Abstract
Cognitive impairment is as an important feature of Multiple Sclerosis (MS), and might be even more relevant to patients than mobility restrictions. Compared to the multitude of studies investigating memory deficits or basic cognitive slowing, executive dysfunction is a rarely studied cognitive domain in MS, and its neural correlates remain largely unexplored. Even rarer are topological studies on specific cognitive functions in MS. Here we used several structural MRI parameters – including cortical thinning and T2 lesion load – to investigate neural correlates of executive dysfunction, both on a global and a regional level by means of voxel- and vertex-wise analyses. Forty-eight patients with relapsing-remitting MS and 48 healthy controls participated in the study. Five executive functions were assessed, i.e. verbal and figural fluency, working memory, interference control and set shifting. Patients scored lower than controls in verbal and figural fluency only, and displayed widespread cortical thinning. On a global level, cortical thickness independently predicted verbal fluency performance, when controlling for lesion volume and central brain atrophy estimates. On a regional level, cortical thinning in the anterior cingulate region correlated with deficits in verbal and figural fluency and did so in a lateralised manner: Left-sided thinning was related to reduced verbal – but not figural – fluency, whereas the opposite pattern was observed for right-sided thinning. We conclude that executive dysfunction in MS patients can specifically affect verbal and figural fluency. The observed lateralised clinico-anatomical correlation has previously been described in brain-damaged patients with large focal lesions only, for example after stroke. Based on focal grey matter atrophy, we here show for the first time comparable lateralised findings in a white matter disease with widespread pathology.
Abbreviations: EDSS, Expanded Disability Status Scale; FDR, false discovery rate; FLAIR, fluid attenuated inversion recovery; MPRAGE, magnetization prepared rapid gradient-echo imaging; MS, multiple sclerosis; TVW, third ventricle width; VLSM, voxel-lesion symptom mapping
Keywords: Multiple sclerosis, Executive function, Fluency, Atrophy, Cortical thinning
Highlights
-
•
Executive dysfunction in Multiple Sclerosis (MS) can specifically affect fluency.
-
•
Fluency deficits in MS correlate with thinning of the anterior cingulate cortex.
-
•
This correlation seems lateralised and modality-specific.
1. Introduction
Multiple Sclerosis (MS) is a chronic demyelinating disease affecting all parts of the central nervous system. It used to be characterised primarily by its sensory, motor and visual symptoms. However, cognitive impairment is now recognised as an important feature of MS, prevalent in 43–70% of patients (Chiaravalloti and DeLuca, 2008). It might even be more relevant to patients than mobility restrictions (Amato et al., 2006). As MS has usually an early onset in young adult life (Calabrese, 2006), cognitive impairment significantly contributes to patients' disability status, is critical for working capacity, and thus negatively affects quality of life (Rao et al., 1991). Processing speed and episodic memory seem the most prominent cognitive features of MS (Chiaravalloti and DeLuca, 2008); however, MS patients often exhibit significant deficits in executive functions too (Drew et al., 2008). Although the latter can have devastating effects on patients' everyday life, their level of independence and societal costs (Amato et al., 2013), studies examining executive deficits in MS patients are relatively rare (Foong et al., 1997, Henry and Beatty, 2006, Radomski et al., 2015), compared to the multitude of studies investigating memory deficits or basic cognitive slowing (e.g. Chiaravalloti and DeLuca, 2008, Rogers and Panegyres, 2007, Roth et al., 2015). Traditionally, MS has been thought of as a white matter disease, with focal demyelinating lesions in the white matter being the pathological hallmark. However, this view has been challenged in recent years. It is now known that grey matter areas including the cerebral cortex can also be affected (Calabrese et al., 2012), a finding demonstrated first in post-mortem analyses (Kidd et al., 1999). In addition to focal lesions, brains of MS patients sometimes show considerable atrophy (Barkhof, 2002), which affects not only white, but also grey matter structures. Atrophy can become manifest, for example, as widespread cortical thickness reduction, with a predominant involvement of temporal and frontal regions (Calabrese et al., 2010, Narayana et al., 2013, Sailer et al., 2003).
Modern MRI techniques and post-processing methods assessing atrophy parameters contribute to the improvement in understanding the mechanisms responsible for physical and cognitive impairment in MS (Filippi and Rocca, 2010). During the last three decades, many studies have demonstrated an association between white matter pathology and cognitive impairment (e.g. Rao et al., 1989). However, only modest correlations were found (e.g. Foong et al., 1997, Swirsky-Sacchetti et al., 1992), so that a majority of variance in cognitive performance remained unexplained. MRI markers of atrophy such as increased third ventricle width or reduced total white and grey matter volumes are consistently found to correlate more strongly with cognitive deficits (Amato et al., 2004, Benedict et al., 2006) than white matter lesion load. In the last decade, grey matter pathology, i.e. cortical and deep grey matter atrophy and cortical lesion load, has been identified as a significant substrate of cognitive impairment, by showing particularly strong associations (e.g. Amato et al., 2004, Calabrese et al., 2009, Nielsen et al., 2013, Zivadinov et al., 2001).
Despite the huge amount of correlation studies between different structural alterations and cognitive dysfunction in MS, focal–topological studies on specific cognitive functions are rare. For example, deficits in sustained attention and working memory were related to lesions in bilateral frontal and parietal white matter (Sperling et al., 2001), and deficits in verbal learning to lesions in the left frontal lobe (Reuter et al., 2011). With regard to atrophy, bilateral hippocampal atrophy has been related to impaired verbal learning (Sicotte et al., 2008).
