfected cells with pEGFP-VP55 and a let-7 targeted luciferase reporter construct. These data found that luciferase expression was diminished by ~60% in the presence of pEGFP but showed no significant repression in the presence of pEGFP-VP55 (Figure 1B).
Figure 1. VP55-mediated degradation of endogenous miRNAs modulates cellular transcriptome.
(A) Small RNA northern blot of 293T cells co-transfected with a plasmid expressing miR-124 and either pEGFP or pEFGP-VP55. Total RNA was collected 24hrs post-transfection. (B) Gaussia luciferase reporter assay containing two perfect let-7 target sites or a control insert in the 3′UTR co-transfected with either pEGFP or pEGFP-VP55. Targeted constructs were normalized to control in each condition. Average of three independent experiments. Error bars denote ± SD. * p < 0.05 from one-tailed Student’s t test. (C) Small RNA northern blot of total RNA from 293T cells treated with either AdV-GFP or AdV-VP55 for 24hrs. (D) Small RNA deep sequencing of 293T cells mock, AdV-GFP, or AdV-VP55 treated for 24hrs. (E) Differentially expressed transcripts from mRNA-Seq of samples in (C) and (D). Graph depicts data from libraries generated from two biological replicates.
To ensure that VP55-mediated activity was specific for miRNAs, we next elucidated the transcriptome in pEGFP- and pEGFP-VP55-expresssing cells lacking endogenous miRNAs. A 293T cell clone in which genome editing was employed to disrupt Dicer expression (NoDice cells (Bogerd et al., 2014b)), was transfected with pEGFP or pEGFP-VP55 for 24hrs, assessed for protein expression (Figure S1A), then subsequently analyzed by mRNA-Seq. Of the 11,777 genes expressed in NoDice cells, we found that VP55 impacted less than 0.35% of the transcriptome (Table S1). Most noteworthy amongst this small subset of genes were histone cluster one transcripts that normally lack a poly A tail yet contain a consensus site for VP55, thus explaining their presence in a oligo dT-based RNAseq assay (Deng et al., 1997; Hentschel and Birnstiel).
Upon demonstrating that VP55 is sufficient for the rapid and specific degradation of miRNAs, we next incorporated the codon optimized EGFP-VP55 construct or EGFP alone as a control into a non-replicative Adenovirus 5 vector (AdV-VP55 or AdV-GFP, respectively). VP55 expression from this vector was sufficient to rapidly degrade the most abundant miRNAs in immortalized fibroblasts (Figure 1C and S1B). Characterization of its activity revealed that miRNA degradation was visible as early as 16hrs (Figure S1C). Furthermore, small RNA sequencing from AdV-GFP or –VP55 –treated cells demonstrated that degradation was uniform upon VP55 treatment (Figure 1D and Table S1). The most abundant miRNAs, comprising more than 50% of the total miRNAs in these cells, were all reduced by ~90% in the presence of VP55 (Table S1). Importantly, we also found that AdV-GFP treatment had no impact on endogenous miRNA biogenesis – a phenotype previously reported for replication competent Adenovirus (Figure 1D and Table S1) (Lu et al., 2004).
In an effort to ascertain the overall impact loss of miRNAs would have in a specific cellular environment, we first determined the impact of AdV-GFP treatment alone. We administered AdV-GFP to immortalized fibroblasts and compared the transcriptome to that of mock treated cells at 24hrs post infection (Table S1). These data revealed only 48 differentially expressed genes between these two cellular cohorts, none of which were known ISGs or components of the intrinsic response to virus infection (Rusinova et al., 2013). Based on these results, we conclude that the AdV vector platform does not elicit an antiviral response and therefore could serve as a tool to study miRNA function in this context.
We next sought to define all of the endogenous transcripts that are impacted by miRNAs by performing mRNA-Seq on AdV-GFP or –VP55 treated fibroblasts. After 24hrs of treatment, we found ~10% of host genes were differentially regulated as a result of VP55 expression (1346 of 13185 transcripts) (Figure 1E and Table S1). Upregulation of the most differentially expressed genes were confirmed by qPCR (Figure S1D). These data suggest that AdV-VP55 can effectively relieve miRNA-mediated control and reveal the extent to which miRNAs can influence transcript levels. Lastly, to ensure that any results obtained from our vectors were not due to toxicity, we also administered our vectors to fibroblasts with increasing inoculate sizes and determined viability by CellTiter Glo analysis (Figure S1E). Even at the highest dose, neither vector showed signs of cellular toxicity. In all, these data demonstrate that our AdV-VP55 vector specifically disrupts miRNA stability and thus provides a unique tool to study their biology.
