Abstract
Background
Representations of objects and actions in everyday speech are usually materialized as nouns and verbs, two grammatical classes that constitute the core elements of language. Given their very distinct roles in singling out objects (nouns) or referring to transformative actions (verbs), they likely rely on distinct brain circuits.
Method
We tested this hypothesis by conducting network-based lesion-symptom mapping in 38 patients with chronic stroke to the left hemisphere. We reconstructed the individual brain connectomes from probabilistic tractography applied to magnetic resonance imaging and obtained measures of production of words referring to objects and actions from narrative discourse elicited by picture naming tasks.
Results
Words for actions were associated with a frontal network strongly engaging structures involved in motor control and programming. Words for objects, instead, were related to a posterior network spreading across the occipital, posterior inferior temporal, and parietal regions, likely related with visual processing and imagery, object recognition, and spatial attention/scanning. Thus, each of these networks engaged brain areas typically involved in cognitive and sensorimotor experiences equivalent to the function served by each grammatical class (e.g. motor areas for verbs, perception areas for nouns).
Conclusions
The finding that the two major grammatical classes in human speech rely on two dissociable networks has both important theoretical implications for the neurobiology of language and clinical implications for the assessment and potential rehabilitation and treatment of patients with chronic aphasia due to stroke.
Keywords: Nouns, Verbs, Discourse, Connectome, Magnetic resonance imaging, Diffusion tensor imaging, Network-symptom mapping, Stroke
Highlights
-
•
Nouns & verbs serve distinct functions in speech, relying on distinct neural networks.
-
•
Production of nouns depends on an occipital–temporal–parietal network.
-
•
Production of verbs depends on a frontal network of structures.
-
•
Network-based lesion-symptom mapping is a useful tool in understanding behavior.
1. Introduction
In the existing literature, there is conflicting evidence regarding the neurobiological mechanisms underlying the production of words used to represent objects (nouns) and words used to represent actions (verbs) during spoken language (Crepaldi et al., 2011, Vigliocco et al., 2011). This separation is important because it embodies how neural systems have evolved to support language. Namely, each speech element may recruit isolated neural circuitries that are shared by other cognitive processes. Thus far, this hypothesis has not been fully corroborated due to methodological limitations to assess neural systems in humans in vivo.
Nouns and verbs constitute the core components of speech across virtually all world languages (Robins, 1952). The acquisition of these basic word classes consolidates towards the first two years of life (Pinker, 1996, Becker, 2014), with nouns usually emerging before verbs (Waxman et al., 2013, Gentner, 1982), albeit with some variability across different languages (Kauschke et al., 2007). In fact, in most languages similar to English, the earliest phrasal structures produced by toddlers (i.e., two/three word structures at around 24 months of age) are composed mainly of nouns and verbs, which represent universal building blocks of language (Pinker, 1996, Chomsky, 1995). Further evidence that these word classes are universal pillars of language is the fact that verbs and nouns are the main constituents of speech among speakers of languages learned informally or instinctively, such as the cases of creole and pidgin (Becker, 2014, Slobin, 1975).
Everyday conversation relies strongly on the use of these grammatical classes. Nouns and verbs serve very distinct communicative functions, representing remarkably different sensorimotor experiences (Kemmerer, 2014). Nouns are used for denotation (i.e., singling out of an object in space) while verbs are used for predication (i.e., attribution of transformative properties/actions of such object). In everyday speech, many variations can occur (e.g. due to the syntactic role of the word, such as “-ing words,” which can be nouns [The shooting was hard to watch] or actions [He is shooting at the target]). For the purposes of the present study, we refer to nouns and verbs as the representation of objects (nouns) and the representations of actions (verbs). Their fundamental conceptual and representational differences may implicate that separate neural networks underlie each of these word classes. In fact, the brain may harness neural circuitry shared by other sensorimotor systems (e.g. movement, spatial attention, etc.) to encode these two distinct communication units.
Several sources of evidence including behavioral, neuropsychological, electrophysiological, neuroimaging and transcranial magnetic stimulation studies have, with considerable controversy, investigated the hypothesis of distinct neural circuitry being used for processing of each of these classes, based either on grammatical or semantic differences (Crepaldi et al., 2011, Vigliocco et al., 2011). However, the existing literature is conflicting as to the extent of the dissociation within the functional systems supporting each element (Crepaldi et al., 2013).
Within cognitive neuroscience, lesion based studies have been central to revealing areas that are crucial for the performance of a given task. Noun deficits have been most commonly linked to lesions in the left ventral middle and posterior temporal lobe. Areas associated with verb deficits have been more variable, with left frontal, temporal, and parietal areas reported across many studies (Vigliocco et al., 2011, Kemmerer, 2014, Matzig et al., 2009). Nonetheless, lesion based methods have been limited by their inability to accurately map beyond regional necrotic damage, i.e., they are unable to identify system networks that support a function. This limitation can now be surmounted by newer neuroimaging and computational techniques to derive individual maps of brain connectivity, i.e. the brain connectome. The brain connectome provides information about individual network architecture, and by examining the topography of network damage, it is possible to map function to connectivity architecture.
