Abstract
Objective
To describe abnormalities in large scale functional networks in unmedicated patients with schizophrenia and to examine effects of risperidone on networks.
Material and methods
34 unmedicated patients with schizophrenia and 34 matched healthy controls were enrolled in this longitudinal study. We collected resting state functional MRI data with a 3T scanner at baseline and six weeks after they were started on risperidone. In addition, a group of 19 healthy controls were scanned twice six weeks apart. Four large scale networks, the dorsal attention network, executive control network, salience network, and default mode network were identified with seed based functional connectivity analyses. Group differences in connectivity, as well as changes in connectivity over time, were assessed on the group's participant level functional connectivity maps.
Results
In unmedicated patients with schizophrenia we found resting state connectivity to be increased in the dorsal attention network, executive control network, and salience network relative to control participants, but not the default mode network. Dysconnectivity was attenuated after six weeks of treatment only in the dorsal attention network. Baseline connectivity in this network was also related to clinical response at six weeks of treatment with risperidone.
Conclusions
Our results demonstrate abnormalities in large scale functional networks in patients with schizophrenia that are modulated by risperidone only to a certain extent, underscoring the dire need for development of novel antipsychotic medications that have the ability to alleviate symptoms through attenuation of dysconnectivity.
Abbreviations: ALFF, amplitude of low frequency fluctuations; BOLD, blood oxygen level dependent signal; BPRS, Brief Psychiatric Rating Scale; DAN, dorsal attention network; DARTEL, diffeomorphic anatomical registration using exponentiated lie algebra algorithm; DMN, default mode network; ECN, executive control network; FD, framewise displacement; FDR, false discovery rate; HC, healthy control; KE, cluster extent; MNI, Montreal Neurological Institute; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SZ, patient with schizophrenia
Keywords: Antipsychotic medication, Default mode network, Dorsal attention network, Executive control network, Salience network
Highlights
-
•
We found widespread functional dysconnectivity in unmedicated patients with schizophrenia.
-
•
Large scale functional networks appear differentially affected in the disorder.
-
•
Attenuation of dysconnectivity with risperidone is seen only to a limited extent.
1. Introduction
The brain's function is thought to be dependent on coordinated activity in complex networks, with the goal of highly efficient information processing (Achard and Bullmore, 2007). With the advent of functional magnetic resonance imaging (fMRI), we have been provided opportunities to gain insights to neural networks in vivo. A growing number of studies suggest schizophrenia to be a disorder of brain network organization, with symptoms likely related to aberrant communication in networks of brain regions (Friston and Frith, 1995).
Resting state fMRI allows examination of intrinsic characteristics of brain networks, by assessing temporal covariation of low frequency fluctuations in the blood oxygen level dependent (BOLD) signal across all voxels in the brain, or functional connectivity (Biswal et al., 1995). Several cortical resting state networks that are reproducible across different study populations have been identified. These include the dorsal attention network (DAN), executive control network (ECN), salience network, and default mode network (DMN). The DAN and ECN are engaged in higher-order cognitive and attentional control (Seeley et al., 2007, Dosenbach et al., 2008). The salience network supports integrative processes (Palaniyappan and Liddle, 2012) and the DMN has been associated with introspection and attention to internal states (Buckner, 2013, Raichle et al., 2001, Whitfield-Gabrieli and Ford, 2012).
Large scale functional networks appear differentially affected (Woodward et al., 2011), and increases, decreases, as well as lack of abnormalities in connectivity reported (Whitfield-Gabrieli and Ford, 2012, Williamson and Allman, 2012, Mamah et al., 2013, Fornito et al., 2011, Whitfield-Gabrieli et al., 2009, Ongur et al., 2010, Repovs et al., 2011), for a summary of studies examining large scale network abnormalities at rest, please see Table 1. The DMN is the most widely investigated large scale network in patients with schizophrenia. Some studies suggest hyperconnectivity of the DMN (Whitfield-Gabrieli et al., 2009, Zhou et al., 2007a, Guo et al., 2015), while others find a mix of hyperconnectivity in posterior regions and hypoconnectivity in anterior regions of the network (Ongur et al., 2010, Mannell et al., 2010, Camchong et al., 2011). An activation likelihood meta-analysis of DMN resting state studies in predominantly medicated patients with schizophrenia discovered hypoconnectivity in the posterior cingulate cortex, hippocampus, and medial prefrontal cortex, as well as hyperconnectivity in the lingual gyrus (Kuhn and Gallinat, 2013). Results are also variable in other large scale networks in schizophrenia. While one study reported no functional abnormalities in the ECN in antipsychotic-naïve, first episode schizophrenia patients (Lui et al., 2009), ECN connectivity has been found reduced in the dorsolateral prefrontal cortex (Woodward et al., 2011, Zhou et al., 2007a), possibly spanning to the parietal aspects of the network (Baker et al., 2014) in medicated patients. Salience network alterations in schizophrenia are inconsistent as well with reductions in connectivity in the anterior cingulate cortex and insula reported in the early stage of the illness (Pu et al., 2012), but increased anterior cingulate together with decreased insula connectivity described in the chronic stage (Woodward et al., 2011, Manoliu et al., 2014). Others reported intact within salience network connectivity (Woodward et al., 2011, Mamah et al., 2013). Data is limited for the DAN in schizophrenia, but connectivity in this network may also be aberrant (Woodward et al., 2011). High variability between studies may be due to several factors, including sample size, image acquisition and analyses methods, data quality, and clinical heterogeneity.
Table 1.
Resting state fMRI studies examining abnormalities in large-scale networks in schizophrenia.
| Study | Year | Sample size (HC/SZ) | Medication status | Illness stage | Field strength (Tesla) | Length of scan (min) | Eyes open/closed | Analysis type | Results |
|---|---|---|---|---|---|---|---|---|---|
| Dorsal attention network | |||||||||
| (Li et al., 2015) | 2015 | 103/121 | Medication-naïve/minimally treated | First episode | 3 | 6.6 | Closed | ICA | No group differences |
| (Woodward et al., 2011) | 2012 | 61/42 | Medicated | Chronic | 3 | 7 | Closed | Seed based | HC > SZ |
| Executive control network | |||||||||
| (Baker et al., 2014) | 2014 | 100/60 | Medicated (4 off medication) | Chronic | 3 | 6.2 | Open | Seed based | HC > SZ |
| (Chang et al., 2014) | 2014 | 25/25 | Medicated (6 off medication) | Chronic | 1.5 | 6 | Closed | ICA | Mixed increase and decrease in SZ |
| (Khadka et al., 2013) | 2013 | 118/70 | Medicated | Chronic | 3 | 5.25 | Open | ICA | No group differences |
| (Li et al., 2015) | 2015 | 103/121 | Medication-naïve/minimally treated | First episode | 3 | 6.6 | Closed | ICA | No group differences |
| (Littow et al., 2015) | 2015 | 43/43 | Medicated | Chronic | 1.5 | 8.4 | ICA | SZ > HC | |
| (Lui et al., 2010b) | 2010 | 34/34 | Medication-naïve | First episode | 3 | 6.6 | ICA | SZ > HC | |
| (Mamah et al., 2013) | 2013 | 33/25 | Medicated | Chronic | 3 | 13.7 | Closed | Seed based | No group differences |
| (Manoliu et al., 2014) | 2014 | 20/18 | Medicated (2 off medication) |
Chronic | 3 | 10 | Closed | ICA | HC > SZ frontal and parietal lobe; SZ > HC temporal and occipital lobe |
| (Repovs et al., 2011) | 2011 | 15/25 | Medicated | Chronic | 3 | 13.7 | Closed | Seed based | No group differences |
