Abstract
Background
Despite the high prevalence of generalized anxiety disorder (GAD) and its negative impact on society, its neurobiology remains obscure. This study characterizes the neurostructural abnormalities associated with key symptoms of GAD, focusing on indicators of impaired emotion regulation (excessive worry, poor concentration, low mindfulness, and physiological arousal).
Methods
These domains were assessed in 19 (16 women) GAD patients and 19 healthy controls matched for age and gender, using questionnaires and a low demand behavioral task performed before and after an induction of perseverative cognition (i.e. worry and rumination). Continuous pulse oximetry was used to measure autonomic physiology (heart rate variability; HRV). Observed cognitive and physiological changes in response to the induction provided quantifiable data on emotional regulatory capacity. Participants underwent structural magnetic resonance imaging; voxel-based morphometry was used to quantify the relationship between gray matter volume and psychological and physiological measures.
Results
Overall, GAD patients had lower gray matter volume than controls within supramarginal, precentral, and postcentral gyrus bilaterally. Across the GAD group, increased right amygdala volume was associated with prolonged reaction times on the tracking task (indicating increased attentional impairment following the induction) and lower scores on the ‘Act with awareness’ subscale of the Five Facets Mindfulness Questionnaire. Moreover in GAD, medial frontal cortical gray matter volume correlated positively with the ‘Non-react mindfulness’ facet. Lastly, smaller volumes of bilateral insula, bilateral opercular cortex, right supramarginal and precentral gyri, anterior cingulate and paracingulate cortex predicted the magnitude of autonomic change following the induction (i.e. a greater decrease in HRV).
Conclusions
Results distinguish neural structures associated with impaired capacity for cognitive, attentional and physiological disengagement from worry, suggesting that aberrant competition between these levels of emotional regulation is intrinsic to symptom expression in GAD.
Abbreviations: ACC, anterior cingulate cortex; BDI, Beck Depression Inventory; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; FFMQ, Five Facets Mindfulness Questionnaire; GAD, generalized anxiety disorder; HC, healthy controls; HRV, heart rate variability; IBI, Inter-beat-intervals; ICV, intra-cranial volume; MNI, Montreal Neurological Institute; mOFC, medial orbitofrontal cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; RMSSD, root mean square successive difference; ROI, region-of-interest; RT, reaction times; SCID, Structured Clinical Interview for DSMIV; STAI, State-Trait Anxiety Inventory; VAS, visual-analogue scales; VBM, voxel-based morphometry
Keywords: Generalized anxiety disorder, Perseverative cognition, Magnetic resonance imaging, Heart rate variability, Mindfulness, Attentional deficit
Highlights
-
•
Neural structures associated with impaired emotion regulation in GAD are described.
-
•
GAD patients had lower supramarginal and pre/postcentral gyrus volume than controls.
-
•
Right amygdala volume was associated with impaired attention after a worry induction.
-
•
Volume of insula predicted autonomic changes following a worry induction.
-
•
Medial frontal cortex volume correlated with the Non-react mindfulness facet in GAD.
1. Introduction
Generalized anxiety disorder (GAD) is the most prevalent anxiety disorder (Kessler et al., 2001, Olesen et al., 2012); yet it remains poorly understood and, as a result, is more difficult to treat (Brown et al., 1994). The most pervasive and distressing symptoms of GAD, associated with significant impairment in daily functioning, are excessive worry, difficulties concentrating, and feelings of physiological arousal (Andor et al., 2008, Stefanopoulou et al., 2014). To date, the extent to which these key features of GAD reflect disruption of common versus unique underlying mechanisms remains unclear (Forster et al., 2015).
One construct that has the potential to integrate these aspects of emotion regulation (and deficits in GAD) is mindfulness (Desrosiers et al., 2013a, Greeson and Brantley, 2009, Roemer et al., 2009). Mindfulness (Brewer et al., 2013) consists of several distinct dimensions (with symptom counterparts), including the ability to pay attention (difficulty concentrating; (Sugiura, 2004)), the ability to notice experiences in the present without judging (worry and rumination; (Hoge et al., 2013)), and the ability to accept negative experience and feelings without reacting (physiological arousal; (Hazlett-Stevens, 2008)). Consequently, mindfulness based theories are increasingly incorporated in psychotherapeutic treatments to foster emotion regulation skills in GAD (Kabat-Zinn, 1990).
Symptoms of emotion dysregulation in GAD, expressed across different psychophysiological axes, may reflect disruption of the degree to which the origin of symptoms overlap, and may ultimately inform the targeting of therapeutic interventions. Within the brain, a network model has been proposed for the integration of autonomic, attentional, and affective control underlying emotion regulation and dysregulation (Thayer and Lane, 2000). Despite the evident role of the brain in core symptomatology of anxiety, the neurobiological studies of GAD patients are sparse. The few published studies concentrate on the role of amygdala, prefrontal cortex (PFC) and anterior cingulate cortex (ACC) (reviewed in (Hilbert et al., 2014)). The amygdala is proposed as the major “fear-circuit” structure (Davis et al., 2010), the PFC as responsible for frontal overcontrol (perseverative cognition; (Thayer and Lane, 2000)), and the ACC implicated in emotion regulation and autonomic control (Critchley et al., 2003). Enlargement of the amygdala and dorsomedial PFC (DMPFC) is reported in adult (Etkin et al., 2009, Schienle et al., 2011), children, and adolescents with GAD (De Bellis et al., 2000). Increased gray matter volume is also reported in the superior temporal gyrus (a region supporting social processing) in pediatric GAD (De Bellis et al., 2002), while decreased gray matter volume is observed within precuneus and precentral, orbitofrontal and posterior cingulate gyrus (PCC) in adolescents (Strawn et al., 2013). One study reported decreased bilateral hypothalamus volumes in GAD adults (Terlevic et al., 2012).
Few studies investigated the neurostructural correlates of core symptoms such as worry. DMPFC and ACC volume appeared to positively correlate with self-report levels of worry and beliefs about the usefulness of worry (Schienle et al., 2011). A second study reported a positive correlation between worry levels and medial orbitofrontal cortex (mOFC), but not dorsolateral PFC (DLPFC) or amygdala volume (Mohlman et al., 2009).
Neurobiological correlates of mindfulness suggest that individuals who are more ‘mindful of the present’ are characterized by increased gray matter volume in right hippocampus/amygdala and bilateral ACC, yet have reduced gray matter volume in bilateral PCC and the left OFC implying that trait mindfulness is determined by brain regions involved in executive attention, emotion regulation, and self-referential processing (Lu et al., 2014). A meta-analysis of morphometric neuroimaging among meditation practitioners found eight brain regions consistently altered in meditators, including key areas for meta-awareness (frontopolar cortex), exteroceptive and interoceptive body awareness (sensory cortices and insula), memory consolidation and reconsolidation (hippocampus), and emotion regulation (anterior and mid cingulate, OFC) (Fox et al., 2014). Holzel and colleagues investigated the neural mechanisms of symptom improvements in GAD following mindfulness training compared to an active control intervention (Hölzel et al., 2013). Mindfulness training was associated with decreased amygdala and enhanced prefrontal activation to emotional stimuli and increased amygdala–prefrontal connectivity, which is known to be crucial to successful emotion regulation. Importantly, these changes correlated with improvements in anxiety symptoms following the mindfulness training.
