Abstract
Chronic intermittent hypoxia (CIH) has been identified as a relevant risk factor for the development of enhanced sympathetic outflow and arterial hypertension. Several studies have highlighted the importance of peripheral chemoreceptors for the cardiovascular changes elicited by CIH. However, the effects of CIH on the central mechanisms regulating sympathetic outflow are not fully elucidated. Our research group has explored the hypothesis that the enhanced sympathetic drive following CIH exposure is, at least in part, dependent on alterations in the respiratory network and its interaction with the sympathetic nervous system. In this report, I discuss the changes in the discharge profile of baseline sympathetic activity in rats exposed to CIH, their association with the generation of active expiration and the interactions between expiratory and sympathetic neurones after CIH conditioning. Together, these findings are consistent with the theory that mechanisms of central respiratory–sympathetic coupling are a novel factor in the development of neurogenic hypertension.
Introduction
Cardiorespiratory homeostasis depends critically on peripheral feedback information that initiates reflex adjustments in response to environmental challenges. Peripheral chemoreceptors, also known as glomus cells, monitor the levels of oxygen in the arterial blood continuously and elicit cardiorespiratory changes to ensure adequate oxygen supply in conditions of low oxygen (Lahiri et al. 2006). In mammals, these oxygen-sensitive cells are mainly located in the carotid and the aortic bodies and release neurotransmitters in response to hypoxia, activating afferent nerves that convey the chemosensory information to the neurones of the nucleus of the solitary tract (NTS) in the brainstem (Machado, 2001). Within the NTS, the carotid body inputs are processed and transmitted to other brain regions engaged in autonomic and respiratory control, evoking responses of enhanced respiratory drive, sympathetic activation and parasympathetic stimulation (Machado, 2001).
Humans with cardiorespiratory diseases commonly present with autonomic dysfunction manifested as elevated sympathetic drive. For example, patients with obstructive sleep apnoea (OSA) develop arterial hypertension associated with high levels of muscle sympathetic nerve activity (Somers, 1995; Pedrosa et al. 2011). There is evidence that the exposure to intermittent hypoxia, as a consequence of the obstruction of upper airways during sleep, is a major risk factor for the development of sympathetic overactivity in OSA patients (Somers, 1995). This notion is strongly supported by experimental studies showing the following findings: (i) animals submitted to chronic intermittent hypoxia (CIH) for 2–5 weeks exhibit high arterial pressures (Fletcher, 2001; Zoccal et al. 2007, 2008); (ii) carotid body denervation prevents the development of hypertension induced by CIH exposure (Fletcher, 2001); (iii) the exposure to intermittent hypercapnic hypoxia produces similar increases in arterial pressure to intermittent normocapnic/hypocapnic hypoxia (Fletcher et al. 1995); (iv) the sensory activity of peripheral chemoreceptors and the cardiorespiratory chemoreflex responses are enhanced after CIH exposure (Peng et al. 2003); and (v) rats exposed to CIH show elevated plasma catecholamine concentrations, augmented sympathetically mediated variability in systolic pressure and larger depressor responses to ganglionic blockade (Zoccal et al. 2007, 2009). These findings indicate that the episodic activation of peripheral chemoreceptors consequent to CIH modifies the chemoreflex control of the sympathetic nervous system. Accumulating evidence indicates that changes in the sensory activity of the carotid body chemoreceptors significantly contributes to the autonomic dysfunction induced by CIH (Prabhakar & Kumar, 2010). However, the effects of CIH on the central nuclei regulating the sympathetic activity are still not fully understood. In this report, I summarize our recent findings, exploring the neural mechanisms leading to the increased sympathetic activity of rats exposed to CIH.
Chronic intermittent hypoxia and the character of sympathetic activity
The importance of the sympathetic nervous system in the maintenance of hypertension in rats exposed to CIH was initially suggested based on indirect evidence, including pharmacological sympathetic denervation, plasma noradrenaline measurements and analysis of arterial pressure variability (Fletcher, 2001; Zoccal et al. 2007, 2009). Using decerebrated in situ preparations of juvenile rats exposed to CIH (6% O2 for 30–40 s, every 9 min for 8 h per day) for 10 days (Zoccal et al. 2008), we performed recordings of the activity of sympathetic nerves and neurones. We found that the in situ preparations of rats exposed to CIH exhibited higher baseline vasoconstrictor sympathetic activity in comparison to preparations of control rats (Zoccal et al. 2008; Moraes et al. 2013), providing direct evidence that baseline sympathetic activity is augmented in rats exposed to CIH. Importantly, the enhanced sympathetic activity of rats exposed to CIH was associated with the presence of novel bursts during the expiratory phase, which were absent in control rats (Fig. 1; Zoccal et al. 2008). These additional expiratory bursts in sympathetic nerves of rats exposed to CIH were not secondary to reductions in baroreflex function (Zoccal et al. 2009) and not eliminated after acute peripheral chemoreceptor denervation (Zoccal et al. 2008), indicating that they were driven by central mechanisms.
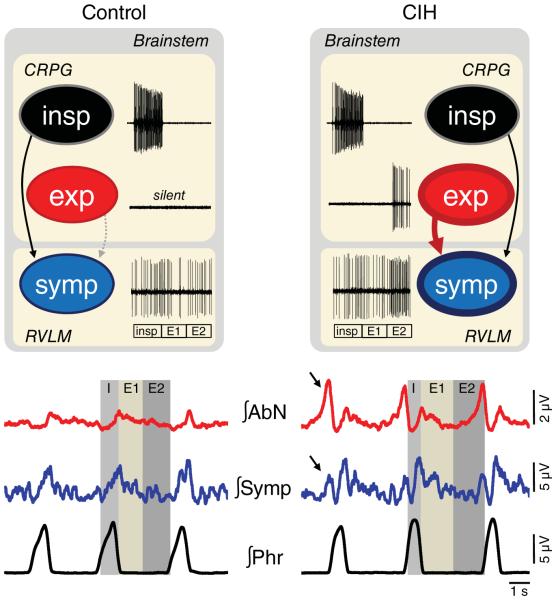
Figure 1. Schematic drawing illustrating the possible changes in the coupling of respiratory and sympathetic neurones after exposure to chronic intermittent hypoxia (CIH).
The traces inside the boxes represent extracellular recordings of inspiratory, expiratory and sympathetic neurones, while the traces at the bottom illustrate the integrated (∫) recordings of abdominal (AbN), sympathetic (Symp) and phrenic (Phr) nerve activities. In control animals (left panels), in conditions of normoxia and normocapnia, sympathetic nerve activity presents a phasic increase during the inspiratory phase (insp), with a peak of activity during late inspiration/early postinspiration (post-I), following by a decline during post-I/expiratory stage 2 (E2). This pattern is formed by inputs from the inspiratory (insp) and expiratory (exp) neurones of the central respiratory pattern generator (CRPG) to the presympathetic neurones (symp) of the rostral ventrolateral medulla (RVLM; Moraes et al. 2012b). In resting conditions, inspiration is an active process, while expiration is a passive process. In rats exposed to CIH, the central mechanisms responsible for the generation of active expiration appear to be activated in resting conditions (Zoccal et al. 2008; Molkov et al. 2011). As a result, the expiratory neurones, which are normally silent in control animals, are active and send excitatory inputs to bulbospinal expiratory neurones and to presympathetic neurones of the RVLM, leading to the emergence of coupled expiratory bursts in abdominal and sympathetic outputs (indicated by the arrows). For more details, please see the main text.
Owing to the synchronization with expiratory phase, we theorized that the sympathetic overactivity of juvenile rats submitted to CIH was linked to changes in the respiratory pattern. This hypothesis was based on the fact that neurones of the central respiratory pattern generator interact with the neurones involved with the generation of sympathetic activity and introduce rhythmical oscillations in sympathetic nerve discharge coupled with respiration (Moraes et al. 2012b). In the in situ preparations of rats exposed to CIH, the expiratory-related sympathetic bursts were coupled with the emergence of high-amplitude bursts in abdominal expiratory motor nerves (Fig. 1; Zoccal et al. 2008), suggesting that CIH exposure alters the resting respiratory pattern, transforming expiration into an active process. Similar results were observed in conscious CIH-treated animals, which presented with higher arterial pressure levels in association with contractions in abdominal expiratory muscles in resting conditions (Moraes et al. 2013). We, therefore, considered that CIH in juvenile rats promotes the following changes: (i) central activation of the mechanisms controlling the expiratory motor activity; and (ii) alteration in the strength of coupling between expiratory and sympathetic neurones in the brainstem. Indeed, the reduction of respiratory drive in CIH-exposed rat preparations (with the exposure to hypocapnia) abolished the expiratory bursts in the abdominal and sympathetic nerves (Molkov et al. 2011), indicating that active expiration and sympathetic overactivity in rats exposed to CIH are coupled events.
The ventral surface of the medulla is a critical site for the generation of respiratory–sympathetic coupling. Within this region, respiratory neurones of the ventral respiratory column intermingle with the presympathetic neurones of the rostral ventrolateral medulla (RVLM), which is a major source of excitation to the preganglionic sympathetic neurones of the spinal cord (Ross et al. 1984). The RVLM neurones also establish connections with other pontine–medullary respiratory nuclei (Moraes et al. 2012b), denoting that the RVLM is an important region for respiratory–sympathetic integration. In animals exposed to CIH, but not in control rats, it was identified a population of non-catecholaminergic RVLM neurones that displayed an augmented firing frequency and increased excitatory postsynaptic currents during the second phase of expiration (E2), coincident with the expiratory bursts in sympathetic nerves (Moraes et al. 2013). These RVLM neurones did not present changes in their intrinsic excitability and input resistance, suggesting that the additional expiratory burst in presympathetic neurones of rats exposed to CIH did not depend on changes in their intrinsic properties (Moraes et al. 2013). Based on these data, it was hypothesized that excitatory synaptic inputs, potentially originating from neurones involved with the generation of active expiration, are critical for the emergence of novel expiratory-related bursts in the RVLM presympathetic neurones as well as in the sympathetic activity of rats exposed to CIH (Fig. 1). The emergence of novel rhythmical bursts in sympathetic vasoconstrictor nerves may be a mechanism to increase the efficacy of neurotransmitter release and probability of summation of other excitatory inputs at the ganglionic and blood vessel levels (Gilbey, 2007). Therefore, the change in the pattern of sympathetic discharge induced by CIH exposure appears to have pathophysiological relevance for the development of arterial hypertension associated with this condition.
Respiratory–sympathetic coupling after exposure to CIH: interactions between peripheral and central chemoreceptors
In resting conditions, breathing is composed of three phases: inspiration, postinspiration (post-I) and E2 (Fig. 1; Richter & Smith, 2014). In mammals, inspiration is an active process and depends on the diaphragmatic contraction, while exhalation occurs passively due to the recoil forces of the chest and the lungs. During metabolic challenges, such as episodes of hypercapnia and hypoxia, expiration turns into an active process, with dilatation of upper airways and the recruitment of abdominal expiratory muscles during the E2 phase. Our in vivo and in situ data demonstrate that juvenile rats exposed to CIH present a pattern of active expiration at rest (Zoccal et al. 2008; Moraes et al. 2013), indicating that the neural mechanisms responsible for the generation of active expiration are hyperactive in conditions of normocapnia and normoxia.
A group of expiratory neurones located in the parafacial respiratory group (pFRG) are suggested to play a major role in the generation of active expiration (Janczewski & Feldman, 2006). The pFRG is located ventral to the facial nucleus, overlapping anatomically with the retrotrapezoid nucleus (RTN; Abdala et al. 2009). During central and peripheral chemoreceptor activation, pharmacological inhibition of the pFRG/RTN region blocks bursts in abdominal motor activity during the E2 phase, and recordings of the pFRG neurones reveal that quiescent expiratory neurones discharge rhythmically during the E2 phase (Fig. 1; Abdala et al. 2009; Moraes et al. 2012a). Thus, the expiratory neurones of the pFRG appear to be an important excitatory source to bulbospinal expiratory neurones of the ventral respiratory column. Anatomical studies have identified projections from the pFRG/RTN to the RVLM region (Rosin et al. 2006). Although it remains unclear whether these projections originate from either the expiratory or the chemosensitive neurones, these observations allowed us to propose that the activation of the pFRG neurones may also drive expiratory-related excitatory inputs to the presympathetic neurones of the RVLM and generate coupled expiratory bursts in abdominal and sympathetic nerves (Molkov et al. 2011). Therefore, the pFRG neurones may be considered as a potential excitatory source that drives active expiration and sympathetic overactivity in rats exposed to CIH (Fig. 1).
Using computational modelling of respiratory–sympathetic interactions in the brainstem (Molkov et al. 2011), we explored the contribution of the pFRG to elicit the changes in the expiratory pattern and in the respiratory–sympathetic coupling of rats exposed to CIH. Modelling simulations showed that an increased excitatory drive to the pFRG is able to activate the expiratory neurones and generate coupled abdominal and sympathetic bursts during the E2 phase (Molkov et al. 2011). Furthermore, according to the model, the augmented excitatory drive to the pFRG expiratory neurones of rats exposed to CIH may originate from the chemosensitive neurones of the RTN (Molkov et al. 2011). Using the in situ preparations, we tested the predictions of the model and confirmed that: (i) central chemoreflex activation in naive rats evoked expiratory bursts in abdominal and sympathetic activities; and (ii) rats exposed to CIH exhibited a lower hypocapnic apnoeic threshold, suggesting an increased sensitivity of the central chemoreceptors in these animals (Molkov et al. 2011). Based on these findings, my co-authors and I concluded that CIH exposure promotes a persistent activation of the pFRG expiratory neurones that is maintained in conditions of normoxia and normocapnia due to a sensitization of the central chemoreceptors.
The hypothesis that the central chemoreceptors are sensitized after CIH still requires further experimental investigation in order to be proved. However, this possibility is in accordance with previous studies demonstrating a synergistic interaction between the mechanisms of peripheral and central chemoreception (Blain et al. 2010). On this subject, I speculate that the episodic activation of peripheral chemoreceptors, consequent to the CIH exposure, may introduce changes in the mechanisms involved with central CO2 sensing, which might explain why acute carotid body denervation after CIH did not eliminate the expiratory bursts in sympathetic activity (Zoccal et al. 2008). The effects of CIH on the central chemoreflex may be associated with the activation of neuromodulatory mechanisms in the pFRG/RTN, such as the serotoninergic system (Mulkey et al. 2007). It may also be related to changes in the neuronal respiratory circuitry that interacts with the pFRG/RTN and contributes to the generation of active expiration, including the pons (Abdala et al. 2009), the ventral respiratory column (Molkov et al. 2011), the dorsal respiratory group (Takakura et al. 2006) and the pulmonary stretch receptors (Lemes & Zoccal, 2014). These possibilities are currently under investigation in our laboratory.
General conclusions
Our studies emphasize that the central mechanisms underpinning interactions between expiratory and sympathetic activities play a relevant role for the development and maintenance of sympathetic overactivity and arterial hypertension in rats submitted to CIH. A recent study demonstrated that untreated OSA patients exhibit a significant change in the temporal profile of the respiratory modulation of muscle sympathetic nerve activity, with higher levels during the postinspiratory phase (Fatouleh et al. 2014). Although the CIH model does not reproduce the pathophysiology of OSA, these clinical data parallel our experimental observations, highlighting that changes in the respiratory–sympathetic coupling mechanisms may be considered relevant to the development of arterial hypertension associated with CIH exposure.
New Findings
-
What is the topic of this review?
Chronic intermittent hypoxia (CIH), as observed in patients with obstructive sleep apnoea, is associated with the development of sympathetically mediated arterial hypertension. Nevertheless, the mechanisms underpinning the augmented sympathetic outflow in CIH still remain under investigation.
-
What advances does it highlight?
In this report, I present experimental evidence supporting the hypothesis that changes in the function of the respiratory network and coupling with the sympathetic nervous system may be considered as a novel and relevant mechanism for the increase in baseline sympathetic outflow in animals submitted to CIH.
Acknowledgments
Funding
This study was supported by the São Paulo Research Foundation (FAPESP, 2013/06077-5 and 2009/54888-7) and the Conselho Nacional de Desenvolvimento Científico e Tecnoló gico (CNPQ, 478640/2013-7).
Footnotes
Competing interests
None declared.
References
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem–spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouleh R, McKenzie DK, Macefield VG. Respiratory modulation of muscle sympathetic nerve activity in obstructive sleep apnoea. Exp Physiol. 2014;99:1288–1298. doi: 10.1113/expphysiol.2013.077511. [DOI] [PubMed] [Google Scholar]
- Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Bao G, Miller CC., 3rd Effect of recurrent episodic hypocapnic, eucapnic, and hypercapnic hypoxia on systemic blood pressure. J Appl Physiol. 1995;78:1516–1521. doi: 10.1152/jappl.1995.78.4.1516. [DOI] [PubMed] [Google Scholar]
- Gilbey MP. Sympathetic rhythms and nervous integration. Clin Exp Pharmacol Physiol. 2007;34:356–361. doi: 10.1111/j.1440-1681.2007.04587.x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol. 2006;91:249–286. doi: 10.1016/j.pbiomolbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lemes EV, Zoccal DB. Vagal afferent control of abdominal expiratory activity in response to hypoxia and hypercapnia in rats. Respir Physiol Neurobiol. 2014;203:90–97. doi: 10.1016/j.resp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci. 2001;940:179–196. doi: 10.1111/j.1749-6632.2001.tb03676.x. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of retrotrapezoid/parafacial respiratory region to the expiratory–sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol. 2012a;108:882–890. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- Moraes DJ, Zoccal DB, Machado BH. Medullary respiratory network drives sympathetic overactivity and hypertension in rats submitted to chronic intermittent hypoxia. Hypertension. 2012b;60:1374–1380. doi: 10.1161/HYPERTENSIONAHA.111.189332. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol. 2010;174:156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Somers VK. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol. 2007;92:79–85. doi: 10.1113/expphysiol.2006.035501. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LGH, Paton JFR, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol. 2009;94:972–983. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LGH, Braga VA, Pickering AE, Paton JFR, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]