Abstract
Learning impairment is a core deficit in schizophrenia that impacts on real-world functioning and yet, elucidating its underlying neural basis remains a challenge. A key issue when interpreting learning-task experiments is that task-independent changes may confound interpretation of task-related signal changes in neuroimaging studies. The nature of these task-independent changes in schizophrenia is unknown. Therefore, we examined task-independent “time effects” in a group of participants with schizophrenia contrasted with healthy participants in a longitudinal fMRI learning-experiment designed to allow for examination of non-specific effects of time. Flanking the learning portions of the experiment with a task-of-no-interest allowed us to extract task-independent BOLD changes. Task-independent effects occurred in both groups, but were more robust in the schizophrenia group. There was a significant interaction effect between group and time in a distributed activity pattern that included inferior and superior temporal regions, frontal areas (left anterior insula and superior medial gyri), and parietal areas (posterior cingulate cortices and precuneus). This pattern showed task-independent linear decrease in BOLD amplitude over the two scanning sessions for the schizophrenia group, but showed either opposite effect or no activity changes for the control group. There was a trend towards a correlation between task-independent effects and the presence of more negative symptoms in the schizophrenia group.
The strong interaction between group and time suggests that both the scanning experience as a whole and the transition between task-types evokes a different response in persons with schizophrenia and may confound interpretation of learning-related longitudinal imaging experiments if not explicitly considered.
Keywords: Schizophrenia, Learning, Cognition, fMRI, Multivariate analysis, Partial least squares
Highlights
-
•
A robust method was used to identify task-independent fMRI BOLD changes in a multiday learning experiment in schizophrenia
-
•
Task-independent effects were apparent in healthy control group and schizophrenia but differed in direction and magnitude
-
•
In schizophrenia they were greater in magnitude and most prominent in areas of the salience and default mode networks
-
•
Unless properly accounted for, these effects will compromise precise interpretation of fMRI learning data in schizophrenia.
1. Introduction
Although schizophrenia is most often recognized by accompanying psychotic symptoms, the cognitive symptoms (and learning difficulties in particular) lead to much of the associated disability in the disorder (Green, 1996, Tandon et al., 2009). Considerable effort is being given to develop cognitive, pharmacological, and brain stimulation strategies for remediation of cognitive impairment in schizophrenia (Minzenberg and Carter, 2012). Identifying those with a high ‘learning potential’ and the capacity to benefit from practice is a crucial aspect of developing targeted remediation treatments (Ohrmann et al., 2008; Pedersen et al.; Raffard et al., 2009). However, an ongoing need to refine the baseline brain target for these interventions remains (Genevsky et al., 2010, Kaneko and Keshavan, 2012). While a number of fMRI studies have examined practice-related learning in schizophrenia (Eyler et al., 2008, Heinze et al., 2006, Koch et al., 2007, Koch et al., 2010, Pedersen et al., 2012, Rowland et al., 2010, Schlosser et al., 2009, van Raalten et al., 2008), many fewer have focussed on how this learning manifests in time and the brain activation underlying practice-related learning across multiple imaging sessions. Thus far, authors have not explicitly accounted for co-occurring nonspecific learning-independent imaging signal changes, which may also undermine efforts to precisely characterize the brain processes underlying learning in schizophrenia.
A significant challenge for imaging learning in general is that there are confounding nonspecific task-independent effects (Petersson et al., 1999, Poldrack, 2000, Rajah et al., 1998, Ross, 2010). These effects, if not taken into consideration, lead to misattribution of brain activation to an experimental learning task when it may be better explained by another task-independent ‘time effect’. These effects are particularly (although by no means exclusively) relevant to learning experiments. “Learning” and “time” are tightly correlated; furthermore, fMRI learning-experiments often occur over lengthier or multiple scanning sessions. For these reasons, learning-experiments are more vulnerable to contamination by nonspecific effects of ‘time’. Randomization or counterbalancing of task order is not always possible in learning experiments, so efforts must be made to explicitly control for these time effects by: modelling them as confounds, modifying the experiment by bracketing the learning task with control tasks that are ‘tasks-of-no-interest’, or a combination of these approaches. Longitudinal learning studies are key for understanding how learning unfolds over time and how practice impacts learning, but may compound these challenges as there is also potential for task-by-session interactions. Thus, extra care must be taken to control for time effects in multisession studies (McGonigle et al., 2000).
Time effects attributed to MRI scanning characteristics (e.g. temporally-correlated low frequency noise, motion artifact, and scanner drift) are typically dealt with in common preprocessing steps (Huettel et al., 2009). However, there are secondary task-independent time effects with presumably a neural basis that are less well characterized and we do not know how these processes interact with task-specific learning processes. Related time-dependent BOLD changes have been attributed to processes such as nonspecific arousal, attentional changes habituation, repetition suppression, and enhancement (Grill-Spector et al., 2006, Henson and Rugg, 2003, Meltzer et al., 2009, Poldrack, 2000, Segaert et al., 2013) in functional imaging studies in healthy adults. Learning researchers have employed several strategies to address nonspecific changes in learning experiments including using tasks-of-no-interest as a baseline or comparator task to isolate learning.
Task- independent time effects have been explicitly examined in healthy participants. Rajah et al. had participants perform a visual activation task for 6 scans bracketed by two baseline scans in a PET study. Linear task-independent rCBF changes spanned all 8 scans. They then compared these findings with two separate PET datasets using different tasks, but with similar designs in that there were ‘baseline scans’ with tasks-of-no-interest bookending a series of scans in which participants performed a cognitive task. In all three studies there were linear task-independent CBF decreases in temporal and occipital regions, and there were task-independent CBF increases in anterior cingulate and pre- and post-central gyri (Rajah et al., 1998). Petersson et al. demonstrated how two analytic approaches to extract task-independent effects (‘time’ as a linear confound approach and an interaction approach) gave overlapping, but non-identical results in a pattern-completion learning PET experiment (Petersson et al., 1999). Although it may not always be practical, careful construction of the experimental paradigm to utilize tasks-of-no-interest flanking the learning component is the most prudent approach to extract potential time confounds.
To our knowledge, there are no imaging studies that have explicitly examined these task-independent effects in schizophrenia. However, there is ample reason to hypothesize that task-independent effects may play a large role in imaging studies of cognition in schizophrenia. For example, a growing body of neuroimaging evidence suggests that inadequate suppression of default mode activity interferes with task engagement in schizophrenia (Anticevic et al., 2012, Whitfield-Gabrieli et al., 2009). This difficulty disengaging with ‘idling’ networks could hypothetically manifest as a linear confound. Additionally, impairment in general cognitive domains, especially attention and arousal networks, may interfere with the ability to ‘isolate’ brain in a given cognitive task (Foucher et al., 2011). Other possible contributors might include aberrant habituation and enhancement of the BOLD signal (Williams et al., 2013), underlying psychotic symptoms, the subjective experience of the scanning environment, and an altered capacity for those with schizophrenia to coordinate events in time (Parsons et al., 2013).