Given these premises, we used voxel-based morphometry (VBM) to characterize gray matter volume differences associated with worry, sustained attention, mindfulness, and autonomic arousal in GAD and controls. We first tested for gray matter volume differences between the two groups, hypothesizing that GAD patients would manifest structural abnormalities in brain (mainly enlarged amygdala and PFC volumes) compared to controls. Subsequently, we ran a series of correlational analyses to explore which brain regions showed a significant association between the gray matter volume and our key variables. In this set of analyses, our approach was first theory-driven, as we primarily focused on the amygdala and PFC, in light of their established role in emotional and autonomic regulation and the structural alterations of these structures often described in GAD (Etkin et al., 2009, Schienle et al., 2011, De Bellis et al., 2000). However, considering that the structural alterations described in GAD go beyond these two structures, we subsequently adopted a data-driven approach and described significant results outside the regions of the amygdala and PFC, which might be even more informative of the biology underlying GAD condition. We used objective measures of attention; i.e. reaction time (RT) to infrequent probes within a low demand attentional task (Ottaviani et al., 2013) and of autonomic control; i.e. HRV derived from pulse oximetry. In both healthy and psychopathological subjects there is evidence for the close mechanistic coupling of autonomic nervous system control and expression of anxiety symptoms, with functional neuroimaging studies highlighting the role of specific regions including amygdala, ACC and insula, in integrating the control and representation of autonomic arousal with neural and behavioral sensitivity to fear signals (Garfinkel et al., 2014, Makovac et al., 2015, Phelps and LeDoux, 2005). To overcome the limitations of the few studies conducted on this topic not only we did not rely on self-report measures of symptoms but we also included an emotion regulation induction.
2. Methods and materials
2.1. Participants
Forty volunteers were recruited by public advertisement to take part in the study. Two participants were excluded because of neuroimaging and peripheral physiology artifacts. The final sample was composed of 19 individuals (16 women, 3 men; mean age = 30.0 ± 6.9 years) who met diagnostic criteria for GAD and 19 healthy controls (16 women, 3 men; age = 29.2 ± 9.8 years). The prevalence of women in the sample reflects the unequal gender distribution in the examined psychopathological disorder. All participants were right-handed, native English speakers, and had normal or corrected-to-normal vision. Exclusion criteria were: age younger than 18 years, past head injury or neurological disorders, prior history of major medical or psychiatric disorder (other than GAD for the patient group), cognitive impairment, history of substance or alcohol abuse or dependence, diagnosis of heart disease, obesity (body mass index > 30 kg/m2), pregnancy, claustrophobia or other general MRI exclusions. Two GAD participants were included who use long-term medications (1 citalopram, 1 pregabalin) at the time of the study. All other patients and controls were medication free. The average disease duration was 16.78 (± 8.01) years and none of our GAD participants had a formal diagnosis of co-morbid major depressive disorder. All participants provided written informed consent. The study was approved by the National Research Ethics Service (NRES) for the National Health Service (NHS) with local approval the Brighton and Sussex Medical School Research Governance and Ethics Committee. Participants were compensated for their time.
2.2. Procedure
The Structured Clinical Interview for DSMIV (SCID) was administered to both patient and controls to confirm/exclude the diagnosis of GAD. Participants then completed a series of questionnaires, were subsequently familiarized with the neuroimaging environment, connected to the physiological recording equipment, and underwent the MRI protocol.
2.3. Questionnaires
Participants completed questionnaires accessing sociodemographic and lifestyle (nicotine, alcohol, and caffeine consumption, physical activity) information, state and trait anxiety (State-Trait Anxiety Inventory, STAI; (Spielberger et al., 1983)), depression (Beck Depression Inventory, BDI; (Beck et al., 1988)), trait worry (Penn State Worry Questionnaire, PSWQ; (Meyer et al., 1990)), and a dispositional measure of mindfulness (Five Facets Mindfulness Questionnaire, FFMQ; (Baer et al., 2006)). The FFMQ measures five mindfulness skills: Non-reactivity to inner experience, Observing/noticing, Acting with awareness, Describing, and Non-judging of experience. Responses are given on a 5-point Likert scale (1 = Never or very rarely true, 5 = Very often or always true). The five subscales have shown adequate to good internal consistency (Baer et al., 2006).
2.4. MRI protocol
After the structural MRI brain scan, participants underwent four repetitions of a 6-min low-demand visuomotor attentional tracking task, each followed by a 5-min resting period (see S1 in the supplementary online material for a visual representation of the experimental design). The visuomotor attentional task (adapted from 29) required participants to track a circle moving horizontally on the screen and press a button as fast as possible each time the circle turned red. The duration of the red circle (target) was 100 ms: if participants did not respond within that time limit, the circle disappeared and the task continued. For each target, accuracy and RT were recorded. The level of difficulty was very low to increase the likelihood of episodes of perseverative cognition. At the end of each tracking task, participants were required to report their thoughts during the preceding period using visual-analogue scales (VAS). After the second or third resting block participants underwent a recorded verbal induction procedure designed to engender perseverative cognition: “Next I would like you to recall an episode that happened in the past year that made you feel sad, anxious, or stressed, or something that may happen in the future that worries you. Then, I would like you to think about this episode in detail, for example about its possible causes, consequences, and your feelings about it. Please keep thinking about this until the end of the next tracking task. Thank you. Please take as much time as you need to recall the episode and press the button whenever you are ready”.
To control for temporal order effects, participants were randomized according to when the induction occurred.
This induction has been proved to be particularly effective in evoking worrisome and ruminative thoughts that have sustained and prolonged physiological consequences and findings have been replicated in different experimental settings in both healthy and clinical samples ((Ottaviani et al., in press) for a meta-analysis).
2.5. Visual analogue scales (VAS)
To assess levels of perseverative cognition occurring prior to and following the induction, participants were asked to rate on four separate visual analogue 100-point scales “How much, for the duration of the task, were you”: 1) focused on the task?; 2) distracted by external stimuli?; 3) ruminating/worrying?; and 4) distracted by internal thoughts?
The last question had the aim to investigate non-pathological mind wandering, in light of recent findings suggesting distinguished physiological signatures of functional mind wandering and maladaptive perseverative cognition (Ottaviani et al., 2013, Ottaviani et al., 2015a, Ottaviani et al., 2015b) and was described to participants in terms of “letting their mind just wander, without getting stuck on any particular thought”.
VAS scores were averaged using before (Pre) and after (Post) induction ratings.
2.6. Physiological data processing
Heartbeats were monitored using MRI-compatible finger pulse oximetry (8600FO; Nonin Medical), recorded digitally as physiological waveforms at a sample rate of 1000 Hz (via a CED power 1401, using Spike2v7 software; Cambridge Electronic Design; Cambridge UK). Inter-beat-intervals (IBI) values were visually inspected and potential artifacts were manually removed. The RHRV 4.0 analysis software (http://rhrv.r-forge.r-project.org/) was used to derive the root mean square successive difference (RMSSD) a measure of vagally mediated HRV (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). RMSSD has been demonstrated to be particularly suited to capture autonomic perturbation in anxiety disorders (Alvares et al., 2013) even from very short (down to 10 s) ECG recordings (Nussinovitch et al., 2012) and to be relatively free of the influences of respiration (Goedhart et al., 2007, Penttilä et al., 2001). Thus, RMSSD was obtained for the 20 s epochs preceding each probe using RHRV 4.0 (http://rhrv.r-forge.r-project.org) and then averaged using before (Pre) and after (Post) induction values.