| (Rotarska-Jagiela et al., 2010) | 2010 | 16/16 | Medicated | Chronic | 3 | 6.6 | Open | ICA | HC > SZ frontal gyrus, SZ > HC parietal cortex |
| (Su et al., 2013) | 2013 | 25/25 | Medicated | Chronic | 3 | 8.4 | Seed based | HC > SZ | |
| (Tu et al., 2013) | 2013 | 36/36 | Medicated | Chronic | 3 | 8.5 | Open | Seed based | HC > SZ |
| (Wolf et al., 2011, Wolf et al., 2007) | 2011 | 14/10 | Medicated | Chronic | 3 | 6 | Closed | ICA | HC > SZ to precuneus, SZ > HC to frontal lobe |
| (Woodward et al., 2011) | 2012 | 61/42 | Medicated | Chronic | 3 | 7 | Closed | Seed based | HC > SZ in frontal regions |
| (Zhou et al., 2007b) | 2007 | 17/17 | Minimally treated | First episode | 1.5 | 6 | Closed | Seed based | Mixed increase and decrease in SZ |
| (Zhou et al., 2007a) | 2007 | 18/18 | Medicated (3 off medication) |
Recent onset | 1.5 | 6 | Closed | Seed based | SZ > HC in frontal regions, HC > SZ in insula and parietal lobe |
| Default mode network | |||||||||
| (Alonso-Solis et al., 2012) | 2012 | 19/19 | Medicated (2 off medication) |
First episode | 3 | 2.6 | Closed | Seed based | HC > SZ |
| (Alonso-Solis et al., 2015) | 2015 | 20/33 | Medicated | Chronic | 3 | 6 | Closed | Seed based | Mixed increase and decrease in SZ |
| (Bluhm et al., 2007) | 2007 | 17/17 | Medicated (2 off medication) |
Chronic | 4 | 5.5 | Closed | Seed based | HC > SZ |
| (Camchong et al., 2011) | 2011 | 29/29 | Medicated (2 off medication) |
Chronic | 3 | 6 | ICA | HC > SZ | |
| (Chang et al., 2014) | 2014 | 25/25 | Medicated (6 off medication) |
Chronic | 1.5 | 6 | Closed | ICA | SZ > HC |
| (Guo et al., 2015) | 2015 | 50/49 | Medication-naïve | First episode | 3 | 8.3 | Closed | Seed based | SZ > HC |
| (Jafri et al., 2008) | 2008 | 25/29 | Medicated | Chronic | 3 | 5 | ICA | Within network differences not reported | |
| (He et al., 2013) | 2013 | 113/115 | Medication-naïve | First episode | 3 | 6.6 | Closed | Seed based | No group differences |
| (Khadka et al., 2013) | 2013 | 118/70 | Medicated | Chronic | 3 | 5.25 | Open | ICA | HC > SZ |
| (Li et al., 2015) | 2015 | 103/121 | Medication-naïve/minimally treated | First episode | 3 | 6.6 | Closed | ICA | SZ > HC |
| (Littow et al., 2015) | 2015 | 43/43 | Medicated | Chronic | 1.5 | 8.4 | ICA | SZ > HC | |
| (Lui et al., 2010b) | 2010 | 34/34 | Medication-naïve | First episode | 3 | 6.6 | ICA | HC > SZ | |
| (Mamah et al., 2013) | 2013 | 33/25 | Medicated | Chronic | 3 | 13.7 | Closed | Seed based | No group differences |
| (Mannell et al., 2010) | 2010 | 16/16 | Medicated | Chronic | 1.5 | 8 | ICA and seed based | SZ > HC in posterior regions, HC > SZ in frontal regions |
|
| (Manoliu et al., 2014) | 2014 | 20/18 | Medicated (2 off medication) |
Chronic | 3 | 10 | Closed | ICA | HC > SZ |
| (Meda et al., 2012) | 2012 | 118/70 | Medicated | Chronic | 3 | 5.25 | Open | ICA | Within network differences not reported |
| (Meda et al., 2012, Meda et al., 2014) | 2014 | 324/296 | Medicated (11 off medication) |
Chronic | 3 | 5 | Open | ICA | HC > SZ |
| (Mingoia et al., 2012) | 2012 | 25/25 | Medicated | Chronic | 3 | 9 | Closed | ICA | Mixed increase and decrease in SZ |
| (Ongur et al., 2010) | 2010 | 15/14 | Medicated | Chronic | 3 | 10 | Open | ICA | HC > SZ in the ACC; SZ > HC in basal ganglia |
| (Orliac et al., 2013) | 2013 | 26/26 | Medicated | Chronic | 3 | 8 | Closed | ICA | HC > SZ |
| (Repovs et al., 2011) | 2011 | 15/25 | Medicated | Chronic | 3 | 13.7 | Closed | Seed based | No group differences |
| (Rotarska-Jagiela et al., 2010) | 2010 | 16/16 | Medicated | Chronic | 3 | 6.6 | Open | ICA | HC > SZ |
| (Skudlarski et al., 2010) | 2010 | 27/27 | Medicated | Chronic | 3 | 5.25 | Seed based | SZ > HC | |
| (Swanson et al., 2011) | 2011 | 28/28 | Medicated | Chronic | 3 | 5 | ICA | Differences in network laterality | |
| (Tang et al., 2013) | 2013 | 32/32 | Medicated (6 off medication) |
Early onset | 1.5 | 6 | Closed | ICA | SZ > HC |
| (Wang et al., 2015) | 2015 | 53/55 | Medicated (34 drug naïve) |
First episode | 3 | 8 | Closed | ICA | SZ > HC |
| (Whitfield-Gabrieli et al., 2009) | 2009 | 13/13 | Medicated (3 off medication) |
Chronic | 1.5 | Seed based | SZ > HC | ||
| (Wolf et al., 2007) | 2011 | 14/10 | Medicated | Chronic | 3 | 6 | Closed | ICA | No group differences |
| (Woodward et al., 2011) | 2012 | 61/42 | Medicated | Chronic | 3 | 7 | Closed | Seed based | SZ > HC |
| (Zhou et al., 2007a) | 2007 | 18/18 | Medicated (3 off medication) |
Recent onset | 1.5 | 6 | Closed | Seed based | SZ > HC |
| Salience network | |||||||||
| (Li et al., 2015) | 2015 | 103/121 | Medication-naïve/minimally treated | First episode | 3 | 6.6 | Closed | ICA | No group differences |
| (Mamah et al., 2013) | 2013 | 33/25 | Medicated | Chronic | 3 | 13.7 | Closed | Seed based | No group differences |
| (Manoliu et al., 2014) | 2014 | 20/18 | Medicated (2 off medication) |
Chronic | 3 | 10 | Closed | ICA | HC > SZ in insula; SZ > HC in frontal regions |
| (Moran et al., 2013) | 2013 | 44/44 | Medicated | Chronic | 3 | 5 | Open | Seed based | HC > SZ |
| (Orliac et al., 2013) | 2013 | 26/26 | Medicated | Chronic | 3 | 8 | Closed | ICA | HC > SZ |
| (Palaniyappan et al., 2013) | 2013 | 35/38 | Medicated (2 off medication) |
Chronic | 3 | 10 | Open | Seed based | Mixed increase and decrease in SZ |
| (Pu et al., 2012) | 2012 | 90/90 | Medicated (16 drug naïve) |
Recent onset | 1.5 | 6 | Closed | Seed based | HC > SZ |
| (Woodward et al., 2011) | 2012 | 61/42 | Medicated | Chronic | 3 | 7 | Closed | Seed based | No group differences |
It is likely that differences in the patient population such as medication management may account for a portion of the variability in findings. However, few attempts have been made to characterize large scale functional networks at rest in unmedicated patients with schizophrenia and to examine systems-level effects of antipsychotic medication on these measures, despite evidence suggesting that functional network architecture is sensitive to neuromodulation (Cole et al., 2013a, van de Ven et al., 2013, Schaefer et al., 2014). Dopamine targeting agents such as L-dopa, methylphenidate, and haloperidol have been shown to affect functional connectivity in healthy subjects (Cole et al., 2013a, Sripada et al., 2013, Cole et al., 2013b). In patients with schizophrenia, our group has reported that abnormally low ventral tegmental area to thalamus connectivity observed in unmedicated patients was restored to more normal patterns after one week of treatment with risperidone (Hadley et al., 2014). Sarpal and colleagues reported increases in connectivity between striatal seed regions and the thalamus in a group of first episode psychosis patients after twelve weeks of treatment with risperidone or aripiprazole and also reported changes in fronto-limbic to striatal connectivity as a function of good clinical response (Sarpal et al., 2015). Olanzapine has been reported to increase functional connectivity in DMN connectivity with the ventromedial prefrontal cortex, but not posterior regions of the DMN between weeks four and eight of treatment (Sambataro et al., 2010). Examining amplitudes of low frequency fluctuations (ALFF), another measure in functional connectivity, another group showed increased ALFF in prefrontal, parietal and temporal areas after six weeks of treatment with various second generation antipsychotic medications (Lui et al., 2010a).
The purpose of this study was to better characterize systems-level effects of risperidone, a commonly prescribed second generation antipsychotic medication, on large scale resting state networks in schizophrenia. We obtained resting state scans before medication was initiated (in unmedicated subjects) and after six weeks of treatment to investigate antipsychotic effects on four large scale networks, the DAN, the ECN, the salience network, and the DMN. Based on the existing literature, we hypothesized large scale functional networks to be differentially affected in unmedicated patients and that network integrity would be, at least partially, restored after six weeks of antipsychotic treatment. We also conducted exploratory analyses to investigate associations between connectivity and clinical variables.