Our study aims to fill this key gap in the literature in the neuroimaging of learning in schizophrenia. We used an established method to “exert statistical control over time-dependent effects” (Petersson et al., 1999, Rajah et al., 1998) by incorporating tasks-of-no-interest flanking our task-of-interest (a lexicon-learning task) to extract monotonic BOLD changes spanning a two-session fMRI experiment. We focussed on monotonic task-independent changes as, given that the prevailing analytic approach to fMRI cognitive data still utilizes the GLM, these would be the ones most likely to confound accurate interpretation of task-related BOLD changes. The use of multiple sessions allowed us to examine the relevance of task-independent BOLD changes spanning multiple days, which is important given that longitudinal paradigms are crucial for understanding learning and the durability of underlying associated brain changes.
The primary goal was to characterize nonspecific task-independent BOLD signal effects that spanned the entire experiment, irrespective of task, in persons with chronic schizophrenia and to contrast these with control participants. The secondary goal was to examine the relationship between these task-independent effects and clinical characteristics on the schizophrenia group. We hypothesized that, based on the extant literature cited above, while both groups would show monotonic task-independent BOLD signal changes across the scanning sessions; these effects would differ in schizophrenia and be distributed across a wide range of brain regions including regions where activity had heretofore assumed to be solely related to either practice of the task or learning per se.
2. Materials and methods
2.1. Participants
Seventeen healthy control participants (HC) and 16 participants with schizophrenia (SZ) were enrolled in the study. HC participants were recruited via local advertisement and the research participant database at the Rotman Research Institute, University of Toronto. SZ participants were recruited from the outpatient clinics and local advertisements at the Centre for Addiction and Mental Health (CAMH), University of Toronto. All participants were native English speakers, right-handed (assessed through the Edinburgh Handedness inventory (Oldfield, 1971)), suitable for MRI scanning, and had no known neurological, or relevant medical illnesses. HC participants had no psychological illnesses (assessed via the MINI-Plus (Sheehan et al., 1998)). SZ participants had a DSM-IV diagnosis of schizophrenia or schizoaffective disorder (confirmed with the MINI-Plus and by a study-MD), had been prescribed an atypical antipsychotic medication at a stable dose for the last three months, and scored less than “2” on the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1989) ratings of “Conceptual Disorganization” and “Uncooperativeness”. All potential participants, including those with schizophrenia, were excluded if there was a lifetime history of substance dependence or a history of substance abuse within the preceding three months.
Participants provided informed consent and were paid a stipend for their participation. The study protocol was approved by the Research Ethics Boards of Baycrest Hospital and the Centre for Addiction and Mental Health according to the guidelines from these hospitals and the University of Toronto.
The final sample was comprised of data from twelve participants in each group. Data from five HC participants and four SZ participants were excluded due to improper task performance (2), technical difficulties with equipment (3), or excessive movement artifact (4) on fMRI scans.
2.2. Stimuli and procedures
The study timeline is illustrated in Fig. 1.
Fig. 1.

Study timeline.
The fMRI experiment was structured to optimize our ability to examine monotonic task-independent BOLD signal changes by bracketing five learning runs with runs of a control task that were of the same duration. This same format was repeated for two scanning sessions over a one week period (Fig. 2). Participants learned a novel lexicon comprised of 30 English pseudowords in an associative learning task developed by Breitenstein and Knecht, but modified by us for native-English speakers (Breitenstein and Knecht, 2002, Breitenstein et al., 2005). Each participant's new vocabulary was comprised of 30 auditory pseudowords arbitrarily paired with object drawings selected from a standardized set of pictures (Snodgrass and Vanderwart, 1980). They were explicitly told that over the course of the two-day task they would be learning a new language and they had practiced the task with a parallel set of stimuli in the MRI simulator on a day prior to the first scanning day. Participants heard a spoken pseudoword (normalized to 600 ms) in their headphones and then 200 ms after the onset of the auditory pseudoword saw a picture of an everyday object (duration 1 s) followed by a fixation cross (duration 2 s). Sometimes the pairings were ‘correct’ and sometimes ‘incorrect’, though this was not conveyed to the subjects. Participants indicated whether or not they thought the pairings were correct by pushing one of two buttons on a response pad. The underlying learning principle was a higher co-occurrence of ‘correct’ pairings with a 20:1 (correct:incorrect) ratio by the end of both scanning sessions. The learning runs across both days were additive in that the vocabulary to be learned for each participant was comprised of the same 30 words-object pairings on each of the days.
Fig. 2.

FMRI experiment format.
Participants completed two fMRI scanning sessions, which were identically structured. Each functional scanning session began with one run of a no-learning (NL) task followed by 5 runs of the learning task and then another NL run. This format repeated on the second day. The learning task was cumulative in that the vocabulary of random pseudoword–picture pairings that the participants were instructed to learn stayed the same across both sessions. The underlying learning principle involved a gradual increasing co-occurrence of correct pairings over the two days. In contrast, each pairing word–picture pairing of the NL task occurred only once and thus there was no underlying learning principle.
The control task-of-no-interest was identical in presentation to the learning task, but used a parallel set of matched stimuli and lacked an underlying learning principle as each pseudoword–object pairing was random and occurred only once and thus there was no lexicon to learn. The participants were instructed to respond simply by pushing the button as quickly as possible after each picture appeared on the screen.
The study took place over three days. On the first day, participants were trained on the tasks in an MRI simulator using parallel sets of stimuli. Each participant was trained until they reached 75% proficiency on the learning-task. Participants completed identically structured fMRI scanning sessions on the second and third study days. Both scanning sessions occurred within a one-week period of time. The functional runs began with the control task (120 trials) followed by five learning runs (600 trials in total), and then another run of the control task (120 trials). Each run lasted approximately 6.5 min.
2.3. MRI data acquisition
MRI images were acquired on a Siemens Trio 3 T MRI scanner. A T1-weighted anatomical scan was obtained using SPGR (TE = 2.6 ms, TR = 2000 ms, FOV = 256 mm, slice thickness = 1 mm). T2* functional images (TE = 30 ms, TR = 2000 ms, flip angle = 70°, FOV = 200 mm, in-plane voxel size = 3.5 mm × 3.5 mm × 5.0 mm) were obtained using an echo-planar image (EPI) acquisition sequence leading to a blood oxygenation level-dependent (BOLD) contrast. Each functional sequence consisted of twenty-eight 5-mm thick slices in the axial–oblique plane positioned to image the entire brain.
Participants' responses were made using the Fibre-Optic Response Pad (FORP) system (http://www.curdes.com/usbforp.htm), which has two four-button response pads and is designed for MR compatibility. Visual stimuli were presented on a rear projection screen placed at the foot of the MR scanner using an LCD projector. The participants viewed the stimuli using a mirror mounted on the head coil. Auditory stimuli were presented using the Silent Scan auditory presentation system (AVOTEC), which uses air conduction to transmit tones to headphones to attenuate the gradient noise. E-Prime Stimulus Presentation Software (http://www.pstnet.com) was used to control stimulus presentation, collect behavioural responses, and to log the precise timing of stimulus events and response for matching to fMRI data.