2.7. MRI acquisition and preprocessing
The present manuscript focuses on the structural neuroimaging analyses. Structural brain MRI scans (0.9 mm isometric voxels, 192 sagittal slices, repetition time 11.4 ms, echo time 4.4 ms, inversion time 300 ms) were acquired using a Siemens Avanto 1.5 T scanner (32-channel head coil, Siemens, Erlangen, Germany).
The T1 weighted (MPRAGE) volumes from all participants were visually reviewed to exclude the presence of macroscopic artifacts. T1-weighted volumes were pre-processed using the VBM protocol implemented in SPM8 (Statistical Parametrical Mapping, http://www.fil.ion.ucl.ac.uk/spm/), which consists of an iterative combination of segmentations and normalizations to produce a gray matter probability map (Ashburner and Friston, 2000, Ashburner and Friston, 2005) in standard space (Montreal Neurological Institute, or MNI coordinates) for each subject. In order to compensate for compression or expansion which might occur during warping of images to match the template, gray matter maps were modulated by multiplying the intensity of each voxel in the final images by the Jacobian determinant of the transformation, corresponding to its relative volume before and after warping (Ashburner and Friston, 2001). For each subject, gray matter, white matter, and cerebrospinal fluid volumes were computed from these probabilistic images. All data were then smoothed using an 8-mm FWHM Gaussian kernel.
2.8. Statistical analyses
Data analysis was performed with SPSS 22.0 for Windows (SPSS Inc, USA).
2.9. Questionnaires, behavioral, and HRV analyses
To test for pre-existing group differences, a series of t and χ2 tests were conducted on self-report socio-demographic and personality measures.
To test for the effects of the induction on cognitive and autonomic variables, a series of Group (GAD vs. HC) × Induction (Pre vs. Post) General Linear Models (GLMs) were performed on RT, RMSSD, and each VAS. Log transformation has been applied to variables that did not meet the criteria of normality and equal variance (i.e., RTs and HRV).
2.10. VBM analysis
Statistical analyses were performed on smoothed gray matter maps within the framework of the general linear model. We used a t-test design implemented in SPM8, to compare gray matter volume in GAD and HC, using total intra-cranial volume (ICV, obtained by adding up white matter volume, gray matter volume, and cerebrospinal fluid volume) as a nuisance covariate to adjust for potential confounding individual differences (e.g. in head size). For RT and HRV, we calculated change scores by subtracting pre induction from post induction scores (Δ[Post − Pre]). Subsequently, these change scores as well as mindfulness facets scores have been entered in SPM8 t-test design as variables of interest, to explore in which regions of the brain gray matter volumes were associated with our emotion (dys) regulation indices. For each participant, we next extracted specific gray matter volume values from statistically significant voxels and reported correlational graphs for illustrative purposes only.
For a priori region-of-interest (ROI) analyses for bilateral amygdala, we constructed anatomical masks using the anatomical toolbox for SPM (Tzourio-Mazoyer et al., 2002). Moreover, we used an anatomically derived (AAL atlas) partial brain mask of entire prefrontal cortex/frontal lobe. Results were accepted as significant at p < 0.05 FWE corrected at cluster level (cluster formed with p value < 0.005).
3. Results
3.1. Descriptive results
Table 1 illustrates baseline characteristics of the samples. The groups did not differ in terms of age, years of education, gender distribution, nicotine consumption, alcohol and caffeine intake, physical activity, and body-mass index (Table 1). As expected, GAD participants reported higher levels of state (t(18) = 4.69; p < 0.0001, d = 2.21) and trait anxiety (t(18) = 6.40; p < 0.0001, d = 1.59), trait worry (t(18) = 6.03, p < 0.0001, d = 2.27), and depression (t(18) = 4.59; p < 0.0001, d = 1.62). The examined questionnaires showed adequate internal consistency; Cronbach's α values in HC and GAD were as follows: 0.89 and 0.92 for STAI state; 0.92 and 0.86 for STAI trait; 0.94 and 0.84 for PSWQ; 0.87 and 0.89 for BDI.
Table 1.
Baseline differences between generalized anxiety disorder (GAD) and healthy controls (HC).
| GAD (n = 19) | HC (n = 19) | p | |
|---|---|---|---|
| Age (years) | 30 ± 6.9 | 29.2 ± 9.8 | 0.81 |
| Gender (M/F) | 3/16 | 3/16 | 0.67 |
| BMI (kg/m2) | 22.80 ± 3.26 | 23.07 ± 2.97 | 0.67 |
| Education (years) | 13 ± 1.89 | 12.16 ± 2.52 | 0.29 |
| Smoking status1 | 7 Y, 12 N | 3 Y, 16 N | 0.27 |
| Cigarettes per day (smokers only) | 1.74 ± 0.47 | 2.88 ± 1.26 | 0.12 |
| Alcohol (units/week) | 4.79 ± 4.38 | 4.04 ± 3.35 | 0.54 |
| Coffee/caffeinated drinks (cups/day) | 2.42 ± 1.74 | 2.16 ± 1.92 | 0.65 |
| Perceived physical fitness2 | 12 H, 5 M, 2 L | 9 H, 9 M, 1 L | 0.39 |
| STAI state | 43 ± 8.76 | 29.84 ± 7.81 | < 0.0001 |
| STAI trait | 55.11 ± 8.02 | 35.89 ± 9.28 | < 0.0001 |
| BDI | 16.53 ± 9.56 | 4.11 ± 5.07 | < 0.0003 |
| PSWQ | 68.11 ± 8.56 | 43.47 ± 13.09 | < 0.0001 |
| HRV (pre-induction) | 44.75 ± 21.85 | 69.92 ± 35.15 | 0.01 |
| HRV (post-induction) | 42.42 ± 16.36 | 74.82 ± 43.36 | 0.01 |
| RT (pre-induction) | 464.58 ± 83.36 | 464.47 ± 72.48 | 0.5 |
| RT (post-induction) | 523.58 ± 121.15 | 505.37 ± 63.0 | 0.28 |
| VAS (pre-induction) | |||
| Ruminating/worrying | 33.11 ± 29.43 | 22.47 ± 22.06 | 0.11 |
| Focused on task | 76.06 ± 19.46 | 64.68 ± 30.78 | 0.1 |
| Distracted by external stimuli | 56.89 ± 24.35 | 44.16 ± 28.17 | 0.07 |
| Distracted by internal thoughts | 55.84 ± 29.22 | 55.16 ± 27.75 | 0.5 |
| VAS (post-induction) | |||
| Ruminating/worrying | 66.42 ± 25.11 | 70.89 ± 21.40 | 0.27 |
| Focused on task | 47.21 ± 25.42 | 57.79 ± 23.12 | 0.09 |
| Distracted by external stimuli | 42.95 ± 24.87 | 36.89 ± 28.66 | 0.24 |
| Distracted by internal thoughts | 84.32 ± 12.65 | 77.16 ± 18.55 | 0.09 |
Note: Generalized anxiety disorder = GAD; healthy controls = HC; body mass index = BMI; State Trait Anxiety Inventory = STAI; Beck Depression Inventory = BDI. Penn State Worry Questionnaire = PSWQ; heart rate variability = HRV; reaction times = RT; Visual Analogue Scale = VAS.
Y = YES, N = NO.
H = High, M = Medium, L = Low.