Defining miRNA function during the intrinsic response to acute virus infection
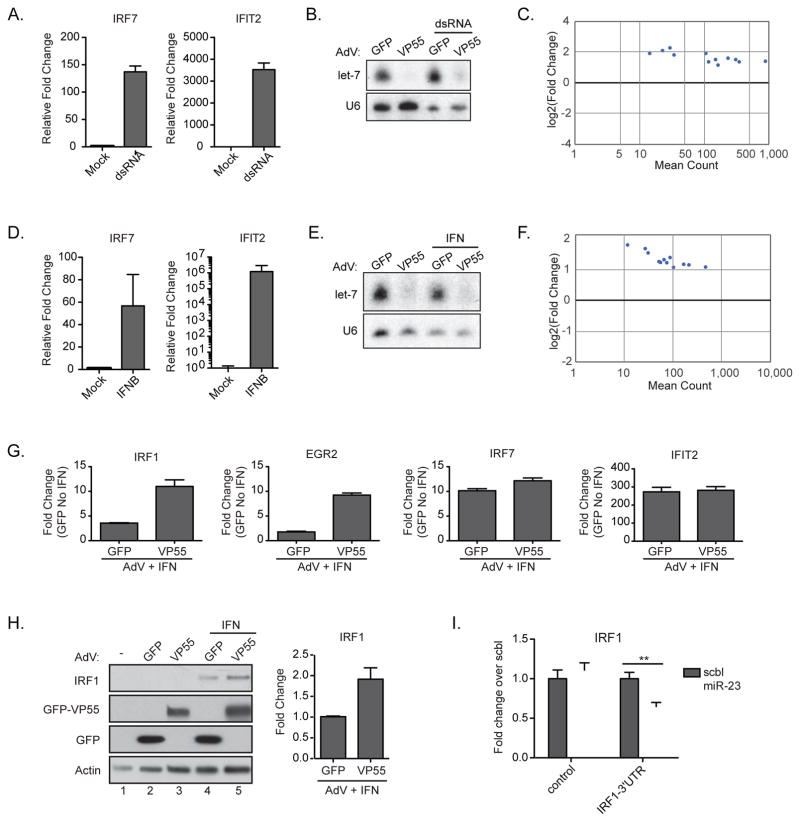
The contribution of miRNAs in the antiviral response is controversial (Cullen, 2006). While some have found that miRNAs had no impact on virus replication (Bogerd et al., 2014a), other reports claim that miRNAs can be directly antiviral and are responsible for as much as 50% of the antiviral activity of IFN-I (Otsuka et al., 2007; Pedersen et al., 2007). In an effort to address the contribution of miRNAs in response to virus or the IFN-I response, we applied our AdV-VP55 tool to primary cell models both ex vivo and in vivo to better reflect the physiological response to virus infection. As such, we used low passage human foreskin fibroblasts (BJ) which we first characterized for their capacity to respond to transfected poly(I:C), a mimetic of viral double strand RNA (dsRNA). Quantitative RT-PCR (qPCR) analysis of treated cells demonstrated robust induction of both IRF7 and IFN-induced protein with tetratricopeptide repeats 2 (IFIT2) (Figure 2A). To ascertain whether miRNAs impact the immediate response to virus infection, we next treated these cells with either AdV-GFP or –VP55 for 24hrs prior to stimulation with dsRNA for 6hrs to induce this intrinsic response to virus infection in the complete absence of miRNAs (Figure 2B). These rapid kinetics were chosen to best reflect the intrinsic response to virus infection prior to cell death, as dsRNA transfection mimics a time during infection when replication would be at its highest level. Control cells treated with AdV-GFP and dsRNA demonstrated a robust response of 1548 differentially expressed genes, defined by a minimum two-fold induction over mock and a statistical p value of less than 0.05 (Table S2). Strikingly, this same stimulation in the absence of miRNAs only statistically impacted 12 of the 1548 genes (Figure 2C and Table S2). The mRNA-Seq results were corroborated by qPCR (Figure S2). This suggests that miRNA-mediated repression during the response to PAMP detection is unlikely to be a physiological contributor to the intrinsic response to a replicating virus.
Figure 2. Depletion of miRNAs does not modulate the intrinsic response to virus.
(A) qPCR of RNA from BJ cells treated with poly(I:C) for 6hrs. All samples were normalized to tubulin. (B) Small RNA northern blot of BJ cells treated with AdV-GFP or AdV-VP55 for 24 hrs prior to transfection with dsRNA. Total RNA collected 6hrs post-transfection. (C) Differentially expressed transcripts with a greater than 2-fold induction from mRNA-Seq of samples in (B). Statistical analysis was performed on 2 biological replicates per condition. (D) qPCR of RNA from BJ cells treated with IFNβ for 6 hrs. All samples were normalized to tubulin. (E) Small RNA northern blot of BJ cells treated with AdV-GFP or AdV-VP55 for 24hrs alone or prior treatment with IFNβ for 6hrs. (F) Differentially expressed transcripts with a greater than 2-fold induction from mRNA-Seq of samples in (E). Analysis was performed on two biological replicates per condition. (G) qPCR validation of selected genes from (F). All samples were normalized to tubulin. Fold change over samples treated with AdV-GFP alone. (H) Western blot (left) and quantification (right) of BJ cells treated with AdV-GFP or AdV-VP55 as described in (E). IRF1 levels were normalized to actin. Fold change over AdV-GFP+IFN. Average of two independent experiments. (I) Gaussia luciferase reporter assay containing either the 3′UTR of IRF1 or the 3′UTR with mutated putative miR-23 target site. NoDice cells were co-transfected with indicated reporter and either a miR-23 or scrambled miRNA mimetic. ** p < 0.005 from a one-tailed Student’s t test. Representative of 3 independent experiments. For all graphs, Error bars denote ± SD.
Defining miRNA function during the response to IFN-I
While the transcriptional profile following dsRNA transfection demonstrated few genes impacted by the loss of miRNAs, this could be due to the length of time required for miRNAs to function and the rapid, often cytotoxic, response elicited by dsRNA. Therefore, we next chose to focus on IFN-I treated cells. IFN-I signaling is not associated with cytopathic effects in isolation and, as such, may be more substantially influenced by miRNA activity (Palombella and Maniatis, 1992). To first ensure our primary cells responded to IFN-I, we administered saturating levels of recombinant IFNβ and analyzed total RNA by qPCR. Comparable to dsRNA treatment, robust induction of IRF7 and IFIT2 was observed (Figure 2D). Based on these results, we again treated cells with AdV-GFP or AdV-VP55 for 24hrs prior to an acute 6hr treatment with IFNβ. VP55-mediated loss of host miRNAs was confirmed by small RNA northern blot for let-7 (Figure 2E). Libraries for mRNA-Seq were then generated. This data revealed that IFNβ treatment, similar to dsRNA, induced a robust transcriptional response to the stimuli resulting in 179 statistically significant genes induced by more than two fold (Table S2). Surprisingly, the lack of miRNAs again had a minimal impact on modulating the ISG profile with only 12 genes showing a significant change in levels, most notably IRF1 (Figure 2F and Table S2). The mRNA-Seq results were corroborated at the level of both RNA (Figure 2G) and protein (Figure 2H). miRNA-mediated regulation was also confirmed by reporter assay in which the 3′ UTR of IRF1 could render a Gausia luciferase reporter susceptible to miR-23 targeting, consistent with previous implications in the silencing of this transcript (Figure 2I) (Liu et al., 2013). Moreover, it should be noted that transcripts found to be differentially regulated by miRNAs were only impacted ~2–4 fold. Prolonged IFN-I stimulation (24hrs) increased the differentially regulated genes to 78, but again the genes impacted by the loss of miRNAs did not include central mediators in the IFN-I response (Table S2). Taken together, the datasets for both PAMP detection and IFN-I signaling strongly suggest that miRNA function demands time to result in significant changes to the host transcriptome.