2.4. Image preprocessing
The images were processed prior to statistical analysis. Slice-timing correction was done using AFNI (http://afni.nimh.nih.gov/afni). Motion correction was completed using AIR (http://bishopw.loni.ucla.edu/AIR5/) by registering functional volumes to the 100th volume within each run, using a rigid-body transformation model. All functional volumes within each motion-corrected run were averaged to create mean functional volumes for each run. Using a rigid body transformation model, this mean functional volume was then registered with each participant's structural volume. The structural data were spatially normalized to the Common Anatomical Template previously described by Grady et al. (2010). Thus, the end result was a direct nonlinear transform from each initial fMRI volume into the Common Template space.
The functional data were smoothed using a 7 mm Gaussian kernel. The voxel time series were further adjusted by regressing out motion correction parameters, white matter (WM) time series, and CSF time series. As in Garrett et al. (2010), time series of the unsmoothed data from small regions-of-interest in the corpus callosum and ventricles of the Common Template were used as the white matter (WM) and cerebrospinal fluid (CSF) regressors, respectively. This last step minimizes the risk of regressing out relevant grey matter signal.
After data analyses, the results were transformed into MNI-space using the FSL/FNIRT registration algorithm to find a nonlinear transform between our anatomical template and the MNI152_T1 template provided with FSL software (www.fmrib.ox.ac.uk/fsl). We used SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) to aid anatomical localization of the relevant MNI coordinates.
2.5. Data analysis
2.5.1. Behavioural data analysis
The learning curves for both groups were analysed with a two-way repeated measures analysis of variance (ANOVA) of accuracy and reaction time (on accurate responses only). Group (HC versus SZ group) was the between-subjects factor and runs were within-subjects factors. The no-learning curves were similarly analysed with reaction time as the performance measure. Cognitive scores and demographic data were compared between the groups using Student's t-tests.
2.5.2. Imaging data analysis
“Partial least squares” (PLS) (McIntosh and Lobaugh, 2004, McIntosh et al., 2004) was used to analyse the fMRI data. PLS is a multivariate statistical method that identifies maximal covariance (latent variables (LVs)) between sets of independent measures and allows for both spatial and temporal interpretation. In contrast to PCA, PLS solutions are constrained to the part of the covariance structure attributable to experimental manipulations (task) or directly related to behaviour.
The data must be in matrix form for PLS. The rows of the data matrix contain the condition blocks (the reaction-time and the learning runs). These rows are stacked vertically and each participant has a row of data within each condition block. The columns of the data matrix contain the signal intensity measure of the BOLD signal for each voxel across the lag window (see below), Given that the hemodynamic response function (HRF) for each condition lasts for several scans, a “lag window” is thus defined as a short signal segment within a given trial that represents the response of each voxel. In our experiment, the lag-window size was 8 (TR = 2, 16 s), beginning at the offset of the auditory pseudoword (600 ms). We chose this approach to best capture language-related activation using the same rationale as Breitenstein et al. (2005).
For the learning task, only correct trials were analysed. For the control task, where there were no ‘correct’ responses, we analysed trials corresponding to the same trial numbers analysed in the adjacent learning runs in order to balance the design.
There are several approaches to analysing imaging data with PLS. In this study, non-rotated task-PLS (NRPLS) was used (McIntosh and Lobaugh, 2004). While ‘mean-centred’ task-PLS uses singular value decomposition to rotate the data matrix in order to identify the strongest effects that differentiate experimental conditions, NRPLS uses a priori contrasts to constrain the results generated by task-PLS. This approach allows for direct testing of hypothesized relationships. The effects of interest were the main effects of task type for both groups (i.e. learning versus the control task), the interaction effects of group by task, the main effects of ‘time’ for both groups (i.e. the linear task-independent effects that spanned both task types), and the interaction between ‘time’ and group. The ‘singular image’ generated is the distributed voxel pattern that best characterizes the effects of interest. It is the cross-product of the contrast and the data matrix. The ‘singular value’ quantifies the strength of the relationship between this singular image and the contrast. Specifically, it is the sum of the squared voxel values for the singular image.
We used two resampling techniques to provide complementary information about the statistical strength of each task contrast and its reliability across participants. The statistical significance of the LVs was determined using permutation testing (Edgington, 1980) (500 permutations). The robustness/stability of the voxel contribution to the effect was then estimated using bootstrap resampling (Efron and Tibshirani, 1986) (500 bootstrap iterations). Bootstrap resampling adds protection against parametric violations as could potentially occur with a smaller sample size. As PLS operates on the entire data structure simultaneously, there is no need to correct for multiple comparisons at the voxel level.
Lastly, correlation analyses were done between the slope of the ‘brain scores’ (i.e. the dot-product of the singular image and the individual's fMRI data) as they changed across scan sessions and accuracy on the learning task, symptom scores (PANSS), neurocognitive scores (RBANS) and medication dosages (chlorpromazine equivalents) for the schizophrenia group. The correlations were assessed for reliability using bootstrap resampling for confidence interval estimation. Since we were not interested in null hypothesis testing, no correction for multiple comparisons was necessary.
3. Results
3.1. Demographics and clinical characteristics
Demographic characteristics are displayed in Table 1. The two groups were similar with regard to: sex, age, and premorbid IQ. They differed with regard to: years of education, number of languages spoken, and cognitive measures from the RBANS. PANSS scores for the schizophrenia group were in the ‘mildly symptomatic’ range (Leucht et al., 2005). All schizophrenia participants were prescribed atypical antipsychotics. The doses were converted to chlorpromazine equivalents using Woods' guidelines (Woods, 2003) and the mean dose for the group was 474 mg.
Table 1.
Demographic and clinical data.
| SZ (mean/count) | SZ (SD/%) | HC (mean/count) | HC (SD/%) | p-Value | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sex (female) | 4 | 33 | 5 | 42 | 0.673 |
| Age (years) | 32.25 | 10.58 | 30.83 | 8.19 | 0.54 |
| Education (years) | 14.33 | 4.05 | 17.33 | 2.46 | 0.039 |
| Number languages spoken | 1.17 | 0.39 | 2.08 | 1 | 0.007 |
| Smoker | 1 | 0.08 | 0 | 0 | 0.307 |
| RBANS (percentile scores) | |||||
| Immediate memory | 29.83 | 28.74 | 55.56 | 29.77 | 0.047 |
| Delayed memory | 31.18 | 20.44 | 50.91 | 27.39 | 0.062 |
| Visuospatial/constructional | 49.17 | 34.95 | 76.27 | 20.93 | 0.037 |
| Language | 34.75 | 26.29 | 57.64 | 19.94 | 0.03 |
| Attention | 38.37 | 34.36 | 68.64 | 27.25 | 0.03 |
| Total RBANS | 32 | 28.53 | 67.55 | 18.48 | 0.002 |
| Clinical characteristics | |||||
| PANSS positive subscale | 11.75 | 2.6 | |||
| PANSS negative subscale | 9.5 | 3.09 | |||
| PANSS general subscale | 23.96 | 3.62 | |||
| PANSS total | 45.21 | 6.17 | |||
| Antipsychotic dose (CPZ equivalents) | 474 | 495 | |||
Note: RBANS = Repeated Battery for the Assessment of Neuropsychological Status, PANSS = Positive and Negative Syndrome Scale, CPZ = chlorpromazine.