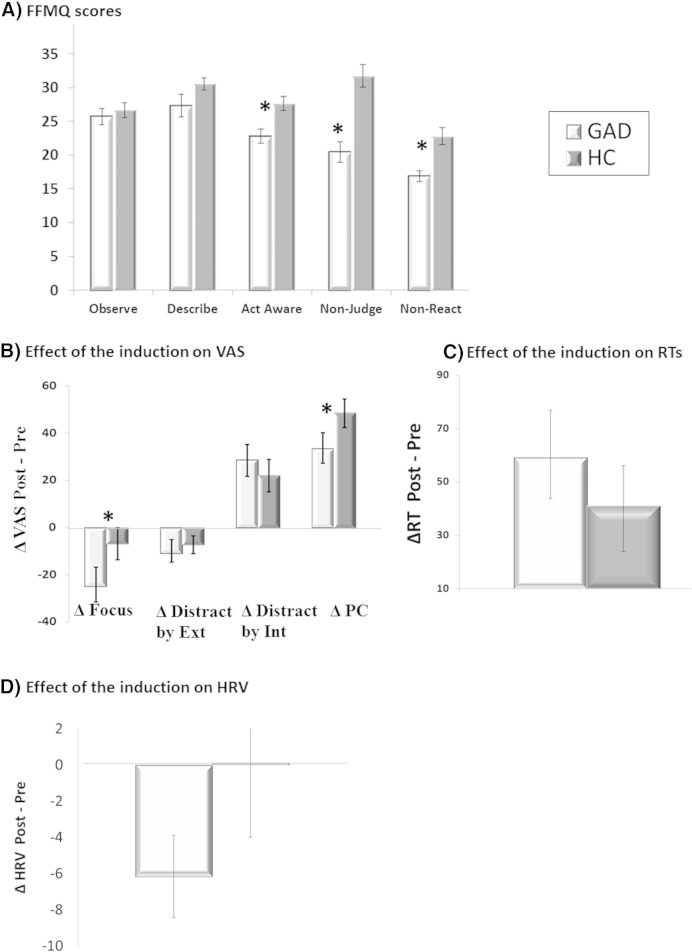
As shown in Fig. 1A, GAD participants had lower scores on the following FFMQ subscales: Act with awareness, t(18) = 3.65, p < 0.01, d = 1.09, Non judging, t(18) = 6.05, p < 0.0001, d = 1.67 and Non reactivity, t(18) = 4.56, p < 0.001, d = 1.35. No differences emerged for the Describing and Observing subscales. Cronbach's α values in the present study in HC and GAD were as follows: 0.79 and 0.85 for Act with awareness; 0.90 and 0.92 for Non judging; 0.63 and 0.83 for Non reactivity; 0.93 and 0.78 for Describing; 0.78 and 0.71 for Observing.
Fig. 1.
Differences in FFMQ subscales between individuals with generalized anxiety disorder (GAD) and healthy controls (HC) (A). Effect of the induction (Δ[Post − Pre]) for GAD and HC on visual analogue scale ratings (VAS; B), reaction times (RT; C), and heart rate variability (HRV; D) measures during the tracking tasks.
Note: Distract by Ext = Distracted by external stimuli; Distract by Int = Distracted by internal thoughts; PC = perseverative cognition. *Although the Group × Induction interaction was not significant for the VAS rumination/worry (PC), ΔPC significantly bigger in HC compared to GAD (due to a higher baseline in GAD).
3.2. Behavioral results
A main effect of Induction emerged, expressed as a decrease in being focused on task (Pre = 70.22 ± 26.18 vs. Post = 51.84 ± 24.55, F(1, 35) = 18.18, p < 0.0001, ƞp2= .34) and distracted by external stimuli (Pre = 50.35 ± 26.80 vs. Post = 40.27 ± 26.93; F(1, 35) = 9.78, p = 0.004, ƞp2 = .22), and an increase in perseverative cognition (Pre = 27.79 ± 26.21 vs. Post = 68.65 ± 23.12, F(1, 36) = 83.47, p < 0.0001, ƞp2 = .69) and being distracted by internal thoughts (Pre = 55.50 ± 28.11 vs. Post = 82.84 ± 17.05; F(1, 36) = 29.53, p < 0.0001, ƞp2 = .45).
A Group × Induction interaction was evident for “being focused on the task” (F(1, 35) = 7.25, p = 0.01, ƞp2 = .17), driven by a stronger decrease in GAD (Pre = 76.10 ± 19.46 vs. Post = 45.55 ± 25.10, t(18) = 5.20, p = 0.001, d = 1.52) compared to HC (Pre = 64.68 ± 30.78 vs. Post = 57.79 ± 23.12, t(18) < 1). The induction also interfered with attention dependent performance, as evident by a main effect of Induction indicating a prolongation of RTs in both groups (Pre = 464.52 ± 77.05 vs. Post = 514.47 ± 95.69, F(1, 36) = 24.24, p < 0.001, ƞp2 = 0.41).
GAD patients had lower HRV when compared to HC, as confirmed by the main effect of Group (GAD = 45.14 ± 16.23 vs. HC = 74.20 ± 41.99; F(1, 36) = 8.12, p < 0.001,, ƞp2 = 0.18). No other significant effects or interactions emerged.
3.3. Morphometry
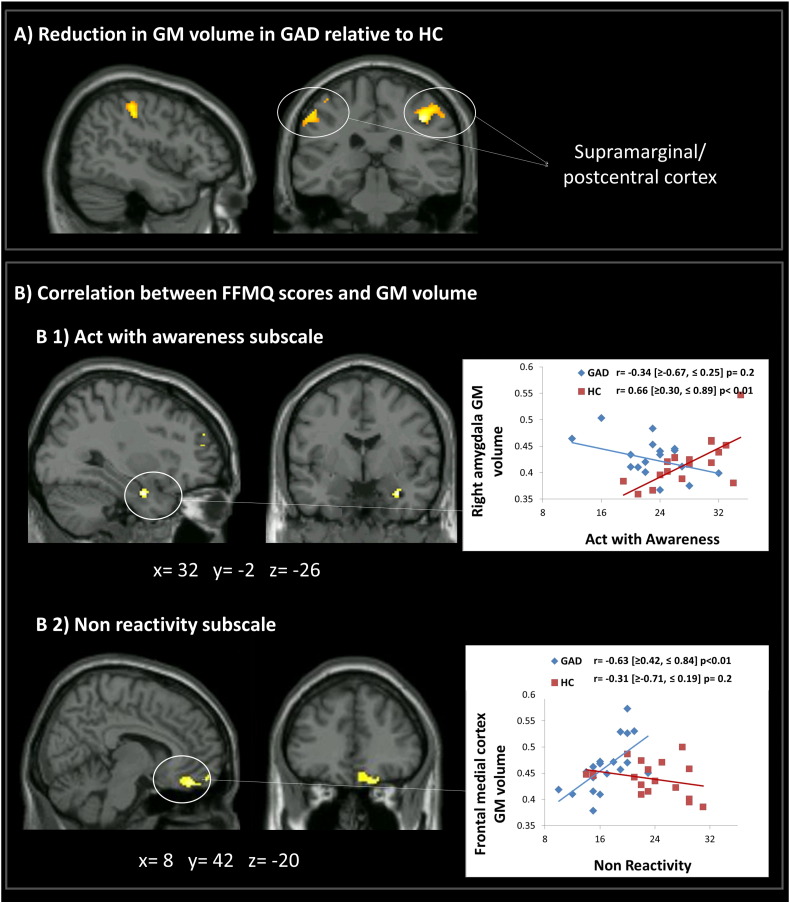
Patients with GAD showed significantly lower gray matter volume in supramarginal gyrus, precentral and postcentral gyrus, bilaterally compared to HC (Fig. 2A, Table 2 (1)). There were no areas of reduced gray matter volume in HC when compared to GAD.
Fig. 2.