The aim of the present study was to investigate executive dysfunction in patients with MS and to analyse its relationship with both global and regional MRI markers. Our methodological focus was on cortical thinning. Only few previous studies have addressed the identification of cortical thinning in MS and its association to global cognitive impairment (Calabrese et al., 2010, Morgen et al., 2006, Tekok-Kilic et al., 2007). And to the best of our knowledge, only a single study so far investigated the impact of regional cortical thinning on specific cognitive disability, showing that focal thinning in the bilateral fusiform gyrus was related to impaired processing of facial expressions (Mike et al., 2013). Here we extend this approach by examining whether similar foci can be found for MS-related executive dysfunction.
2. Materials and methods
2.1. Participants
Forty-eight patients with a definite diagnosis of MS according to the McDonald 2010 criteria (Polman et al., 2011) and a relapsing–remitting course were recruited at the Multiple Sclerosis Centre of the University Hospital of Zurich. Forty-seven patients (98%) were treated with an immunomodulatory drug — 32 with natalizumab, ten with beta-interferons, three with fingolimod, one with glatiramer acetat, one with dimethylfumarate, and one patient had no disease-modifying therapy. We applied the following exclusion criteria: a) relapse or steroid-treatment during the last two months, b) current or past neurological disorder in addition to multiple sclerosis, c) psychiatric disorder apart from multiple sclerosis-related depressive mood state, d) a reading-relevant visual acuity deficit, e) a writing- and/or drawing-relevant upper limb sensorimotor impairment of the dominant hand and f) dysarthria. Furthermore, 48 age-, gender-, handedness-, and education-matched healthy controls without previous or present history of neurological or psychiatric dysfunction were included. The study was approved by the regional Ethics Committee. All participants provided written informed consent. Control participants received financial compensation for their attendance.
2.2. Neuropsychological and neurological examination
Neuropsychological and neurological (only for the patient group) examinations were performed within one month of the MRI scan described below by experienced clinicians of the Multiple Sclerosis Centre. Five executive functions were examined. First, verbal-phonematic fluency was assessed with the Regensburger verbal fluency test (Aschenbrenner et al., 2000). Participants were required to generate as many words as possible beginning with the letter “s” in two minutes. Repetitions of word stems or deviations from test rules were regarded as errors; the number of correct answers was further analysed. The HAMASCH-five-points-test (Haid et al., 2002) was applied to assess figural fluency (Regard et al., 1982). This test required participants to create as many unique designs as possible through connecting at least two dots of a five-dot pattern with straight lines within three minutes. The total number of unique designs entered further analyses. Furthermore, working memory was assessed by a two-back task (Zimmermann and Fimm, 2007), where the total number of errors (number of misses plus number of false positives) was further analysed. The Colour-Word-Interference subtest of the Delis–Kaplan Executive Function System (Delis et al., 2001) was used to investigate response inhibition and set shifting. In the interference condition, participants had to name the colour of the ink in which the word was printed – which is at conflict with the word meaning – while inhibiting the propensity to read the word. In the switching condition, participants had to irregularly alternate between reading the word and naming the ink, depending on the presence or absence of a box surrounding the word. Again, ink colour and word meaning were always at conflict with one another. Furthermore, a third condition of the D-KEFS colour-word interference test, i.e. the colour naming condition, served as a control parameter for basic information processing speed. As this study focuses on executive functions rather than on basic cognitive slowing, corrected time-to-completion indices were calculated with this latter parameter, according to the procedure specified by Delis and colleagues (2001). In other words, and concerning both interference control and set shifting, two variables entered further analyses, i.e. the number of correct answers and the corrected time-to-complete. Moreover, participants had to complete a German version (Hautzinger and Bailer, 1992) of the CES-D Depression questionnaire (Radloff, 1977) and the Würzburg Fatigue Inventory (WEIMuS) (Flachenecker et al., 2008) to self-assess depressive symptoms as well as cognitive and physical fatigue during the last week. Similar to other studies (Sumowski et al., 2014), cognitive reserve was examined with vocabulary knowledge. In our study, the Multiple-Choice Word Test-B (Lehrl, 2005) was applied. Additionally, Expanded Disability Status Scale (EDSS) scores (Kurtzke, 1983) were obtained in all patients.
2.3. MR image acquisition protocol
All images were acquired with a neuro-optimised 1.5 Tesla MR scanner (Siemens Magnetom AvantoTM) equipped with a SQ-engine gradient (45 m/T/m @ 200 T/m/s) using a dedicated 32-channel head coil. The sequences acquired in each subject included a T1-weighted Magnetisation Prepared Rapid Gradient Echo (MPRAGE; voxel size = 1 x 1 x 1 mm, slice thickness = 1 mm, repetition time = 2420 ms, echo time = 4.18 ms, inversion time = 960 ms) and a T2-weighted Fluid Attenuated Inversion Recovery sequence (FLAIR; voxel size = 0.9 x 0.9 x 2.0 mm, slice thickness = 2 mm, repetition time = 5000 ms, echo time = 342 ms, inversion time = 1800 ms).
2.4. Image analysis
2.4.1. T2-hyperintense lesion volume
FLAIR-hyperintense lesions were identified and manually delineated with MRIcron (http://sph.sc.edu/comd/rorden/mricron), which was also used to measure total lesion volume. An experienced rater (TP), supervised by a neuroradiologist (BS), performed this lesion analysis. Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) was used to co-register and normalise individual lesion maps to MNI (Montreal Neurological Institute and Hospital) standard space. Normalised lesion maps were then mapped on a template brain with MRIcron to create lesion overlap plots. Included in the MRIcron package, the non-parametric mapping software (Rorden et al., 2007) was used to analyse possible associations of behavioural performance with the spatial distribution of lesions, i.e. voxel-based lesion symptom mapping (VLSM).