3.2. Behavioural data
Repeated measures ANOVA showed a significant learning effect for both groups over the ten learning runs (F(4,89) = 116.43 p ≪ 0.001). There was a main group effect (F(1,22) = 7.82, p = 0.011), but no significant learning run by group interaction, suggesting that the overall learning rates were similar between groups. Fig. 3 displays the learning curves. Similarly, analysis of the reaction time data for the learning task showed a significant decrease in reaction time for correct responses across the ten runs for both groups (F(2.8,61.69) = 11.79, p ≪ 0.0001). There were differences between the groups (F(1,22) = 18.43, p ≪ 0.0001) with the HC having faster reaction times overall, but no significant interaction between group and learning. Lastly, for the no-learning control task, performance did not change significantly over time, did not differ between groups and there was no group by time interaction.
Fig. 3.

Behavioural learning curves.
3.3. Learning vs. no-learning PLS results
There was a significant main effect of task that differentiated a spatiotemporal brain pattern between the learning and the no-learning control task for both groups (p < 0.005) (Fig. 4 and Table 2). Areas of the brain that showed more activity in the learning task versus the control task included: frontal regions (left pars triangularis, bilateral middle frontal gyri, bilateral precentral gyri, bilateral rolandic operculums), temporal regions (left middle temporal gyrus, bilateral superior temporal gyri, right hippocampus), parietal regions (left postcentral gyrus), occipital regions (right superior and middle occipital gyri, left fusiform gyrus, left inferior occipital gyrus), and bilateral dorsal striatum. Bilateral cerebellar areas and the left lingual gyrus were more active in the control task compared to the learning task. There was no significant group by task interaction.
Fig. 4.
Results from the non-rotated task PLS analysis contrasting modulation of activity between no-learning and learning tasks across both groups: main effects of task. The bar graph (top) displays the mean brain scores for both groups on this latent variable (LV) (p < 0.005) for all runs across both days (grey = no-learning task runs and green = learning task runs). The error bars represent the 95% confidence intervals derived from the bootstrap estimations. The singular image (bottom) identifies brain areas in this LV showing reliable maximal differentiation between the two task types for both participant groups. The y-axis indexes activity across time from event-onset. The x-axis shows ventral (left) through dorsal (right) axial slices. Warm (yellow/red) areas showed more activity during the learning task for both groups, whereas cool (blue) areas showed more activity during the no-learning task. A bootstrap ratio threshold of 3 (roughly 99% confidence interval) was used to display the activity of this LV. Brains are displayed in neurological convention (L = L).
Table 2.
Local maxima for learning vs. no-learning analysis: main effects.
| Lag | MNI X (mm) | MNI Y (mm) | MNI Z (mm) | BSR | Cluster size (voxels) | Region |
|---|---|---|---|---|---|---|
| 1 | − 44 | − 30 | 3 | 13.6 | 226 | Left superior temporal gyrus |
| 1 | 59 | − 11 | − 4 | 9.9 | 422 | Right superior temporal gyrus |
| 1 | − 38 | − 33 | 46 | 9.6 | 750 | Left postcentral gyrus |
| 1 | − 5 | 1 | 49 | 7.7 | 432 | Left supplementary motor area |
| 1 | − 18 | − 101 | − 8 | 7.2 | 70 | Left inferior occipital gyrus |
| 1 | − 3 | 28 | 24 | 6.5 | 54 | Left anterior cingulate cortex |
| 1 | 15 | 13 | − 10 | 5.8 | 116 | Right caudate |
| 1 | − 23 | 82 | − 15 | 5.7 | 147 | Right middle occipital gyrus |
| 1 | − 34 | 41 | 10 | 4.1 | 52 | Left inferior frontal gyrus (p. triangularis) |
| 2 | − 31 | − 9 | 36 | 7.2 | 238 | Left precentral gyrus |
| 2 | 32 | − 25 | 20 | 7.0 | 341 | Right insula lobe |
| 2 | − 5 | 53 | − 15 | 6.0 | 38 | Left rectal gyrus |
| 2 | 24 | 24 | 8 | 5.6 | 137 | Right caudate |
| 2 | − 10 | 30 | − 2 | 4.8 | 41 | Left middle orbital gyrus |
| 3 | − 46 | − 61 | − 18 | 8.4 | 1968 | Left fusiform gyrus |
| 3 | − 42 | − 17 | 56 | 7.3 | 196 | Left precentral gyrus |
| 3 | 20 | 14 | − 5 | 6.8 | 611 | Right putamen |
| 3 | − 33 | 11 | 53 | 5.7 | 93 | Left middle frontal gyrus |
| 3 | 16 | − 14 | − 29 | 5.1 | 23 | Right hippocampus |
| 3 | 38 | 7 | 53 | 4.8 | 23 | Right middle frontal gyrus |
| 4 | − 42 | 26 | 8 | 9.0 | 237 | Right superior temporal gyrus |
| 4 | − 8 | 6 | 7 | 8.1 | 813 | Left caudate |
| 4 | − 48 | − 25 | 2 | 8.0 | 142 | Left superior temporal gyrus |
| 4 | − 38 | − 26 | 48 | 7.9 | 595 | Left postcentral gyrus |
| 4 | 27 | − 76 | 32 | 5.0 | 45 | Right superior occipital gyrus |
| 4 | − 19 | − 72 | − 2 | − 4.5 | 52 | Left lingual gyrus |
| 4 | 34 | − 54 | − 28 | − 6.0 | 37 | Right cerebellum (VI) |
| 5 | − 16 | − 23 | 62 | 5.1 | 30 | Left postcentral gyrus |
| 5 | 38 | − 19 | 52 | 5.0 | 82 | Right precentral gyrus |
| 5 | 29 | − 56 | − 33 | − 5.2 | 62 | Right cerebellum (VI) |
| 6 | − 48 | − 30 | 3 | 10.7 | 226 | Left middle temporal gyrus |
| 6 | 54 | − 29 | 8 | 8.3 | 240 | Right superior temporal gyrus |
| 6 | − 45 | 2 | 16 | 8.2 | 453 | Left rolandic operculum |
| 6 | − 53 | − 10 | 42 | 8.2 | 203 | Left postcentral gyrus |
| 6 | 45 | 3 | 17 | 6.5 | 220 | Right rolandic operculum |
| 6 | − 31 | − 57 | − 18 | 5.6 | 56 | Left fusiform gyrus |
| 6 | 10 | − 5 | 3 | 5.5 | 163 | Right putamen |
| 6 | − 12 | 9 | 1 | 5.4 | 49 | Left caudate |
| 6 | 39 | − 90 | 0 | 5.2 | 162 | Right middle occipital gyrus |
| 6 | − 23 | − 105 | − 2 | 4.6 | 123 | Left fusiform gyrus |
| 6 | 27 | − 76 | 32 | 4.6 | 35 | Right superior occipital gyrus |
| 7 | − 42 | 26 | 8 | 8.1 | 101 | Left inferior frontal gyrus (p. triangularis) |
| 7 | 37 | 1 | 21 | 6.6 | 613 | Right rolandic operculum |
| 7 | − 31 | − 9 | 36 | 6.1 | 177 | Left postcentral gyrus |
| 7 | − 44 | − 30 | 3 | 5.7 | 66 | Left superior temporal gyrus |
| 7 | − 11 | − 51 | 47 | 5.1 | 64 | Left precuneus |
| 7 | − 1 | 44 | − 8 | 5.1 | 125 | Left mid − orbital gyrus |
| 7 | − 32 | − 71 | − 29 | − 4.2 | 22 | Left cerebellum (Crus 1) |
| 7 | − 19 | − 72 | − 2 | − 5.4 | 58 | Left lingual gyrus |
| 7 | 34 | − 55 | − 32 | − 6.4 | 73 | Right cerebellum (VI) |
Note: Lag refers to the period, in seconds, after stimulus onset during which the peak occurred. X, Y, and Z are the voxel coordinates of the peak in each cluster in MNI space. BSR (bootstrap ratio) represents each peak voxel's PLS parameter estimate divided by its standard error and is a measure of robustness. A bootstrap ratio threshold of 3 (roughly 99% confidence interval) was used as a cut-off to identify cluster-peaks for this table. Cluster size refers to the number of contiguous voxels included in the cluster.
