Abstract
Neuroblastoma is the most common extra-cranial solid tumor in childhood; and patients in stage IV of the disease have a high propensity for tumor recurrence. Retinoid therapy has been utilized as a means to induce differentiation of tumor cells and to inhibit relapse. In this study, the expression of a common neuronal differentiation marker [neurofilament M (NF-M)] in human SK-N-SH neuroblastoma cells treated with 10 μM all-trans retinoic acid (ATRA) showed significantly increased expression in accordance with reduced cell number. This was accompanied by an increase in MitoSOX and DCFH2 oxidation that could be indicative of increased steady-state levels of reactive oxygen species (ROS) such as O2•− and H2O2, which correlated with increased levels of MnSOD activity and immuno-reactive protein. Furthermore PEG-catalase inhibited the DCFH2 oxidation signal to a greater extent in the ATRA-treated cells (relative to controls) at 96 h indicating that as the cells became more differentiated, steady-state levels of H2O2 increased in the absence of increases in peroxide-scavenging antioxidants (i.e., glutathione, glutathione peroxidase, and catalase). In addition, ATRA-induced stimulation of NF-M at 48 and 72 h was enhanced by decreasing SOD activity using siRNA directed at MnSOD. Finally, treatment with ATRA for 96 h in the presence of MnSOD siRNA or PEG-catalase inhibited ATRA induced increases in NF-M expression. These results provide strong support for the hypothesis that changes in steady-state levels of O2•− and H2O2 significantly contribute to the process of ATRA-induced differentiation in neuroblastoma, and suggest that retinoid therapy for neuroblastoma could potentially be enhanced by redox-based manipulations of superoxide metabolism to improve patient outcome.
Keywords: MnSOD, Retinoic acid, Reactive oxygen species, Neuroblastoma, Differentiation
Graphical abstract
Highlights
-
•
A role for ROS is proposed for retinoid-differentiation of neuroblastoma cells.
-
•
Superoxide and hydrogen peroxide coordinate with increased MnSOD activity.
-
•
Hydrogen peroxide is a potential signaling molecule to promote differentiation.
-
•
Preventing H2O2 degradation may improve retinoid based neuroblastoma therapies.
1. Introduction
Children diagnosed with high-risk neuroblastoma are highly susceptible to recurrent tumors, even after initial treatments such as surgery, radiation and/or chemotherapy. Retinoids are a successful therapeutic option for these patients. All-trans retinoic acid (ATRA; tretinoin) and 13-cis-retinoic acid (13-cis RA; isotretinoin), metabolites of Vitamin A, not only stimulate differentiation [34], but also inhibit cellular proliferation, induce apoptosis [2], and promote cell cycle arrest [9]. Although 13-cis RA is currently administered clinically for neuroblastoma, ATRA is the ultimate metabolite in vivo and one of the most potent differentiation inducers for human neuroblastoma in vitro [37]. The “cis” isoform likely has effects independent of ATRA; however, anti-proliferative capabilities are suggested to be due to isomerization to the “trans” form in human neuroblastoma cells [44]. Despite its ability to differentiate cancer cells, the drawback of long-term retinoid treatment is that patients acquire resistance to chemotherapeutic drugs such as adriamycin and cisplatin [20], [11]. Nonetheless, retinoids are a much safer, less toxic alternative. Studies indicate retinoids not only stimulate the production of reactive oxygen species (ROS), but also upregulate antioxidant defense pathways during neuroblastoma differentiation [36], [27], [35]. Nonetheless, to our knowledge, this is the first study performing a comprehensive analysis of the antioxidant response system (ROS and antioxidants) during all-trans retinoic acid- (ATRA) induced differentiation of a neuroblastoma cell line (SK-N-SH). Given the therapeutic benefits of retinoid treatment for differentiating neuroblastoma cells it is necessary to further characterize retinoids' influence on specific signaling pathways and identify the ROS responsible for the anti-proliferative activity in order to develop a biochemical rationale for enhancing therapeutic responses.
The current study was designed to determine if the mitochondrial manganese containing superoxide dismutase enzyme (MnSOD) was essential to ATRA-mediated differentiation in the SK-N-SH neuroblastoma model. The results showed 10 μM ATRA induced a significant increase in the differentiation marker, neurofilament M (NF-M) prior to induction of MnSOD activity in neuroblastoma cells. Furthermore suppressing the induction of MnSOD activity using an siRNA, enhanced NF-M expression in the presence of ATRA for 48 or 72 h. Finally polyethene glycol conjugated catalase (PEG-CAT) as well as siRNA against MnSOD were both able to suppress ATRA-induced increases in NF-M protein at 96 h of treatment with retinoids. Taken together, these data support the hypothesis that superoxide is essential for inducing the differentiation of neuroblastoma cells in the early phase (0–72 h) of ATRA treatment, whereas both superoxide and hydrogen peroxide play a role in modulating levels of NF-M at 96 h.
2. Materials and methods
2.1. Cell culture and treatment
For all experiments, the human neuroblastoma cell line (SK-N-SH) obtained from the American Type Culture Collection (Manassas, VA) was maintained in minimal essential medium (MEM; Sigma, St. Louis, MO) supplemented with 10% heat-inactivated bovine serum (Invitrogen, Carlsbad, CA), 1% penicillin/streptomycin/neomycin (Invitrogen), 1% non-essential amino acids (Invitrogen), and 1 mM sodium pyruvate (Sigma-Aldrich). Cells were grown at 37 °C in a humidified atmosphere containing 5% CO2. Dimethyl sulfoxide (DMSO) and all-trans retinoic acid (ATRA) were obtained from Sigma-Aldrich. DMSO (0.05%) treatment served as the control and followed the same regimen as ATRA treatment (10 μM). The concentration of ATRA used is consistent with previous reports to induce differentiation in this cell type [37], [30]. Representative pictures were obtained by use of an Olympus CKX41 Inverted Microscope with Camera and MicroSuite V Imaging software (10× magnification).
2.2. Growth rate analysis
On day one, cells were plated at a density of 2×103 cells/cm2. On day two, 2 plates were counted as the 24 h control. Two plates per treatment were counted and averaged each subsequent day for the duration of the experiment. ATRA-containing culture media was replenished every 48 h. The data are represented as the average log of cell numbers vs. time (hrs).
2.3. Western blot analysis
Cells were plated 24 h prior to initiating treatments at a density of 1–5×104 cells/cm2. Whole cell lysates were collected via scraping in cold phosphate buffered saline (PBS; 137 mM sodium chloride, 3 mM potassium chloride, 1 mM potassium phosphate monobasic, 10 mM sodium phosphate dibasic; pH 7.4) and centrifuged at 1 500 rpm×5 min at 4 °C. Pellets were subjected to at least one freeze-thaw cycle at −80 °C and resuspended in HALT protease inhibitor cocktail (Thermo Scientific, Rockford, IL) to be analyzed via SDS-PAGE. Proteins were transferred to nitrocellulose membranes and transfer efficiency was assessed by staining with 0.1% Ponceau S. Membranes were incubated overnight with primary antibodies neurofilament-M (1: 2000; Invitrogen), or glyceraldehye 3-phosphate dehydrogenase (GAPDH) (1:50,000; Trevigen, Gaithersburg, MD), followed by a horseradish peroxidase conjugated secondary antibody (1: 3000; Santa Cruz, Santa Cruz, CA). Detection of antibody-protein interactions were made via the enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ). All specific protein expression was normalized to levels of GAPDH and reported as fold change vs. control (CON: DMSO treated cells).