2.4.2. Central atrophy
Central brain atrophy was examined by measuring the width of the third ventricle (TVW), applying the procedure specified by Benedict and colleagues (Benedict et al., 2006). Accordingly, a line was drawn through the long axis of the third ventricle parallel to the interhemispheric fissure on the axial MPRAGE slice on which the third ventricle was best visible. Then a second line, perpendicular to the first one, was drawn through the midpoint of the first line and measured with the Jim software (Xinapse Systems Ltd., Northants (UK); http://www.xinapse.com).
2.4.3. Cortical thickness analyses
Cortical thickness evaluation was performed with the Freesurfer image analysis suite, which is documented and freely available online (http://surfer.nmr.mgh.harvard.edu). It is a set of automated processing procedures, requiring 3D MPRAGE MRI images and comprising skull-stripping, registration, intensity normalisation, Talairach transformation, tissue segmentation and surface parcellation. Further information and technical details of the procedures are described in prior publications (Fischl et al., 1999). To detect possible misclassifications of white and grey matter, e.g. due to multiple sclerosis lesions, all images were visually inspected after the white/grey matter segmentation. In two patients, a semi-automated correction of topological defects was required by manually adding control points.
2.5. Statistical analysis
Statistical analyses of the demographic and cognitive data were performed in SPSS (IBM, Chicago, USA, Version, 21.0, http://spss.com). Unless otherwise stated, a p-level below 5% was considered statistically significant. Assumptions for normality were tested for all continuous data with Kolmogorov–Smirnov tests, and parametric (t-test, Pearson correlation) or non-parametric tests (Mann–Whitney-U, Spearman rank correlation) were used when appropriate. Chi-Square tests were conducted to test for differences in frequency distributions between the two groups. Whole-brain vertex-wise statistical analyses of cortical thickness were performed with the Query Design Estimate Contrast (Qdec) module implemented in Freesurfer.
As a first step, we analysed significant group differences in demographic variables (age, cognitive reserve, gender, handedness), fatigue, depressive symptoms, global cortical thickness, third ventricle width, and executive functions. Further analyses were restricted to those executive functions for which significant impairments were observed. In order to analyse whole-brain vertex-wise differences in cortical thickness between the two groups a general linear model (GLM) was used. Key demographics (e.g. age, education) were not controlled for, as these did not statistically differ between groups. Group differences of the cortical thickness were separately calculated for each hemisphere. A false discovery rate (FDR) of 0.05 was used to control for multiple comparisons.
As a next step, we analysed the association of impaired executive functions with demographic and disease variables (age, disease duration, EDSS, education, fatigue, depressive symptoms) within the patient group. Any variable significantly associated with executive impairment were controlled for in subsequent analyses. In a third step, the relative contribution of global MRI variables on executive performance was examined by hierarchical regression analyses. Multicollinearity between MRI variables was assessed using the variance inflation factor.
In a final step, the relationship between regional structural alterations and executive dysfunction was examined. A general linear model at each vertex point of the cortical surface model was used to investigate the correlation between areas of regional cortical thickness with executive impairment. Statistical surface maps showing significant correlations between cortical thinning and executive performance were generated by thresholding the images of t-statistics at a 0.05 significance level, corrected for FDR. For the VLSM we performed Brunner–Munzel tests (Brunner and Munzel, 2000) at a threshold of 5% FDR to identify lesioned voxels associated with executive deficits. Only voxels affected in at least two patients were considered for analysis. For all vertex- and voxel-wise analyses, only clusters with a continuous extent of 10 mm2 were reported.
3. Results
3.1. Demographic, clinical and conventional MRI assessment
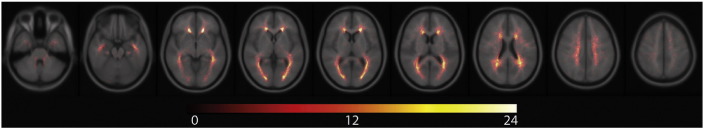
Age (p = 0.691), education (p = 0.398), gender (p > 0.99), and handedness (p = 0.506) did not significantly differ between controls and patients. An overview of the main demographic, clinical and MRI features of patients and controls is given in Table 1. Lesion probability maps are shown in Fig. 1, displaying the expected accumulation (Rossi et al., 2012) of T2-hyperintense lesions in periventricular areas.
Table 1.
Demographic and disease characteristics.
| RRMS patients (n = 48) |
Healthy controls (n = 48) |
Z-/t-/Χ2 score |
p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (mean, SD) a | 38.8 (9.3) | 38.0 (9.6) | t = 0.398 | 0.691 |
| Females/males (number) | 37/11 | 37/11 | Χ2 = 0.000 | > 0.999 |
| Years of education (median, range) | 13 (11–22) | 14 (11–20) | Z = − 0.846 | 0.398 |
| Disease duration, years since diagnosis (mean, SD) | 6.5 (5.4) | n/a | n/a | n/a |
| EDSS score (mean, SD) | 2.69 (1.9) | n/a | n/a | n/a |
| MRI metrics | ||||
| T2- hyperintense lesion volume, cm3 (mean, SD) | 8.82 (9.24) | n/a | n/a | n/a |
| Cortical thickness global, mm (mean, SD) a | 2.33 (0.13) | 2.42 (0.11) | t = − 3.435 | < 0.010⁎⁎ |
| Third ventricle width, mm (median, range) | 3.70 (1.2–12.7) | 2.40 (1.2–7.4) | Z = − 4.785 | < 0.010⁎⁎ |
Abbreviations: SD = standard deviation
Unless otherwise noted statistical comparisons are based on Mann–Whitney U tests.