(A) Generalized anxiety disorder (GAD) reported significant gray matter atrophy in bilateral supramarginal/postcentral gyrus compared to healthy controls (HC). (B) Significant association between gray matter volume of (B1) the right amygdala and the Act with awareness facet and (B2) medial frontal cortex volume and the Non-react mindfulness facet. Values inside square brackets refer to Bootstrap Confidence Intervals.
Table 2.
Brain areas showing significant correlation between gray matter volume and GAD core symptoms.
| Brain region |
Cluster |
Voxel |
|||
|---|---|---|---|---|---|
| Side | k | p FWE | Z | MNI xyz | |
| (1) Reduction in GM volume in GAD relative to HC | |||||
| Supramarginal gyrus/postcentral gyrus | R | 549 | 0.02 | 4.83 | 38 − 34 44 |
| Precentral gyrus | R | 2.95 | 52 − 6 52 | ||
| Postcentral gyrus | L | 469 | 0.04 | 3.65 | − 54 − 22 53 |
| Supramarginal gyrus | L | 3.54 | − 54 − 42 32 | ||
| Postcentral gyrus | L | 2.88 | − 56 − 20 36 | ||
| (2) Relationship between FFMQ facets and GM volume (FFMQ × Group) | |||||
| Act with awareness | |||||
| Amygdala | R | 22 | 0.021, 2 | 3.60 | 32 − 2 − 26 |
| Non-react | |||||
| Medial frontal cortex | R | 282 | 0.0371, 2 | 3.52 | 8 42 − 20 |
| (3) Relationship between GM volume and RT (ΔRT × Group) | |||||
| Amygdala | R | 87 | 0.011, 2 | 3.83 | 30 − 6 − 24 |
| L | 78 | 0.031, 2 | 3.42 | − 20 0 − 26 | |
| L | 15 | 0.031, 2 | 3.42 | − 28 − 8 − 10 | |
| (4) Relationship between GM volume and HRV (ΔHRV × Group) | |||||
| Insula | R | 1619 | 0.0001 | 4.22 | 40 − 16 2 |
| L | 592 | 0.0001 | 3.11 | − 36 18 0 | |
| Precentral gyrus | R | 3.97 | 58 2 12 | ||
| Supramarginal gyrus/superior temporal gyrus | R | 3.94 | 66 − 30 20 | ||
| Central opercular cortex/postcentral gyrus | R | 3.64 | 56 − 6 16 | ||
| Planum polare | L | 3.70 | − 48 2 − 8 | ||
| Frontal opercular cortex | L | 3.39 | − 42 22 0 | ||
| Central opercular cortex | L | 3.96 | − 42 4 8 | ||
| Frontal orbital cortex/insular cortex | L | 3.16 | − 28 26 − 4 | ||
| Anterior cingulate gyrus | R | 640 | 0.0001 | 3.50 | 6 26 24 |
| Paracingulate gyrus | R | 3.48 | 8 26 42 | ||
| L | 3.14 | − 4 26 32 | |||
Note: Generalized anxiety disorder = GAD; healthy controls = HC; GM = gray matter; Five Facet Mindfulness Questionnaire = FFMQ; reaction times = RT; heart rate variability = HRV.
Small-volume correction.
Peak-level.
3.4. Relationship between gray matter volume and mindfulness facets
The volume of right amygdala reflected a between-group interaction with the ‘Acting with awareness’ mindfulness facet. This interaction was driven by a positive correlation in the group of HC, where smaller amygdala volume was associated with a decreased ability to act with awareness (Fig. 2, B1).
Moreover, a significant Group × Non reactivity interaction emerged driven by a positive correlation in GAD where higher levels of this mindfulness skill were associated with increased medial frontal cortex volume (Fig. 2, B2).
No significant associations emerged between structural brain abnormalities and the Non judging facet in our sample.
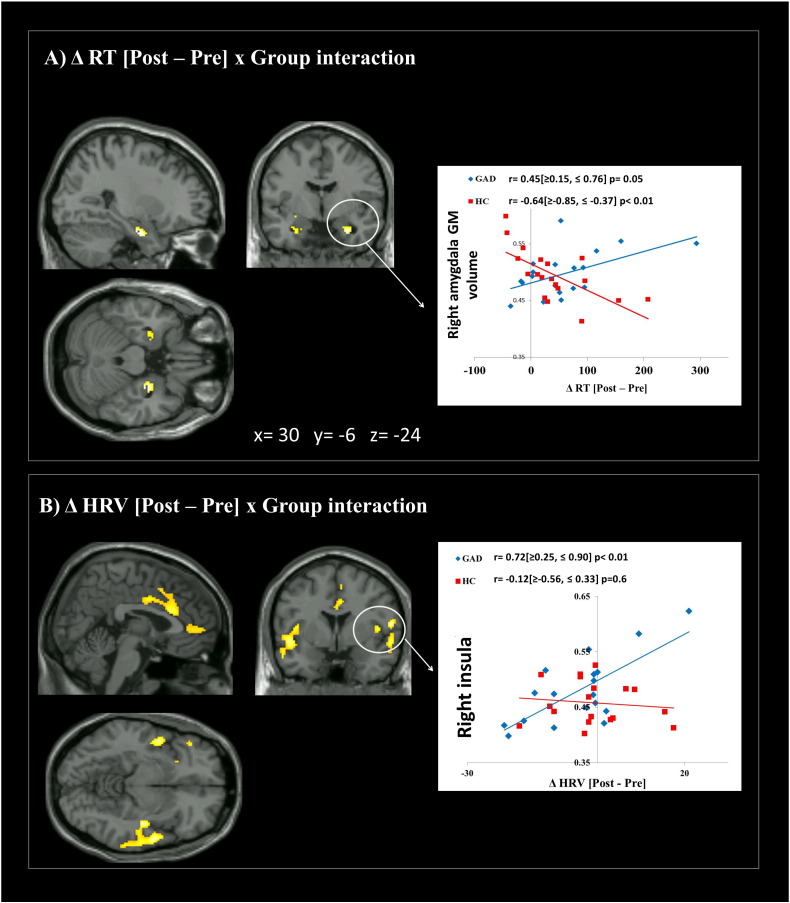
3.5. Relationship between gray matter volume and attentional deficits (RTs)
As illustrated in Fig. 3A, the prolongation in RTs induced by the induction (ΔRT) was associated with gray matter volume in bilateral amygdala. Particularly, a Group × ΔRT interaction emerged, driven by a positive correlation in GAD, and a negative correlation in HC. In other words, the RTs prolongation caused by the induction was associated with augmented volume of the amygdala in GAD and with a diminished volume of the amygdala in HC. Results did not change when a non-parametric test was used or extreme outliers were removed from the analyses (ps < 0.01).
Fig. 3.
Brain regions presenting significant correlations between gray matter density and RTs and HRV changes in response to the induction. (A) A group × ΔRT[Post − Pre] interaction emerged for bilateral amygdala. The interaction was driven by a positive correlation between ΔRT and gray matter in GAD and a negative correlation between ΔRT and gray matter in HC. (B) A positive correlation was evident between the shift in HRV (ΔHRV) and gray matter volume in the bilateral insula, bilateral opercular cortex, right precentral/supramarginal gyrus, anterior cingulate cortex and paracingulate gyrus in GAD only. Values inside square brackets refer to Bootstrap Confidence Intervals.