2.4. 5(and-6)-chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate (DCF) and MitoSOX fluorescence
Oxidant generation was assessed by monitoring the oxidation of 5(and-6)-chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate, acetyl ester (CM-H2DCFDA, “DCFH2”, Invitrogen) and MitoSOX™ Red (Invitrogen). For DCFH2 oxidation assays, SK-N-SH cells were plated 48 h before initiation of treatments at a density of 5×104 cells/cm2. At the completion of treatments (or siRNA transfections), cells were washed 1× with serum free MEM (SF-MEM) and incubated in the presence of 2.5 µM CM-H2DCFDA in SF-MEM for 5 min at 37 °C, 5% CO2. Cells cultured in parallel were incubated with 0.1–10 µM H2O2 after DCFH2 incubation as a positive control. All cells were then washed 1× with PBS, trypsinized, and collected for FACS analysis (excitation at 493 nm, emission at 525 nm). Carboxy-DCFDA (DCF, Molecular Probes, Eugene, OR) (2.5 µM) is an oxidation insensitive dye that was used as a control for changes in uptake, ester cleavage, and efflux so that changes in fluorescence seen with the oxidation-sensitive dye could be directly attributed to changes in steady-state levels of DCFH2 oxidation. Median fluorescence intensity of 10,000 cells was analyzed in each sample. For MitoSOX oxidation assays, cells were plated 48 h before initiation of treatments at a density of 8×104 cells/cm2. At the completion of treatments, cells were washed with PBS and incubated in the presence of 5 μM MitoSOX for 10 min at 37 °C, 5% CO2. Cells cultured in parallel were incubated with rotenone (1–200 µM) and MitoSOX (described above) as a positive control. Fluorescence was then measured (excitation at 510 nm, emission at 580 nm) at 10 and 40 min. The relative change in fluorescence between 10 and 40 min is expressed as the fold change of treated vs. control. All absorbance values were also normalized according to protein content.
2.5. Glutathione (GSH) levels
Cells were plated at a density of 3×104 cells/cm2. At the completion of treatments, whole cell lysates were collected via scraping cells in cold PBS and centrifuging at 2000 rpm for 5 min at 4 °C. The resulting cell pellet was resuspended in potassium phosphate buffer (50 mmol/L, pH 7.8) and aliquots were taken for quantification of protein concentration. Sulfosalicylic acid was added to the remaining solution at a concentration required to achieve a final value of 5%. Samples were incubated on ice for at least 10 min, centrifuged at 2000 rpm for 5 min at 4 °C, and supernatants were saved and stored immediately at −80 °C. Levels of GSH and GSSG were distinguished by adding a mixture of 2-vinylpyridine and triethanolamine (TEA) (2:1), incubated for 30 min, and assayed as described by Griffith and Anderson [15], [3]. All values were normalized according to protein content and reported as fold change vs. control.
2.6. Glutathione peroxidase and catalase activity
Cells were plated at a density of 3×104 cells/cm2. At the completion of treatments, whole cell lysates were collected via scraping cells in cold PBS and centrifuging at 2000 rpm for 5 min at 4 °C. The resulting cell pellet was stored at −80 °C until being resuspended in potassium phosphate buffer (50 mmol/L, pH 7.8) with DETAPAC (1.34 mM diethylenetriaminepentaacetic acid). We utilized an adaptation of methods by Zhang et al. [47] by incubating samples in the presence of 15 mM H2O2 and immediately analyzing spectrophotometrically for 90 s at 240 nm. The change in absorbance within 60 s was recorded to reflect catalase activity in each sample. Glutathione peroxidase (GPx) activity was determined according to the method of [21]. Briefly, samples were added to a solution of phosphate buffer (100 mM Tris/HCl pH 7.6, 5 mM EDTA, 1 mM sodium azide), reduced glutathione (3 mM), glutathione reductase (600 U/mL), and NADPH (0.1 mM); then incubated at 30 °C for 5 min. The enzymatic reaction was started by adding H2O2 (final concentration of 1.5 mM) and evaluated by measurement of NADPH consumption at 340 nm. All values were normalized according to protein content and reported as milliunits (munits)/mg protein.
2.7. MnSOD activity
Activity of SOD was measured in whole cell lysates using an indirect competition assay between SOD and the indicator compound, nitroblue tetrazolium, for superoxide produced by xanthine/xanthine oxidase [39]. The activity of the MnSOD isoform was measured in the presence of sodium cyanide, an inhibitor of copper zinc SOD. The data were normalized to protein content as measured by the Lowry colorimetric assay utilizing bovine serum albumin as a control and reported as units/mg protein.
2.8. SOD2 siRNA treatment
Small interfering RNA against the human SOD2 gene was used to silence expression of MnSOD. Cells were plated 24 h before initiation of treatments at a density of 3×104 cells/cm2. Cells were then rinsed with serum free MEM (SF-MEM) prior to transfection with either ~100 nM SOD2 small interfering RNA (SOD2 siRNA) (ON-TARGET plus SMART pool, Dharmacon Inc, Chicago, IL) or Control siRNA (Ctrl siRNA) (siCONTROL non-targeting siRNA, Dharmacon Inc, Chicago, IL) using Hiperfect transfection reagent (Qiagen, Valencia, CA). Transfected cells were treated with either vehicle or ATRA (10 μM) 24 h later. Following treatments, whole cell lysates were collected via scraping in cold PBS, and centrifuged at 1500 rpm×5 min at 4 °C. Pellets were subjected to at least one freeze-thaw cycle at −80 °C and resuspended in HALT protease inhibitor cocktail (Thermo Scientific, Rockford, IL) to be analyzed for protein expression (SDS-PAGE), or in DETAPAC buffer for MnSOD activity.
2.9. Statistical analysis
All experiments were replicated at least in triplicate and values reported as mean ±S.E.M. When comparing two means, Student's t-test was used, p<0.05. When comparing multiple means, the results were analyzed either by one way ANOVA or one way ANOVA on ranks followed by the Student Newman–Keuls test at a 95% confidence interval.
3. Results
3.1. ATRA promoted differentiated morphology, increased NF-M expression, and slowed proliferation of the SK-N-SH cells
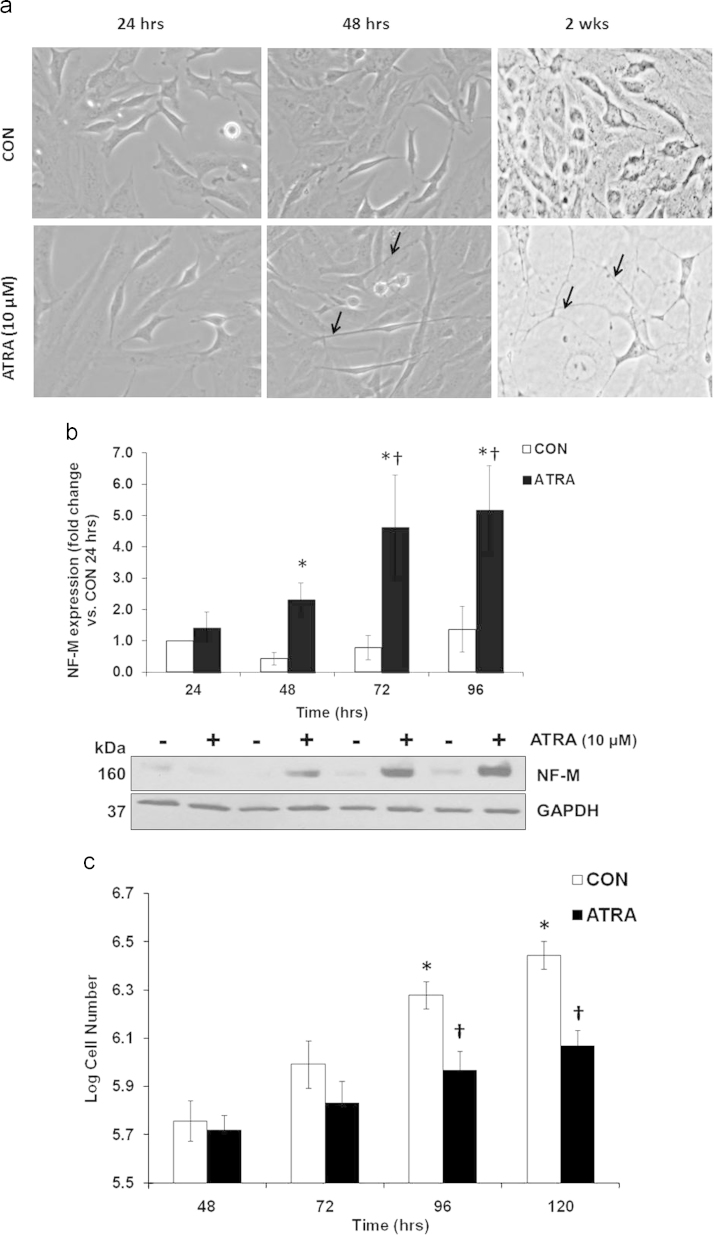
The common mechanism by which retinoids antagonize carcinogenesis is by inducing differentiation and suppressing the ability of the cells to proliferate. To validate these therapeutic effects, SK-N-SH neuroblastoma cells were treated with 10 uM ATRA and cellular morphology, growth rate, and expression of neurofilament-M (NF-M; an early marker of neuronal differentiation) were assessed. Fig. 1a demonstrates a change in morphology characteristic of differentiated neurons as early as 48 h with ATRA treatment. The initiation of neurite formation (arrows) supports an early effect of ATRA to promote differentiation. Images captured after 2 weeks of continuous ATRA treatment (10 µM) display fully differentiated cells with very prominent neurite processes (arrows). These early changes in morphology were accompanied by increased expression of NF-M upon ATRA treatment (Fig. 1b). Cell numbers were also decreased in ATRA treated cells, with significant changes observed at 96 h (Fig. 1c), subsequent to increased NF-M expression.