Defining the kinetics of miRNA-mediated modulation of the host transcriptome
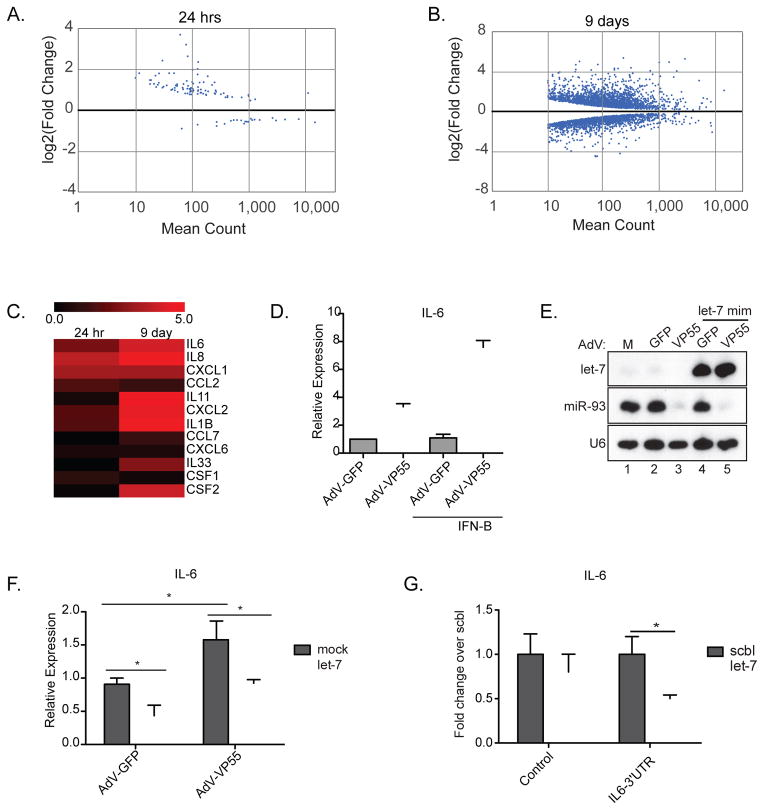
Given that miRNA depletion had a greater impact on the host following prolonged periods of IFN-I stimulation (6hrs vs. 24hrs), we examined the impact of miRNA depletion in the absence of virus-related signaling to define what genes might be impacted should an infected cell persist beyond the acute phase. As the resolution of mRNA-Seq allows us to detect low levels of canonical ISGs even in the absence of infection (e.g. MX1), we felt this strategy would be the most comprehensive way to define any possible antiviral miRNA targetome. To this end, we compared VP55-mediated loss of miRNA silencing at either one or nine days post-AdV administration by mRNA-Seq. Interestingly, this approach clearly revealed the necessity for prolonged miRNA activity in order to influence the host transriptome. At 24hrs, 49 genes were significantly induced two fold or more as a result of the loss of miRNA signaling (Figure 3A, S3A and Table S3). This phenotype was further enhanced when the lack of posttranscriptional regulation was extended to nine days (1058 genes with at least a two fold increase, 699 with at least a two fold decrease) illustrating the potential impact miRNAs can impart on the transcriptome of a cell (Figure 3B, S3B and Table S3).
Figure 3. Loss of miRNA silencing directly alters cellular cytokine production.
(A) Differentially expressed transcripts from mRNA-Seq of BJ cells treated for 24hrs with AdV-GFP or AdV-VP55. Analysis was performed on two biological replicate samples per condition. (B) Differentially expressed transcripts from mRNA-Seq of BJ cells treated for 9 days with AdV-GFP or AdV-VP55. Analysis was performed on two biological replicate samples per condition. (C) Heat map depicting log2 fold induction over AdV-GFP of cytokines differentially expressed in at least one of the indicated time points (in A or B). (D) qPCR of IL6 expression in BJ fibroblasts treated with AdV-GFP or AdV-VP55 for 24hrs alone or prior to treatment with IFNβ for 6hrs. Graph depicts relative expression over AdV-GFP. (E) Small RNA northern blot of RNA from 293T cells mock treated or transfected with protected let-7f miRNA mimetic 4hrs prior to treatment with AdV-GFP or AdV-VP55. Total RNA was collected 24hrs post-transfection. (F) BJ cells transfected with a let-7 mimetic for 1hr then infected with indicated AdV. Total RNA was collected 24hrs post-infection and analyzed by qPCR for IL6 expression. All samples were normalized to tubulin. Average of 3 independent experiments. * p < 0.05 from a one-tailed Student’s t test. (G) Gaussia luciferase reporter assay utilizing either the 3′UTR of IL6 or the 3’UTR with mutated putative let-7 target site. NoDice cells co-transefected with indicated reporter and either a let-7 or scrambled miRNA mimetic. * p < 0.05 from a one-tailed Student’s t test. Representative of three independent experiments. For all graphs, Error bars denote ± SD.