Because of normal distribution in both samples statistical comparisons are based on t-tests.
p < 0.01.
Fig. 1.
Spatial distribution of T2-hyperintense lesions.
All normalised T2-hyperintense lesions of all patients are shown in the lesion maps, illustrating pronounced periventricular accumulation. Image orientation follows the radiological convention (right on left side). The colour bar indicates the number of overlapping lesions.
3.2. Cognitive findings
Test and questionnaire results are displayed in Table 2. Cognitive reserve was nearly identical in the two groups. Performances in the working memory, response inhibition and set shifting tests did not significantly differ between controls and patients (all p > 0.05). However, differences were found in the number of items generated during the verbal and figural fluency tasks (both p < 0.001), with patients generating fewer items than controls in both tasks. Patients were significantly slower in colour naming condition of the D-KEFS word-colour-interference test, indicating slowed basic information processing (p < 0.05). Moreover, patients showed higher levels of cognitive (p = 0.003) and physical (p < 0.001) fatigue, as well as a higher level of depressive mood state (p = 0.025), relative to controls.
Table 2.
Neuropsychological test and questionnaire results.
| RRMS patients | Healthy controls | t-/Z-Score | p-Value | |
|---|---|---|---|---|
| Executive functions | ||||
| Verbal fluency, no. correct (median, range) | 18.5 (9–34) | 25 (15–45) | Z = − 4.821 | < 0.010⁎⁎ |
| Figural fluency, no. correct (mean, SD) a | 32.42 (8.3) | 43.23 (8.6) | t = − 6.266 | < 0.010⁎⁎ |
| Working memory, errors total (median, range) | 3.0 (0–16) | 2.0 (0–11) | Z = − 1.611 | 0.107 |
| Response inhibition, hits (median, range) | 49 (43–50) | 50 (45–50) | Z = − 1.805 | 0.071 |
| Response inhibition, corrected time-to-complete (median, range) 1 | 1 (− 3–5) | 1 (− 8–5) | Z = − 1.268 | 0.205 |
| Set shifting, hits (median, range) | 49.0 (41–50) | 49 (45–50) | Z = − 0.756 | 0.450 |
| Set shifting, corrected time-to-complete (median, range) 2 | 0 (− 6–5) | 0 (− 7–5) | Z = − 1.024 | 0.306 |
| Questionnaires | ||||
| Cognitive fatigue (median, range) | 5.5 (0–30) | 2.0 (0–21) | Z = − 3.216 | < 0.010⁎⁎ |
| Physical fatigue (median, range) | 6.0 (0–15) | 1.5 (0–32) | Z = − 3.005 | < 0.010⁎⁎ |
| Total fatigue (median, range) | 13.5 (0–55) | 4.0 (0–36) | Z = − 3.332 | < 0.010⁎⁎ |
| Depressive symptoms (median, range) | 9.0 (0–37) | 6.5 (0–23) | Z = − 2.243 | 0.025⁎ |
| Cognitive reserve | ||||
| MWT-B (median, range) | 30.5 (23–37) | 31.0 (25–36) | Z = − 0.589 | 0.556 |
Abbreviations: MWT-B = Multiple-Choice Word Test-B; SD = Standard deviation.
Unless otherwise noted statistical comparisons are based on Mann–Whitney U tests.
Because of normal distribution in both samples statistical comparisons are based on t-tests.
p < 0.05.
p < 0.01.
Here, corrected time-to-complete means the difference of the scaled scores between the interference condition and the basic colour naming condition, as proposed by Delis et al. (2001).
Here, corrected time-to-complete means the difference of the scaled scores between the interference condition and set shifting condition.
Verbal and figural fluency performances were not significantly associated with age, disease duration, depressive symptoms, and cognitive or physical fatigue (all p > 0.05). However, our measure of cognitive reserve was related to verbal fluency (r = 0.298; p = 0.02), while EDSS scores correlated with figural fluency performance (r = − 0.310; p = 0.03). Therefore, these two variables were controlled for in further analyses.
3.3. MRI findings
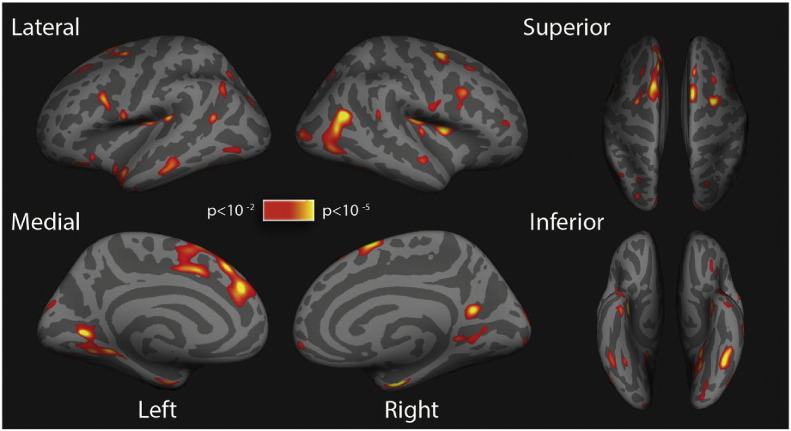
Mean global cortical thickness was reduced in multiple sclerosis patients compared to controls (Table 1; p < 0.001). Whole-brain vertex-wise analysis revealed multiple brain areas of significant cortical thinning in both hemispheres in patients compared with healthy controls (Fig. 2). Cortical thinning was spread over broad regions of both hemispheres. Anatomical locations of clusters of decreased cortical thickness in the patients are summarised in Supplementary Table 1.