2. Material and methods
Unmedicated patients with schizophrenia or schizoaffective disorder (SZ) were recruited from various outpatient clinics, the inpatient unit and emergency room at the University of Alabama at Birmingham (UAB). Healthy controls (HC) matched on age, sex, parental occupation and smoking status were recruited by advertisements in flyers and the university's newspaper. Approval for this investigator-initiated study was obtained by the UAB Institutional Review Board. Written informed consent to participate in the study was obtained after subjects were deemed competent to provide consent (Carpenter et al., 2000).
Diagnoses were established by review of medical records and consensus of two clinicians and then confirmed with the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994). All SZ were off antipsychotic medication for at least ten days and agreed to treatment; medication was not discontinued to meet this criterion. Subjects were excluded if they had a major medical or neurological condition, history of head trauma with loss of consciousness, met criteria for a moderate or severe substance use disorder other than nicotine use disorder within six months of imaging, were prescribed medications known to affect brain function, were pregnant or breastfeeding, or had any MRI contraindications. A personal or family history in a first-degree relative of an Axis I disorder was an exclusion criterion for HC.
Subjects who were either medication naïve or had been off antipsychotic medications were enrolled in a six week trial with risperidone using a flexible dosing regimen. Medication was managed by two psychiatrists (ACL and NVK), and dose determinations were based on therapeutic and side effects. Starting doses were 1–3 mg; titration was done in 1–2 mg increments. Compliance was monitored by pill counts at each visit. Concomitant antidepressant or mood stabilizing medication was allowed to be used as indicated. Resting state scans were obtained prior to treatment (off medication) and after six weeks of treatment with risperidone.
Of the 68 subjects enrolled, one HC was excluded from baseline analysis because of poor scan quality. In the SZ group 6 subjects dropped out of the study prior to the second scan. Additionally, no resting state data was available for 6 subjects at week 6 (resting state scans not obtained for 5 subjects, poor scan quality in 1 subject), leaving data for 34 SZ at baseline and 22 SZ at week 6 in the final analysis. Additionally, we scanned a group of 19 HC twice six weeks apart to give us the ability to assess connectivity changes over time. Resting state data from some subjects have been included in earlier reports (Hadley et al., 2014, Kraguljac et al., 2014).
2.1. Clinical variables
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to characterize general cognitive function (Randolph et al., 1998). The Brief Psychiatric Rating Scale (BPRS) was used for weekly assessments of symptom severity (Overall and Gorham, 1962).
2.2. MRI acquisition
All imaging was performed on a 3T head-only scanner (Magnetom Allegra, Siemens Medical Solutions), equipped with a circularly polarized transmit/receive head coil. Resting state fMRI scans were acquired during a five-minute gradient recalled echo-planar imaging sequence (repetition time/echo time [TR/TE] = 2000/30 msec, flip angle = 70°, field of view = 192 × 192 mm2, 64 × 64 matrix, 6-mm slice thickness, 1-mm gap, 30 axial slices, 150 acquisitions). During the scan, subjects were instructed to keep their eyes open and stare passively ahead. High-resolution structural scans were acquired using the three-dimensional T1-weighted magnetization prepared rapid acquisition gradient-echo sequence (TR/TE/inversion time [TI] = 2300/3.93/1100 msec, flip angle = 12°, 256 × 256 matrix, 1 mm isotropic voxels). All MRI scans were reviewed for abnormalities by a neuroradiologist.
2.3. Functional connectivity analysis
Using the statistical parametric mapping package SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK), resting state data were slice timing corrected, realigned using rigid-body motion transforms, co-registered to the high-resolution structural scan, normalized to 1.5 mm3 Montreal Neurologic Institute (MNI) space, and spatially smoothed employing the diffeomorphic anatomical registration using exponentiated lie algebra algorithm (DARTEL) with a 6-mm at full-width half-maximum three-dimensional Gaussian kernel (Asami et al., 2012).
To remove physiological noise from data, a nuisance regression was first conducted using the six motion parameters identified during the realignment step and their first derivatives as regressors. Next, a step-wise data scrubbing procedure was followed to reduce the effects of time points severely contaminated by motion (Power et al., 2012). Framewise displacements from each time point, i, to the next (FDi) were computed from the six realignment parameters from SPM8. A radius r = 50 mm, approximately the mean distance from the center of MNI space to the cortex, was used to convert angle rotations (radians) to displacements (mm). Time points with FDi > 0.5 mm were considered to be severely contaminated with motion or other artifact. These contaminated time points were first interpolated prior to bandpass filtering of the data (0.009 < f < 0.08 Hz) (Carp, 2013), and then excluded from subsequent analyses (Power et al., 2013). To assess motion effects on connectivity data, we calculated mean absolute framewise displacement of the brain from one timeframe to the next for each subject. There were no significant differences in mean absolute framewise displacement between HC and unmedicated SZ, or between time points in SZ (HC: 0.17 +/− 0.23 mm; SZ baseline: 0.23 mm +/− 0.23 mm; SZ week 1: 0.17 +/− 0.12 mm; SZ week 6: 0.23 mm +/− 0.23 mm; all p > .05), however, more timepoints were scrubbed in SZ than HC (t = − 2.781; p < .01). Following data scrubbing, a principle component analysis was used to extract the components of white matter and cerebral spinal fluid necessary to explain 90% of signal variance from those regions. These extracted components were used as regressors in a second nuisance regression (Behzadi et al., 2007).
Spherical seeds with 6 mm radius were placed at the following MNI coordinates to define resting state networks as described in previous studies (Seeley et al., 2007, Woodward et al., 2011, Vincent et al., 2006, Achard and Bullmore, 2007) − 25/− 53/52 and 25/− 57/52 (intraparietal sulcus and superior parietal lobe) for the DAN, (Friston and Frith, 1995) − 42/34/20 and 44/36/20 (left and right dorsolateral prefrontal cortex) for the ECN, (Biswal et al., 1995) − 32/26/− 14 and 38/22/− 10 (left and right fronto-insular cortex) for the salience network, and (Seeley et al., 2007) 1/− 55/17 (posterior cingulate cortex) for the DMN.
The first eigenvariate of the BOLD time series from each seed was extracted and correlated to the time series of all other voxels in the brain to produce functional connectivity maps (units of Pearson's r correlation). For networks with bilateral seeds, these were averaged to form a single functional connectivity map of each network for each subject. Maps were converted to normally distributed values using Fisher's r-to-Z transform.
Group-level functional connectivity maps were obtained by performing one-sample t-tests on each group's participant-level functional connectivity maps. Group differences in functional connectivity, as well as differences between medication-naïve and previously medicated patients, were assessed using two-sample t-tests on the groups' participant-level functional network connectivity maps. To restrict analyses to include only those brain regions that were recruited by at least one of the groups, we created thresholded t-maps (PFDR < .01) combining groups. We computed these by creating one-sample t-tests for each resting state network and both diagnostic groups (both points in SZ) that were binarized and combined, thus producing masks of the combined connected areas. These masks were used in between group comparisons and comparisons across time within each large scale network. All analyses were corrected for multiple comparisons using the false discovery rate and are reported at PFDR < 0.001 for main effects within group and at PFDR < 0.05 for group differences and change over time.
To assess connectivity changes over time in healthy controls we implemented two statistical tests. First, we used paired-sample t-tests on the participant-level functional network connectivity maps for each network to examine change over time at the group level. Second, we calculated intra-subject reliability at the network level implemented in the ICC toolbox (http://brainmap.co.uk). Intra voxel intra-class correlation coefficients (ICC) were calculated for each subject using the contrast values of the voxels within the networks. The median ICC and standard errors were obtained from a bootstrapped ICC distribution with 1000 re-samples per subject (Caceres et al., 2009).
In an exploratory fashion, we also examined the relationships between connectivity and clinical variables. To assess the relationship between functional connectivity of each network and symptom burden at baseline in patients with schizophrenia, we used a general linear model that included BPRS total scores and one that included RBANS total scores. To restrict analyses to each network, above described masks were used in the general linear model. Treatment response was defined as (BPRS positive score at baseline − BPRS positive score at 6 weeks) / BPRS positive score at baseline. We were able to assess treatment response for 28 subjects at week 6, and carried forward week 5 data from two subjects, totaling of 30 subjects with treatment response scores.
3. Results
3.1. Demographics and behavioral results
Of the 34 SZ subjects, 22 were chronically ill and 12 experienced their first episode. 17 SZ were antipsychotic naïve (five had not received prior treatment despite a prolonged period since illness onset), and 17 have had prior exposure to antipsychotic medication. Average dose of risperidone at endpoint was 4.36 +/− 1.45 mg. Twelve subjects were concomitantly treated with benztropine, two with trazodone, one each was prescribed mirtazapine, amitriptyline, and valproic acid.