3.6. Relationship between gray matter volume and autonomic arousal (HRV)
Although no significant associations emerged within the amygdala, the shift in HRV after the worry induction (ΔHRV) correlated positively with the gray matter volume within bilateral insula, bilateral opercular cortex, anterior cingulate cortex, and paracingulate gyrus (see Table 2 for a detailed list of brain areas) in GAD patients only, indicating that smaller gray matter volumes in these areas were associated with a stronger decrease in HRV after the induction (Fig. 3B). Results did not change when a non-parametric test was used or extreme outliers were removed from the analyses (ps < 0.01).
4. Discussion
We combined structural neuroimaging analyses with self-report questionnaires, behavioral measures of attention, and physiological indices of autonomic control before and after a perseverative cognition induction to provide enriched insight into the neural substrates associated with core symptoms of emotion dysregulation in GAD. Results confirm the presence of neurobiological abnormalities in these patients, mainly involving regions implicated in top–down control of directed attention. In addition to this group effect, more specific gray matter volume abnormalities were associated with dispositional measures of mindfulness, impaired attention, and autonomic dysregulation.
In line with previous studies, the induction was effective in increasing state levels of perseverative cognition as well as prolonging RTs, indexing an impairment of attention-dependent perceptual response (Ottaviani et al., 2013). Results on baseline differences between the two groups (although with a slightly different sample size) as well as induction effects at behavioral and physiological levels have been commented on elsewhere (Ottaviani et al., under review) and will not be repeated here.
In line with previous studies conducted in adolescents (Strawn et al., 2013), GAD patients were characterized by relative decreases in the volume of bilateral supramarginal gyrus, postcentral, and precentral brain regions, compared to HC. Most of these areas are associated with top–down control of attentional focus (Hopfinger et al., 2000), hence abnormalities in their structural integrity might be associated with attentional deficits linked to excessive worry, as often observed in patients with anxiety disorders (Eysenck et al., 2007). Interestingly, aberrant functional activity within these areas is observed in the context of other unwanted intrusive cognitive processes, an extreme example being the experience of auditory verbal hallucinations in both psychotic and nonpsychotic patients (Diederen et al., 2012). Whereas previous neuroimaging studies of GAD report more pronounced volume changes within the right cerebral hemisphere (Hilbert et al., 2014), we observed no systematic group differences in laterality; the present findings encompass bilateral areas.
Unexpectedly, we did not find differences in amygdala volume when comparing GAD to controls. Although increased gray matter volume was found in the bilateral amygdala in adults (Etkin et al., 2009, Schienle et al., 2011), children, and adolescents (De Bellis et al., 2000) with a diagnosis of GAD, this result has not always been replicated (Strawn et al., 2013, Mohlman et al., 2009), suggesting the influence of clinical factors such as disease duration. Although comparable with previous investigations, it is possible that our null finding was simply due to the small sample size of the present study.
As predicted, regional brain volume abnormalities were associated with key symptoms of emotion dysregulation in GAD. In line with previous studies on anxiety disorders, our clinical sample showed lower levels of ‘Act with awareness’, ‘Non-judging’, and ‘Non-reactivity’ mindfulness facets, yet no differences were observed on the Describe and Observe subscales (Desrosiers et al., 2013a). Such replication highlights the potential utility of targeting specific aspects of mindfulness in therapeutic interventions for anxiety. We also replicated the results of brain correlates of mindfulness previously described in healthy participants showing a positive correlation between mindfulness facets and gray matter volume of the right amygdala (Lu et al., 2014, Murakami et al., 2012). There are, however, some controversies regarding this point. For example, a study of 155 healthy community adults showed a negative correlation between amygdala volume and dispositional mindfulness (Taren et al., 2013). Moreover, a reduction in the gray matter density of the right amygdala has been observed following an 8-week mindfulness-based intervention (Hölzel et al., 2010). Although many results on general trait measures of mindfulness are contradictory, increased amygdala volume is otherwise consistently associated with the expression of specific mindfulness facets across healthy individuals. It may be particularly relevant that in our study ‘act with awareness’ and “non reactivity” emerged to be the only two facets differently associated with structural differences in GAD and controls. The presence of this mindfulness facet is particularly beneficial for reducing negative repetitive thoughts and has been found to be inversely associated with worry (Delgado et al., 2010, Fisak and von Lehe, 2012) and general distress-anxiety (Desrosiers et al., 2013b). Interestingly, a positive correlation was obtained between Non reactivity and medial frontal cortex volume in GAD whereas no associations emerged in healthy controls. This is an intriguing result as this brain area has been implicated in thought suppression (Wyland et al., 2003) and proactive control (Stuphorn and Emeric, 2012), which are impaired in GAD and likely need a larger gray matter volume cortical area to perform well. On the contrary, in healthy individuals, the adaptive pruning process has likely improved the efficacy of that region during early development, allowing a better performance with a more mature (i.e., with smaller gray matter volume) cortical area.
Effective interventions aimed to enhance control over perseverative cognition, such as mindfulness based cognitive therapy (MBCT; (Teasdale, 1999)) have focus on the present moment as their key component. In our study, the amygdala was differently associated with ‘Act with awareness’ scores in the two groups, where larger volumes were correlated with higher scores in HC and this association was lost in GAD. This is in agreement with the reported correlation between amygdala volume and ‘sensitivity to punishment’, a measure of behavioral inhibition in response to novelty or punishment cues, which plays an important role in anxiety. Sensitivity to punishment can arguably be considered as the opposite of the ‘Act with awareness’ facet (Barrós-Loscertales et al., 2006).
A similar pattern was observed for our measure of attentional engagement (RT). Not only the induction increased RTs during the attentional task in both GAD and HC but also RT changes from pre to post induction correlated with the gray matter volume in bilateral amygdala. In GAD, larger volumes of amygdala were associated with slower RTs after the induction, whereas in HC the opposite pattern was observed, whereby the stronger impact of the induction on RTs was associated with smaller volumes of the amygdala. Larger volume in amygdala has been repeatedly described in anxious children, adolescents (De Bellis et al., 2000, MacMillan et al., 2003) and adults (Etkin et al., 2009, Schienle et al., 2011), in whom amygdala volume often correlates with reported levels of anxiety (Barrós-Loscertales et al., 2006). The observed difference in the structural integrity of the amygdala might be the consequence of a disorder-related hyper-responsiveness of this structure. Normally correlated activity within the vmPFC and amygdala becomes inversely related during the extinction of conditioned fear (Motzkin et al., 2015). Disruption of this inverse coupling is commonly observed in patients with mood and anxiety disorders (Johnstone et al., 2007, Milad et al., 2006). Speculatively, the enhanced responsivity of the amygdala during aversive anticipation (Nitschke et al., 2009) might engender an enlargement of amygdala volume. In fact, activity-dependent changes in gray matter are already widely described in structural imaging studies (Draganski et al., 2004). Nevertheless, the opposite patterns that emerged for GAD and HC groups suggest a potentially unique neurobiology of this psychopathological condition, likely reflecting disorder-specific symptomatology. Further investigation is warranted to clarify the contribution of amygdala in these distinctive GAD symptoms.
A positive correlation was evident between the greater attenuation of HRV caused by the induction and smaller gray matter volume of bilateral insula, bilateral opercular cortex, right precentral/supramarginal gyrus, anterior cingulate cortex, and paracingulate gyrus in GAD patients only. Thus, a negative shift in HRV after the induction was associated with lower gray matter volumes in these areas. Although studies on the structural correlates of the HRV are limited, recent evidence suggests insular atrophy in conditions such as postural tachycardia syndrome, which is characterized by a wide range of autonomic and psychiatric symptoms such as palpitations, lightheadedness, clouding of thought, blurred vision, fatigue, anxiety and depression (Umeda et al., 2015). The insula is considered a pivotal brain region for these phenomena, due to its role in receiving and integrating all bodily inputs (Critchley, 2009).