3.4. Task-independent ‘time effects’
The first design contrast examined the main effects of ‘time’ for both groups, defined as monotonic task-independent changes in the BOLD signal across both task-types on both scanning sessions. The resulting latent variable was statistically significant (permutation p-value < 0.005). Areas showing high activity at the beginning that diminished linearly over time included: frontal regions (bilateral inferior frontal gyri (triangularis and operculum)), cerebellar vermis, left putamen, and bilateral pallidum. Areas in this pattern that showed higher activity in later stages included: bilateral superior temporal regions and left inferior and superior parietal regions. The plot displaying group-mean brain scores illustrates that, although there were similarities between groups, the linear contrast representing ‘time’ was a better fit for the schizophrenia group (Fig. 5 and Table 3). The HC group, on the other hand showed monotonic changes primarily across the ten learning runs, and the initial control run did not contribute to this brain pattern.
Fig. 5.
Results from the non-rotated task PLS analysis contrasting the monotonic task-independent effects of time for both groups: main effects of time. The format used is similar to Fig. 4. The bar graph of the mean brain scores and the associated 95% confidence intervals for the LV (p < 0.005) illustrates that, although there are linear changes for both groups, the contrast is a better fit for the schizophrenia group. On the singular image, cool colours represent areas where brain activity was higher at the beginning and decreased linearly across time and warm colours represent brain areas showing linear increase in activity across time. A bootstrap ratio threshold of 3 was used to create this figure.
Table 3.
Local maxima for task-independent linear time effects analysis: main effects of “time”.
| Lag | MNI X (mm) | MNI Y (mm) | MNI Z (mm) | BSR | Cluster size (voxels) | Region |
|---|---|---|---|---|---|---|
| 1 | − 62 | − 27 | 16 | 5.3 | 33 | Left superior temporal gyrus |
| 1 | 27 | − 26 | 20 | 3.9 | 10 | Right superior temporal gyrus |
| 1 | 26 | − 49 | − 38 | 3.8 | 26 | Left inferior parietal lobule (angular gyrus) |
| 2 | − 38 | − 41 | 56 | 6.9 | 116 | Left superior parietal lobule |
| 2 | 34 | − 75 | 24 | 5.0 | 19 | Right middle occipital gyrus |
| 2 | 46 | − 26 | 39 | 4.5 | 10 | Right postcentral gyrus |
| 3 | − 27 | 12 | − 10 | − 5.6 | 45 | Left insula lobe |
| 3 | 2 | − 61 | 2 | − 5.0 | 32 | Cerebellar vermis (4/5) |
| 3 | − 28 | − 100 | 2 | − 4.8 | 19 | Left middle occipital gyrus |
| 3 | − 7 | − 52 | 33 | − 4.6 | 58 | Left posterior cingulate cortex |
| 3 | − 58 | 19 | 17 | − 4.0 | 10 | Left inferior frontal gyrus (p. triangularis) |
| 3 | 38 | 9 | 32 | − 3.9 | 10 | Right inferior frontal gyrus (p. opercularis) |
| 4 | − 22 | 5 | 1 | − 4.6 | 42 | Left putamen |
| 4 | − 53 | 12 | 25 | − 3.7 | 10 | Left inferior frontal gyrus (p. opercularis) |
| 5 | − 31 | 11 | − 15 | − 5.4 | 15 | Left insula lobe |
| 5 | 18 | 1 | − 3 | − 4.6 | 16 | Right pallidum |
| 6 | − 17 | 0 | − 3 | − 5.5 | 52 | Left pallidum |
| 6 | 2 | − 60 | 6 | − 3.6 | 10 | Cerebellar vermis (4/5) |
| 7 | 43 | − 79 | 15 | 5.9 | 18 | Right middle occipital gyrus |
| 7 | 11 | − 24 | 61 | 5.1 | 31 | Right supplementary motor area |
A bootstrap ratio threshold of 3 (roughly 99% confidence interval) was used as a cut-off to identify cluster-peaks for this table.
The second design contrast examined the time by group interaction. The latent variable that characterized this disordinal contrast was statistically significant (p < 0.04).
There was a time by group interaction in: frontal areas (left anterior insula and superior medial gyrus), bilateral temporal regions (inferior, middle, and superior temporal gyri), and left parietal regions (superior parietal lobule, posterior cingulate, precuneus, and postcentral gyrus) (Fig. 6 and Table 4).
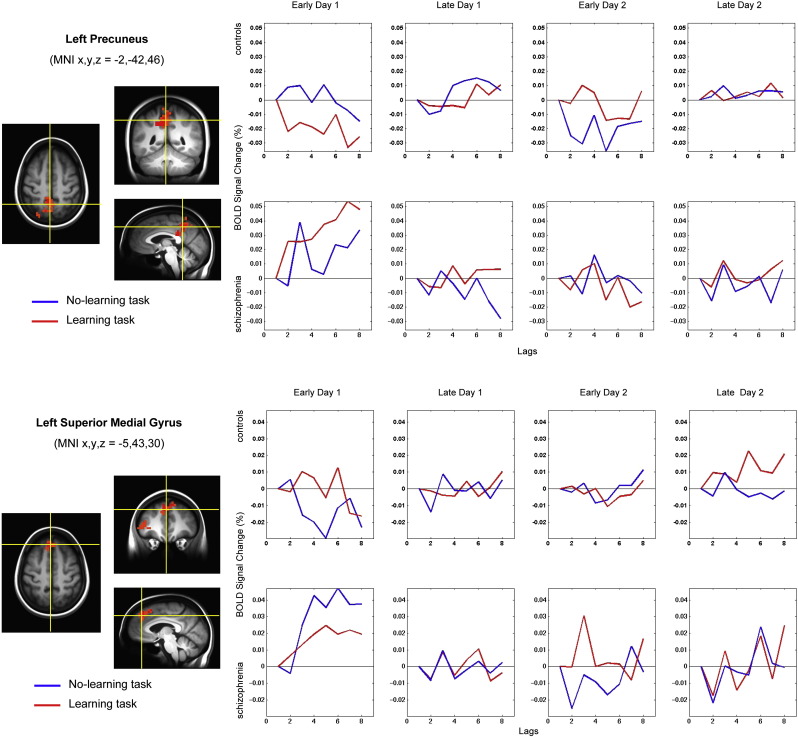
Fig. 6.