Fig. 1.
ATRA promoted differentiated morphology, increased NF-M expression, and decreased number of the SK-N-SH cells. (A) Representative images from SK-N-SH cells treated with ATRA (10 μM) for 24 h, 48 h, and 2 wks. Arrows denote neurite formation. (B) Western Blot analysis of neurofilament M (NF-M) expression after treatment with ATRA (10 μM) for 24, 48, 72, and 96 h. n=5. *p<0.05, significant difference between CON and ATRA at each time point. †p<0.05, significant difference between ATRA 72, 96 h vs. 24, 48 h. Kruskal–Wallis One way ANOVA on Ranks; Student-Newman–Keuls. Results are expressed as fold changes vs. 24 h CON±SEM. (C) Cell number after treatment with ATRA (10 μM) for 24, 48, 72, and 96 h. n=4. *p<0.05, significant difference vs. 24 h CON; †p<0.05, significant difference between ATRA and CON at respective time point. One way ANOVA; Student-Newman–Keuls. Results are expressed as changes in log cell number±SEM.
3.2. ATRA modulates DCFH2 and MitoSOX oxidation as well as induction of MnSOD
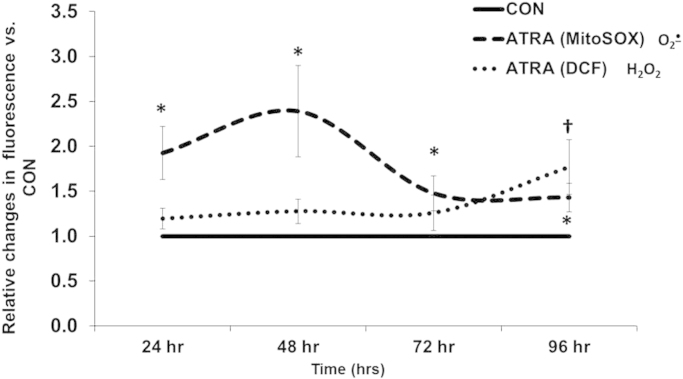
Although previous reports have investigated the generation of ROS in neuroblastoma cell lines (including a subclone of the SK-N-SH's) [10], [27], the role of either O2•− or H2O2 in ATRA-induced differentiation of this cancer cell type is unknown. Our previous publication demonstrates a significant up-regulation of MnSOD activity with 72 h ATRA treatment of SK-N-SH cells [17]. The MnSOD enzyme scavenges O2•− at the mitochondrial level; yielding hydrogen peroxide as the product (H2O2). Therefore, evaluation with both MitoSOX (an indicator of mitochondrial O2•−) and CM-H2DCFDA (an indicator of intracellular H2O2) was performed to assess steady-state levels of ROS. Treatment with ATRA significantly increased MitoSOX oxidation in comparison to controls at all time points (Fig. 2); with a major peak seen at 48 h, preceding the ATRA-mediated increase in MnSOD activity in these cells. Parallel cultures were incubated with rotenone (1–200 µM as a positive control), and exhibited a concentration-dependent increase in MitoSOX oxidation (data not shown).
Fig. 2.
ATRA modulated DCFH2 oxidation and MitoSOX oxidation in SK-N-SH cells. MitoSOX (n=5) and DCF (n=4) fluorescence of SK-N-SH cells after treatment with ATRA (10 μM) for 24, 48, 72, and 96 h. MitoSOX: *p<0.05 vs. CON. DCF: †p<0.05 vs. CON. T-test performed for each time point vs. CON. Results are expressed as fold changes vs. CON at each time point±SEM.
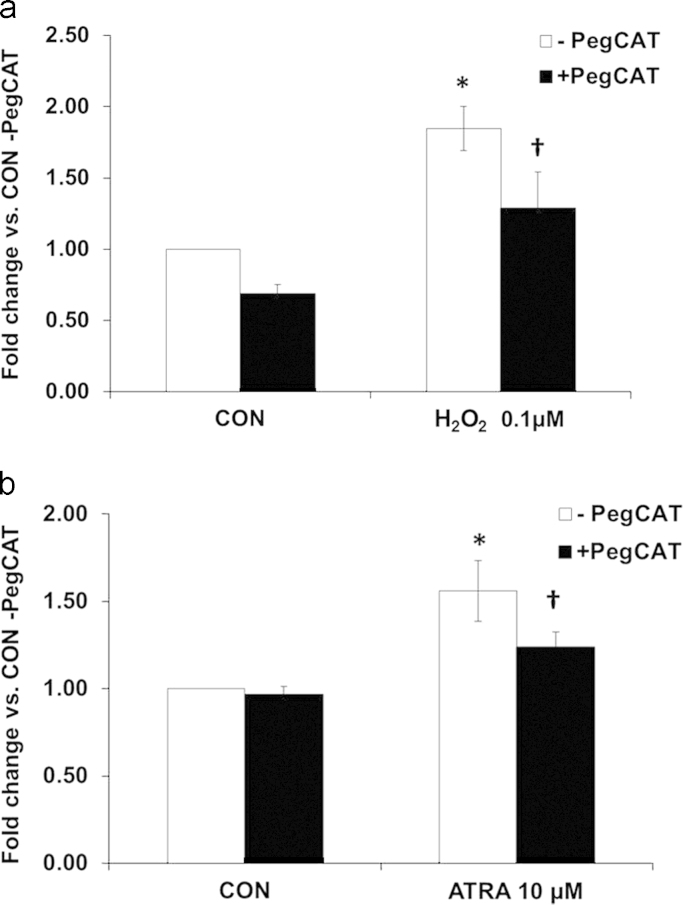
As MitoSOX fluorescence returned towards baseline levels (72–96 h); ATRA significantly increased DCFH2 oxidation at 96 h. Hydrogen peroxide was used as a positive control and also increased DCFH2 oxidation over that of control cells (data not shown). Since DCFH2 oxidation is not specific for H2O2 (i.e. peroxynitrite) [22], polyethylene glycol-conjugated catalase (PEG-Cat-100 U/mL) was used to confirm the increase in fluorescence was indicative of changes in steady-state levels of H2O2. Pretreatment of cells with PEG-Cat significantly attenuated H2O2-induced (Fig. 3a) as well as ATRA-induced (Fig. 3b) increases in DCFH2 oxidation, supporting the conclusion that the increased dye oxidation seen at 96 h of ATRA treatment was caused by H2O2.
Fig. 3.
PegCat-mediated reductions in DCFH2 oxidation. DCF oxidation was assessed in SK-N-SH cells after treatment with (A) H2O2 (0.1 μM, 20 min, n=4) or (B) ATRA (10 μM, 96 h, n=3). Cells were pretreated with PegCat (100 U/ml) for 3 h prior to DCFH2 (2.5 μM) incubation. *p<0.05 vs. CON (-)PegCat; †p<0.05 vs. *. One-way ANOVA; Student-Newman–Keuls. Results are expressed as fold changes vs. CON (-)PegCat±SEM.