To the best of our knowledge, this is the first study to combine MRI and peripheral physiology monitoring with the aim of examining the link between neurostructural abnormalities and different facets of emotion dysregulation in GAD. Taken together, results support the view of the disruption of common underlying mechanisms, with an important role played by the amygdala.
The major limitation of the present study is the small sample size, which increases the likelihood that the whole-brain analyses are capitalizing on chance or that the estimate of the magnitude of a significant effect is exaggerated (Button et al., 2013). Another limitation is that mindfulness was solely assessed by self-rating instruments (Park et al., 2013). For example, even if none of our GAD participants had a comorbid depression disorder, BDI scores were significantly higher in the pathological sample, suggesting the presence of depressive symptoms that might lead to a subjective underestimation of mindfulness self-rating. Lastly, some recent reviews have raised a note of caution on the use of VBM methodology in the study of psychiatric conditions (Weinberger and Radulescu, in press), which might misinform the readers on the biological abnormalities underlying psychiatric conditions. Limitations notwithstanding, results inform us about fundamental changes in brain organization in GAD patients and link them to the core symptoms of this psychopathological disease. Giving the prevalence and chronicity of this disorder in the general population and the lack of integrative studies on the topic, the elucidation of dysfunctional brain–body interactions is necessary to increase existing treatment effectiveness and contribute to the development of targeted interventions.
The following are the supplementary data related to this article.
Graphical representation of the experimental design. Each participant underwent a structural MRI scanning (sMRI). Consequently, participants performed a series of three 6-min easy visuo-motor tracking task (Task block) interspersed by resting state periods. Prior to either the second or third performance of the tracking task, participants underwent a verbal induction procedure designed to engender perseverative negative cognition. Participants were randomized according to when the induction occurred to permit control of temporal order effects.
Financial disclosure
No financial interests to disclose.
Acknowledgments
This work was supported by the Italian Ministry of Health Young Researcher Grant awarded to Dr. Cristina Ottaviani (GR-2010-2312442).
References
- Alvares G.A., Quintana D.S., Kemp A.H., Van Zwieten A., Balleine B.W., Hickie I.B., Guastella A.J. Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andor T., Gerlach A.L., Rist F. Superior perception of phasic physiological arousal and the detrimental consequences of the conviction to be aroused on worrying and metacognitions in GAD. J. Abnorm. Psychol. 2008;117:193–205. doi: 10.1037/0021-843X.117.1.193. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. Using self-report assessment methods to explore facts of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A., Meseguer V., Sanjuan A., Belloch V., Parcet M.A., Torrubia R., Avila C. Behavioral inhibition system activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. Neuroimage. 2006;33:1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Garbin M.G.J. Psychometric properties of the Beck depression inventory twenty-five years of evaluation. Clin. Psychol. Rev. 1988;8:77–100. [Google Scholar]
- Brewer J.A., Davis J.H., Goldstein J. Why is it so hard to pay attention, or is it? Mindfulness, the factors of awakening and reward-based learning. Mindfulness. 2013;4:75–80. doi: 10.1007/s12671-012-0164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.A., Barlow D.H., Liebowitz M.R. The empirical basis of generalized anxiety disorder. Am. J. Psychiatry. 1994;151:1272–1280. doi: 10.1176/ajp.151.9.1272. [DOI] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Josephs O., O'Doherty J., Zanini S., Dewar B.K., Cipolotti L., Shallice T., Dolan R.J. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int. J. Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis M.D., Casey B.J., Dahl R.E., Birmaher B., Williamson D.E., Thomas K.M., Axelson D.A., Frustaci K., Boring A.M., Hall J., Ryan N.D. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol. Psychiatry. 2000;48:51–57. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Keshavan M.S., Shifflett H., Iyengar S., Dahl R.E., Axelson D.A., Birmaher B., Hall J., Moritz G., Ryan N.D. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol. Psychiatry. 2002;51:55–562. doi: 10.1016/s0006-3223(01)01375-0. [DOI] [PubMed] [Google Scholar]
- Delgado L.C., Guerra P., Perakakis P., Vera M.N., Reyes del Paso G., Vila J. Treating chronic worry: psychological and physiological effects of a training programme based on mindfulness. Behav. Res. Ther. 2010;48:873–882. doi: 10.1016/j.brat.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Desrosiers A., Klemanski D.H., Nolen-Hoeksema S. Mapping mindfulness facets onto dimensions of anxiety and depression. Behav. Ther. 2013;44:373–384. doi: 10.1016/j.beth.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers A., Klemanski D.H., Nolen-Hoeksema S. Mapping mindfulness facets onto dimensions of anxiety and depression. Behav. Ther. 2013;44:373–384. doi: 10.1016/j.beth.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen K.M., Daalman K., de Weijer A.D., Neggers S.F., van Gastel W., Blom J.D., Kahn R.S., Sommer I.E. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr. Bull. 2012;38:1074–1082. doi: 10.1093/schbul/sbr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., Gaser C., Busch V., Schuierer G., Bogdahn U. Neuroplasticity: changes in gray matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., Menon V., Greicius M.D. Disrupted amygdalar subregion functional connectivity and evidence for a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck M.W., Derakshan N., Santos R., Calvo M.G. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fisak B., von Lehe A.C. The relation between the five facets of mindfulness and worry in anon-clinical sample. Mindfulness. 2012;3:15–21. [Google Scholar]
- Forster S., Nunez Elizalde A.O., Castle E., Bishop S.J. Unraveling the anxious mind: anxiety, worry, and frontal engagement in sustained attention versus off-task processing. Cereb. Cortex. 2015;25:609–618. doi: 10.1093/cercor/bht248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C., Nijeboer S., Dixon M.L., Floman J.L., Ellamil M., Rumak S.P., Sedlmeier P., Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Minati L., Gray M.A., Seth A.K., Dolan R.J., Critchley H.D. Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J. Neurosci. 2014;34:6573–6582. doi: 10.1523/JNEUROSCI.3507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhart A.D., van der Sluis S., Houtveen J.H., Willemsen G., de Geus E.J. Comparison of time and frequency domain measures of RSA in ambulatory recordings. Psychophysiology. 2007;44:203–215. doi: 10.1111/j.1469-8986.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Greeson J., Brantley J. Mindfulness and anxiety disorders: developing a wise relationship with the inner experience of fear. In: Didonna F., editor. Clinical Handbook of Mindfulness. Springer Science + Business Media; New York: 2009. pp. 171–188. [Google Scholar]
- Hazlett-Stevens H. Relaxation strategies. In: Hazlett-Stevens H., editor. Psychological Approaches to Generalized Anxiety Disorder. Springer; 2008. pp. 81–105. (A Clinician's Guide to Assessment and Treatment). [Google Scholar]