Results from the non-rotated task PLS analysis contrasting the monotonic task-independent effects of time for both groups: interaction by group. The bar graph of the brain scores illustrates the LV (p < 0.04) that expresses the disordinal contrast between time and group. The cardinal sections and HRF plots highlight activity in regions where the contrast was reliably expressed (see Table 4). The HRF plots capture BOLD activity differences between groups for the no-learning and learning tasks. The top row of plots for each area pertains to the control group and the bottom row to the schizophrenia group. The y-axis is the percent BOLD signal change and the x-axis is time (expressed in lags in TRs (TR = 2 s)) from event onset. Blue lines represent the HRFs for no-learning (NL) runs and red lines for the learning (L) runs. The columns highlight different sections of the fMRI experiment: Early Day 1 = Run 1 (NL) and Run 2 (L); Late Day 1 = Run 6 (L) and Run 7 (NL); Early Day 2 = Run 8 (NL) and Run 9 (L); Late Day 2 = Run 13 (L) and Run 14 (NL).
Table 4.
Local maxima for task-independent linear time effects analysis: interaction of time by group.
| Lag | MNI X (mm) | MNI Y (mm) | MNI Z (mm) | BSR | Cluster size (voxels) | Region |
|---|---|---|---|---|---|---|
| 3 | − 32 | 22 | 2 | 5.2 | 29 | Left insula lobe |
| 3 | − 12 | − 52 | 32 | 4.3 | 15 | Left precuneus |
| 3 | − 5 | 43 | 30 | 4.1 | 13 | Left superior medial gyrus |
| 4 | − 24 | − 61 | 54 | 4.3 | 15 | Left superior parietal lobule |
| 5 | − 31 | 7 | − 14 | 5.1 | 56 | Left insula lobe |
| 5 | − 56 | − 55 | 13 | 4.8 | 26 | Left middle temporal gyrus |
| 5 | 50 | − 25 | 8 | 4.2 | 12 | Right superior temporal gyrus |
| 5 | − 46 | − 3 | 44 | 3.9 | 10 | Left precentral gyrus |
| 6 | − 2 | − 42 | 46 | 5.2 | 94 | Left posterior cingulate/precuneus |
| 6 | 47 | − 67 | − 9 | 5.2 | 20 | Right inferior temporal gyrus |
| 6 | − 32 | − 20 | 0 | 4.9 | 16 | Left superior temporal sulcus |
| 6 | − 14 | − 59 | 57 | 4.4 | 38 | Left precuneus |
| 6 | − 28 | − 32 | 54 | 4.1 | 10 | Left postcentral gyrus |
A bootstrap ratio threshold of 3 (roughly 99% confidence interval) was used as a cut-off to identify cluster-peaks for this table.
Lastly, the correlation analyses showed a significant relationship between the magnitude of negative symptoms and the likelihood of expressing learning-independent linear BOLD effects for the SZ group at the 90th confidence interval (r = 0.41, CI90 = 0.11–0.61). There was no significant correlation with the other behavioural or symptoms measures. Of note, there was no relationship between antipsychotic dose and time effects as assessed via bootstrap resampling for confident interval estimation (r = 0.24, CI95 = − 0.58–0.22).
3.5. Results summary
In summary, these analyses showed that the two tasks (i.e. the learning task and the control task) elicited very different patterns of brain activity for both groups spanning multiple scanning sessions and there was no detectable interaction between task and group. However, there were significant monotonic changes in brain activity across both tasks over the two scanning sessions that were independent of task type for both groups. The statistical significance and stability of these linear changes were more robust for the SZ group. Interaction analyses demonstrated that several key areas differentiated between groups, particularly inferior and superior temporal regions, frontal areas including left anterior insula and superior medial gyri, posterior cingulate, and precuneus. These were all areas showing high task-independent BOLD activity that diminished linearly over the two scanning sessions for the SZ group, but showed either opposite patterns of activity or no activity changes for the HC group. Lastly, there was a trend towards a correlation between these effects and the presence of more negative symptoms in the SZ group.
4. Discussion
4.1. Overview
The primary goal of this study was to examine task-independent ‘time’ changes to the BOLD signal in a multisession learning experiment in schizophrenia. Indeed, the results support our hypothesis that nonspecific time effects are more prominent and differentially distributed in schizophrenia and could potentially confound conclusions drawn about the learning process if not taken into consideration. Although there were monotonic BOLD signal changes spanning the learning and control tasks across both sessions in both groups, the changes in the healthy control group were slight and would not obscure conclusions drawn about learning manifest in analyses that did not take time effects into consideration. It is possible that these time effects in healthy controls are subtle and require larger numbers to detect. Regardless, the situation was quite different for those with schizophrenia in this study. Here, linear time effects were considerable and show different anatomic distribution than in the controls.
4.2. Rationale for approach and task findings
We first established that the two tasks (i.e. learning and control tasks) showed different patterns of BOLD activity. The results showed that both groups differentiated between the task types with similar activity patterns. This analysis established that, although the stimuli used were identical in nature, the two tasks were quite unique with regard to brain areas activated. Thus, based on this finding, linear changes that incorporated both tasks would not be solely attributable to brain-task similarities per se and could be considered to be task-independent as per the aim of the study. As would be expected, relative to the control task, the learning task activated a widely distributed set of cortical and striatal regions whereas the control task showed greater activity primarily in cerebellar regions. Importantly, there was no task by group interaction, suggesting that on a gross level of inspection, similar brain areas were engaged, thus making the approach to analysing time suitable for both groups. As per Rajah et al. (Rajah et al., 1998), we then extracted monotonic BOLD signal changes that traversed both task types across both scanning days using the control tasks as bookends bracketing the learning task on each day.
4.3. Time effects across tasks
There was a main effect of time for both groups. Notably, these effects were considerably more variable in the control group whereas the schizophrenia group contributed much more stably to the pattern. Given that in the majority of neuroimaging studies of schizophrenia, the schizophrenia group shows larger standard errors and greater brain heterogeneity than in comparative control groups in linear analyses of the BOLD signal (Javitt et al., 2008, Winterer et al., 2006); this finding in itself is an interesting finding that merits further follow-up.
The main effects included brain areas wherein, if these monotonic changes across all tasks were not considered, one might solely attribute the activity changes in the learning runs to lexicon learning per se. For example, there were task-independent decreases in BOLD activity for both groups in bilateral inferior fronto-opercular and left lateralized triangularis regions and task-independent BOLD activity increases in bilateral superior temporal gyri. An understanding of how these ‘classic’ language brain regions contribute to language acquisition is constantly being refined. There remains debate as to the specificity of their contribution across various aspects of lexicon and language acquisition (Friederici, 2011, Graves et al., 2008, Hickok and Poeppel, 2007). Here, our results suggest that cumulative changes in activity across both scanning sessions may relate to general processing of verbal stimuli as part of what has been referred to as a ‘default language network’(Friederici, 2011, Lohmann et al., 2010) rather than directly attributable to the acquisition of the specific lexicon.