While the sustained loss of miRNAs impacted a substantial number of transcripts, many genes spanning a number of biological processes, including metabolism and mRNA maturation, remained unaltered even at nine days post treatment. It is also noteworthy that many of the central players in the host response to virus infection were unvarying in the prolonged absence of miRNAs. Included amongst the genes that were not significantly impacted were IFIH1, IRF3, IRF7, RELA, RELB, IFNβ, IFNAR1, STAT2, and IRF9 (Table S3). Exceptions to this included RIG-I, IRF1, and STAT1 whose levels all changed in a significant, albeit modest, level in response to sustained periods of miRNA depletion.
Despite the lack of major changes in RNA levels of the components of the intrinsic antiviral defense, we found that loss of miRNA function had a significant impact on an expansive list of chemokines that were notably not present in our NoDice data (Table S1 and S3). Amongst the list of miRNA-targeted chemokines were those responsible for the recruitment of antigen presenting cells (APCs) and neutrophils (IL8, CXCL1, CXCL2, CXCL6, CCL2, CCL7), immune regulators of hematopoeisis (IL1B, IL11, IL33, CSF1, and CSF2), and pro-inflammatory cytokines, including interleukin 6 (IL6) (Figure 3C and Table S3). It should be noted that IL6 was also found to be induced six fold following miRNA depletion in response to 24hrs of IFN-I stimulation (Table S2). This was confirmed by qPCR following administration of AdV-VP55 in the absence or presence of IFN-I (Figure 3D). We next sought to determine whether introduction of a single miRNA could restore the post-transcriptional silencing of IL6. To this end, we chose to introduce let-7, which had been previously implicated in regulating IL6 levels (Iliopoulos et al., 2009). In agreement with our previous work (Backes et al., 2012), transfection of a let-7 mimetic containing methyl groups on the 2′ hydroxyl ends of the ribose moieties, resulted in robust expression even in the presence of VP55 (Figure 3E). qPCR for IL6 under these conditions confirmed that let-7 was a significant contributor to transcript expression (Figure 3F). Additionally, the presence of a functional let-7 target site in the 3′UTR of IL6 was confirmed by Gaussia luciferase reporter assay (Figure 3G). In all, these data illustrate the importance of miRNA function over extended periods of time and suggest they may exert a constitutive level of repression on a diverse group of cytokines.
Cytokine expression as a result of in vivo loss of miRNA silencing
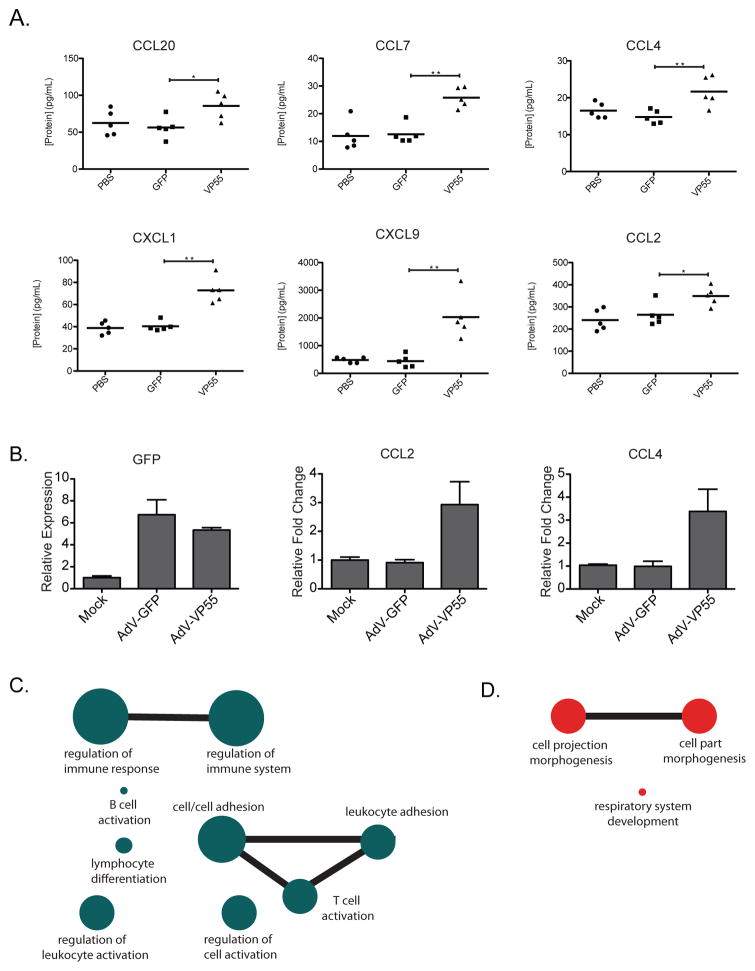
Upon determining that loss of miRNA regulation resulted in the selective induction of cytokines, we next wanted to determine if this was evident in vivo. We thus administered vehicle (PBS), AdV-GFP, or AdV-VP55 intranasally to mice and analyzed whole lung tissue for cytokine production at 48hrs post treatment (Figure 4A). Using a Luminex-based assay, we found six cytokines that were significantly increased in response to VP55 expression and twelve that remained unchanged (Figure 4A and Figure S4). It is noteworthy that the upregulated chemokines could collectively recruit lymphocytes, APCs, and neutrophils to the site of infection and have all been predicted to be subjects of miRNA targeting (Bystry et al., 2001; Carr et al., 1994; Girkin et al., 2015; Hartmann et al., 2015; Hieshima et al., 1997; Kwak et al., 2005; Moser et al., 1990).
Figure 4. Loss of miRNA silencing induces cytokine expression in vivo.