3.3. ATRA effects on glutathione (GSH) levels; glutathione peroxidase (GPx), and catalase activity
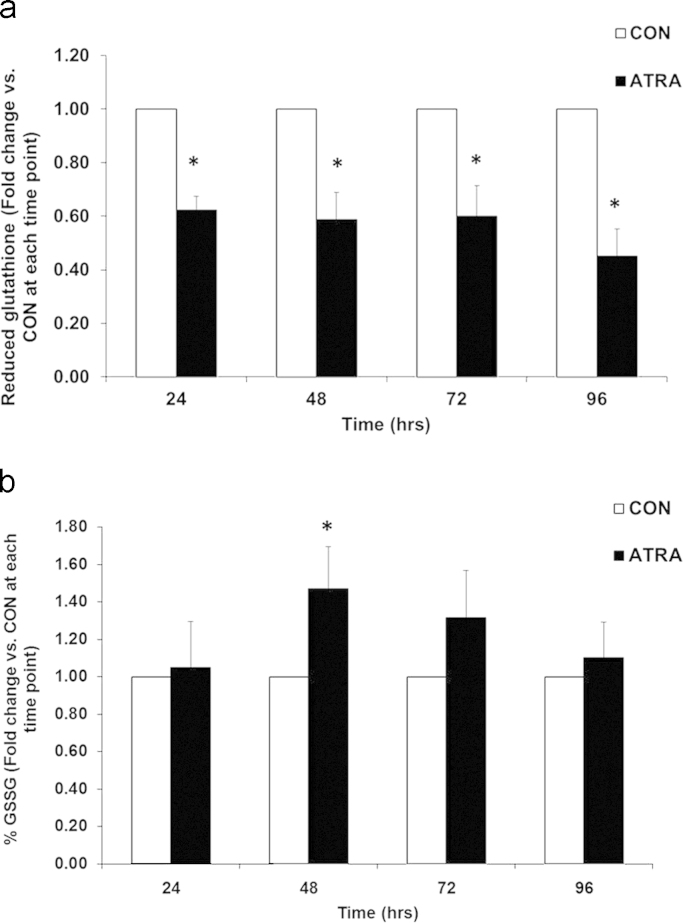
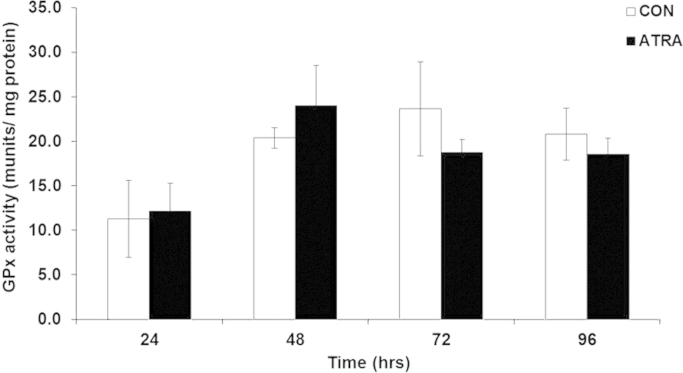
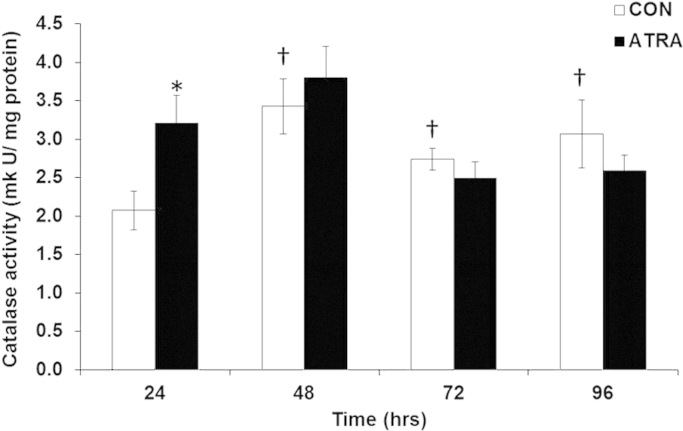
Given that ATRA increased DCFH2 oxidation, the subsequent antioxidant response by peroxide scavengers such as glutathione (GSH), glutathione peroxidase (GPx), and catalase was determined. GSH levels were significantly decreased following ATRA treatment in comparison to non-treated cells at all time points (Fig. 4a). Furthermore, oxidized GSH (%GSSG) was significantly increased in the presence of ATRA at 48 h (Fig. 4b). GSH is a substrate used by GPx to catalyze the degradation of H2O2. However, ATRA treatment of SK-N-SH cells did not alter GPx activity in comparison to controls at any time point (Fig. 5). ATRA significantly increased catalase activity at 24 h; but this subsided to levels comparable to control cells by 48–96 h (Fig. 6). The significant increase found in GPx and catalase activity in non-treated cells during the course of the experiment is consistent with previous reports demonstrating the ability of cells to increase activity of these enzymes as a function of time and cell density when cultured in vitro [28]. Both GPx and catalase activity follow the same trend as the control cells over time when treated with ATRA. Altogether, increased MnSOD, increased DCFH2 oxidation, decreased GSH, and the lack of either GPx or catalase upregulation correlated with elevated H2O2 at the 96 h time point of ATRA treatment.
Fig. 4.
ATRA decreased glutathione (GSH) levels. (A) Reduced GSH and (B) glutathione disulfide (%GSSG) levels were assessed in SK-N-SH cells after treatment with ATRA (10 μM) after 24–96 h. n=7. *p<0.05 vs. CON per time point. T-test performed for each time point. Results are expressed as fold change vs. CON per time point±SEM.
Fig. 5.
ATRA did not significantly alter glutathione peroxidase (GPx) activity. Glutathione peroxidase (GPx) activity in SK-N-SH cells after treatment with ATRA (10 μM) after 24 (n=5), 48, 72 and 96 (n=9) hrs. T-test performed for each time point. One- way ANOVA amongst CON and ATRA. Results are expressed as GPx activity (munits/mg protein)±SEM.
Fig. 6.
ATRA's effects on catalase activity. Catalase activity of SK-N-SH cells after treatment with ATRA (10 μM) for 24, 48, 72, and 96 h. n=6. *p<0.05 vs. CON at respective time point. T-test performed for each time point. †p<0.05 vs. CON 24 h. One way ANOVA amongst CON. Results are expressed as mkUnits/mg protein±SEM.