There were no significant differences between HC and SZ in age, sex, parental socioeconomic status, or smoking status (Table 2). As expected, SZ scored significantly lower on the RBANS compared to HC. In SZ, BPRS total scores decreased from 48.29 +/− 9.38 at baseline to 30.57 +/− 8.47 after six weeks of treatment.
Table 2.
Demographics and clinical measures.a
| SZ (n = 34) | HC (n = 34) | t/X2 | p value | |
|---|---|---|---|---|
| Gender (% male) | 67.6 | 67.6 | 0.000 | 1.0 |
| Age | 32.38 (10.43) | 32.00 (9.02) | −.382 | .87 |
| Parental occupationb | 7.26 (6.39) | 6.21 (4.35) | − 0.80 | .43 |
| Smoking status (% smokers) | 76.5 | 64.7 | 1.133 | .42 |
| Smoking (packs per day) | 0.59 (0.53) | 0.65 (0.60) | 0.447 | .66 |
| Diagnosis | ||||
| Schizophrenia | 31 | |||
| Schizoaffective disorder | 3 | |||
| Illness characteristics | ||||
| Illness duration (years) | 9.59 (9.94) | |||
| First episode | 12 | |||
| Prior antipsychotic treatment | ||||
| Antipsychotic naive | 17 | |||
| Antipsychotic free interval (months) | 23.08 (44.41) | |||
| BPRSc | ||||
| Total | ||||
| Baseline | 48.29 (9.38) | |||
| Week 6d | 30.57 (8.47) | |||
| Positive | ||||
| Baseline | 9.53 (3.04) | |||
| Week 6d | 4.86 (2.38) | |||
| Negative | ||||
| Baseline | 6.79 (2.51) | |||
| Week 6d | 5.39 (2.42) | |||
| RBANSe | ||||
| Total index | 70.21 (13.76) | 94.47 (13.99) | 6.773 | <.01 |
| Immediate memory | 74.68 (16.86) | 96.53 (12.78) | 4.962 | <.01 |
| Visuospatial | 71.41 (15.48) | 89.18 (19.15) | 5.549 | <.01 |
| Language | 84.71 (12.85) | 96.68 (13.92) | 3.663 | <.01 |
| Attention span | 79.03 (20.32) | 100.82 (18.92) | 5.001 | <.01 |
| Delayed memory | 72.53 (19.10) | 93.53 (11.72) | 5.348 | <.01 |
SZ, schizophrenia; HC, healthy control.
Mean (SD) unless indicated otherwise.
Ranks determined from Diagnostic Interview for Genetic Studies (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status.
Brief Psychiatric Rating Scale (1–7 scale); positive (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative (emotional withdrawal, motor retardation, and blunted affect).
n = 28.
Repeatable Battery for the Assessment of Neuropsychological Status.
3.2. Imaging results
3.2.1. Functional connectivity in HC and unmedicated SZ
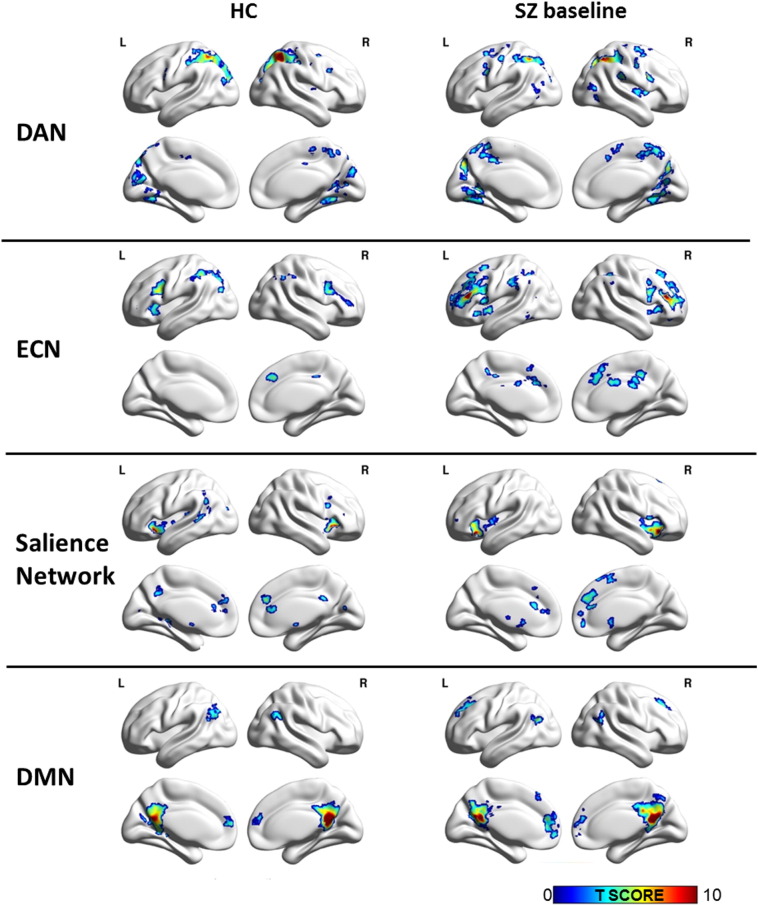
In HC, analyses revealed functional network architecture of all four large scale networks consistent with prior reports (Fig. 1); connectivity patterns at the group level did not significantly change between baseline and week six in any of the networks in the 19 HC scanned twice. Median intra-voxel ICC in HC between the two scans was 0.44 +/− 0.03 for the DAN, 0.35 +/− 0.07 for the ECN, 0.38 +/− 0.08 for the salience network, and 0.33 +/− 0.07 for the DMN.
Fig. 1.
Resting state networks in healthy controls (HC; n = 33; left column) and unmedicated patients with schizophrenia (SZ baseline; n = 34; right column). Statistical maps are significant at PFDR < .001. Results are projected on BrainNet Viewer surface map, medium view. Abbreviations: DAN: dorsal attention network; ECN: executive control network; DMN: default mode network; L: left; R: right.
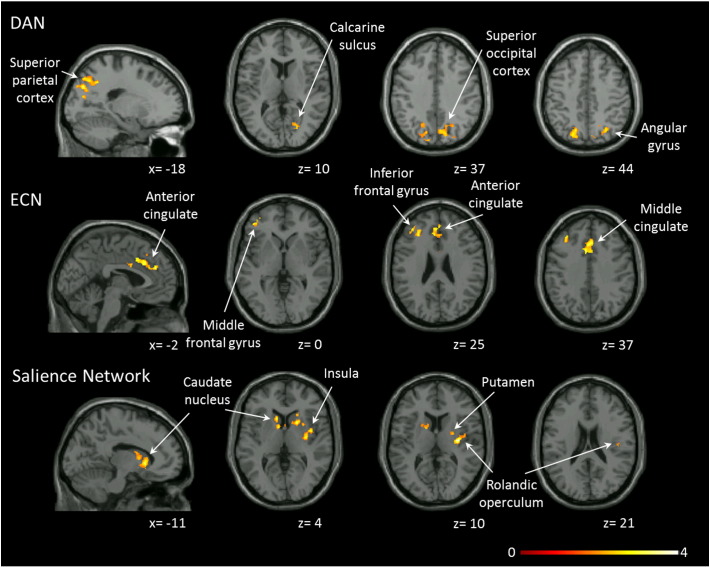
In unmedicated SZ, we found increased functional connectivity in the DAN, ECN, and salience networks, but not the DMN when compared to HC (Fig. 2). There were no areas of decreased connectivity in any of the resting state networks. Connectivity patterns did not significantly differ between the 17 medication naïve and 17 previously medicated SZ in any network. DAN connectivity was increased in the angular gyrus, middle temporal gyrus, superior and inferior parietal gyri, cuneus/precuneus, superior and middle occipital gyrus, and calcarine sulcus. In the ECN, we noted elevated connectivity in the superior frontal, middle frontal, and inferior frontal gyri, supplemental motor cortex, as well as anterior and middle cingulate cortices. Connectivity was also increased in the rolandic operculum, insula, basal ganglia and amygdala in the salience network (Table 3). Using more stringent corrections we found that ECN connectivity to the inferior frontal gyrus was increased (t = 4.37, PFDR < .01, kE [cluster extent, number of voxels] = 493, MNI coordinates: x = − 30, y = 52, z = 14), but we no longer found increased connectivity in the DAN or salience networks. To better understand the increase in connectivity (and because the number of voxels functionally connected to the seed region was 11% higher in the DAN and 17% higher in the ECN, salience network, and DMN in unmedicated SZ compared to HC), we conducted a post-hoc analysis limiting analyses of connectivity differences between HC and unmedicated SZ only to areas included in HC network (i.e. all voxels that functionally connected to the seed in HC). We found no abnormalities in any of the networks (all PFDR > .05) or differences in mean signal amplitude (DAN: t = − 0.102; p = .91; ECN: t = − 0.293; p = .77; salience network: t = 1.357; p = .18; DMN: t = 0.544; p = .59), suggesting that the increase in functional connectivity seen in SZ may be related to topographical expansion of large scale networks rather than abnormally increased connectivity within areas that are typically part of the networks.