Fig. 2.
Differences in cortical thickness.
Statistical maps representing significant cortical thickness differences between patients and controls on lateral, medial, inferior and superior view of inflated brains. A colour scale indicates statistical significance and shows p-values. Red/yellow areas represent clusters of significant cortical thinning in patients, relative to controls.
3.4. Global analyses
The hierarchical regression model accounted for 45.7% of the variance in verbal fluency performance. No predictor indicated multicollinearity (all variance inflation factors < 4). After controlling for education in block one, lesion volume retained in block two, with larger lesion volume predicting worse performance in verbal fluency (R2 = 0.288, p = 0.003). In block three, TVW as an indicator of central brain atrophy accounted for an additional 7% of variance in verbal fluency performance, with more atrophy predicting worse verbal fluency performance (R2 = 0.364, p = 0.026). Finally, in block four global cortical thickness accounted for additional 9% of variance in verbal fluency, with a thinner cortex being associated with worse verbal fluency performance (R2 = 0.457, p = 0.010). In other words, cortical thickness independently predicted verbal fluency, even when controlling for lesion volume and central brain atrophy estimates. Regarding figural fluency, the full hierarchical regression model accounted for 30.2% of variance in figural fluency performance. After controlling for EDSS in block one, there was a negative effect of lesion volume on figural fluency (R2 = 0.285, p = 0.009) in block two, but no effect of brain atrophy (TVW) in block three (p = 0.335) or cortical thinning in block four (p = 0.729). Within the control group, neither global cortical thickness nor the TVW significantly correlated with verbal or figural fluency performance (all p > 0.05).
3.5. Regional analyses
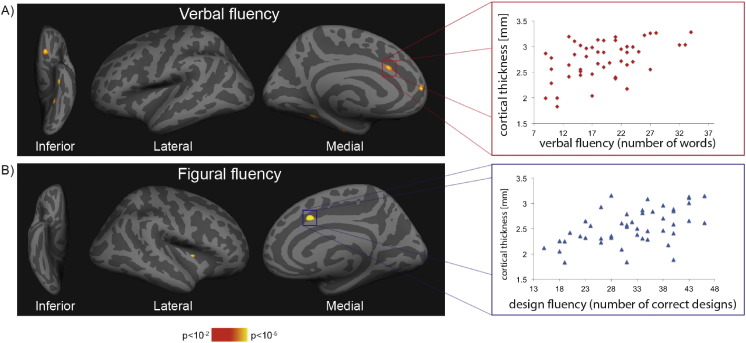
Clusters of significant correlations between regional cortical thickness and verbal or figural fluency performance within the patient group are shown in Fig. 3 and Table 3. Poorer performance in the verbal fluency task correlated with a thinner cortex within the left anterior cingulate, superior frontal, lateral orbitofrontal, fusiform and superior parietal region. These clusters survived FDR-correction. Performance in the figural fluency task was associated with cortical thickness in the right anterior cingulate and insula. No significant correlations were observed in the control group. Concerning the relation of T2-hyperintense lesion location with fluency, VLSM revealed no clusters of lesioned voxels associated with performance in the two fluency tasks.
Fig. 3.
Association of cortical thickness and fluency performance.
Correlation of fluency performance and cortical thickness within patients.
On the left side, statistical maps show clusters of significant correlation between verbal (A) and figural (B) fluency performance and cortical thickness in the patient groups. On the right side, scatterplots illustrate the correlation between cortical thickness in the anterior cingulate cortex cluster and verbal (A), respectively fluency (B) performance.
Table 3.
Regions of cortical thinning significantly associated with verbal and figural fluency in the patient group.
| Cluster number |
Talairach coordinates x, y, z |
Surface extension (mm2) | -log (p) | Structures |
|---|---|---|---|---|
| Left hemisphere, medial surface | ||||
| Verbal fluency | ||||
| 1 | − 35.5, 17.1, − 19.6 | 109.6 | 5.412 | Fusiform |
| 2 | − 10.5, 52.3, 9.6 | 50.75 | 4.999 | Superior frontal |
| 3 | − 14.2, 23.8, − 16.4 | 85.84 | 4.956 | Lateral orbitofrontal |
| 4 | − 34.0,-48.4, − 10.2 | 58.35 | 4.739 | Fusiform |
| 5 | − 8.3, 21.8, 27.9 | 44.60 | 5.676 | Caudal anterior cingulate |
| Right hemisphere, medial and lateral surfaces | ||||
| Figural fluency | ||||
| 1 | 13.1, 10.6, 37.3 | 46.01 | 5.386 | Anterior cingulate |
| 2 | 34.1, 3.3, 7.6 | 17.61 | 4.664 | Insula |
All p-values corrected for multiple comparison using FDR = 0.05. Small significant clusters are not shown (Cluster size < 10 mm2). Anatomical terms are used according to the Desikan–Killiany template.
4. Discussion
The results of the present study indicate that executive dysfunction in patients with multiple sclerosis can specifically affect verbal and figural fluency. For both deficits, side- and site-specific cortical correlates in the anterior cingulate region were found by means of cortical thickness analysis. Although several clusters of cortical thinning were associated with verbal and figural fluency, only one of them was congruent across the two hemispheres, i.e. the one in the anterior cingulate cortex. The main finding of the present study is the lateralized characteristic of the correlational analyses: Thinning in the left anterior cingulate cortex predicted verbal – but not figural – fluency, while the opposite pattern was found for thinning in the right anterior cingulate cortex. This lateralisation corresponds well with lesion studies in patients with focal brain damage linking verbal fluency with the left prefrontal cortex and figural fluency with the right prefrontal cortex (Robinson et al., 2012, Schwartz and Baldo, 2001). These studies were based on patients with relatively large focal lesions, for example due to stroke or brain tumours. On the basis of focal grey matter atrophy, our study demonstrates the same lateralised clinico-anatomical correlation pattern in MS, which is traditionally seen as a white matter disease with widespread pathology.
Previous studies showed that tests of verbal fluency are amongst the most sensitive neuropsychological instruments to assess cognitive impairment in multiple sclerosis, relative to other measures of executive functioning (Henry and Beatty, 2006). Fluency tasks in general have a long tradition in the examination of one of the most important executive function, i.e. the voluntary generation of new series of responses. Sustained activation for the duration of the task is required in order to generate non-overlearned responses for all types of fluency tasks (Robinson et al., 2012). Performance deficits on fluency tasks are due to a failure of “energisation” (Stuss and Alexander, 2007), that is, the process of initiating and sustaining any response, which is required for the generation of new responses.
In this framework, the fluency deficits found in the present study could be re-labelled “cognitive hypoenergisation”. It might be tempting to view this latter concept as identical to that of cognitive fatigue. However, in line with previous studies showing that self-reported fatigue is not associated with cognitive deficits in multiple sclerosis (Morrow et al., 2009), we did not find a significant correlation between fluency performance and self-reported cognitive fatigue in our patient sample.
Patients with large medial frontal lobe lesions that involve the cingulate cortex often show deficits in spontaneous initiation of speech and movement and/or an inability to suppress externally triggered subroutines (Paus, 2001). Akinetic mutism, which is caused by bilateral medial frontal lesions, represents an extreme example of this clinico-anatomical association (Barris and Schuman, 1953). Robinson and colleagues (Robinson et al., 2012) conclude that energisation processes – necessary for fluency tasks – are bilaterally represented in the medial frontal region. To the best of our knowledge, we here show for the first time that the relation between anterior cingulate pathology and hypoenergetic cognitive functioning can also be found in patients with multiple sclerosis.
With regard to the clinical assessment of executive functions in multiple sclerosis, we propose that the inclusion of fluency tasks is particularly relevant. These brief tests are sensitive to executive impairment and – as suggested by our findings – cortical involvement, providing clinicians' efficient tools in situations when examination time is limited.
Comparable to previous studies, we could only find modest association between T2-hyperintense lesion burden and cognitive test performance. Roosendaal and colleagues (2009) suggest that one main reason of this is that pathology outside the focal white matter lesions, in the so-called normal appearing white matter (NAWM), remains largely undetected by conventional MRI. By now, there is evidence that advanced MRI sequences, such as diffusion tensor imaging (DTI) exhibit more robust relationship with cognitive variables (e.g. Roosendaal et al., 2009, Van Hecke et al., 2010), as it provides in-vivo information about the orientation and integrity of WM fibre bundles. Thus, NAWM changes should be investigated as an additional mechanism related to cognitive dysfunction beside T2-hyperintense lesion volume, central and cortical atrophy. Furthermore, DTI – potentially in combination with fMRI – would allow analyses of neuroconnectivity and neuronal networks. As cognitive functions and thus also fluency performances are likely based on a network of brain regions – rather than on a single region – additional knowledge could be gained with such analyses. Based on previous and our findings, we propose that the AC is an important node of the fluency network.
The present study is not without limitations: First, only MS patients suffering from the relapsing-remitting subtype were included, so that the generalisation of the findings to other forms of multiple sclerosis such as the primary progressive variant is debatable. Furthermore, the cross-sectional design of the study does not allow causal interpretation of the observed association between structural MRI changes and cognitive impairment.
The following are the supplementary data related to this article.
Clusters of cortical areas (left and right hemisphere) showing significant cortical thinning in relapsing-remitting MS patients compared to healthy controls.
Conflict of Interest
ML received grants, funding or honoraria from Bayer, Biogen, Genzyme, Merck, Novartis and Teva. The other authors declare that they have no conflict of interest.
Acknowledgment
This work was supported by the Swiss Multiple Sclerosis Society and Bayer Switzerland (Bseva/SGSZJX313B21/6890000).
Contributor Information
Olivia Geisseler, Email: Olivia.geisseler@usz.ch.
Tobias Pflugshaupt, Email: Tobias.pflugshaupt@luks.ch.
Ladina Bezzola, Email: l.bezzola@psychologie.uzh.ch.
Katja Reuter, Email: katja.reuter@usz.ch.
David Weller, Email: david.weller@usz.ch.
Bernhard Schuknecht, Email: image-solution@ggaweb.ch.
Peter Brugger, Email: peter.brugger@usz.ch.
Michael Linnebank, Email: michael.linnebank@helios-kliniken.de.
References
- Amato M., Bartolozzi M., Zipoli V., Portaccio E., Mortilla M., Guidi L., Siracusa G., Sorbi S., Federico A., De Stefano N. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- Amato M., Zipoli V., Portaccio E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J. Neurol. Sci. 2006;245:41–46. doi: 10.1016/j.jns.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Amato M.P., Langdon D., Montalban X., Benedict R.H.B., DeLuca J., Krupp L.B., Thompson A.J., Comi G. Treatment of cognitive impairment in multiple sclerosis: position paper. J. Neurol. 2013;260:1452–1468. doi: 10.1007/s00415-012-6678-0. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner A., Tucha O., Lange K. Hogrefe Verlag; Göttingen: 2000. Regensburger wortflüssigkeits-test. Handanweisung. [Google Scholar]
- Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr. Opin. Neurol. 2002;15:239–245. doi: 10.1097/00019052-200206000-00003. [DOI] [PubMed] [Google Scholar]
- Barris R.W., Schuman H.R. Bilateral anterior cingulate gyrus lesions; syndrome of the anterior cingulate gyri. Neurology. 1953;3:44–52. doi: 10.1212/wnl.3.1.44. [DOI] [PubMed] [Google Scholar]
- Benedict R., Bruce J.M., Dwyer M.G., Abdelrahman N., Hussein S., Weinstock-Guttman B., Garg N., Munschauer F., Zivadinov R. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch. Neurol. 2006;63:1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- Brunner E., Munzel U. The nonparametric Behrens–Fisher problem: asymptotic theory and a small-sample approximation. Biom. J. 2000;42:17–25. [Google Scholar]
- Calabrese P. Neuropsychology of multiple sclerosis—an overview. J. Neurol. 2006;253(Suppl. 1):I10–I15. doi: 10.1007/s00415-006-1103-1. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Agosta F., Rinaldi F., Mattisi I., Grossi P., Favaretto A., Atzori M., Bernardi V., Barachino L., Rinaldi L., Perini P., Gallo P., Filippi M. Cortical lesions and atrophy associated with cognitive impairment in relapsing–remitting multiple sclerosis. Arch. Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Rinaldi F., Mattisi I., Grossi P., Favaretto A., Atzori M., Bernardi V., Barachino L., Romualdi C., Rinaldi L., Perini P., Gallo P. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology. 2010;74:321–328. doi: 10.1212/WNL.0b013e3181cbcd03. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Poretto V., Favaretto A., Alessio S., Bernardi V., Romualdi C., Rinaldi F., Perini P., Gallo P. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–2961. doi: 10.1093/brain/aws246. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N., DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Delis D., Kaplan E., Kramer J. The Psychological Corporation; San Antonio (TX): 2001. Delis–Kaplan Executive Function System (D–KEFS) [Google Scholar]
- Drew M., Tippett L., Starkey N., Isler R. Executive dysfunction and cognitive impairment in a large community-based sample with multiple sclerosis from New Zealand: a descriptive study. Arch. Clin. Neuropsychol. 2008;23:1–19. doi: 10.1016/j.acn.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Filippi M., Rocca M.A. MRI and cognition in multiple sclerosis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2010;31:S231–S234. doi: 10.1007/s10072-010-0367-5. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Flachenecker P., König H., Meissner H., Müller G. Fatigue bei Multipler Sklerose: Validierung des “Würzburger Erschöpfungs-Inventar bei Multipler Sklerose (WEIMuS).”. Neuro Rehabil. 2008;14:299–306. [Google Scholar]
- Foong J., Rozewicz L., Quaghebeur G., Davie C., Kartsounis L., Thompson A., Miller D., Ron M. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain. 1997;120:15–26. doi: 10.1093/brain/120.1.15. [DOI] [PubMed] [Google Scholar]
- Haid T., Martl C., Schubert F., Wenzl M., Kofler M., Saltuari L. Der “HAMASCH 5 Punkt Test” — erste Normierungsergebnisse. Z. Für Neuropsychol. 2002;13:233. [Google Scholar]
- Hautzinger M., Bailer M. Beltz Test GmbH; Göttingen: 1992. Allgemeine Depressionsskala ADS. [Google Scholar]
- Henry J.D., Beatty W.W. Verbal fluency deficits in multiple sclerosis. Neuropsychologia. 2006;44:1166–1174. doi: 10.1016/j.neuropsychologia.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kidd D., Barkhof F., McConnell R., Algra P.R., Allen I.V., Revesz T. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lehrl S. 5th ed. Spitta Verlag; Balingen: 2005. Mehrfachwahl–Wortschatz-intelligenz MWT-B. [Google Scholar]
- Mike A., Strammer E., Aradi M., Orsi G., Perlaki G., Hajnal A., Sandor J., Banati M., Illes E., Zaitsev A., Herold R., Guttmann C.R.G., Illes Z. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: a structural MRI study. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgen K., Sammer G., Courtney S., Wolters T., Melchior H., Blecker C., Oschmann P., Kaps M., Vaitl D. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing–remitting MS. Neuroimage. 2006;30:891–898. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Morrow S.A., Weinstock-Guttman B., Munschauer F.E., Hojnacki D., Benedict R.H.B. Subjective fatigue is not associated with cognitive impairment in multiple sclerosis: cross-sectional and longitudinal analysis. Mult. Scler. 2009;15:998–1005. doi: 10.1177/1352458509106213. [DOI] [PubMed] [Google Scholar]
- Narayana P.A., Govindarajan K.A., Goel P., Datta S., Lincoln J.A., Cofield S.S., Cutter G.R., Lublin F.D., Wolinsky J.S. Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. NeuroImage Clin. 2013;2:120–131. doi: 10.1016/j.nicl.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A.S., Kinkel R.P., Madigan N., Tinelli E., Benner T., Mainero C. Contribution of cortical lesion subtypes at 7 T MRI to physical and cognitive performance in MS. Neurology. 2013;81:641–649. doi: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O'Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Radomski A.D., Power C., Purdon S.E., Emery D.J., Blevins G., Warren K.G., Fujiwara E. Decision-making under explicit risk is impaired in multiple sclerosis: relationships with ventricular width and disease disability. BMC Neurol. 2015;15:61. doi: 10.1186/s12883-015-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Leo G., Haughton V., St Aubin-Faubert P., Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology. 1989;39:161–166. doi: 10.1212/wnl.39.2.161. [DOI] [PubMed] [Google Scholar]
- Rao S., Leo G., Ellington L., Nauertz T., Bernardin L., Unverzagt F. Cognitive dysfunction in multiple sclerosis II. Impact on employment and social functioning. Neurology. 1991;41:692–696. doi: 10.1212/wnl.41.5.692. [DOI] [PubMed] [Google Scholar]
- Regard M., Strauss E., Knapp P. Children's production on verbal and non-verbal fluency tasks. Percept. Mot. Skills. 1982;55:839–844. doi: 10.2466/pms.1982.55.3.839. [DOI] [PubMed] [Google Scholar]
- Reuter F., Zaaraoui W., Crespy L., Faivre A., Rico A., Malikova I., Confort-Gouny S., Cozzone P.J., Ranjeva J.-P., Pelletier J., Audoin B. Cognitive impairment at the onset of multiple sclerosis: relationship to lesion location. Mult. Scler. Houndmills Basingstoke Engl. 2011;17:755–758. doi: 10.1177/1352458511398265. [DOI] [PubMed] [Google Scholar]
- Robinson G., Shallice T., Bozzali M., Cipolotti L. The differing roles of the frontal cortex in fluency tests. Brain. 2012;135:2202–2214. doi: 10.1093/brain/aws142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.M., Panegyres P.K. Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J. Clin. Neurosci. 2007;14:919–927. doi: 10.1016/j.jocn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Roosendaal S.D., Geurts J.J.G., Vrenken H., Hulst H.E., Cover K.S., Castelijns J.A., Pouwels P.J.W., Barkhof F. Regional DTI differences in multiple sclerosis patients. NeuroImage. 2009;44:1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Rorden C., Karnath H.-O., Bonilha L. Improving lesion-symptom mapping. J. Cogn. Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rossi F., Giorgio A., Battaglini M., Stromillo M.L., Portaccio E., Goretti B., Federico A., Hakiki B., Amato M.P., De Stefano N. Relevance of brain lesion location to cognition in relapsing multiple sclerosis. PLoS ONE. 2012;7; e44826 doi: 10.1371/journal.pone.0044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A.K., Denney D.R., Lynch S.G. Information processing speed and attention in multiple sclerosis: reconsidering the Attention Network Test (ANT) J. Clin. Exp. Neuropsychol. 2015:1–12. doi: 10.1080/13803395.2015.1037252. [DOI] [PubMed] [Google Scholar]
- Sailer M., Fischl B., Salat D., Tempelmann C., Schonfeld M., Busa E., Bodammer N., Heinze H., Dale A. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Baldo J. Distinct patterns of word retrieval in right and left frontal lobe patients: a multidimensional perspective. Neuropsychologia. 2001;39:1209–1217. doi: 10.1016/s0028-3932(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Sicotte N., Kern K., Giesser B., Arshanapalli A., Schultz A., Montag M., Wang H., Bookheimer S. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Guttmann C.R., Hohol M.J., Warfield S.K., Jakab M., Parente M., Diamond E.L., Daffner K.R., Olek M.J., Orav E.J., Kikinis R., Jolesz F.A., Weiner H.L. Regional magnetic resonance imaging lesion burden and cognitive function in multiple sclerosis: a longitudinal study. Arch. Neurol. 2001;58:115–121. doi: 10.1001/archneur.58.1.115. [DOI] [PubMed] [Google Scholar]
- Stuss D.T., Alexander M.P. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski J.F., Rocca M.A., Leavitt V.M., Dackovic J., Mesaros S., Drulovic J., DeLuca J., Filippi M. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology. 2014;82:1776–1783. doi: 10.1212/WNL.0000000000000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirsky-Sacchetti T., Mitchell D., Seward J., Gonzales C., Lublin F., Knobler R., Field H. Neuropsychological and structural brain lesions in multiple sclerosis: a regional analysis. Neurology. 1992;42:1291–1295. doi: 10.1212/wnl.42.7.1291. [DOI] [PubMed] [Google Scholar]
- Tekok-Kilic A., Benedict R., Weinstock-Guttman B., Dwyer M., Carone D., Srinivasaraghavan B., Yella V., Abdelrahman N., Munschauer F., Bakshi R., Zivadinov R. Independent contributions of cortical gray matter atrophy and ventricle enlargement for predicting neuropsychological impairment in multiple sclerosis. Neuroimage. 2007;36:1294–1300. doi: 10.1016/j.neuroimage.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Van Hecke W., Nagels G., Leemans A., Vandervliet E., Sijbers J., Parizel P.M. Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J. Magn. Reson. Imaging JMRI. 2010;31:1492–1498. doi: 10.1002/jmri.22198. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Fimm B. Psytest; Herzogenrath: 2007. TAP Testbatterie zur Aufmerksamkeitsprüfung. [Google Scholar]
- Zivadinov R., Sepcic J., Nasuelli D., De Masi R., Bragadin L., Tommasi M., Zambito-Marsala S., Moretti R., Bratina A., Ukmar M., Pozzi-Mucelli R., Grop A., Cazzato G., Zorzon M. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2001;70:773–780. doi: 10.1136/jnnp.70.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clusters of cortical areas (left and right hemisphere) showing significant cortical thinning in relapsing-remitting MS patients compared to healthy controls.