3.4. Reducing MnSOD activity decreased DCFH2 oxidation and increased MitoSOX oxidation at 96 h
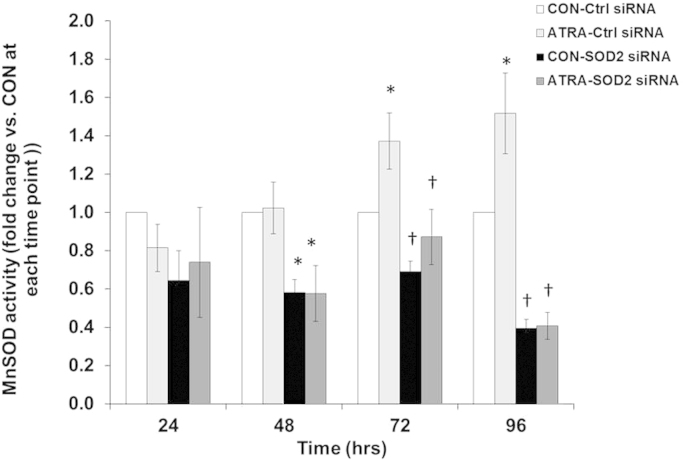
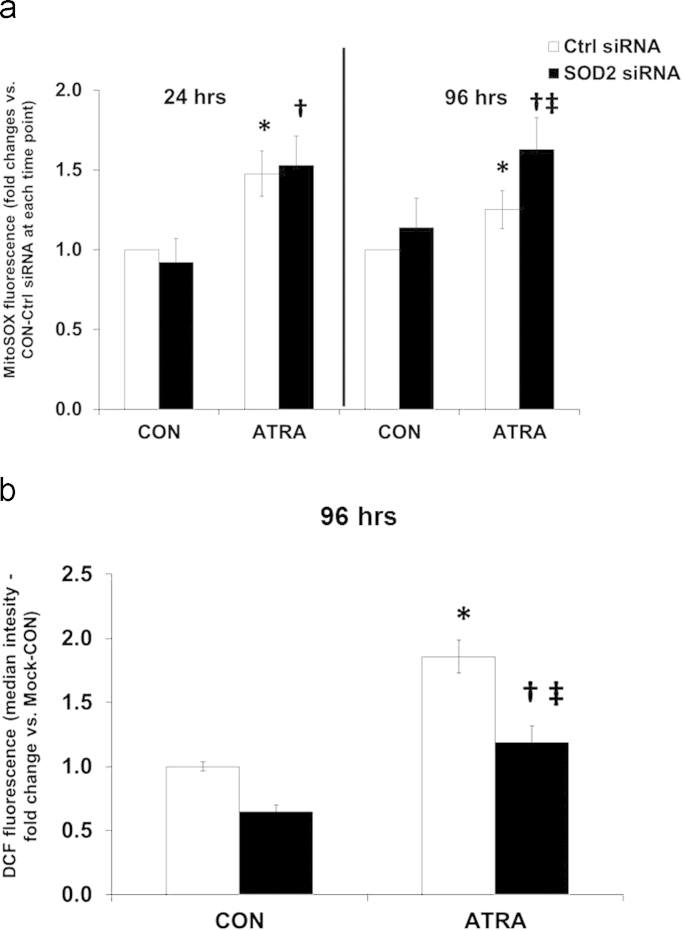
Several studies have investigated ROS production and antioxidant expression for differentiated neural cell types [41], [10], [29], [27], but few provide a time-course analysis of these effects during the early process of differentiation. Our next step was to determine the effect of altering steady-state levels of ROS by reducing MnSOD activity. A significant increase in MnSOD activity was noted when treating SK-N-SH cells with ATRA for 72 and 96 h (Fig. 7). In both control and ATRA-treated cells, activity was significantly decreased in the presence of SOD2 siRNA versus Ctrl siRNA for 48, 72 and 96 h. MitoSOX oxidation was also measured to evaluate if reducing MnSOD activity influenced the steady-state levels of O2•− (Fig. 8a). Again, at 24 h, ATRA significantly increased MitoSOX oxidation versus control cells in the presence of SOD2 siRNA. At 96 h, MitoSOX oxidation in both Ctrl and SOD2 siRNA transfected cells was significantly increased by ATRA in comparison to controls. Furthermore, there was a significant increase in MitoSOX oxidation when comparing ATRA-SOD2 siRNA to ATRA-Ctrl siRNA. In contrast, at 96 h, the reduction of MitoSOX oxidation towards baseline levels in control cells with ATRA treatment is apparent. ATRA-stimulated DCFH2 oxidation was significantly attenuated when MnSOD activity was reduced with siRNA (SOD2 siRNA) (Fig. 8b). These results suggest that MnSOD may modulate increases in steady-state levels of H2O2 during ATRA treatment at 96 h. These findings support the conclusion that a reduction in MnSOD activity caused by siRNA treatment of neuroblastoma cells caused an increase in steady-state levels of O2•− at 48–96 h.
Fig. 7.
MnSOD activity decreased with SOD2 siRNA treatment. MnSOD activity was assessed in SK-N-SH cells after treatment with ATRA (10 μM) for 24, 48, 72, and 96 h. 24: n=3; 48–96: n=4. *p<0.05 vs. CON at respective time point.. †p<0.05 vs. * Two-way ANOVA per timepoint; Student-Newman–Keuls. Results are expressed as fold change vs. CON±SEM per timepoint.
Fig. 8.
Reducing MnSOD activity increased MitoSOX and decreased DCFH2 oxidation at 96 h of exposure to ATRA. MitoSOX (A) and DCFH2 (B) oxidation in SK-N-SH cells after incubation with either a control vector (Ctrl-siRNA) or one targeting the MnSOD gene (SOD2-siRNA) and subsequent treatment with ATRA (10 μM). DCF: n=3, MitoSOX: n=8. MitoSOX: *p<0.01 vs. CON-Ctrl siRNA; †p<0.05 vs. CON-SOD2 siRNA; ‡p<0.05 vs †. T-test. DCF: *p<0.001 vs. CON-Ctrl siRNA; †p<0.05 vs. CON-SOD2 siRNA; ‡p<0.05 vs *. One way ANOVA; Student-Newman–Keuls. Results are expressed as fold changes vs. Mock-CON (DCF: not shown) or CON-Ctrl siRNA (MitoSOX)±SEM.
3.5. Reducing MnSOD activity enhanced expression of NF-M at 48 h but led to diminished levels of NF-M at 96 h of treatment with ATRA
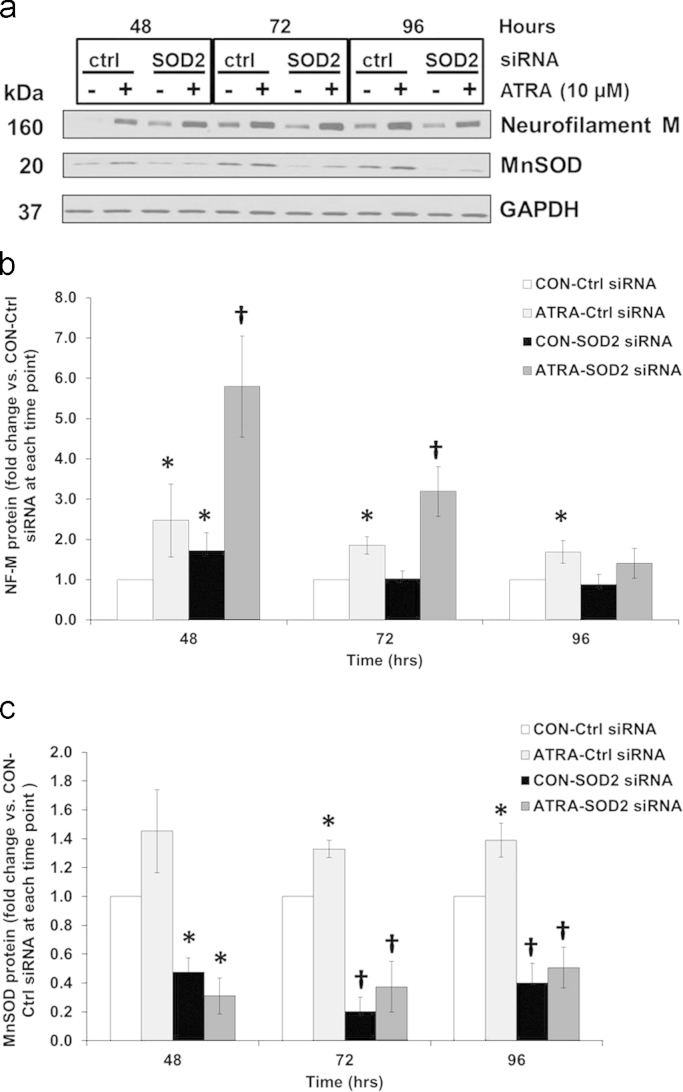
Having identified 1) an increase in MnSOD that correlated with increased levels of the neuronal differentiation marker NF-M (Fig. 1b), and 2) similar changes in steady-state levels of O2•− and H2O2 caused by reducing MnSOD activity (Fig. 8a and b); we next determined if altering MnSOD influenced the expression of NF-M. At 48 h of treatment with 10 uM ATRA, immuno-reactive NF-M increased prior to the significant induction of MnSOD activity at 72 h (Fig. 9a and b). Furthermore, inhibiting MnSOD activity with siRNA not only enhanced ATRA-induced expression of NF-M (ATRA-SOD2 siRNA), but it also significantly increased its expression over that of the cells receiving only the control siRNA vector (CON-Ctrl siRNA) at 48 h. This effect was diminished at 96 h. Fig. 9a and c demonstrates the significant increase in MnSOD protein expression in the presence of ATRA at 72 and 96 h, and the significant reduction of this antioxidant enzyme in the presence of the SOD2 siRNA at 48 and 96 h. In agreement with protein expression, MnSOD activity was also reduced to ~50% of normal values at each time point (Fig. 6a – 96 h). Furthermore, the reduction in DCFH2 oxidation (Fig. 7a) and increase in MitoSOX oxidation (Fig. 7b) in the presence of SOD2 siRNA were consistent with decreases in MnSOD activity. These data suggest that MnSOD is not critical for initiating the differentiation process by modulation of NF-M expression at 48 h in the SK-N-SH cells but that superoxide is the inducer of these biological effects.