(A) Mice were mock treated or transduced with 2.5×108 pfu of either AdV-GFP or AdV-VP55 for 48hrs. Cytokine concentrations from homogenized whole lung tissue are indicated. Significance was determined using a one-tailed Student’s t test, * p < 0.05 and ** p < 0.005. n=5 mice per cohort. (B) qPCR of RNA from lungs in (A) for indicated genes. All samples normalized to tubulin. Fold change over mock-treated cohort. (C) Diagram depicting significantly enriched gene ontology categories of the 139 differentially expressed genes from (A) that are induced by two or more log2 fold change. Connecting lines denote 25 or more shared genes between ontology categories. Circle size represents the number of genes in each indicated category. (D) Diagram as described in (C) for the 138 differentially expressed genes decreased by two or more log2 fold change.
To corroborate the observed increased cytokine expression and to ascertain whether increased protein expression was the result of enhanced mRNA stability or translation, we performed qPCR on the lungs of mice described above. GFP levels confirmed comparable AdV treatments between animal cohorts (Figure 4B). Interestingly, at the level of mRNA, we observe two patterns of cytokine expression. While qPCR confirmed elevated levels of CCL2 and CCL4 that corresponded to the Luminex data, we also found examples where RNA transcripts remained unchanged despite protein induction (Figure 4B and S4B). In contrast, CXCL1 and CCL7 mRNA levels were stable or even diminished in the presence of VP55, yet showed significant induction by Luminex assay (Figure 4B and S4B). These results suggest that the alleviation of miRNA targeting results in both enhanced mRNA stability as well as increased translation.
To further investigate the significance of these findings, we analyzed whole lung of PBS, AdV-GFP, and AdV-VP55-treated mice by mRNA-Seq. Of the 12,045 genes constituting the lung transcriptome, only 50 were differentially expressed in response to AdV-GFP as compared to PBS-treated mice (Table S4). In contrast, the addition of VP55 induced significant changes in 2,427 genes (Table S4). RNA-Seq data not only corroborated our qPCR results, we further identified 139 differentially expressed genes that demonstrated a two or more log2 fold change (Table S4). Inputting these genes into an unbiased pathway prediction software (Kamburov et al., 2013) indicated that all of these transcripts were implicated in the activation of the immune response (Figure 4C). General gene ontology categories showing the greatest enrichment included: immune cell adhesion, regulation of the immune processes, and lymphocyte activation and differentiation pathways (Figure 4C). Interestingly, when looking at the 138 differentially expressed genes that demonstrated a log2 fold change of less than two, enriched gene ontology categories were largely limited to morphogenesis and development, consistent with the role of miRNA involvement in these pathways (Figure 4D and Table S4)(Nguyen et al., 2015; Zhao and Srivastava, 2007).
DISCUSSION
The discovery that small RNAs could elicit a level of posttranscriptional control on complementary RNA targets revealed an additional layer of complexity to the parameters that define protein levels. Perfectly complementary small RNAs are employed in antiviral/anti-transposon defenses in bacteria, archaea, and even a wide range of eukaryotes, yet their utilization in mammals appears restricted to germline cells in the form of PIWI-interacting RNAs and possible RNAi in stem cells (Cullen et al., 2013). One significant finding that would suggest small RNA-based antiviral strategies have been largely (if not entirely) abandoned in mammals was the discovery that RISC function is lost in response to both cell stress and virus infection (Leung et al., 2011; Seo et al., 2013). Further, new reports suggest that functional components of a bona fide antiviral RNAi system antagonize the innate immune response in mammalian cells, indicating an incompatibility between these two systems (Girardi et al., 2015). While such findings strongly suggest that a canonical RNAi response is absent or irrelevant in mammals, here we investigate whether endogenous miRNAs participate in the host response to pathogens. Expanding on an earlier discovery that poxviruses rapidly tail and degrade miRNAs (Backes et al., 2012), we developed a system that could elicit the same activity independent of a virus infection. This method provided significant advantages over an earlier design in which we delivered VP55 using a recombinant VSV strain (Backes et al., 2014). While that study allowed us to ascertain whether RNAi impacted virus infection directly, the inflammatory environment induced by the virus itself made it unsuitable to study miRNA function as it related to cytokine signaling. Indeed, in that work we concluded that the attenuation induced by VP55 was due to reduced repression of the host antiviral response. This conclusion was made because, consistent with our results in this study, we observed differentially expressed cytokines in response to VSV-VP55. However, because VSV was replicating, cytokine production also resulted in a secondary response of ISG induction that masked the selective action of VP55 alone. In an effort to eliminate the variables created by a replicating virus, we expressed VP55 using an Adenovirus vector that elicits almost no response from the host (Table S1 and S4). This methodology allowed us to examine how the acute response to virus replication or IFN-I could be modulated by miRNA-mediated regulation independent of any confounding variables. These experimental models demonstrated almost no influence on the resulting transcriptional response to either stimulus. The lack of modulation is likely due to the inherently subtle activity of miRNAs coupled to the robust transcriptional nature of the antiviral response. While acute modulation may be ill-suited for the transcriptional response to dsRNA or IFN-I, loss of miRNAs did demonstrate dramatic changes in the cellular profile – especially in regard to cytokines. This is particularly noteworthy as the kinetics are in perfect agreement with recent work demonstrating that, beyond 8hrs of infection, RISC function is compromised as a result of ribosylation (Seo et al., 2013). These two observations would suggest that while miRNA silencing may not be directly involved in the innate response to viruses, the shut down of this regulatory pathway may itself provide a rapid means to de-repress translation of essential inflammatory transcripts. The idea that the host response to virus requires loss of miRNA function as a means to generate a burst of cytokine translation is further supported by in vivo data. Administration of AdV-VP55 resulted in a significant increase in a wide array of cytokines, ultimately resulting in immune cell activation.