Fig. 2.
Resting state connectivity abnormalities in unmedicated patients with schizophrenia (n = 34) compared to healthy controls (n = 33). Clusters indicate areas with significantly increased connectivity in unmedicated patients compared to controls. No clusters with decreased connectivity in unmedicated patients compared to controls are present. Statistical maps are significant at PFDR < .05. Clusters are overlaid on the Xjview single subject T1 template, left columns show a sagittal slice, all others are axial. Numbers below the slices indicate x and z coordinates in MNI convention. Color bar indicates t values. Abbreviations: DAN: dorsal attention network; ECN: executive control network.
Table 3.
Increased functional connectivity in unmedicated patients with schizophrenia compared to healthy controls.
| Brain regions | Voxels in cluster | Hem. | Voxels in region | Peak coordinatesa |
Peak t | PFDRb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Dorsal attention network | ||||||||
| Cluster 1 | 2971 | 24 | − 54 | 51 | 3.891 | |||
| Superior parietal gyrus | B | 468 | ||||||
| Inferior parietal gyrus | B | 228 | ||||||
| Cuneus/precuneus | B | 665 | ||||||
| Superior and middle occipital gyrus | L | 534 | ||||||
| Cluster 2 | 2234 | 15 | − 70.5 | 31.5 | 3.673 | |||
| Middle temporal gyrus | R | 119 | ||||||
| Angular gyrus | R | 56 | ||||||
| Superior parietal gyrus | R | 66 | ||||||
| Cuneus/precuneus | R | 741 | ||||||
| Superior and middle occipital gyrus | R | 701 | ||||||
| Calcarine sulcus | R | 174 | ||||||
| Executive control network | ||||||||
| Cluster 1 | 1450 | − 30 | 52.5 | 13.5 | 4.369 | |||
| Superior frontal gyrus | L | 141 | ||||||
| Middle frontal gyrus | L | 1195 | ||||||
| Inferior frontal gyrus | L | 109 | ||||||
| Cluster 2 | 1344 | − 9 | 31.5 | 30 | 3.779 | |||
| Superior medial frontal gyrus | B | 364 | ||||||
| Supplemental motor area | B | 77 | ||||||
| Anterior cingulate cortex | B | 269 | ||||||
| Middle cingulate cortex | L | 734 | ||||||
| Salience network | ||||||||
| Cluster | 2615 | 34.5 | − 13.5 | 12 | 4.613 | |||
| Rolandic operculum | R | 118 | ||||||
| Pallidum/putamen | B | 765 | ||||||
| Caudate | B | 580 | ||||||
| Insula | R | 253 | ||||||
| Amygdala | R | 32 | ||||||
| Default mode network | ||||||||
| No clusters | ||||||||
Abbreviations: Hem., hemisphere; L, left; R, right.
Reported in Montreal Neurologic Institute coordinates (X, Y, and Z).
All comparisons were FDR-corrected (PFDR ≤ 0.05).
3.2.2. Relationship between abnormalities in large scale networks and symptom burden
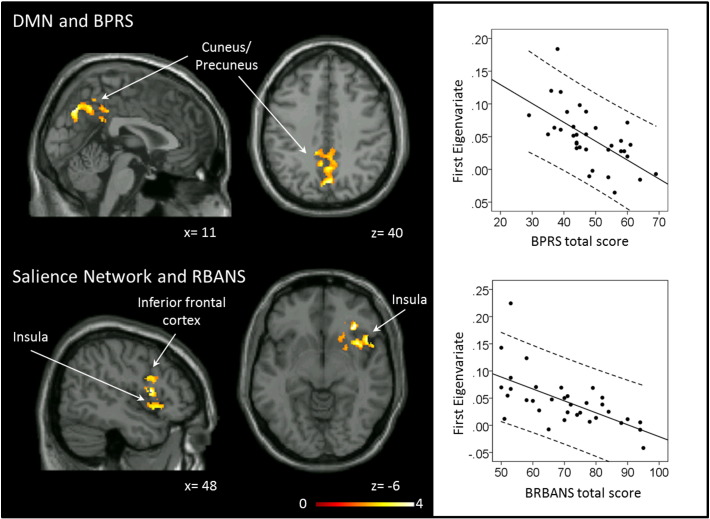
Exploratory analyses to investigate associations between connectivity and clinical variables were also conducted. We found that higher BPRS total scores in unmedicated SZ were related to higher DMN functional connectivity strength in one cluster in the bilateral precuneus/posterior cingulate cortex (t = 3.86, PFDR < .05, kE = 2029, MNI coordinates: x = 0, y = − 72, z = 39; Fig. 3), but this association was not unique to symptom dimension. Lower RBANS scores were associated with higher salience network connectivity strength in one cluster in the right insula and inferior frontal cortex (t = 5.61, PFDR < .05, kE = 2665, MNI coordinates: x = 34, y = 30, z = − 4; Fig. 3), no relationship between RBANS scores and salience network connectivity strength was found in HC. Greater baseline DAN connectivity in two clusters spanning the cuneus/precuneus, superior and inferior parietal lobes, lingual gyrus, middle occipital lobe, and calcarine sulcus was associated with eventual clinical response at week six (Cluster 1: t = 4.91, PFDR < .05, kE = 1677, MNI coordinates: x = 8, y = − 34, z = 51; Cluster 2: t = 4.42, PFDR < .05, kE = 2701, MNI coordinates: x = − 36, y = − 84, z = 20; Fig. 4).
Fig. 3.
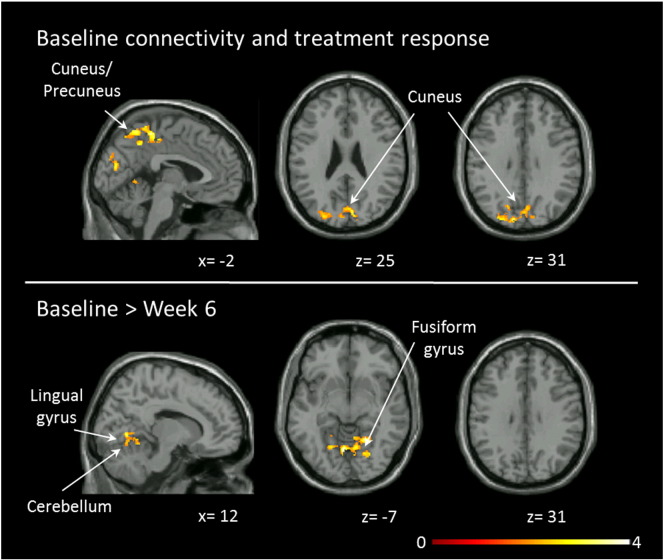
Dorsal attention network connectivity, effects of risperidone and treatment response. Top row: Clusters indicate regions where connectivity strength in unmedicated patients with schizophrenia is related to clinical response after six weeks of treatment with risperidone (n = 30). Bottom row: Clusters indicate regions showing changes in functional connectivity in patients with schizophrenia between baseline and week 6 (n = 22). Statistical maps are significant at PFDR < .05. Clusters were overlaid on the Xjview single subject T1 template, left columns show a sagittal slice, all others are axial. Numbers below the slices indicate x and z coordinates in MNI convention. Color bar indicated t values. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Relationship between resting state functional connectivity and clinical variables in patients with schizophrenia (n = 34). Left: Clusters indicate regions where connectivity strength within the network where is correlated with symptom severity. Statistical maps are significant at PFDR < .05. Clusters are overlaid on the Xjview single subject T1 template, left column shows a sagittal slice, middle column shows an axial slice. Numbers below the slices indicate x and z coordinates in MNI convention. Color bar indicates t values. Right: Correlations between baseline functional connectivity strength and symptom severity. Abbreviations: DMN: default mode network; BPRS: Brief Psychiatric Rating Scale; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Change in functional connectivity over time with risperidone treatment
We observed resting state functional connectivity changes after six weeks of treatment with risperidone only in the DAN, but none of the other networks. Contrasting baseline and week six of treatment, an overall decrease in connectivity was seen in the lingual gyrus, fusiform gyrus, precuneus, calcarine sulcus and cerebellum. No areas within the DAN showed increased connectivity compared to baseline (Table 4, Fig. 4). Using more stringent corrections (PFDR < .01) we observed no change in connectivity over time in the DAN. Median intra-voxel ICC in SZ between the two scans was 0.48 +/− 0.06 for the DAN, 0.38 +/− 0.03 for the ECN, 0.36 +/− 0.06 for the salience network, and 0.70 +/− 0.04 for the DMN.