Similarly, the linear increases we observed in the left inferior parietal regions (supramarginal gyrus) have been previously attributed to mapping cortical representations of newly learned information in the original study by Breitenstein et al. (2005), using this same task. Although Breitenstein et al. incorporated a similar control task in their experiment; they did not directly measure linear changes across both task-types. Our results suggest that the increasing activation across all runs may be also due to (at least in part) task-independent processes. That being said, the existence of a ‘time effect’ does not preclude the inclusion of any given area in a learning effect. Looking more closely at the hemodynamic response functions from this region illustrates this point clearly (Fig. 7). For the controls, the contribution of the supramarginal gyrus to the LV is, in fact, mainly driven by linear changes in the learning conditions and some, but less, from the no-learning conditions. In contrast, for the schizophrenia group, the monotonic changes are expressed quite clearly throughout the two days. Therefore, this suggests that this region shows monotonic changes in the BOLD signal primarily due to learning in the controls and the situation with the task-independent component is less compelling. However, the linear increase in activity in the inferior parietal cortex seems to play a different role for those with schizophrenia.
Fig. 7.
Hemodynamic response function (HRF) from the left inferior parietal lobe (IPL) region at the first lag (2 s after event onset) across no-learning and learning runs for both groups. This lag is where the linear contrast for main effects of time for both groups in the non-rotated PLS analysis was most reliably expressed in this brain region. The cardinal sections are taken from the singular image from the time effects analysis discussed in Fig. 5. The cross-hairs are at the peak local maxima of activity from this region (MNI coordinates (mm): X = 26, Y = − 49, Z = − 38). The graph top-right displays change in BOLD activity across all fourteen runs (green line = group; orange line = schizophrenia group). The bottom two graphs parse the same activity by task to illustrate those monotonic changes in BOLD activity in left IPL occur primarily during the learning runs for the control group, whereas the schizophrenia group shows monotonic changes that span both types of task.
A recent study of structural brain changes in schizophrenia has shown significant morphological differences in the supramarginal gyri (e.g. reduced gyrification, cortical thinning, and contraction in surface area) and a reversal of the normal L > R asymmetry (Palaniyappan and Liddle, 2012). These structural changes may underlie differences in functional contributions from this region, particularly relating to navigating language learning tasks. To further disambiguate this possibility, analyses using direct measurements of brain–behaviour relationships and further network analyses must be done. Our study illustrates how taking into consideration BOLD activity during the learning task only and ignoring potential time confounds can obscure the underlying complexity of the relationships by not fully accounting for the ‘neural context’ in which that activity is embedded (McIntosh and Korostil, 2008).
4.4. Time by group interaction
As predicted in our initial hypothesis, there was a significant time by group interaction. The schizophrenia group showed task-independent effects that differed in magnitude and directionality to those expressed by the control group. The effects were distributed across frontal, temporal, and parietal regions. The most statistically robust differences occurred in the left anterior insula (AI). After initial activation during the first run on day 1, the controls showed little activity modulation in this region until the first learning run on day 2. In contrast, the schizophrenia group showed initial activation during the first run, sustained and relative hyperactivation at the start of learning, and then a task-independent linear reduction in activation over the course of the two scanning sessions (Fig. 6). The AI has been repeatedly demonstrated to show functional and structural anomaly in schizophrenia (Manoliu et al., 2013). It has been postulated that this area is part of the so-called Salience Network (SN), which is comprised of coordinated activity between the AI and anterior cingulate cortices. This network facilitates switching between the Default Mode Network (DMN) and the Central Executive Network (CEN) (Menon and Uddin, 2010). Recent work of Manoliu et al. has suggested that dysfunction in the AI as part of the SN directly relates to psychotic and negative symptoms in the disorder (Manoliu et al., 2013, Manoliu et al., 2014). They have found that reduced intra–intrinsic functional connectivity of the left AI within the SN relates to both an increase in negative symptoms and hyperconnectivity between the SN and the CEN. It is possible that the aberrant modulation of BOLD activity in the same region in our data may also contribute to deficits in cognitive control leading to abnormal switching between the control and learning tasks.
The interaction contrast also incorporated robust activity changes in a distributed set of regions commonly associated with DMN functioning including: precuneus, posterior cingulate cortices, superior medial frontal gyri, and inferior and middle temporal gyri (Andrews-Hanna et al., 2010, Broyd et al., 2009, Mingoia et al., 2012, Raichle et al., 2001). Current research on the DMN suggests that in a normally functioning system there is a task-negative DMN component that attenuates during goal-directed tasks (Anticevic et al., 2012, Broyd et al., 2009). A recent theory hypothesizes that if there is lack of appropriate attenuation of these regions when transitioning to active task-specific processing, spontaneous low-frequency oscillations will persist and interfere with attentional resources. This theory of bottom-up DMN interference is in contrast to traditional ideas wherein attentional lapses are the failure of top-down processes (Sonuga-Barke and Castellanos, 2007). Consistent with this hypothesis, a growing body of literature suggests that persons with schizophrenia fail to attenuate these low frequency components during task-processing and that this failed attenuation may be associated with cognitive impairments as well as other symptoms of the disorder (Anticevic et al., 2013, Metzak et al., 2012, Nejad et al., 2011, Nygard et al., 2012, Whitfield-Gabrieli et al., 2009). In our results, the schizophrenia group showed marked persistent hyperactivation relative to the control group as they transitioned from the less cognitively demanding control task to the more demanding learning task. There was then a monotonic task-independent decrease in activity in these regions over the course of the two days. Towards the end of the second scanning session there was deactivation in DMN regions in the schizophrenia group, whereas the controls began to show linear increases in these same regions, presumably as the tasks became automated.
4.5. Relationship between time effects and symptomatology
We also examined the potential relationship between task-independent effects, symptom status, and measured neurocognitive deficits. There was a stable relationship (at the 90th CI) between the presence of time effects and negative symptoms such that those with more negative symptoms were more likely to manifest linear task-independent BOLD effects. Other authors have suggested a link between negative symptoms, task switching, and attentional modulation relating to impaired functioning in the AI, prefrontal cortex, and default mode regions (Manoliu et al., 2013, Menon and Uddin, 2010). It is possible that our findings are the result of similar underlying relationships between negative symptoms and reduced modulation of brain states resulting in the linear time confound observed in our study.
We did not find a statistically significant relationship between task-independent effects and other measures. However, due to our small sample size and the entry-criterion of ‘clinical stability’ there was little variance on behavioural measures, which likely reduced our discriminatory power. While these findings should therefore be considered exploratory, they are nevertheless intriguing and warrant further investigation.