In this study, we examined a cohort of subjects with left hemisphere damage as a result of stroke. In contrast to the vast majority of previous work that is based on isolated picture naming, we evaluated their abilities to produce nouns and verbs within narrative speech, thus assessing their language production in situations akin to daily life, a context of high ecological value. We mapped the individual structural brain connectome from brain Magnetic Resonance Imaging (MRI), using methodological steps to accurately preserve the anatomical authenticity of lesion location. The pattern of individual neural architecture loss was statistically related to verb and noun production in order to examine crucial networks supporting production of each word category.
2. Methods
The University of South Carolina (USC) Institutional Review Board approved this study.
2.1. Participants
We recruited 38 individuals who had sustained a unique left-hemisphere stroke at least six months prior to participating in this study. At time of assessment, 12 patients had Broca's aphasia, 10 had anomic aphasia, 3 had conduction aphasia, 1 had Wernicke's aphasia, 1 had global aphasia, and 11 patients presented as not having aphasia per standardized language assessment. Participants were native speakers of English and demonstrated adequate hearing and vision for the main tasks. They were seizure-free, had no history of other neurological or psychiatric diseases, and no history or imaging evidence of other past strokes. Patients were enrolled in the study after signing an informed consent and were subsequently tested at the University of South Carolina.
2.2. Language assessment
To obtain a measure of aphasia severity, all participants were tested with the revised version of the Western Aphasia Battery [(WAB) (Kertesz, 2007)]. We then presented participants with three pictures placed sequentially in front of them in random order; namely, the Cookie Theft scene from the Boston Diagnostic Aphasia Examination [(BDAE) (Goodglass and Kaplan, 1983)], the picnic scene from the WAB (Kertesz, 2007), and the circus scene from the Apraxia Battery for Adults [(ABA-2) (Dabul, 2000)].
For each picture, participants described the contents of the scene in as much detail as possible during a two-minute period, and their discourse was recorded and transcribed verbatim. We assessed each transcript for correct information units (CIU), that is, words that were both intelligible in context and accurate in relation to the scene they were describing. Taking into account the syntactic role of the CIUs elicited by each patient, we recorded the number of distinct nouns and verbs produced for each scene (i.e. number of different words for each category), and derived an average score per minute for each grammatical category.
Importantly, a priori analyses of the recorded transcripts revealed that, likely due to the concrete nature of the three scenes in the picture description tasks, elicited nouns were tangible objects (e.g. tiger, swing, rope), while elicited verbs referred largely, indeed, to actions (e.g. washing, escape, catching). Auxiliary verbs were computed as part of the total number of verbs since, in the context of these picture descriptions, they were always used in reference to a tangible aspect of the scene (e.g. “The mother has a plate in her hands”). Similarly, empty nouns such as “thing(s)” and “stuff” were computed as part of the total number of nouns since they always referred to tangible objects in the picture, with patients usually pointing at it on the scene. We note that both auxiliary verbs and empty nouns can be very frequent especially in aphasic patients, the reason why, as explained before, the outcome measure was the total number of different nouns/verbs produced, thus attenuating the confounding effect of repetition and circumlocutions classically seen in aphasic patients. For the purposes of this study, we thus employ “noun” and “verb” as equivalents of words referring to objects and actions but acknowledge that this is a far more complex issue.
2.3. MRI scanning and connectome construction
Brain scanning took place in a 3 T Siemens Trio equipped with a 12-channel head coil employing the following specifications: (a) T1-weighted images (3D MP-RAGE, TR = 2250 ms, TE = 4.15 ms, 256 × 256matrix, 256 × 256 mm FOV, parallel imaging GRAPPA = 2, 80 reference lines, TA = 377 s; slice thickness = 1 mm); (b) Diffusion EPI scan (30-directions with b = 1000 s/mm2 and b = 2000 s/mm2, TR = 6100 ms, TE = 101 ms, 82 × 82 matrix, 222 × 222 mm FOV, parallel imaging GRAPPA = 2, 80, 45 contiguous 2.7 mm axial slices, TA = 390 s).