- Hilbert K., Lueken U., Beesdo-Baum K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. J. Affect. Disord. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Hoge E.A., Bui E., Marques L., Metcalf C.A., Morris L.K., Robinaugh D.J., Worthington J.J., Pollack M.H., Simon N.M. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J. Clin. Psychiatry. 2013;74:786–792. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., Gard T., Creswell J.D., Brown K.W., Barrett L.F., Schwartz C., Vaitl D., Lazar S.W. Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clin. 2013;2:448–458. doi: 10.1016/j.nicl.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Evans K.C., Hoge E.A., Dusek J.A., Morgan L., Pitman R.K., Lazar S.W. Stress reduction correlates with structural changes in the amygdala. Soc. Cogn. Affect. Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger J.B., Buonocore M.H., Mangun G.R. The neural mechanisms of top–down attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top–down prefrontal-subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Dell Publishing; New York: 1990. Full Catastrophe Living: Using the Wisdom of your Mind to Face Stress, Pain and Illness. [Google Scholar]
- Kessler R.C., Keller M.B., Wittchen H.U. The epidemiology of generalized anxiety disorder. Psychiatr. Clin. N. Am. 2001;24:19–39. doi: 10.1016/s0193-953x(05)70204-5. [DOI] [PubMed] [Google Scholar]
- Lu H., Song Y., Xu M., Wang X., Li X., Liu J. The brain structure correlates of individual differences in trait mindfulness: a voxel-based morphometry study. Neuroscience. 2014;272:21–28. doi: 10.1016/j.neuroscience.2014.04.051. [DOI] [PubMed] [Google Scholar]
- MacMillan S., Szeszko P.R., Moore G.J., Madden R., Lorch E., Ivey J., Banerjee S.P., Rosenberg D.R. Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J. Child Adolesc. Psychopharmacol. 2003;13:65–73. doi: 10.1089/104454603321666207. [DOI] [PubMed] [Google Scholar]
- Makovac E., Garfinkel S., Bassi A., Basile B., Macaluso E., Cercignani M., Calcagnini G., Mattei E., Agalliu D., Cortelli P., Caltagirone C., Bozzali M., Critchley H. Effect of parasympathetic stimulation on brain activity during appraisal of fearful expressions. Neuropsychopharmacology. 2015;40:1649–1658. doi: 10.1038/npp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L., Borkovec T.D. Development and validation of the Penn State Worry Questionnaire. Behav. Res. Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mohlman J., Price R.B., Eldreth D.A., Chazin D., Glover D.M., Kates W.R. The relation of worry to prefrontal cortex volume in older adults with and without generalized anxiety disorder. Psychiatry Res. 2009;173:121–127. doi: 10.1016/j.pscychresns.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Nakao T., Matsunaga M., Kasuya Y., Shinoda J., Yamada J., Ohira H. The structure of mindful brain. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke J.B., Sarinopoulos I., Oathes D.J., Johnstone T., Whalen P.J., Davidson R.J., Kalin N.H. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am. J. Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinovitch U., Cohen O., Kaminer K., Ilani J., Nussinovitch N. Evaluating reliability of ultra-short ECG indices of heart rate variability in diabetes mellitus patients. J. Diabet. Complicat. 2012;26:450–453. doi: 10.1016/j.jdiacomp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Olesen J., Gustavsson A., Svensson M., Wittchen H.U., Jönsson B., CDBE2010 study group, European Brain Council The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J.F., Verkuil B., Lonigro A., Medea B., Couyoumdjian A., Brosschot J.F. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 2015 doi: 10.1037/bul0000036. (in press) [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Shapiro D., Couyoumdjian A. Flexibility as the key for somatic health: from mind wandering to perseverative cognition. Biol. Psychol. 2013;94:38–43. doi: 10.1016/j.biopsycho.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Medea B., Lonigro A., Tarvainen M., Couyoumdjian A. Cognitive rigidity is mirrored by autonomic inflexibility in daily life perseverative cognition. Biol. Psychol. 2015;107:24–30. doi: 10.1016/j.biopsycho.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Shahabi L., Tarvainen M., Cook I., Abrams M., Shapiro D. Cognitive, behavioral, and autonomic correlates of mind wandering and perseverative cognition in major depression. Front. Neurosci. 2015;8:433. doi: 10.3389/fnins.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C., Watson D.R., Meeten F., Makovac E., Garfinkel S.N., Critchley H.D. Neurobiological substrates of cognitive rigidity and autonomic inflexibility in generalized anxiety disorder. Biol. Psychol. 2015 doi: 10.1016/j.biopsycho.2016.06.009. (under review) [DOI] [PubMed] [Google Scholar]
- Park T., Reilly-Spong M., Gross C.R. Mindfulness: a systematic review of instruments to measure an emergent patient-reported outcome (PRO) Qual. Life Res. 2013;22:2639–2659. doi: 10.1007/s11136-013-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttilä J., Helminen A., Jartti T., Kuusela T., Huikuri H.V., Tulppo M.P., Coffeng R., Scheinin H. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin. Physiol. 2001;21:365–376. doi: 10.1046/j.1365-2281.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Roemer L., Lee J.K., Salters-Pedneault K., Erisman S.M., Orsillo S.M., Mennin D.S. Mindfulness and emotion regulation difficulties in generalized anxiety disorder: preliminary evidence for independent and overlapping contributions. Behav. Ther. 2009;40:142–154. doi: 10.1016/j.beth.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A., Ebner F., Schäfer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2011;261:303–307. doi: 10.1007/s00406-010-0147-5. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Stefanopoulou E., Hirsch C.R., Hayes S., Adlam A., Coker S. Are attentional control resources reduced by worry in generalized anxiety disorder? J. Abnorm. Psychol. 2014;123:330–335. doi: 10.1037/a0036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn J.R., Wehry A.M., Chu W.J., Adler C.M., Eliassen J.C., Cerullo M.A., Strakowski S.M., Delbello M.P. Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: a voxel-based morphometry study. Depress. Anxiety. 2013;30:842–848. doi: 10.1002/da.22089. [DOI] [PubMed] [Google Scholar]
- Stuphorn V., Emeric E.E. Proactive and reactive control by the medial frontal cortex. Front. Neuroeng. 2012;5:9. doi: 10.3389/fneng.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y. Detached mindfulness and worry: a meta-cognitive analysis. Personal. Individ. Differ. 2004;37:169–179. [Google Scholar]
- Taren A.A., Creswell J.D., Gianaros P.J. Dispositional mindfulness co-varies with smaller amygdala and caudate volumes in community adults. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Terlevic R., Isola M., Ragogna M., Meduri M., Canalaz F., Perini L., Rambaldelli G., Travan L., Crivellato E., Tognin S., Como G., Zuiani C., Bazzocchi M., Balestrieri M., Brambilla P. Decreased hypothalamus volumes in generalized anxiety disorder but not in panic disorder. J. Affect. Disord. 2012;146:390–394. doi: 10.1016/j.jad.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Teasdale J.D. Emotional processing, three modes of mind and the prevention of relapse in depression. Behav. Res. Ther. 1999;37:S53–S77. doi: 10.1016/s0005-7967(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Umeda S., Harrison N.A., Gray M., Mathias C., Critchley H. Structural brain abnormalities in postural tachycardia syndrome: a VBM-DARTEL study. Front. Neurosci. 2015;9:34. doi: 10.3389/fnins.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger D.R., Radulescu E. Finding the elusive psychiatric “lesion” with 21st-century neuroanatomy: a note of caution. Am. J. Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15060753. (in press) [DOI] [PubMed] [Google Scholar]
- Wyland C.L., Kelley W.M., Macrae C.N., Gordon H.L., Heatherton T.F. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical representation of the experimental design. Each participant underwent a structural MRI scanning (sMRI). Consequently, participants performed a series of three 6-min easy visuo-motor tracking task (Task block) interspersed by resting state periods. Prior to either the second or third performance of the tracking task, participants underwent a verbal induction procedure designed to engender perseverative negative cognition. Participants were randomized according to when the induction occurred to permit control of temporal order effects.