Table 4.
Change in dorsal attention network functional connectivity over six weeks of treatment with risperidone.
| Brain regions | Voxels in cluster | Hem. | Voxels in region | Peak coordinatesa |
Peak t | PFDRb | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Baseline > week 6 | ||||||||
| Cluster | 1454 | − 6 | − 64.5 | − 9 | 4.217 | |||
| Lingual gyrus | B | 754 | ||||||
| Fusiform gyrus | B | 132 | ||||||
| Precuneus | B | 47 | ||||||
| Calcarine sulcus | B | 22 | ||||||
| Cerebellum | L | 380 | ||||||
Abbreviations: Hem., hemisphere; L, left; R, right.
Reported in Montreal Neurologic Institute coordinates (X, Y, and Z).
All comparisons were FDR-corrected (PFDR ≤ 0.05).
4. Discussion
To our knowledge, this is the first longitudinal study characterizing four large-scale brain networks in unmedicated patients with schizophrenia and the effects of risperidone on resting state functional connectivity. We report functional architecture of each network in healthy controls that is principally consistent with the existing literature and show increased resting state connectivity in the DAN, ECN, and salience networks, but not DMN, in unmedicated patients; abnormalities did not differ between medication-naïve subjects and those with prior exposure to antipsychotic medications. Additionally, we demonstrate attenuation of dysconnectivity in the DAN after six weeks of treatment with risperidone and a possible link between baseline DAN connectivity strength and eventual clinical response.
Our findings are in agreement with the concept of schizophrenia as a disorder of brain network organization and a prior report of resting state networks being differentially affected in schizophrenia (Woodward et al., 2011). A number of studies suggest widespread abnormalities in large scale networks in schizophrenia (Khadka et al., 2013, Meda et al., 2012, Sheffield et al., 2015, Orliac et al., 2013, Moran et al., 2013, Wolf et al., 2011), but disturbances vary by network, and neither directionality (increase or decrease) nor spatial patterns of dysconnectivity appear consistent (Williamson and Allman, 2012, Yu et al., 2012). For example, Rotarska-Jagiela and colleagues who reported decreased DMN connectivity and increased ECN connectivity to the left parietal cortex in patients with schizophrenia compared to healthy controls, but observed no group differences in other large scale networks (Rotarska-Jagiela et al., 2010). In a large group of medication-naïve first episode schizophrenia patients, resting state ALLFs were found to be decreased in areas of the DMN and ECN (Ren et al., 2013). Examining topological properties of functional network connectivity using graph metrics, another study reports abnormalities in areas of the brain that are typically conceptualized as part of the DMN, ECN, and motor network in medicated patients with schizophrenia compared to healthy controls (Yu et al., 2011). We report an increase in resting state connectivity in networks that typically show activation in response to goal directed tasks. The negative correlation between salience network connectivity strength and RBANS scores is consistent with prior reports linking aberrant connectivity in this network with information processing deficits in schizophrenia (White et al., 2010). Similar to Baker et al. who examined resting state ECN integrity across the psychosis spectrum, we did not see a relationship between connectivity disruption and clinical variables (Baker et al., 2014). Somewhat surprisingly, we did not observe dysconnectivity in the DMN, a network that is often reported abnormal in medicated patients with schizophrenia (Williamson and Allman, 2012), and possibly even in unaffected relatives of patients with schizophrenia (Meda et al., 2014, Chang et al., 2014) [but also see (Wolf et al., 2011), which did not find aberrant DMN connectivity]. To our knowledge, all resting state studies examining the DMN to date have enrolled medicated subjects, which may explain discrepancies in findings.
Our findings suggest that increased network connectivity seen in unmedicated patients with schizophrenia may be related to topographical expansion of networks. Examining the spatial extent of resting state networks, wider connectivity in the ECN and greater spatial dispersion of the DMN in schizophrenia compared to healthy controls have been reported (Woodward et al., 2011, Littow et al., 2015). However, the cellular level mechanisms of aberrant resting state connectivity remain unclear. Postmortem studies report abnormalities in both excitatory and inhibitory neuronal components, such as reduced NMDA-receptor expression (Beneyto et al., 2007), disrupted glutamate transport protein complex integrity (Shan et al., 2014), reductions in parvalbumin containing interneurons (Lewis et al., 2012), and lower glutamic-acid-decarboxylase levels, the rate-limiting enzyme of GABA production (Akbarian and Huang, 2006). Disruption in the excitation/inhibition balance may lead to increase of excitability in cortical microcircuitry, impact connectivity in large-scale networks, and result in behavioral abnormalities (Krystal et al., 2003, Uhlhaas, 2013, Deco et al., 2014). Consistent with this, administration of ketamine, a NMDA receptor antagonist, has been found to globally increase functional connectivity during rest (Driesen et al., 2013). In a complementary experiment, examining large scale networks during a working memory task, Anticevic and colleagues demonstrated a disruption in the reciprocal relationship between networks and interpreted this as evidence of cortical disinhibition secondary to NMDA receptor hypofunction (Anticevic et al., 2012). In light of these findings, it is tempting to speculate that the increase in connectivity we see in schizophrenia could be secondary to an aberrant excitation/inhibition balance.
Our findings extend the existing literature by showing that risperidone, a commonly prescribed antipsychotic medication, may affect resting state functional connectivity in patients with schizophrenia. Thus far, the majority of studies have considered antipsychotic medications as confounding variable rather than modulating factor. Only few studies have explicitly investigated systems-level effects of antipsychotic medications on resting state connectivity. Lui et al. examined connectivity in medication-naïve, first-episode patients using seven seed regions defined as areas in which patients showed abnormalities in regional amplitudes of low frequency fluctuations (ALFF), a different measure of spontaneous synchronous neuronal activity. They report the impact of antipsychotics to be rather non-specific, restoring connectivity patterns in some areas but also affecting connectivity to areas that were not altered before treatment (Lui et al., 2010b). Our group recently examined the effects of risperidone on resting state connectivity patterns of ventral tegmental area (VTA), the origin of mesocorticolimbic dopamine projections and reported that risperidone partially attenuates VTA dysconnectivity after one week of treatment. Interestingly, we had found that connectivity between the VTA and DMN as well as the dorsal anterior cingulate cortex at baseline, while unmedicated, correlated with eventual clinical response to medication (Hadley et al., 2014). Now, we again report a relationship between functional connectivity (in this case the DAN) in unmedicated patients and eventual clinical response to antipsychotic medication. In a recent seed-based resting state study with a similar clinical design to ours, Sarpal and colleagues have examined longitudinal effects of treatment with second generation antipsychotic medications (risperidone and aripiprazole) on striatum functional connectivity in patients with first episode psychosis and report a cortico-striatal connectivity increase as a function of clinical symptom improvement (Sarpal et al., 2015). Contrasting to our study, the authors did not find differences in connectivity at baseline between patients and healthy controls, which may due to not all of their subjects having an antipsychotic free interval prior to the baseline scan.
Our finding of attenuation in DAN hyperconnectivity with medication is consistent with recent drug challenge studies demonstrating that dopamine antagonism may reduce connectivity in task positive networks (Cole et al., 2013a). The DAN controls goal-oriented, top-down deployment of attention. In medication-naïve patients with schizophrenia, reduced activation of the DAN during an antisaccade task has been reported to be partially restored by risperidone (Keedy et al., 2015). One may speculate that the extent of increased functional connectivity in the DAN may reflect the system's ability to maintain integrated (mal)adaptive functional systems and neuroplasticity, and thus be related to better treatment response (Gerretsen et al., 2014). Though it remains unclear how antipsychotic drugs modulate functional connectivity, several mechanisms have been proposed. Antipsychotic medications principally act via blockage of the dopamine D2 receptor. However, it is unlikely that observed changes were due solely to changes in dopaminergic transmission, given the indirect nature of the BOLD response in relation to neuronal activity (Logothetis and Wandell, 2004). Evidence from both cross-sectional and longitudinal studies suggests that abnormally elevated glutamate levels may decrease with antipsychotic treatment, potentially attenuating excitation/inhibition imbalances and restoring functional connectivity (Kraguljac et al., 2012, Kraguljac et al., 2013, de la Fuente-Sandoval et al., 2013, Kegeles et al., 2012). Work in animal models suggests that antipsychotics may have effects on white matter volumes, myelination, and numbers of oligodendrocytes (Bartzokis et al., 2009, Dorph-Petersen et al., 2005, Konopaske et al., 2008). Consistent with this, diffusion tensor imaging (DTI) studies in first episode schizophrenia report a decrease in fractional anisotropy (FA), a marker of white matter structural integrity, after twelve weeks of treatment with antipsychotic medication (Szeszko et al., 2014). It is possible that some of the changes in functional networks seen with treatment are secondary to antipsychotic induced changes in underlying structural networks.