In all, the data presented in this paper strongly suggest that the acute response to virus infection functions independently of the miRNA system. This is in agreement with other independent studies that found that loss of Dicer has no impact on RNA virus titers in vitro (Bogerd et al., 2014a; Shapiro et al., 2010). While miRNAs may not have the capacity to influence a dsRNA- or IFN-I-mediated transcriptional response, sustained virus replication in the absence of cell death does provide an environment in which cells can utilize the miRNA pathway through a loss of function event (Seo et al., 2013). Here we provide in vivo evidence that the miRNA machinery is used in this way to induce inflammatory cytokines, which may reflect some of the final actions of the dying cell. One of the unique aspects of this biological action is that it would not require any de novo transcription and would therefore be a difficult system to antagonize. Interestingly, influenza A virus, infamous for its capacity to induce significant morbidity and mortality encodes an NS1 protein which has been found to block RISC ribosylation (Seo et al., 2013). Based on the results presented here, this antagonistic activity of NS1 would serve to reduce the expression of cytokines and dampen both innate and adaptive immune response to infection.
EXPERIMENTAL PROCEDURES
Viruses
Human codon optimization of the Vaccinia virus VP55 sequence, synthesis of, and cloning into a pUC57 vector to generate pUC57-VP55 was performed by GenScript. In this construct, the open reading frame of the codon optimized VP55 was flanked by a N-terminal EcoRI site, upstream of a kozak sequence, and a C-terminal KpnI site (see supplemental material for complete gene sequence). VP55 was digested out of pUC57 and cloned into pEGFP-C1 (Clontech) using EcoRI and KpnI to generate an N-terminal EGFP-tagged VP55. The EGFP-tagged, codon optimized VP55 or EGFP alone were then inserted into a non-replicative Adenovirus type 5 E1/E3 deleted vector behind an EF1a promoter (AdV-EF1a-EGFP-VP55 and AdV-EF1a-EGFP, respectively) as described by the manufacturer (www.vectorbiolabs.com). The fiber protein was modified to contain an Arg-Gly-Asp (RGD) motif to permit broad use in primary cells (Wickham et al., 1997). Vector Biolabs produced all Adenovirus rescue constructs and titered viral stocks. Adenoviral vectors were used at an MOI of 500 unless otherwise indicated.
in vivo infections
Six to eight week old C57BL/6 mice were anesthetized with ketamine/xylazine and intranasally administered 2.5×108 pfu of either AdV-GFP or AdV-VP55 in 50μL of PBS. All experiments involving animals were performed in accordance with the Icahn School of Medicine Animal Care and Use Committee.
mRNA Sequencing
mRNA-Seq libraries from 2 biological replicates per experimental condition were prepared using 1μg (for HEK 293T) or 150ng (for BJ) total RNA. The TruSeq RNA Library Preparation Kit v2 was used according to the manufacture’s protocol. Briefly, mRNA was purified via oligo-dT beads, fragmented, and reverse transcribed with SuperScript II (Invitrogen), followed by second strand synthesis, end repair, A-tailing, and adapter ligation. Quantification of barcoded samples and pooled libraries was done using the Universal Complete KAPA Library Quantification Kit (KAPA Biosystems). Pooled libraries were run on either an Illumina MiSeq platform using the MiSeq Reagent Kit v3 (Illuminia) or an Illumina HiSeq 2500. Read mapping, statistical analysis on two independent libraries from biological replicates, and dot plot generation was performed using BaseSpace RNA Express application which determines expression based on the negative binomial distribution, with variance and mean linked by local regression (DESeq, (Anders and Huber)) (Illumina). Cytokine heatmap was generated using MultiExperiment Viewer (TM4). The raw data are available under the accession numbers: GSM1901280, GSM1901281, GSM1901282, GSM1901283, GSM1901284, GSM1901285, GSM1901286, GSM1901287, GSM1901288, GSM1901289, GSM1901290, GSM1901291, GSM1901292, GSM1901293, GSM1901294, GSM1901295, GSM1901296, GSM1901297, GSM1901298, GSM1901299, GSM1901300, GSM1901301, GSM1901302, GSM1906440, GSM1906441, GSM1906442, GSM1906443, GSM1906444, GSM1906445, GSM1906456, GSM1906457, GSM1906458, GSM1906459.
Supplementary Material
Supplemental Figure 1. Characterization of pEGFP-VP55 and Adenoviral vectors. Related to Figure 1.
(A) Western blot for EGFP expression in NoDice cells transfected with either pEGFP or pEGFP-VP55 for 24hrs. (B) Western blot for EGFP expression of 293T cells treated with Vaccinia-GFP, AdV-GFP, or AdV-VP55 at the indicated time points. (C) Small RNA northern blot of 293T cells treated with AdV-VP55 for the indicated time points. (D) qPCR of 293T cells treated with AdV-GFP or AdV–VP55 for 24 hrs for indicated genes. (E) CellTiterGlo Assay of 293T cells treated with AdV-GFP or AdV-VP55 at indicated MOIs 24hrs post-transduction.
Supplemental Figure 2. Validation of deep sequencing results from immortalized fibroblasts and primary fibroblasts treated with dsRNA. Related to Figure 2.
qPCR of BJ cells treated with AdV-GFP or AdV-VP55 for 24 hrs prior to transfection with dsRNA. Total RNA collected 6hrs post-transfection. All samples were normalized to tubulin. Fold changes over samples treated with AdV-GFP alone. Error bars denote ± SD.
Supplemental Figure 3. Validation of deep sequencing results from Adeno vector-treated primary fibroblasts. Related to Figure 3.
qPCR of BJ fibroblasts treated with AdV-GFP or AdV–VP55 for 24 hrs (A) or 9 days (B) for indicated genes. All samples were normalized to tubulin. Fold changes over samples treated with AdV-GFP alone. Error bars denote ± SD.
Supplemental Figure 4. Characterization of the cytokine response to loss of miRNA silencing in vivo. Related to Figure 4.