We built the individual connectome for each patient in accordance with the following pre-processing steps: 1) segmentation of the probabilistic gray matter map from T1-weighted images; 2) division of the probabilistic gray matter map into 189 regions of interest (ROIs) based on the Johns Hopkins University (JHU) atlas; 3) segmentation of the probabilistic white matter map from T1-weighted images; 4) registration of the individual white matter map and cortical ROIs into the individual diffusor tensor imaging (DTI) space; 5) probabilistic DTI fiber tracking, 6) iterative evaluation of the number of tractography streamlines connecting each possible pair of gray matter ROIs generated in step 2 above; and 7) correction of each pair-wise connection strength (i.e., number of streamlines between two ROIs) based on the volume of the connected ROIs and the distance traveled by the streamlines. Because of the major anatomical distortions occurring as a result of stroke-related necrotic changes, we employed methods designed and previously used by our group (Bonilha et al., 2014) to preserve the anatomical authenticity of gray and white matter without computing fibers embedded in these necrotic areas. Please refer to the online supplementary methods section to obtain details about the neuroimaging preprocessing steps.
We consequently constructed a weighted adjacency matrix M for each patient of size 189 × 189, where Mi,j represented the weighted link between ROI i and ROI j. Importantly, we corrected this value for the volume of both i and j regions as well as the distance traveled by the streamlines to connect these two areas.
2.4. Statistical analyses
We analyzed data using the NiiStat package developed by our group (http://www.nitrc.org/projects/niistat/) which computes continuous imaging (link weight between all possible ROIs) and behavior (number of different nouns and verbs) data using the general linear model by applying a least squares linear regression. Using this mass univariate approach, this algorithm yields more positive standardized z values when increased weight (i.e., stronger connection between two ROIs) is associated with increased behavioral scores (i.e., larger number of nouns and verbs per minute). The alpha value for this mass univariate analysis was set at 0.05, one-tailed, as we predicted injured tissue to cause poorer performance. Importantly, we controlled for familywise error rates by means of permutation thresholding (5000 permutations).
3. Results
3.1. Discourse
Participants elicited an average of 69.3 ± 41.0 total words per minute and 33.4 ± 19.1 different words per minute. Specifically, they produced 10.1 ± 6.1 different nouns and 8.9 ± 6.1 different verbs per minute. Their mean aphasia quotient (AQ), a measure of aphasia severity where lower scores mean more severe aphasia, on the WAB-R was 79.1 (SD = 20.7) and it was significantly associated with noun (r = .77, p < .001) and verb (r = .64, p < .001) production (Fig. 1).
Fig. 1.
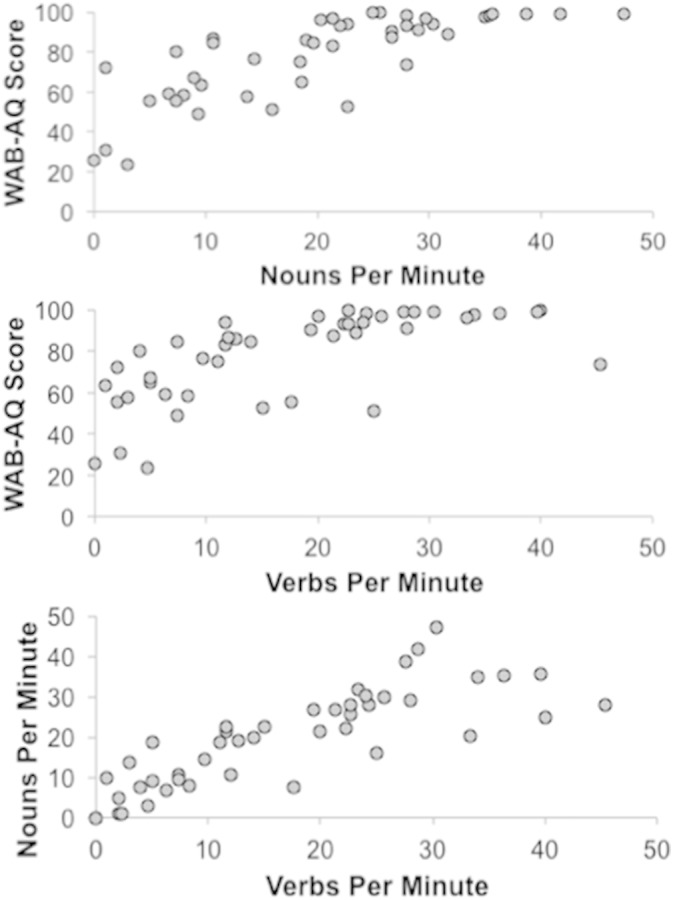
We observed a strong association between the severity of aphasia (WAB-AQ) and the number of nouns or verbs produced per minute during spontaneous discourse.
3.2. Lesion extension and connectome
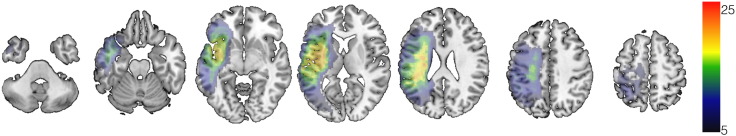
Lesion overlap revealed a clear involvement of left-hemisphere temporal and frontal areas (Fig. 2). The connectome was built for each participant (Fig. 3) and frequently disrupted links were identified especially in posterior frontal, temporal, and parietal regions (Fig. 4).