4.6. Notes for study comparisons
While numerous studies have found brain activation differences during verbal and other relational learning paradigms in schizophrenia (Eyler et al., 2008, Heinze et al., 2006, Murray et al., 2010, Wadehra et al., n.d) (see (Barch and Ceaser, 2012) and (Li et al., 2009) for reviews), to the best of our knowledge, no studies have directly measured potentially confounding effects of time in their analyses. Similarly, task-independent BOLD changes have not been calculated in the studies that have explicitly examined practice-related learning in schizophrenia (Koch et al., 2007, Koch et al., 2010, Pedersen et al., 2012, Rowland et al., 2010, Schlosser et al., 2009, van Raalten et al., 2008). However, some of the findings in these studies overlap with ours and, we would suggest, may be tapping into the same underlying brain processes, albeit with a different interpretation from the authors. For example, using a modified Sternberg paradigm with repeated presentation of verbal material, Koch et al. demonstrated that a group of persons with schizophrenia were able to benefit from practice and showed an associated frontoparietal BOLD signal decrease with learning similar to the control group (Koch et al., 2007). They found a relatively stronger signal decrease in several brain areas associated with ‘hyperactivation’ in the schizophrenia group at the start of learning that normalized with practice. There was overlap with our findings, notably the BOLD hyperactivity in superior and middle temporal gyri, pre-motor, SMA, and precuneus. Without disentangling the time component, we would argue that it might oversimplify the picture to attribute these findings to task-related differences. In their data, this hyperactivity at the beginning related to an exponential signal decrease across learning runs, whereas in our data this hyperactivity at the beginning was the start of a monotonic decrease across learning and no-learning runs in two scanning sessions.
In a follow-up study, the group used the same paradigm, but divided their schizophrenia cohort into ‘more successful’ and ‘less successful’ learners using a median split (Koch et al., 2010). The less successful learners were found to have more hyperactivation at the beginning of the task in superior temporal gyrus, inferior frontal regions, superior parietal, and cingulate regions and these activation differences were also associated with more psychopathology. Again, we would argue that by not incorporating time as a potential confound in their analyses, the ability to draw inferences about the underlying learning mechanisms is necessarily constrained.
Finally, although authors have incorporated multisession scanning to compare brain activation across time, these experiments have generally been pre- and post-training scans as opposed to scanning during the learning process itself (Rowland et al., 2010). To our knowledge, there is no other study that has examined the unfolding of learning across multiple scanning sessions in schizophrenia, and thus the presence/absence of task-independent BOLD effects in schizophrenia fMRI studies spanning multiple scans is virtually unknown.
4.7. Limitations
Our final sample size was small and thus these results should be considered preliminary and followed up with larger studies. However, as several authors have recently commented, a robust effect found in a neuroimaging study despite a small sample size is compelling and worth reporting assuming that one has constructed an accurate model and minimized sampling bias (Friston, 2012, Lindquist et al., 2013). Mitigating factors include: resampling statistics with very robust effects (evidenced by bootstrapping with 500 randomizations) particularly in the clinical sample; consistency with prior findings in the literature and the within-subject power of the study with measurement of the BOLD signal across two days and ten learning runs for each subject.
Lastly, due to previous findings in the literature on time effects in neuroimaging learning studies, we chose to model monotonic task-independent changes. However, it is possible that there may also be nonlinear task-independent effects that we were unable to capture through our analyses. Our focus on linear time effects was motivated by previous findings in the literature and our primary objective to highlight task-independent effects most likely to interfere with proper interpretation of task-related effects given that the vast majority of fMRI analyses of cognitive task-related data still utilize the general linear method as a framework.
4.8. Conclusions
Time and learning are tightly correlated. Learning studies must control for this potential confounder. We ascribed to a similar approach for disentangling these two phenomena as have been described in the past: with using a task-of-no-interest to bookend the learning runs. We showed that this approach can also be used in multisession imaging studies, which are crucial for understanding practice-related learning. Our results suggest that understanding time effects may be even more relevant for learning studies in schizophrenia as the magnitude and direction of these effects differed markedly from the healthy control group and extended across multiple scanning sessions. Isolating the brain substrates underlying learning in schizophrenia and understanding how practice may or may not modify them continues to challenge the field. Our findings highlight the importance of careful experimental design in neuroimaging learning experiments in schizophrenia in order to maximize precision in data interpretation and minimize the confound of task-unrelated signal changes.
Acknowledgements
The authors would like to acknowledge and thank: Dr. Shitij Kapur for guidance regarding task development and financial support; Ms. Heidi Marcon for assistance with data collection; Ms. Maria Karachalios for help with data collection and manuscript proof-reading; and Dr. Catarina Breitenstein for generously sharing expertise about the learning task.
This work was funded through a Canadian Institute of Health Research (CIHR) Operating Grant to Dr. S. Kapur and a James S. McDonnell Foundation Grant (JSMF: 220020255) to Dr. A.R. McIntosh.
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Barch D.M. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr. Bull. 2013;39:168–178. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C., Knecht S. Development and validation of a language learning model for behavioral and functional-imaging studies. J. Neurosci. Methods. 2002;114:173–179. doi: 10.1016/s0165-0270(01)00525-8. [DOI] [PubMed] [Google Scholar]
- Breitenstein C., Jansen A., Deppe M., Foerster A.F., Sommer J., Wolbers T., Knecht S. Hippocampus activity differentiates good from poor learners of a novel lexicon. Neuroimage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Edgington E.S. Marcel Dekker; New York: 1980. Randomization Tests. [Google Scholar]
- Efron B., Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Stat. Sci. 1986;1:54–77. [Google Scholar]
- Eyler L.T., Jeste D.V., Brown G.G. Brain response abnormalities during verbal learning among patients with schizophrenia. Psychiatry Res. 2008;162:11–25. doi: 10.1016/j.pscychresns.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher J.R., Luck D., Marrer C., Pham B.T., Gounot D., Vidailhet P., Otzenberger H. fMRI working memory hypo-activations in schizophrenia come with a coupling deficit between arousal and cognition. Psychiatry Res. 2011;194:21–29. doi: 10.1016/j.pscychresns.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61:1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Garrett D.D., Kovacevic N., McIntosh A.R., Grady C.L. Blood oxygen level-dependent signal variability is more than just noise. J. Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevsky A., Garrett C.T., Alexander P.P., Vinogradov S. Cognitive training in schizophrenia: a neuroscience-based approach. Dialogues Clin. Neurosci. 2010;12:416–421. doi: 10.31887/DCNS.2010.12.3/agenevsky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., Protzner A.B., Kovacevic N., Strother S.C., Afshin-Pour B., Wojtowicz M., Anderson J.A., Churchill N., McIntosh A.R. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves W.W., Grabowski T.J., Mehta S., Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J. Cogn. Neurosci. 2008;20:1698–1710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K., Henson R., Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn. Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Heinze S., Sartory G., Muller B.W., de Greiff A., Forsting M., Juptner M. Neural activation during successful and unsuccessful verbal learning in schizophrenia. Schizophr. Res. 2006;83:121–130. doi: 10.1016/j.schres.2005.12.852. [DOI] [PubMed] [Google Scholar]
- Henson R.N.A., Rugg M.D. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41:263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Huettel S.A., Song A.W., McCarthy G. Sinauer Associates; Sunderland, Mass: 2009. Functional Magnetic Resonance Imaging. [Google Scholar]
- Javitt D.C., Spencer K.M., Thaker G.K., Winterer G., Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Keshavan M. Cognitive remediation in schizophrenia. Clin. Psychopharmacol. