While microRNAs are well known for their roles in the regulation of mRNA stability and translation in the cytoplasm, their presence and potential function in the mitochondria have only been recently realized. How specific microRNAs enter the lumen of mitochondria remains unknown. Jagannathan et al.1 now reports that some microRNAs may even specifically target to different populations of mitochondria in the same cells upon diabetic insult, suggesting a potential new disease mechanism, but the finding appears to require further technical and mechanistic refinement.
MicroRNA was initially detected in the mitochondria during analysis of the mitochondrial transcriptome, and at the time, were thought to be contaminants of purified mitochondria2. However, several later studies based on qPCR analysis and confocal microscopy suggest that the presence of microRNAs in the mitochondria is real3,4. The most vigorous proof is achieved through the membrane protection assay on purified mitochondria to show that mitochondrial microRNAs (mitomiRs) are insensitive to nuclease treatment even after the outer membrane is stripped, which is an essential step to eliminate contamination of endoplasmic reticulum and other single membraned vesicles5. These studies open a new chapter in understanding potential role(s) of mitomiRs in development and disease.
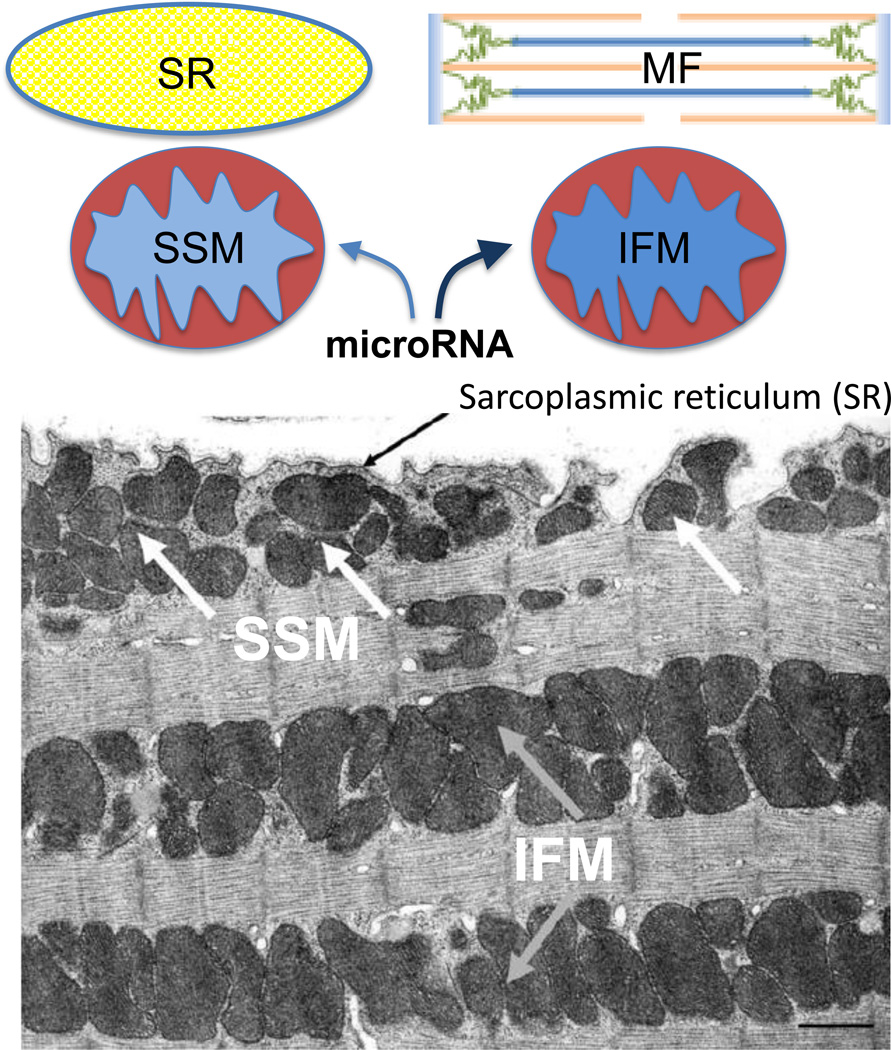
Jagannathan et al., went further to provide evidence that specific mitomiRs may be targeted to different populations of mitochondria within the same compartment of cardiac muscle1. It was initially observed more than 4 decades ago that cardiomyocytes contain two populations of mitochondria, one beneath sarcoplasmic reticulum (called subsarcoplasmic mitochondria or SSM) and the other residing between myofibrils (called interfibrillar mitochondria or IFM) (Figure), which could be separately extracted and then purified6. Various studies have linked such distinct mitochondria populations to heart failure and other diseases7,8. In the present study, using heart from mice insulted with a drug to induce type I diabetes, the authors isolated those two populations of mitochondria using a further improved methodology, and then subjected purified small RNA to microarray analysis. Coupled with validation by qPCR, they identified a large number of nuclear genome-derived microRNAs in the mitochondria, some of which appear to be differentially targeted to SSM versus IFM upon diabetic insult (Figure). One in particular, miR-378, was chosen for detailed analysis, as the mitomiR appears to selectively target IFM upon diabetes mellitus, despite unaltered total levels in normal and diseased hearts. Because the purification procedure involves Polytron treatment and enzymatic digestion, which may be prone for some unintended artifacts, validation of the data will require in situ analysis by high-resolution microscopy for specific mitomiRs, certain key protein factors involved (such as FXR1, see below), and ideally, functional alternations (such as membrane potential and ROS production) in the affected population of mitochondria in future studies.
Figure 1.
Diagram of Sarcoplemmal mitochondria (SSM) and Interfibrillar mitochondria (IFM) in cardiac muscle (the EM image is from reference 6 with authors’ permission). Diabetic insult is currently reported to induce redistribution of specific microRNAs in these mitochondrial subpopulations, which may influence critical signaling events in sarcoplasmic reticulum (SR, the major Ca2+ storage site) and/or the contractile activity of myofibrils (MF) in cardiomyocytes.
Based on bioinformatics prediction, coupled with UV-induced crosslinking of Ago2 (a key component of the RNA-Induced Silencing Complex or RISC), the authors went to great lengths to identify mitomiR-378 as a putative regulator of ATP synthase F0 subunit 6 (ATP6), a mitochondrial encoded protein responsible for transporting H+ protons across the inner mitochondrial membrane during aerobic respiration. A reporter-based assay was used to show the ability of miR-378 to target the sequence containing there deduced microRNA regulatory elements (MREs) in transfected HEK293 cells, though it remains unclear which specific MRE or all are responsible for the observed effect. This experiment would need to be repeated with mutant reporters to help validate the modest base-pairing potential in the seed region of the effective MRE(s).
Interestingly, the authors discovered that, while Ago2 went down in both SSM and IFM, the Ago2-associated RNA binding protein FXR1 was dramatically increased in IFM upon diabetic insult. Concordantly, ATP6 was selectively down regulated at both the transcript and protein levels in IFM of diabetic mice. These observations raised a series of mechanistic questions. First, as both ATP6 mRNA and protein were decreased, it remains to be determined whether decreased mRNA could entirely account for the reduction of protein, and thus, it is unclear whether mitomiR-378 is involved in the regulation of translation, as implied. This question is pertinent to the current study, as FXR1 has been long known for its role in the regulation of mRNA stability9,10, which also begs the question of whether ATP6 mRNA down-regulation is mediated by Ago2, FXR1, or both in conjunction with mitomiR-378. Most importantly, it is critical to determine direct versus indirect effects (e.g. via some miR-378 targets in the cytoplasm).
Sorting out the mechanistic questions raised above will require a cellular model in which the putative mitomiR-mediated effect(s) observed in the heart could be recapitulated. The authors accomplished this on HL-1 cells, observing that transfected miR-378 could indeed down regulate ATP6 mRNA and protein. Although not further pursued, this cellular model would allow for further testing of the regulation at mRNA stability and/or translation levels as well as for the functional requirement of Ago2, FXR1, or both in the process. The most challenging question is to differentiate between direct or indirect effect. However, a recent study has provided a diagnostic criterion for microRNA-mediated function in the cytoplasm versus mitochondria5. In this study, it was demonstrated that Ago2, but not GW182 (an essential co-factor of Ago2 in the RISC complex), is present in the mitochondria, which was now further confirmed by Jagannathan et al. in the current study1. Because GW182 is known to be required for microRNA-mediated mRNA decay and translational repression in the cytoplasm11, knockdown of GW182 would affect microRNA-mediated functions in the cytoplasm, but not in the mitochondria. This critical experiment remains to be carried out in order to validate the direct effect of mitomiR-378 in the mitochondria, and is also one of the most important functional tests for studying any mitomiR in future studies.
In summary, the findings of Jagannathan et al. raise an interesting possibility that diabetic insult may trigger microRNA redistribution among spatially organized mitochondria in cardiac cells, thus suggesting a novel mechanism for disease-induced cardiomyopathy. The findings are timely, as we are at the beginning to understand an expanded role of microRNAs in different cellular compartments, not just the cytoplasm. Although there still remain an array of questions regarding the function and mechanism of mitomiRs in mitochondria, as addressed above, the authors have established a biological system to study not just miR-378, but also the many other microRNAs that seem to be perturbed in diabetic heart. This opens the door for future studies aimed at characterizing the mechanism of signal-induced uptake of specific mitomiRs into spatially distinct organelles. Additionally, due to an apparent improvement of cardiac contractile activity with intraperitoneally-injected antagomir-378 reported in the current study, this observation points to an exciting new therapeutic route for treating heart malfunction in diabetics patients.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Stricker JC, Croston TL, Baseler WA, Lewis SE, Martinez I, Hollander JM. Translational regulation of the mitochondrial genome following redistribution of mitochondrial microRNA (mitomiR) in the diabetic heart. Circ Cardiovasc Genet. 2015;8 doi: 10.1161/CIRCGENETICS.115.001067. XXX-XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, Shearwood AM, Haugen E, Bracken CP, Rackham O, Stamatoyannopoulos JA, Filipovska A, Mattick JS. The human mitochondrial transcriptome. Cell. 2011;146:645–658. doi: 10.1016/j.cell.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandiera S, Matégot R, Girard M, Demongeot J, Henrion-Caude A. MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic Biol Med. 2013;64:12–19. doi: 10.1016/j.freeradbiomed.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Nouws J, Shadel GS. MicroManaging mitochondrial translation. Cell. 2014;158:477–478. doi: 10.1016/j.cell.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, Huang J, Zhao X, Zhou J, Yan Y, Zhang H, Guo P, Sun H, Guo L, Zhang Y, Fu XD. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158:607–619. doi: 10.1016/j.cell.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 7.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new layer in cardiac pathophysiology. Cardiovasc Res. 2010;88:30–39. doi: 10.1093/cvr/cvq225. [DOI] [PubMed] [Google Scholar]
- 8.Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X mental retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan S, Steitz JA. AU-rich-melement-mediated upregualtion of translation by RXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol. 2013;768:147–163. doi: 10.1007/978-1-4614-5107-5_9. [DOI] [PubMed] [Google Scholar]