Of course, our results have to be interpreted in context of several limitations. Resting state functional MRI is prone to contamination by head motion and non-neuronal signals such as heart beat. To minimize the chance of spurious correlations, we used well-validated preprocessing techniques and motion “scrubbing”. Medication compliance was monitored with pill counts at each visit, but we did not have serum risperidone levels available to confirm adherence. Also, because known effective treatments cannot be withheld, we did not have a placebo group in this study, rendering it impossible to definitively attribute DAN connectivity changes over six weeks in the SZ group to medication effects rather than time, or lack of stability/reliability in functional connectivity measures. However, given that we did not find significant functional connectivity changes in the subgroup of HC we scanned twice six weeks apart and that within network test-retest reliability was fair to high (Wang et al., 2013), it is likely that changes seen in patients are due to effects of treatment. Our SZ group included five subjects who were chronically ill, but had no prior exposure to antipsychotic treatment. Because of the limited number of subjects, we were unable to examine connectivity patterns in this subgroup, but it will be important for future studies to determine if patients with an extended duration of untreated psychosis are more similar to medication-naïve subjects with shorter illness duration or to chronically ill patients with prior exposure to antipsychotics. An unresolved issue in the literature lays in accounting for multiple comparisons when using several seed regions. We attempted to mitigate this by limiting comparisons to voxels that were recruited by their respective networks, substantially reducing the likelihood of type I errors. However, future studies are warranted to investigate the replicability of our findings.
In summary, our results demonstrate abnormalities in large scale functional networks in patients with schizophrenia that are modulated by risperidone only to a certain extent, highlighting the need for development of novel antipsychotic medications that have the ability to alleviate symptoms through attenuation of dysconnectivity. Given the considerable overlap in clinical presentation and management across the spectrum of psychotic disorders, it would be important for future studies to examine if antipsychotic medication effects on connectivity are specific to schizophrenia or extend to other diagnostic constructs.
Disclosures and acknowledgments
Medication for this study was donated by Janssen Pharmaceuticals, Inc. Dr. Lahti has received investigator-initiated research grants from Pfizer and Janssen Pharmaceuticals, Inc. The research was supported by the National Institutes of Health (R01MH081014; ACL).
References
- Achard S., Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007;3 doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S., Huang H.S. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res. Rev. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Alonso-Solis A., Corripio I., de Castro-Manglano P., Duran-Sindreu S., Garcia-Garcia M., Proal E., Nunez-Marin F., Soutullo C., Alvarez E., Gomez-Anson B., Kelly C., Castellanos F.X. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr. Res. 2012;139:13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solis A., Vives-Gilabert Y., Grasa E., Portella M.J., Rabella M., Sauras R.B., Roldan A., Nunez-Marin F., Gomez-Anson B., Perez V., Alvarez E., Corripio I. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 2015;161:261–268. doi: 10.1016/j.schres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Anticevic A., Gancsos M., Murray J.D., Repovs G., Driesen N.R., Ennis D.J., Niciu M.J., Morgan P.T., Surti T.S., Bloch M.H., Ramani R., Smith M.A., Wang X.J., Krystal J.H., Corlett P.R. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T., Bouix S., Whitford T.J., Shenton M.E., Salisbury D.F., McCarley R.W. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. NeuroImage. 2012;59:986–996. doi: 10.1016/j.neuroimage.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.T., Holmes A.J., Masters G.A., Yeo B.T., Krienen F., Buckner R.L., Ongur D. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Lu P.H., Stewart S.B., Oluwadara B., Lucas A.J., Pantages J., Pratt E., Sherin J.E., Altshuler L.L., Mintz J., Gitlin M.J., Subotnik K.L., Nuechterlein K.H. In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophr. Res. 2009;113:322–331. doi: 10.1016/j.schres.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M., Kristiansen L.V., Oni-Orisan A., McCullumsmith R.E., Meador-Woodruff J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Miller J., Lanius R.A., Osuch E.A., Boksman K., Neufeld R.W., Theberge J., Schaefer B., Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr. Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. The brain's default network: origins and implications for the study of psychosis. Dialogues Clin. Neurosci. 2013;15:351–358. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A., Hall D.L., Zelaya F.O., Williams S.C., Mehta M.A. Measuring fMRI reliability with the intra-class correlation coefficient. NeuroImage. 2009;45:758–768. doi: 10.1016/j.neuroimage.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Camchong J., MacDonald A.W., 3rd, Bell C., Mueller B.A., Lim K.O. Altered functional and anatomical connectivity in schizophrenia. Schizophr. Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: comment on Power et al. NeuroImage. 2013;76:436–438. doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Carpenter W.T., Jr., Gold J.M., Lahti A.C., Queern C.A., Conley R.R., Bartko J.J., Kovnick J., Appelbaum P.S. Decisional capacity for informed consent in schizophrenia research. Arch. Gen. Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- Chang X., Shen H., Wang L., Liu Z., Xin W., Hu D., Miao D. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014;1562:87–99. doi: 10.1016/j.brainres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Beckmann C.F., Oei N.Y., Both S., van Gerven J.M., Rombouts S.A. Differential and distributed effects of dopamine neuromodulations on resting-state network connectivity. NeuroImage. 2013;78:59–67. doi: 10.1016/j.neuroimage.2013.04.034. [DOI] [PubMed] [Google Scholar]
- Cole D.M., Oei N.Y., Soeter R.P., Both S., van Gerven J.M., Rombouts S.A., Beckmann C.F. Dopamine-dependent architecture of cortico-subcortical network connectivity. Cereb. Cortex. 2013;23:1509–1516. doi: 10.1093/cercor/bhs136. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C., Leon-Ortiz P., Azcarraga M., Stephano S., Favila R., Diaz-Galvis L., Alvarado-Alanis P., Ramirez-Bermudez J., Graff-Guerrero A. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–1066. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Ponce-Alvarez A., Hagmann P., Romani G.L., Mantini D., Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J. Neurosci. 2014;34:7886–7898. doi: 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen K.A., Pierri J.N., Perel J.M., Sun Z., Sampson A.R., Lewis D.A. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen N.R., McCarthy G., Bhagwagar Z., Bloch M., Calhoun V., D'Souza D.C., Gueorguieva R., He G., Ramachandran R., Suckow R.F., Anticevic A., Morgan P.T., Krystal J.H. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol. Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clin. Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Gerretsen P., Menon M., Mamo D.C., Fervaha G., Remington G., Pollock B.G., Graff-Guerrero A. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr. Res. 2014;160:43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Liu F., Xiao C., Liu J., Yu M., Zhang Z., Zhang J., Zhao J. Increased short-range and long-range functional connectivity in first-episode, medication-naive schizophrenia at rest. Schizophr. Res. 2015;166:144–150. doi: 10.1016/j.schres.2015.04.034. [DOI] [PubMed] [Google Scholar]
- Hadley J.A., Nenert R., Kraguljac N.V., Bolding M.S., White D.M., Skidmore F.M., Visscher K.M., Lahti A.C. Ventral tegmental area/midbrain functional connectivity and response to antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2014;39:1020–1030. doi: 10.1038/npp.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Deng W., Li M., Chen Z., Jiang L., Wang Q., Huang C., Collier D.A., Gong Q., Ma X., Zhang N., Li T. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychol. Med. 2013;43:769–780. doi: 10.1017/S0033291712001638. [DOI] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy S.K., Reilly J.L., Bishop J.R., Weiden P.J., Sweeney J.A. Impact of antipsychotic treatment on attention and motor learning systems I first-episode schizophrenia. Schizophr. Bull. 2015;41(2):355–365. doi: 10.1093/schbul/sbu071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles L.S., Mao X., Stanford A.D., Girgis R., Ojeil N., Xu X., Gil R., Slifstein M., Abi-Dargham A., Lisanby S.H., Shungu D.C. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch. Gen. Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Khadka S., Meda S.A., Stevens M.C., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., O'Neil K., Schretlen D., Pearlson G.D. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol. Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopaske G.T., Dorph-Petersen K.A., Sweet R.A., Pierri J.N., Zhang W., Sampson A.R., Lewis D.A. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol. Psychiatry. 2008;63:759–765. doi: 10.1016/j.biopsych.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N.V., Reid M.A., White D.M., den Hollander J., Lahti A.C. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. doi: 10.1038/npp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N.V., White D.M., Reid M.A., Lahti A.C. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac N.V., White D.M., Hadley J., Reid M.A., Lahti A.C. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus. 2014;24:1524–1532. doi: 10.1002/hipo.