Fig. 9.
Reducing MnSOD activity enhanced NF-M expression at 48 and 72 h but diminished it at 96 h. Western Blot analysis of (A,B) NF-M and MnSOD (A,C) in SK-N-SH cells after incubation with either a control vector (Ctrl siRNA) or one targeting the MnSOD gene (SOD2 siRNA) and subsequent treatment with ATRA (10 μM) for 48, 72, and 96 h. n=6. *p<0.05 vs. CON-Ctrl siRNA per time point, †p<0.05 vs *. Kruskal–Wallis one way ANOVA on ranks. Results are expressed as fold changes vs. CON-Ctrl siRNA per time point±SEM.
3.6. PegCat treatment influenced the expression of NF-M at 96 h of treatment with ATRA
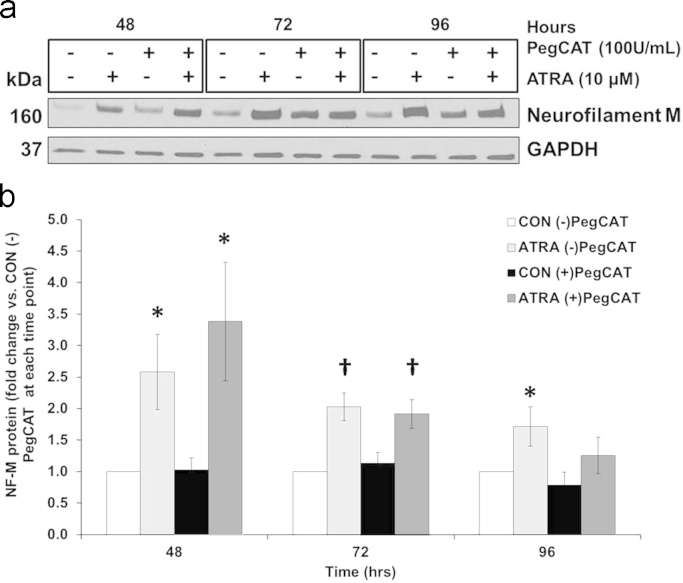
To evaluate the role of H2O2 in ATRA-mediated induction of NF-M expression, we incubated the cells continuously in the presence of PegCat for 0–96 h. Western blot analysis demonstrated that PegCat treatment influenced the expression of NF-M in a similar manner as did the SOD2 siRNA construct (Fig. 10a and b). Although there was no significant enhancement of NF-M expression at 48 h by PegCat treatment, it is apparent that any significant up-regulation of NF-M expression by ATRA was lost by 96 h in the presence of PEG-Cat. These findings parallel those found by reducing MnSOD activity at 96 h of ATRA treatment.
Fig. 10.
PegCat treatment influenced NF-M expression in a similar manner to reducing MnSOD activity at 96 h of ATRA. (A,B) Western Blot analysis of neurofilament M (NF-M) expression in SK-N-SH cells after 24 h pre-incubation with PegCat (100 U/mL) and subsequent treatment with ATRA (10 μM) for 48, 72, and 96 h. n=6. *p<0.05, significant difference vs. CON (-)PegCat; Kruskal–Wallis one way ANOVA on ranks. †p<0.05, significant difference vs. CON (-)PegCat; One way ANOVA; Student-Newman-Keuls. Results are expressed as fold changes vs. CON (-)PegCat per time point±SEM.
4. Discussion
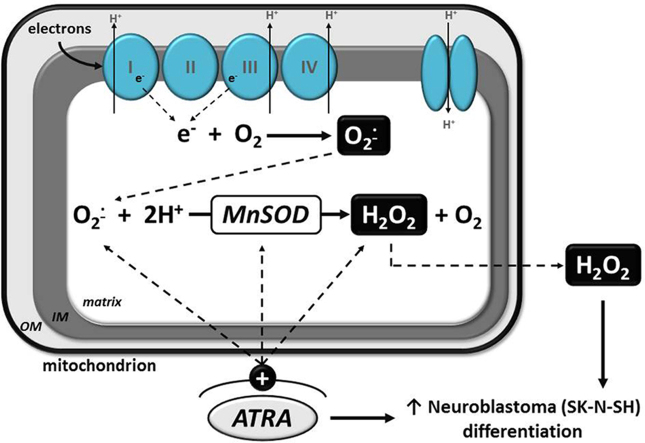
Many studies have demonstrated that retinoid-induced neuronal differentiation results in increased steady-state levels of reactive oxygen species (ROS) as well as altered levels of antioxidant enzymes [12], [18], [27], [35]. About 1–3% of oxygen consumed by the body is converted into ROS; three of the most prominent being superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radical (•OH) [38]. Therefore, cells have developed a means to limit excess accumulation of ROS by induction of antioxidant defense systems (i.e. superoxide dismutases, catalase, glutathione peroxidase, glutathione) [6]. We have previously reported a time-dependent ATRA-mediated increase in manganese superoxide dismutase (MnSOD) mRNA, protein and activity in the SK-N-SH cells [17]; and the current study identifies a rise in ROS levels that likely initiates this adaptive response by MnSOD. The aims of this study were to determine the role of ROS and/or MnSOD activity in ATRA-mediated differentiation.
In PC12 cells, generation of O2•− via xanthine/xanthine oxidase induces neurite outgrowth, which is characteristic of differentiation of this and other neuronal cell types [14]. Kamata and others suggest that ROS (i.e. H2O2) mediate the actions of nerve growth factor (NGF) to promote neuron survival and axonal regeneration also in PC12 cells [16], [41]. We now provide a time-course analysis of ROS generation and antioxidant expression in differentiating SK-N-SH neuroblastoma cells treated with 10 uM ATRA (Fig. 2, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
MnSOD is a mitochondrial enzyme upregulated in the presence of O2•− [31], [8], and has been shown by Fridovich and others to be necessary for survival in oxygen-metabolizing cells [25]. Homozygous MnSOD null mice die within the first 10–30 days of life due to dilated cardiomyopathy and neurodegeneration [24], [23]. This essential antioxidant enzyme not only protects against ROS accumulation, but is important in the process of differentiation in some cell types [40], [5]. Transfection of human MnSOD cDNA into mouse 10T1/2 embryonic cells enhanced 5-azacytidine mediated differentiation [40]. Differentiation of IMR-32 neuroblastoma cells also resulted in an increase in MnSOD activity, which correlated with an increase in ROS [10].
Human neuroblastoma cell lines (i.e., SK-N-SH) are composed of three distinct cell types (epithelial, neuronal, and intermediate) and are sensitive to retinoid-induced growth inhibition and formation of neurite extensions. Sidell et al. demonstrate that growth inhibition and differentiation are commonly associated with a decreased malignant phenotype [37]. We confirm these observations by identifying NF-M as an early neuronal differentiation marker whose protein expression increases two days prior to growth suppression by ATRA. In parallel to these changes, ATRA caused the upregulation of the essential mitochondrial antioxidant enzyme, MnSOD. The importance of the mitochondria in differentiation is supported in other neuronal models. In HN9.10e hippocampal neuroblasts, retinoic acid-induced neurite formation resulted in remodeling of the mitochondrial network, fusion of mitochondria and collective distribution throughout the neurites, and an increase in mitochondrial potential [45]. It is well established that there is a strong correlation between an increase in mitochondrial membrane potential and the production of ROS [19], [42]. Schneider et al. support this finding by showing an increase in mitochondrial membrane potential during ATRA-induced differentiation of SH-SY-5Y cells (a subclone of the SK-N-SH population) [35]. Furthermore, they also demonstrated distribution of the mitochondrial network throughout the neurites and, in accordance with our findings, reported increased MnSOD expression.