Fig. 2.
Voxel based map demonstrating the anatomical distribution of the post-stroke necrotic lesion across all patients. Each voxel is colored in accordance with the number of subjects that had a lesion including that voxel. The color bar represents the number of subjects.
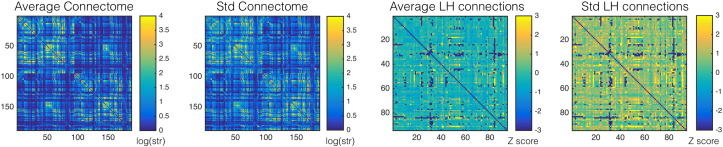
Fig. 3.
This panel demonstrates raw connectome data from all patients. The leftmost matrix demonstrates the average connectome and the second matrix left-to-right shows the connectome standard deviation across all subjects. Within each of these matrices, each cell represents the weighted connectivity between the corresponding ROI listed in the column, and the ROI listed in the row. The numbering of the ROIs is equivalent to the numbering system of the JHU anatomical atlas. The two rightmost matrices display the average and standard deviation Z scores for connections on the left side, based on their comparison with the homologous right hemisphere connection.
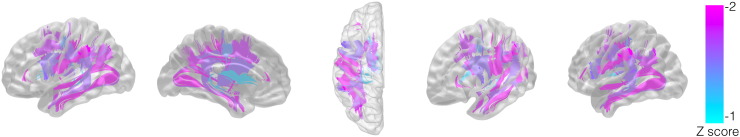
Fig. 4.
This figure demonstrates a tractography-based representation of white matter pathways most commonly affected by the stroke across all subjects. Each white matter track represents a connectome link and it is color-coded in accordance with the average Z score obtained by comparing the number of streamlines in the left hemisphere with the right hemisphere.
3.2.1. Brain connectivity
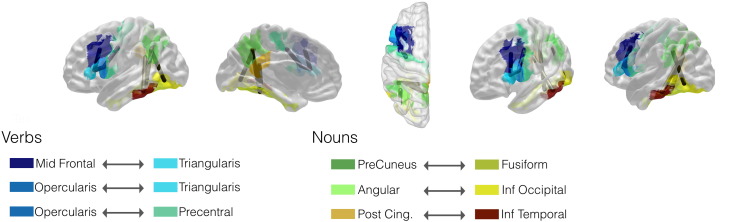
We found a clear dissociation of networks sub-serving noun and verb production, with all significant links in the left hemisphere (Fig. 5).
Fig. 5.
This diagram demonstrates the cortical brain regions (displayed as colored projections on the brain surface) and the connections (subcortical cylinders linking the centers of mass of each cortical region) associated with nouns and verb production. The legend provides a summary of the relevant connections and their related regions that were associated with noun or verb production.
Three links were specific for nouns: (1) left precuneus and fusiform gyrus (z = 4.01); (2) angular gyrus and inferior occipital gyrus (z = 3.94); and (3) posterior cingulate gyrus and posterior inferior temporal gyrus (z = 4.19).
Three links were specific for verbs: (1) middle frontal gyrus (posterior segment) and the pars triangularis of the inferior frontal gyrus (z = 4.54); (2) the pars opercularis and the pars triangularis in the inferior frontal gyrus (z = 4.78); and (3) the pars opercularis of the inferior frontal gyrus and the precentral gyrus (z = 4.57).
4. Discussion
In this study, we demonstrated the existence of two distinct networks underlying the production of words used for representations of objects and representations of actions among patients with chronic stroke and varying degrees of aphasia. Each of these networks engaged brain areas that have been previously implicated in cognitive and sensorimotor experiences equivalent to the function served by each grammatical class (e.g. motor areas for actions, perception areas for objects). These results thus provide novel evidence about the distinct white matter tracts that are involved in the production of nouns and verbs, which were found to connect gray-matter areas previously identified as important for these word types in lesion, functional neuroimaging, and TMS studies. Our study therefore confirms the role of these cortical regions in production of nouns and verbs, but it also provides evidence that these areas are likely working in a concerted manner via their relevant physical connections in the brain.
We elicited fluent speech by using picture description tasks. Importantly, everyday discourse makes use of language “beyond the boundaries of isolated sentences” (Ulatowska and Olness, 2004) and can yield rich information about a person's communicative abilities, including the appropriate, relevant, and coherent use of words from different grammatical classes. In clinical and laboratory settings, picture description tasks have been used to elicit narrative and descriptive discourse samples in neurologically intact adults (Capilouto et al., 2005, Kemper et al., 1990, Marini et al., 2005), as well as patients with language difficulties, especially aphasia (Armstrong, 2000, Olness et al., 2010). For instance, lexical diversity, which among other factors contemplates the number of different words produced in a given timeframe, has been shown to be a sensitive measure of aphasia across different discourse-eliciting tasks (Fergadiotis and Wright, 2011). Among anomic aphasic speakers, picture description tasks reveal decreased speech rates and mean length of utterance, as well as increased agrammatical sentences and semantic paraphasias (Andreetta et al., 2012). More specifically, studies have found impaired noun and verb production among patients with aphasia when describing pictures relative to healthy controls (Edwards and Bastiaanse, 1998, Gordon, 2008). Previous studies investigating brain processes underlying speech production, however, have most frequently relied on isolated picture-naming. Thus, our use of descriptive discourse derived from pictures represents a more ecological way to elicit speech production resembling everyday linguistic demands in a more reliable manner. Also importantly, the use of these pictures led to our participants eliciting nouns that were referring to concrete objects (as opposed to abstract concepts, e.g. “peace”) and verbs that referred to specific actions in the scenes (as opposed to verbs that do not involve motor actions (e.g. “daydreaming”)). We recognize however that the issue of what constitutes a noun and what constitutes a verb is far more complex. In fact, we believe that future studies should use different stimuli (e.g. picture description vs. storytelling) capable of eliciting, for instance, abstract vs. concrete nouns and motion vs. non-motion verbs in order to study whether the white matter connections supporting each type of word within each grammatical class can help elucidate the ongoing controversies of noun and verb processing (Crepaldi et al., 2011, Vigliocco et al., 2011).
Using whole-brain connectivity allowed us to perform network-based lesion-symptom mapping. This is a novel approach that can provide complementary information to voxel-based techniques [e.g. (Bates et al., 2003)] because it focuses on the integrity of white matter tracts connecting all areas of the brain. As such, it contemplates the possibility that the connectivity between two spared cortical structures may have been affected due to brain injury (e.g. necrosis due to stroke). Each gray matter structure may indeed contribute independently to the production of nouns and verbs, but it is also possible that they exert a collaborative influence on behavior, for which the white matter streamlines connecting both structures must be spared. Accordingly, the findings of the present study reveal a series of specific connections, which among others, include connections between cortical areas previously identified as contributory to the production of nouns and verbs on voxel-based approaches, including inferior temporal areas in the production of nouns and left prefrontal areas for verbs (Piras and Marangolo, 2004). The combination of probabilistic tractography and special imaging processing steps aimed at attenuating the effect of cortical necrosis make the fiber counts used to build each patient's connectome in our study highly reliable. Analyzing the strength of the connections throughout the entire brain, our data demonstrated that spared connectivity between certain areas was associated with more prolific outputs for words referring to objects and actions.
Our findings have implications that are relevant from both a theoretical and clinical perspective. Theoretically, we found that a network relying exclusively on left frontal regions, namely the connections between middle frontal gyrus, the precentral gyrus, and the pars opercularis and triangularis within the inferior frontal gyrus, served production of words referring to actions. Importantly, studies with a functional approach to language have revealed the motor role of these regions (Chouinard and Paus, 2006, Hickok, 2012, Kubler et al., 2006). Among a variety of functions, these structures play a fundamental role in motion, and have been related to a phenomenon referred to as embodied cognition (Borghi and Cimatti, 2010, Fischer and Zwaan, 2008). For example, several studies have demonstrated that comprehension of action verbs results in the activation of motor areas in the brain (Binder and Desai, 2011, Meteyard et al., 2012). Additionally, studies among patients with neurological disorders characterized by motor impairment have systematically found more prominent impairment for verb than noun processing (Bak and Hodges, 2004, Fernandino et al., 2013, York et al., 2014).
We found that production of nouns, on the other hand, relied on a more posterior left network involving the connections between the parietal lobe (precuneus and angular gyrus), the temporal lobe (fusiform gyrus and posterior portion of the inferior temporal gyrus), and the inferior occipital area. This more widely distributed network for objects highlights an essential property of noun processing in human discourse: our brain's ability to map nouns to real-life objects. For instance, several studies across different languages propose that children produce nouns earlier than verbs because it is easier to associate a specific word to a tangible object that one can perceive and with which one can potentially experiment and interact (Waxman et al., 2013). The network identified in the present study as the basis for noun production can help explain, at least partially, our brain's ability to generate noun–object associations.
In this sense, it is now well established that the functions of the inferior occipital gyrus exceed those purely related to visual processing of external stimuli; for example, its activity has been found to contribute to visual mental imagery (Platel et al., 1997). The more ventral-posterior aspects of the temporal lobe, in turn, have been associated with language functions, especially in word retrieval (Abrahams et al., 2003) and generation (Friedman et al., 1998), but also shown to play a role in visual and semantic knowledge about objects (Kellenbach et al., 2005), especially those with which we are most familiar (Bilalic et al., 2011, Gauthier et al., 2000). In addition to these functions, the parietal components of the network for nouns are associated with visuospatial processing, spatial imagery, and the spatial focusing of attention (Buchsbaum et al., 2006, Knauff et al., 2002, Lloyd et al., 2006). All of these functions are necessary to become aware of the presence of objects in our physical space and, subsequently, process their properties in order to be able to map them to specific words, i.e., nouns (Gleichgerrcht et al., in press). Thus, the network we found for the production of words associated with objects may be engaging areas necessary for the spatial processing of the pictures as well as mental imagery.
Previous studies among patients with brain damage investigating noun and verb production in response to picture-naming tasks have also reported dissociations between the two major word categories, with lesions to the left temporal cortex leading to more prominent deficits in noun production and lesions to the frontal areas affecting verbs more markedly (Berndt et al., 1997, Damasio and Tranel, 1993, Daniele et al., 1994, Laiacona and Caramazza, 2004, Miceli et al., 1984). Production of nouns and verbs in the context of short sentences, a more natural form of speech than isolated naming, has also revealed distinct cortical signatures for each grammatical class, with nouns relying on the left anterior fusiform gyrus and verbs associated with activity in the left prefrontal cortex, left superior parietal lobe, and left superior temporal gyrus (Shapiro et al., 2006). Again, our whole-brain approach to noun and verb production in fluent speech builds on these previous studies and provides a more complete picture of the neural underpinnings of discourse.
Grammatical class and semantics are inherent confounders of spontaneous speech analyses used here, and hence we do not attempt to separate the effects of semantics from those of grammatical class as we cannot determine based on this approach whether differences stem either from semantic or from production deficits involving one class more than the other in different patients. An argument could also be made that the distinct networks relating to noun and verb production are reflective of other differences between these word categories, such as their different position in sentences, or even different morphosyntactic properties of each class. For this reason, it will be important for future studies to replicate this network-based approach using different tasks (e.g. story-telling) as well as a diversity of languages with varying syntax and morphology. It will also be crucial to broaden the spectrum of types of aphasia in patients studied. Here, we were only able to include one patient with Wernicke aphasia. We did, however, include many chronic stroke patients with no aphasia, which allowed us to analyze speech production with a large spectrum of task performance. Taken together, however, the evidence reviewed above is consistent with a division based on the semantic differences between nouns and verbs and adds evidence from a structural connectivity standpoint to findings reported in previous functional and lesion studies.
From a clinical perspective, our findings can provide a valuable source of information in understanding changes to the network configuration as a result of tissue damage due to stroke. Selective difficulties in the production of either verbs or nouns may be indicative of disrupted white matter tracts in different sub-networks. The latter may be especially relevant if standard structural neuroimaging reveals no obvious cortical damage. In vascular etiologies, impaired noun and/or verb production with spared cortical appearance may be reflective of connectional diaschisis, which refers to the re-organization of the brain's structural and functional features as an indirect result of brain damage (Carrera and Tononi, 2014). A similar approach may also be considered for etiologies affecting white matter such as demyelinating disease or traumatic brain injury. Additionally, these findings can potentially provide the basis for the design of new approaches to aphasia treatment and rehabilitation, by which specific deficits in coding objects or actions could be differentially targeted.
In conclusion, we found that the two major grammatical classes in human speech rely on two dissociable networks by employing a whole-brain approach to the correlation between spared brain connections and production of verbs and nouns. Verbs were associated with a frontal network strongly dependent on motor control and programming. Nouns, instead, were related to a posterior network spreading across the occipital, posterior inferior temporal, and parietal regions, likely related with visual processing and imagery, object recognition, and spatial attention/scanning.
Acknowledgments
This study was supported by the following research grants: National Institute on Deafness and Other Communication Disorders DC014021 (LB), DC008355 (JF) and DC009571 (JF).
Footnotes
Disclosure: The authors report no financial or nonfinancial conflicts of interest associated with this study.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.11.013.
Appendix A. Supplementary data
Supplementary material.
References
- Abrahams S., Goldstein L.H., Simmons A. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit overt responses. Hum. Brain Mapp. 2003;20(1):29–40. doi: 10.1002/hbm.10126. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreetta S., Cantagallo A., Marini A. Narrative discourse in anomic aphasia. Neuropsychologia. 2012;50(8):1787–1793. doi: 10.1016/j.neuropsychologia.2012.04.003. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Armstrong E. Aphasic discourse analysis: the story so far. Aphasiology. 2000;14(9):875–892. [Google Scholar]
- Bak T.H., Hodges J.R. The effects of motor neurone disease on language: further evidence. Brain Lang. 2004;89(2):354–361. doi: 10.1016/S0093-934X(03)00357-2. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P. Voxel-based lesion-symptom mapping. Nat. Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Becker M. Cambridge University Press; Cambridge, UK: 2014. The Acquisition of Syntactic Structure: Animacy and Thematic Alignment. [Google Scholar]
- Berndt R.S., Mitchum C.C., Haendiges A.N., Sandson J. Verb retrieval in aphasia. 1. Characterizing single word impairments. Brain Lang. 1997;56(1):68–106. doi: 10.1006/brln.1997.1727. [DOI] [PubMed] [Google Scholar]
- Bilalic M., Langner R., Ulrich R., Grodd W. Many faces of expertise: fusiform face area in chess experts and novices. J. Neurosci. 2011;31(28):10206–10214. doi: 10.1523/JNEUROSCI.5727-10.2011. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L., Rorden C., Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45(4):988–993. doi: 10.1161/STROKEAHA.113.004137. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi A.M., Cimatti F. Embodied cognition and beyond: acting and sensing the body. Neuropsychologia. 2010;48(3):763–773. doi: 10.1016/j.neuropsychologia.2009.10.029. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Buchsbaum M.S., Buchsbaum B.R., Chokron S., Tang C., Wei T.C., Byne W. Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neurosci. Lett. 2006;404(3):282–287. doi: 10.1016/j.neulet.2006.05.063. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Capilouto G., Wright H.H., Wagovich S.A. CIU and main event analyses of the structured discourse of older and younger adults. J. Commun. Disord. 2005;38(6):431–444. doi: 10.1016/j.jcomdis.2005.03.005. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Carrera E., Tononi G. Diaschisis: past, present, future. Brain. 2014;137(Pt 9):2408–2422. doi: 10.1093/brain/awu101. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Chomsky N. MIT Press; Cambridge, MA: 1995. The Minimalist Program. [Google Scholar]
- Chouinard P.A., Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12(2):143–152. doi: 10.1177/1073858405284255. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Crepaldi D., Berlingeri M., Paulesu E., Luzzatti C. A place for nouns and a place for verbs? A critical review of neurocognitive data on grammatical-class effects. Brain Lang. 2011;116(1):33–49. doi: 10.1016/j.bandl.2010.09.005. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Crepaldi D., Berlingeri M., Cattinelli I., Borghese N.A., Luzzatti C., Paulesu E. Clustering the lexicon in the brain: a meta-analysis of the neurofunctional evidence on noun and verb processing. Front. Hum. Neurosci. 2013;7:303. doi: 10.3389/fnhum.2013.00303. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabul B.L. Austin, TX; PRO-ED: 2000. Apraxia Battery for Adults, Second Edition (ABA-2) [Google Scholar]
- Damasio A.R., Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc. Natl. Acad. Sci. U. S. A. 1993;90(11):4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A., Giustolisi L., Silveri M.C., Colosimo C., Gainotti G. Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia. 1994;32(11):1325–1341. doi: 10.1016/0028-3932(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Edwards S., Bastiaanse R. Diversity in the lexical and syntactic abilities of fluent aphasic speaker. Aphasiology. 1998;12(2):99–117. [Google Scholar]
- Fergadiotis G., Wright H.H. Lexical diversity for adults with and without aphasia across discourse elicitation tasks. Aphasiology. 2011;25(11):1414–1430. doi: 10.1080/02687038.2011.603898. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandino L., Conant L.L., Binder J.R. Parkinson's disease disrupts both automatic and controlled processing of action verbs. Brain Lang. 2013;127(1):65–74. doi: 10.1016/j.bandl.2012.07.008. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.H., Zwaan R.A. Embodied language: a review of the role of the motor system in language comprehension. Q. J. Exp. Psychol. 2008;61(6):825–850. doi: 10.1080/17470210701623605. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Friedman L., Kenny J.T., Wise A.L. Brain activation during silent word generation evaluated with functional MRI. Brain Lang. 1998;64(2):231–256. doi: 10.1006/brln.1998.1953. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Gauthier I., Skudlarski P., Gore J.C., Anderson A.W. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 2000;3(2):191–197. doi: 10.1038/72140. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Gentner D. Why nouns are learned before verbs: linguistic relativity versus natural partitioning. In: Kuczaj S., editor. Language Development: Language, Cognition, and Culture. Erlbaum; Hillsdale, NJ: 1982. pp. 301–334. [Google Scholar]
- Gleichgerrcht E., Fridriksson J., Bonilha L. Neuroanatomical foundations of naming impairments across different neurological conditions. Neurology. 2015;85(3):284–292. doi: 10.1212/WNL.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E. Lea & Febiger; Boston: 1983. The Boston Diagnostic Aphasia Examination. [Google Scholar]
- Gordon J.K. Measuring the lexical semantics of picture description in aphasia. Aphasiology. 2008;22(7–8):839–852. doi: 10.1080/02687030701820063. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat. Rev. Neurosci. 2012;13(2):135–145. doi: 10.1038/nrn3158. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauschke C., Lee H., Pae S. Similarities and variation in noun and verb acquisition: a crosslinguistic study of children learning German, Korean, and Turkish. Lang. Cogn. Process. 2007;22(7):1045–1072. [Google Scholar]
- Kellenbach M.L., Hovius M., Patterson K. A pet study of visual and semantic knowledge about objects. Cortex. 2005;41(2):121–132. doi: 10.1016/s0010-9452(08)70887-6. [DOI] [PubMed] [Google Scholar]
- Kemmerer D. Word classes in the brain: implications of linguistic typology for cognitive neuroscience. Cortex. 2014;58:27–51. doi: 10.1016/j.cortex.2014.05.004. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Kemper S., Rash S., Kynette D., Norman S. Telling stories: the structure of adults' narratives. Eur. J. Cogn. Psychol. 1990;2:204–228. [Google Scholar]
- Kertesz A. Grune & Stratton; New York: 2007. The Western Aphasia Battery — Revised. [Google Scholar]
- Knauff M., Mulack T., Kassubek J., Salih H.R., Greenlee M.W. Spatial imagery in deductive reasoning: a functional MRI study. Brain research. Cogn. Brain Res. 2002;13(2):203–212. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Kubler A., Dixon V., Garavan H. Automaticity and reestablishment of executive control—an fMRI study. J. Cogn. Neurosci. 2006;18(8):1331–1342. doi: 10.1162/jocn.2006.18.8.1331. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Laiacona M., Caramazza A. The noun/verb dissociation in language production: varieties of causes. Cogn. Neuropsychol. 2004;21(2):103–123. doi: 10.1080/02643290342000311. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Lloyd D., Morrison I., Roberts N. Role for human posterior parietal cortex in visual processing of aversive objects in peripersonal space. J. Neurophysiol. 2006;95(1):205–214. doi: 10.1152/jn.00614.2005. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Marini A., Boewe A., Caltagirone C., Carlomagno S. Age-related differences in the production of textual descriptions. J. Psycholinguist. Res. 2005;34(5):439–463. doi: 10.1007/s10936-005-6203-z. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Matzig S., Druks J., Masterson J., Vigliocco G. Noun and verb differences in picture naming: past studies and new evidence. Cortex. 2009;45(6):738–758. doi: 10.1016/j.cortex.2008.10.003. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Meteyard L., Cuadrado S.R., Bahrami B., Vigliocco G. Coming of age: a review of embodiment and the neuroscience of semantics. Cortex. 2012;48(7):788–804. doi: 10.1016/j.cortex.2010.11.002. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Miceli G., Silveri M.C., Villa G., Caramazza A. On the basis for the agrammatic's difficulty in producing main verbs. Cortex. 1984;20(2):207–220. doi: 10.1016/s0010-9452(84)80038-6. [DOI] [PubMed] [Google Scholar]
- Olness G.S., Metteson S.E., Stewart C.T. “Let me tell you the point”: how speakers with aphasia assign prominence to information in narratives. Aphasiology. 2010;24(697–708) [Google Scholar]
- Pinker S. Harvard University Press; Cambridge, MA: 1996. Language Learnabiliy and Language Development. [Google Scholar]
- Piras F., Marangolo P. Independent access to phonological and orthographic lexical representations: a replication study. Neurocase. 2004;10(4):300–307. doi: 10.1080/13554790490507614. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Platel H., Price C., Baron J.C. The structural components of music perception. A functional anatomical study. Brain. 1997;120(Pt 2):229–243. doi: 10.1093/brain/120.2.229. [DOI] [PubMed] [Google Scholar]
- Robins R.H. Noun and verb in universal grammar. Language. 1952;28:289–298. [Google Scholar]
- Shapiro K.A., Moo L.R., Caramazza A. Cortical signatures of noun and verb production. Proc. Natl. Acad. Sci. U. S. A. 2006;103(5):1644–1649. doi: 10.1073/pnas.0504142103. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin D.I. Vol. 10. 1975. The more it changes… on understanding language by watching it move through time; pp. 1–30. (Papers and Reports on Child Language Development). [Google Scholar]
- Ulatowska H.K., Olness G.S. Discourse. In: Kent R.D., editor. The MIT Encyclopedia of Communication Disorders. MIT Press; Cambridge, MA: 2004. pp. 300–302. [Google Scholar]
- Vigliocco G., Vinson D.P., Druks J., Barber H., Cappa S.F. Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neurosci. Biobehav. Rev. 2011;35(3):407–426. doi: 10.1016/j.neubiorev.2010.04.007. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- Waxman S., Fu X., Arunachalam S., Leddon E., Geraghty K., Song H.J. Are nouns learned before verbs? Infants provide insight into a longstanding debate. Child Dev. Perspect. 2013;7(3) doi: 10.1111/cdep.12032. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- York C., Olm C., Boller A. Action verb comprehension in amyotrophic lateral sclerosis and Parkinson's disease. J. Neurol. 2014;261(6):1073–1079. doi: 10.1007/s00415-014-7314-y. (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.