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal J.H., D'Souza D.C., Mathalon D., Perry E., Belger A., Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39:358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Deng W., He Z., Wang Q., Huang C., Jiang L., Gong Q., Ziedonis D.M., King J.A., Ma X., Zhang N., Li T. A splitting brain: imbalanced neural networks in schizophrenia. Psychiatry Res. 2015;232:145–153. doi: 10.1016/j.pscychresns.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littow H., Huossa V., Karjalainen S., Jaaskelainen E., Haapea M., Miettunen J., Tervonen O., Isohanni M., Nikkinen J., Veijola J., Murray G., Kiviniemi V.J. Aberrant functional connectivity in the default mode and central executive networks in subjects with schizophrenia — a whole-brain resting-state ICA study. Frontiers in Psychiatry. 2015;6:26. doi: 10.3389/fpsyt.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K., Wandell B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Lui S., Deng W., Huang X., Jiang L., Ma X., Chen H., Zhang T., Li X., Li D., Zou L., Tang H., Zhou X.J., Mechelli A., Collier D.A., Sweeney J.A., Li T., Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am. J. Psychiatry. 2009;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Lui S., Li T., Deng W., Jiang L., Wu Q., Tang H., Yue Q., Huang X., Chan R.C., Collier D.A., Meda S.A., Pearlson G., Mechelli A., Sweeney J.A., Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lui S., Li T., Deng W., Jiang L., Wu Q., Tang H., Yue Q., Huang X., Chan R.C., Collier D.A., Meda S.A., Pearlson G., Mechelli A., Sweeney J.A., Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Mamah D., Barch D.M., Repovs G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J. Affect. Disord. 2013;150:601–609. doi: 10.1016/j.jad.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannell M.V., Franco A.R., Calhoun V.D., Canive J.M., Thoma R.J., Mayer A.R. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum. Brain Mapp. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Muhlau M., Schwerthoffer D., Scherr M., Peters H., Zimmer C., Forstl H., Bauml J., Wohlschlager A.M., Sorg C. Dependence of default mode/ central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014;40(2):428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Gill A., Stevens M.C., Lorenzoni R.P., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., Thaker G., Pearlson G.D. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biol. Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda S.A., Ruano G., Windemuth A., O'Neil K., Berwise C., Dunn S.M., Boccaccio L.E., Narayanan B., Kocherla M., Sprooten E., Keshavan M.S., Tamminga C.A., Sweeney J.A., Clementz B.A., Calhoun V.D., Pearlson G.D. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2066–E2075. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G., Wagner G., Langbein K., Maitra R., Smesny S., Dietzek M., Burmeister H.P., Reichenbach J.R., Schlosser R.G., Gaser C., Sauer H., Nenadic I. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr. Res. 2012;138:143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Moran L.V., Tagamets M.A., Sampath H., O'Donnell A., Stein E.A., Kochunov P., Hong L.E. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol. Psychiatry. 2013;74:467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger J.I., Jr., Blehar M.C., Kaufmann C.A., York-Cooler C., Simpson S.G., Harkavy-Friedman J., Severe J.B., Malaspina D., Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch. Gen. Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Ongur D., Lundy M., Greenhouse I., Shinn A.K., Menon V., Cohen B.M., Renshaw P.F. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliac F., Naveau M., Joliot M., Delcroix N., Razafimandimby A., Brazo P., Dollfus S., Delamillieure P. Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr. Res. 2013;148:74–80. doi: 10.1016/j.schres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Overall J., Gorham D. The brief psychiatric rating scale. Psychol. Rep. 1962;10:799–812. [Google Scholar]
- Palaniyappan L., Liddle P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L., Simmonite M., White T.P., Liddle E.B., Liddle P.F. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W., Li L., Zhang H., Ouyang X., Liu H., Zhao J., Li L., Xue Z., Xu K., Tang H., Shan B., Liu Z., Wang F. Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr. Res. 2012;141:15–21. doi: 10.1016/j.schres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C., Tierney M.C., Mohr E., Chase T.N. The repeatable battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Ren W., Lui S., Deng W., Li F., Li M., Huang X., Wang Y., Li T., Sweeney J.A., Gong Q. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am. J. Psychiatry. 2013;170:1308–1316. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain network connectivity in individuals with schizophrenia and their siblings. Biol. Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knochel V., Uhlhaas P.J., Vogeley K., Linden D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Sambataro F., Blasi G., Fazio L., Caforio G., Taurisano P., Romano R., Di Giorgio A., Gelao B., Lo Bianco L., Papazacharias A., Popolizio T., Nardini M., Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal D.K., Robinson D.G., Lencz T., Argyelan M., Ikuta T., Karlsgodt K., Gallego J.A., Kane J.M., Szeszko P.R., Malhotra A.K. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Burmann I., Regenthal R., Arelin K., Barth C., Pampel A., Villringer A., Margulies D.S., Sacher J. Serotonergic modulation of intrinsic functional connectivity. Curr. Biol. 2014;24:2314–2318. doi: 10.1016/j.cub.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan D., Mount D., Moore S., Haroutunian V., Meador-Woodruff J.H., McCullumsmith R.E. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr. Res. 2014;154:1–13. doi: 10.1016/j.schres.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J.M., Repovs G., Harms M.P., Carter C.S., Gold J.M., MacDonald Iii A.W., Daniel Ragland J., Silverstein S.M., Godwin D., Barch D.M. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P., Jagannathan K., Anderson K., Stevens M.C., Calhoun V.D., Skudlarska B.A., Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada C.S., Kessler D., Welsh R., Angstadt M., Liberzon I., Phan K.L., Scott C. Distributed effects of methylphenidate on the network structure of the resting brain: a connectomic pattern classification analysis. NeuroImage. 2013;81:213–221. doi: 10.1016/j.neuroimage.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.W., Lan T.H., Hsu T.W., Biswal B.B., Tsai P.J., Lin W.C., Lin C.P. Reduced neuro-integration from the dorsolateral prefrontal cortex to the whole brain and executive dysfunction in schizophrenia patients and their relatives. Schizophr. Res. 2013;148:50–58. doi: 10.1016/j.schres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Swanson N., Eichele T., Pearlson G., Kiehl K., Yu Q., Calhoun V.D. Lateral differences in the default mode network in healthy controls and patients with schizophrenia. Hum. Brain Mapp. 2011;32:654–664. doi: 10.1002/hbm.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P.R., Robinson D.G., Ikuta T., Peters B.D., Gallego J.A., Kane J., Malhotra A.K. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39:1324–1331. doi: 10.1038/npp.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Liao Y., Song M., Gao J.H., Zhou B., Tan C., Liu T., Tang Y., Chen J., Chen X. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P.C., Lee Y.C., Chen Y.S., Li C.T., Su T.P. Schizophrenia and the brain's control network: aberrant within- and between-network connectivity of the frontoparietal network in schizophrenia. Schizophr. Res. 2013;147:339–347. doi: 10.1016/j.schres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr. Opin. Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- van de Ven V., Wingen M., Kuypers K.P., Ramaekers J.G., Formisano E. Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0068355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Snyder A.Z., Fox M.D., Shannon B.J., Andrews J.R., Raichle M.E., Buckner R.L. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wang X., Jiao Y., Tang T., Wang H., Lu Z. Investigating univariate temporal patterns for intrinsic connectivity networks based on complexity and low-frequency oscillation: a test-retest reliability study. Neuroscience. 2013;254:404–426. doi: 10.1016/j.neuroscience.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Wang H., Zeng L.L., Chen Y., Yin H., Tan Q., Hu D. Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci. Rep. 2015;5:14655. doi: 10.1038/srep14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.P., Joseph V., Francis S.T., Liddle P.F. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res. 2010;123:105–115. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P.C., Allman J.M. A framework for interpreting functional networks in schizophrenia. Front. Hum. Neurosci. 2012;6:184. doi: 10.3389/fnhum.2012.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D.H., Gur R.C., Valdez J.N., Loughead J., Elliott M.A., Gur R.E., Ragland J.D. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf N.D., Sambataro F., Vasic N., Frasch K., Schmid M., Schonfeldt-Lecuona C., Thomann P.A., Wolf R.C. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J. Psychiatry Neurosci. 2011;36:366–374. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Sui J., Rachakonda S., He H., Gruner W., Pearlson G., Kiehl K.A., Calhoun V.D. Altered topological properties of functional network connectivity in schizophrenia during resting state: a small-world brain network study. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0025423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q., Allen E.A., Sui J., Arbabshirani M.R., Pearlson G., Calhoun V.D. Brain connectivity networks in schizophrenia underlying resting state functional magnetic resonance imaging. Curr. Top. Med. Chem. 2012;12:2415–2425. doi: 10.2174/156802612805289890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Tian L., Wang K., Hao Y., Liu H., Liu Z., Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr. Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Jiang T., Tian L., Liu Y., Liu Z., Liu H., Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci. Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]