Using an indicator of steady-state levels of mitochondrial O2•−, MitoSOX oxidation was found to increase in the presence of ATRA treatment. Leukocytes also produce ROS, in particular O2•−, which is correlated with their differentiation [46], [7]. We observed that the increase in MitoSOX oxidation in the SK-N-SH cells is sustained for at least 96 h. However, a closer examination of the changes in steady-state levels of MitoSOX oxidation over time suggests that O2•− levels return to baseline levels at the same time point (72 h) that a significant increase in MnSOD activity occurred following treatment with ATRA [17]. The increase in DCFH2 oxidation and subsequent attenuation by PegCat treatment at 96 h further suggests that production of H2O2 parallels an increase in MnSOD activity. These studies provide a time-course analysis of both steady-state levels of O2•− and H2O2 that appears to be coordinately regulated by induction of MnSOD activity. Furthermore, specifically identifying that abrogation of H2O2 limits the expression of NF-M at 96 h of treatment with ATRA supports the hypothesis that H2O2 is as an important signaling molecule in this classic model of neuronal differentiation.
In order to get a more complete view of the redox status of the SK-N-SH cells in the presence of ATRA, we also assessed GSH content, GPx and catalase activity. Each of these endogenous antioxidant defense systems is integral in the handling of H2O2. Many studies also implicate GSH as an essential mediator of cellular proliferation [32], [33]. In this study, we made the novel finding that ATRA significantly reduces GSH levels in neuroblastoma cells. Although others show that cAMP treatment of the IMR-32 neuroblastoma cell line resulted in a reduction of GSH, which was enhanced by ATRA, there was no significant change in GSH levels in the presence of ATRA alone [4]. The GPx enzyme was also assessed due to the fact that it uses GSH as a substrate for scavenging peroxides. However, we discovered no significant change in GPx activity in the presence of ATRA at any time point (24–96 h). These data implicate that ATRA functions by a different mechanism to decrease GSH.
Decreased catalase activity is associated with increased differentiation of IMR-32 neuroblastoma cells [10], and addition of catalase decreased NGF-stimulated neuronal differentiation of PC12 cells [41]. In the current study, we demonstrated an initial increase in catalase activity by ATRA within 24 h. Differentiation of Neuro2A mouse neuroblastoma cells with ATRA has been shown to increase catalase activity by a similar magnitude [36]. A lack of increased catalase activity at 96 h in the current study suggests that either H2O2 is not scavenged or that there are insufficient levels to elicit catalase activity. Accordingly, there is growing evidence for the role of H2O2 to be a key signaling molecule and regulate gene expression in many cellular models (as reviewed in [1]).
We have demonstrated that ATRA up-regulates the expression of NMDAR1 [17] and NF-M (at 48 h) prior to a significant increase in MnSOD activity (at 72 h). This suggests that MnSOD is not essential to initiate differentiation. However, an interesting finding is that a reduction of MnSOD activity enhanced NF-M expression at 48 h. Rather than directly affecting target proteins, slight changes in MnSOD may alter the redox status (i.e. through altering steady-state levels of O2•− and H2O2) to influence, for example, the polymerization of NF-M. The stability and structure of intermediate filament proteins such as NF-M have been shown to be susceptible to damage by oxidative stress [13]. The relative ratios of these ROS may therefore affect NF-M stability and function. Attenuation of ATRA-induced NF-M expression by reducing MnSOD at 96 h suggests a role for MnSOD (and possibly H2O2) in differentiation at the later time point. Additionally, the current study investigates relative changes in ROS (O2•− and H2O2) during the differentiation process, whereas many studies examine one time point and may overlook the unique roles each of these ROS play as the differentiation process proceeds. The generation of H2O2 by MnSOD might function to further the differentiation process at 96 h and beyond. Otherwise, considering that MnSOD is well known to promote cell survival, differentiated cells (at 48 h) may subsequently undergo apoptosis (at 96 h). Long-term retinoid treatment of the SK-N-SH cell line, comprised of both epithelial (S-type) and neuronal (N-type) cell types, induces death in the S-type and differentiation of the N-type [26]. Therefore, in this model, future work should investigate if the N-type cell population is subject to apoptosis by reducing MnSOD activity. These findings would hold potential to improve retinoid therapy by augmenting the differentiation process earlier and subjecting differentiated cells to apoptosis by manipulations of SOD activity.
5. Conclusions
Reactive oxygen species have received a lot of attention in the past few decades because they are generated by all aerobic organisms and are known to be involved in a variety of cellular responses, both harmful and beneficial. Moreover, the various antioxidant defense systems within cells have been intensely studied in light of their capacities to influence gene expression in many in vitro and in vivo models. Although the generation of ROS is often associated with damaging effects, they also play an essential role in key signal transduction pathways that are involved in the processes of cellular proliferation, apoptosis, cell cycle arrest, and differentiation (as reviewed in [43]). Here we closely examine the redox status of the SK-N-SH cells as determined by steady-state levels of dye oxidation and cellular antioxidant expression. We have identified H2O2 as a potential signaling molecule to promote ATRA-mediated neuronal differentiation. Our previous work demonstrated NF-κB regulation of both MnSOD and differentiation; therefore, current findings compel us to investigate if this is mediated by H2O2 generation. We hypothesized that reducing MnSOD activity early on in retinoid treatments may stimulate expression of neuronal differentiation markers in neuroblastoma cells. If MnSOD-mediated H2O2 generation is important to promote differentiation at the later time points in therapy, early interventions preventing H2O2 degradation or inhibiting SOD activity could provide potential benefits for improving responses to retinoid based neuroblastoma therapies.
Acknowledgments
We thank the lab of Dr. Monica Valentovic (Marshall University) for mentorship and help with antioxidant assays (GSH, GPx, and catalase). We also extend gratitude to Drs. Douglas Spitz and Michael McCormick for determining SOD activity and providing editorial assistance on the manuscript. This project was supported by the Centers for Biomedical Research Excellence grant, 1P20RR020180 (KKK), WV-NASA Space Grant Consortium (AMS) (Grant Contract # NNX10AK62H) and P30 CA086862 (MLM, DRS).