(A) Cytokine concentrations from mice treated in Figure 4A. Graphs depict cytokines that were detectable in the assay, but not significantly altered across treatment conditions. Significance was determined using an one-tailed Student’s t test. p-values < 0.05 were considered statistically significant. (B) qPCR of RNA from lungs in Figure 4A for indicated genes. All samples normalized to tubulin. Fold change over mock-treated cohort.
mRNA-Seq analysis of NoDice cells transfected with pEGFP or pEGFP-VP55 for 24hrs and small RNA and mRNA-Seq analysis of 293T cells mock treated or treated with either AdV-GFP or AdV-VP55 for 24 hours. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiments and additional tabs contain the subsets of genes determined to be differentially expressed (q<0.05). Tab for small RNA deep sequencing contains the name, sequence, read numbers for each condition, and fold changes for annotated mature miRNAs.
Analysis of BJ fibroblasts treated with AdV-GFP of AdV-VP55 for 24 hours prior to mock, dsRNA transfection for 6 hours, or IFN treatment for 6 or 24 hours prior to mRNA-Seq. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiments. Additional tabs contain the subsets of all genes that were differentially expressed (q<0.05) as well as the subsets of differentially expressed genes that were also induced 2-fold or more for each experimental condition.
Analysis of BJ fibroblasts treated with AdV-GFP or AdV-VP55 for 24 hours or 9 days prior to mRNA-Seq. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiment. Additional tabs contain the subsets that were differentially expressed (q<0.05) and the subsets of differentially expressed genes that were induced 2-fold or more for each time point.
mRNA Seq analysis of whole lung tissue from mice treated intranasally with 2.5 × 108pfu of AdV-GFP or AdV-VP55 for 48 hours. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiment. Subsequent tabs contain the subset determined to be differentially expressed (q<0.05), differentially expressed genes from AdV-VP55 murine lung tissue up- or down-regulated by log2 2-fold or more, and the ConsensusPathDB interaction database output for the ten most significant categories from the over-representation analysis of induced genes (in black) and the four most significant categories for repressed genes (red).
Acknowledgments
We wish to thank Dr. B. Cullen (Duke University) for NoDice cells. This material is based upon work supported in part by the Burroughs Wellcome Fund. BRT is also supported by the National Institute of Allergy and Infectious Diseases grants R01AI110575 and 5R01AI093571. JKL is supported by the National Institute of Allergy and Infectious Diseases grant 1R01AI108715. LCA is partially supported by an American Heart Association Predoctoral Fellowship (15PRE24930012).
Footnotes
Supplemental Information includes four supplemental figures, four supplemental tables, supplemental experimental procedures, and references.
AUTHOR CONTRIBUTION
LCA and SS designed, executed, and analyzed experiments. DS provided bioinformatics support. JVS generated all HiSeq libraries described. LCA and JKL conducted and analyzed the Luminex panel. LCA, SS, and BRT wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes S, Shapiro JS, Sabin LR, Pham AM, Reyes I, Moss B, Cherry S, tenOever BR. Degradation of host microRNAs by poxvirus poly(A) polymerase reveals terminal RNA methylation as a protective antiviral mechanism. Cell Host Microbe. 2012;12:200–210. doi: 10.1016/j.chom.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Skalsky RL, Kennedy EM, Furuse Y, Whisnant AW, Flores O, Schultz KL, Putnam N, Barrows NJ, Sherry B, et al. Replication of many human viruses is refractory to inhibition by endogenous cellular microRNAs. J Virol. 2014a;88:8065–8076. doi: 10.1128/JVI.00985-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerd HP, Whisnant AW, Kennedy EM, Flores O, Cullen BR. Derivation and characterization of Dicer- and microRNA-deficient human cells. Rna. 2014b doi: 10.1261/rna.044545.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR. Is RNA interference involved in intrinsic antiviral immunity in mammals? Nat Immunol. 2006;7:563–567. doi: 10.1038/ni1352. [DOI] [PubMed] [Google Scholar]
- Cullen BR, Cherry S, tenOever BR. Is RNA interference a physiologically relevant innate antiviral immune response in mammals? Cell Host Microbe. 2013;14:374–378. doi: 10.1016/j.chom.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Deng L, Beigelman L, Matulic-Adamic J, Karpeisky A, Gershon PD. Specific recognition of an rU2-N15-rU motif by VP55, the vaccinia virus poly(A) polymerase catalytic subunit. J Biol Chem. 1997;272:31542–31552. doi: 10.1074/jbc.272.50.31542. [DOI] [PubMed] [Google Scholar]
- Girardi E, Lefevre M, Chane-Woon-Ming B, Paro S, Claydon B, Imler JL, Meignin C, Pfeffer S. Cross-species comparative analysis of Dicer proteins during Sindbis virus infection. Sci Rep. 2015;5:10693. doi: 10.1038/srep10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin J, Hatchwell L, Foster P, Johnston SL, Bartlett N, Collison A, Mattes J. CCL7 and IRF-7 Mediate Hallmark Inflammatory and IFN Responses following Rhinovirus 1B Infection. J Immunol. 2015;194:4924–4930. doi: 10.4049/jimmunol.1401362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann P, Schober A, Weber C. Chemokines and microRNAs in atherosclerosis. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-1925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel CC, Birnstiel ML. The organization and expression of histone gene families. Cell. 1981;25:301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamburov A, Stelzl U, Lehrach H, Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013;41:D793–800. doi: 10.1093/nar/gks1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HB, Lee SW, Jin HM, Ha H, Lee SH, Takeshita S, Tanaka S, Kim HM, Kim HH, Lee ZH. Monokine induced by interferon-gamma is induced by receptor activator of nuclear factor kappa B ligand and is involved in osteoclast adhesion and migration. Blood. 2005;105:2963–2969. doi: 10.1182/blood-2004-07-2534. [DOI] [PubMed] [Google Scholar]
- Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA, Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ru J, Zhang J, Zhu LH, Liu M, Li X, Tang H. miR-23a targets interferon regulatory factor 1 and modulates cellular proliferation and paclitaxel-induced apoptosis in gastric adenocarcinoma cells. PLoS One. 2013;8:e64707. doi: 10.1371/journal.pone.0064707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutrophil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990;171:1797–1802. doi: 10.1084/jem.171.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TA, Jo MH, Choi YG, Park J, Kwon SC, Hohng S, Kim VN, Woo JS. Functional Anatomy of the Human Microprocessor. Cell. 2015;161:1374–1387. doi: 10.1016/j.cell.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Jing Q, Georgel P, New L, Chen J, Mols J, Kang YJ, Jiang Z, Du X, Cook R, et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity. 2007;27:123–134. doi: 10.1016/j.immuni.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Maniatis T. Inducible processing of interferon regulatory factor-2. Mol Cell Biol. 1992;12:3325–3336. doi: 10.1128/mcb.12.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova I, Forster S, Yu S, Kannan A, Masse M, Cumming H, Chapman R, Hertzog PJ. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo GJ, Kincaid RP, Phanaksri T, Burke JM, Pare JM, Cox JE, Hsiang TY, Krug RM, Sullivan CS. Reciprocal inhibition between intracellular antiviral signaling and the RNAi machinery in mammalian cells. Cell Host Microbe. 2013;14:435–445. doi: 10.1016/j.chom.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JS, Varble A, Pham AM, Tenoever BR. Noncanonical cytoplasmic processing of viral microRNAs. RNA. 2010;16:2068–2074. doi: 10.1261/rna.2303610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tenOever BR. RNA viruses and the host microRNA machinery. Nat Rev Microbiol. 2013;11:169–180. doi: 10.1038/nrmicro2971. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Tzeng E, Shears LL, 2nd, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32:189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Characterization of pEGFP-VP55 and Adenoviral vectors. Related to Figure 1.
(A) Western blot for EGFP expression in NoDice cells transfected with either pEGFP or pEGFP-VP55 for 24hrs. (B) Western blot for EGFP expression of 293T cells treated with Vaccinia-GFP, AdV-GFP, or AdV-VP55 at the indicated time points. (C) Small RNA northern blot of 293T cells treated with AdV-VP55 for the indicated time points. (D) qPCR of 293T cells treated with AdV-GFP or AdV–VP55 for 24 hrs for indicated genes. (E) CellTiterGlo Assay of 293T cells treated with AdV-GFP or AdV-VP55 at indicated MOIs 24hrs post-transduction.
Supplemental Figure 2. Validation of deep sequencing results from immortalized fibroblasts and primary fibroblasts treated with dsRNA. Related to Figure 2.
qPCR of BJ cells treated with AdV-GFP or AdV-VP55 for 24 hrs prior to transfection with dsRNA. Total RNA collected 6hrs post-transfection. All samples were normalized to tubulin. Fold changes over samples treated with AdV-GFP alone. Error bars denote ± SD.
Supplemental Figure 3. Validation of deep sequencing results from Adeno vector-treated primary fibroblasts. Related to Figure 3.
qPCR of BJ fibroblasts treated with AdV-GFP or AdV–VP55 for 24 hrs (A) or 9 days (B) for indicated genes. All samples were normalized to tubulin. Fold changes over samples treated with AdV-GFP alone. Error bars denote ± SD.
Supplemental Figure 4. Characterization of the cytokine response to loss of miRNA silencing in vivo. Related to Figure 4.
(A) Cytokine concentrations from mice treated in Figure 4A. Graphs depict cytokines that were detectable in the assay, but not significantly altered across treatment conditions. Significance was determined using an one-tailed Student’s t test. p-values < 0.05 were considered statistically significant. (B) qPCR of RNA from lungs in Figure 4A for indicated genes. All samples normalized to tubulin. Fold change over mock-treated cohort.
mRNA-Seq analysis of NoDice cells transfected with pEGFP or pEGFP-VP55 for 24hrs and small RNA and mRNA-Seq analysis of 293T cells mock treated or treated with either AdV-GFP or AdV-VP55 for 24 hours. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiments and additional tabs contain the subsets of genes determined to be differentially expressed (q<0.05). Tab for small RNA deep sequencing contains the name, sequence, read numbers for each condition, and fold changes for annotated mature miRNAs.
Analysis of BJ fibroblasts treated with AdV-GFP of AdV-VP55 for 24 hours prior to mock, dsRNA transfection for 6 hours, or IFN treatment for 6 or 24 hours prior to mRNA-Seq. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiments. Additional tabs contain the subsets of all genes that were differentially expressed (q<0.05) as well as the subsets of differentially expressed genes that were also induced 2-fold or more for each experimental condition.
Analysis of BJ fibroblasts treated with AdV-GFP or AdV-VP55 for 24 hours or 9 days prior to mRNA-Seq. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiment. Additional tabs contain the subsets that were differentially expressed (q<0.05) and the subsets of differentially expressed genes that were induced 2-fold or more for each time point.
mRNA Seq analysis of whole lung tissue from mice treated intranasally with 2.5 × 108pfu of AdV-GFP or AdV-VP55 for 48 hours. Table contains the gene symbol, read status, mean read count, log2 fold change, standard error of the log2 fold change, and the multiple-testing adjusted p-value (q value) for all genes detected in the experiment. Subsequent tabs contain the subset determined to be differentially expressed (q<0.05), differentially expressed genes from AdV-VP55 murine lung tissue up- or down-regulated by log2 2-fold or more, and the ConsensusPathDB interaction database output for the ten most significant categories from the over-representation analysis of induced genes (in black) and the four most significant categories for repressed genes (red).