References
- 1.Allen R.G., Tresini M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000;28(3):463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 2.Altucci L., Gronemeyer H. The promise of retinoids to fight against cancer. Nat. Rev. Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M.E. CRC Press; Boca Raton, Florida: 1985. Handbook of Methods for Oxygen Radical Research. [Google Scholar]
- 4.Carystinos G.D., Alaoui-Jamali M.A., Phipps J., Yen L., Batist G. Upregulation of gap junctional intercellular communication and connexin 43 expression by cyclic-AMP and all-trans-retinoic acid is associated with glutathione depletion and chemosensitivity in neuroblastoma cells. Cancer Chemother. Pharmacol. 2001;47(2):126–132. doi: 10.1007/s002800000231. [DOI] [PubMed] [Google Scholar]
- 5.Cassano S., Agnese S., D'Amato V., Papale M., Garbi C., Castagnola P., Ruocco M.R., Castellano I., De Vendittis E., Santillo M., Amente S., Porcellini A., Avvedimento E.V. Reactive oxygen species, Ki-Ras, and mitochondrial superoxide dismutase cooperate in nerve growth factor-induced differentiation of PC12 cells. J. Biol. Chem. 2010;285(31):24141–24153. doi: 10.1074/jbc.M109.098525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 7.Chomienne C., Ballerini P., Balitrand N., Daniel M.T., Fenaux P., Castaigne S., Degos L. All-trans retinoic acid in acute promyelocytic leukemias. II. In vitro studies: structure-function relationship. Blood. 1990;76(9):1710–1717. [PubMed] [Google Scholar]
- 8.Culotta V.C., Yang M., O'Halloran T.V. Activation of superoxide dismutases: putting the metal to the pedal. Biochim. Biophys. Acta. 2006;1763(7):747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donato L.J., Suh J.H., Noy N. Suppression of mammary carcinoma cell growth by retinoic acid: the cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res. 2007;67(2):609–615. doi: 10.1158/0008-5472.CAN-06-0989. [DOI] [PubMed] [Google Scholar]
- 10.Erlejman A.G., Oteiza P.I. The oxidant defense system in human neuroblastoma IMR-32 cells predifferentiation and postdifferentiation to neuronal phenotypes. Neurochem. Res. 2002;27(11):1499–1506. doi: 10.1023/a:1021600522299. [DOI] [PubMed] [Google Scholar]
- 11.Freemantle S.J., Spinella M.J., Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22(47):7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 12.Gelain D.P., Moreira J.C. Evidence of increased reactive species formation by retinol, but not retinoic acid, in PC12 cells. Toxicol. In Vitro. 2008;22(3):553–558. doi: 10.1016/j.tiv.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Gelinas S., Chapados C., Beauregard M., Gosselin I., Martinoli M.G. Effect of oxidative stress on stability and structure of neurofilament proteins. Biochem. Cell Biol. 2000;78(6):667–674. [PubMed] [Google Scholar]
- 14.Gopalakrishna R., Gundimeda U., Schiffman J.E., McNeill T.H. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J. Biol. Chem. 2008;283(21):14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 16.Kamata H., Tanaka C., Yagisawa H., Matsuda S., Gotoh Y., Nishida E., Hirata H. Suppression of nerve growth factor-induced neuronal differentiation of PC12 cells. N-acetylcysteine uncouples the signal transduction from ras to the mitogen-activated protein kinase cascade. J. Biol. Chem. 1996;271(51):33018–33025. doi: 10.1074/jbc.271.51.33018. [DOI] [PubMed] [Google Scholar]
- 17.Kiningham K.K., Cardozo Z.A., Cook C., Cole M.P., Stewart J.C., Tassone M., Coleman M.C., Spitz D.R. All-trans-retinoic acid induces manganese superoxide dismutase in human neuroblastoma through NF-kappaB. Free Radic. Biol. Med. 2008;44(8):1610–1616. doi: 10.1016/j.freeradbiomed.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konopka R., Kubala L., Lojek A., Pachernik J. Alternation of retinoic acid induced neural differentiation of P19 embryonal carcinoma cells by reduction of reactive oxygen species intracellular production. Neuro Endocrinol. Lett. 2008;29(5):770–774. [PubMed] [Google Scholar]
- 19.Korshunov S.S., Skulachev V.P., Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416(1):15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 20.Lasorella A., Iavarone A., Israel M.A. Differentiation of neuroblastoma enhances Bcl-2 expression and induces alterations of apoptosis and drug resistance. Cancer Res. 1995;55(20):4711–4716. [PubMed] [Google Scholar]
- 21.Lawrence R.A., Burk R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 22.LeBel C.P., Ischiropoulos H., Bondy S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5(2):227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 23.Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Jr., Dionne L., Lu N., Huang S., Matzuk M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93(18):9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y., Huang T.T., Carlson E.J., Melov S., Ursell P.C., Olson J.L., Noble L.J., Yoshimura M.P., Berger C., Chan P.H., Wallace D.C., Epstein C.J. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11(4):376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 25.McCord J.M., Keele B.B., Jr., Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc. Natl. Acad. Sci. USA. 1971;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melino G. Discussion: differentiation. In: Evans A.E., Biedler G.M., Brodeur G., D’Angio G.J., Nakagawara A., editors. Advances in Neuroblastoma Research. Wiley-Liss; New York: 1994. p. 215. [Google Scholar]
- 27.Nitti M., Furfaro A.L., Cevasco C., Traverso N., Marinari U.M., Pronzato M.A., Domenicotti C. PKC delta and NADPH oxidase in retinoic acid-induced neuroblastoma cell differentiation. Cell. Signal. 2010;22(5):828–835. doi: 10.1016/j.cellsig.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Oberley T.D., Schultz J.L., Li N., Oberley L.W. Antioxidant enzyme levels as a function of growth state in cell culture. Free Radic. Biol. Med. 1995;19(1):53–65. doi: 10.1016/0891-5849(95)00012-m. [DOI] [PubMed] [Google Scholar]
- 29.Oh J.E., Karlmark Raja K., Shin J.H., Hengstschlager M., Pollak A., Lubec G. The neuronal differentiation process involves a series of antioxidant proteins. Amino Acids. 2005;29(3):273–282. doi: 10.1007/s00726-005-0214-9. [DOI] [PubMed] [Google Scholar]
- 30.Pizzi M., Boroni F., Bianchetti A., Moraitis C., Sarnico I., Benarese M., Goffi F., Valerio A., Spano P. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur. J. Neurosci. 2002;16(12):2342–2350. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- 31.Privalle C.T., Fridovich I. Induction of superoxide dismutase in Escherichia coli by heat shock. Proc. Natl. Acad. Sci. USA. 1987;84(9):2723–2726. doi: 10.1073/pnas.84.9.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy N.M., Kleeberger S.R., Cho H.Y., Yamamoto M., Kensler T.W., Biswal S., Reddy S.P. Deficiency in Nrf2-GSH signaling impairs type II cell growth and enhances sensitivity to oxidants. Am. J. Respir. Cell Mol. Biol. 2007;37(1):3–8. doi: 10.1165/rcmb.2007-0004RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy N.M., Kleeberger S.R., Yamamoto M., Kensler T.W., Scollick C., Biswal S., Reddy S.P. Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genom. 2007;32(1):74–81. doi: 10.1152/physiolgenomics.00126.2007. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds C.P., Lemons R.S. Retinoid therapy of childhood cancer. Hematol. Oncol. Clin. N. Am. 2001;15(5):867–910. doi: 10.1016/s0889-8588(05)70256-2. [DOI] [PubMed] [Google Scholar]
- 35.Schneider L., Giordano S., Zelickson B.R., M S.J., G A.B., Ouyang X., Fineberg N., Darley-Usmar V.M., Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radic. Biol. Med. 2011;51(11):2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinjyo N., Kita K. Relationship between reactive oxygen species and heme metabolism during the differentiation of Neuro2a cells. Biochem. Biophys. Res. Commun. 2007;358(1):130–135. doi: 10.1016/j.bbrc.2007.04.071. [DOI] [PubMed] [Google Scholar]
- 37.Sidell N., Altman A., Haussler M.R., Seeger R.C. Effects of retinoic acid (RA) on the growth and phenotypic expression of several human neuroblastoma cell lines. Exp. Cell Res. 1983;148(1):21–30. doi: 10.1016/0014-4827(83)90184-2. [DOI] [PubMed] [Google Scholar]
- 38.Sohal R.S., Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spitz D.R., Oberley L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179(1):8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 40.St Clair D.K., Oberley T.D., Muse K.E., St Clair W.H. Expression of manganese superoxide dismutase promotes cellular differentiation. Free Radic. Biol. Med. 1994;16(2):275–282. doi: 10.1016/0891-5849(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 41.Suzukawa K., Miura K., Mitsushita J., Resau J., Hirose K., Crystal R., Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J. Biol. Chem. 2000;275(18):13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- 42.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Veal G.J., Errington J., Redfern C.P., Pearson A.D., Boddy A.V. Influence of isomerisation on the growth inhibitory effects and cellular activity of 13-cis and all-trans retinoic acid in neuroblastoma cells. Biochem. Pharmacol. 2002;63(2):207–215. doi: 10.1016/s0006-2952(01)00844-9. [DOI] [PubMed] [Google Scholar]
- 45.Voccoli V., Colombaioni L. Mitochondrial remodeling in differentiating neuroblasts. Brain Res. 2009;1252:15–29. doi: 10.1016/j.brainres.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 46.Yen A., Albright K.L. Evidence for cell cycle phase-specific initiation of a program of HL-60 cell myeloid differentiation mediated by inducer uptake. Cancer Res. 1984;44(6):2511–2515. [PubMed] [Google Scholar]
- 47.Zhang Y.T., Zheng Q.S., Pan J., Zheng R.L. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 2004;95(2):53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]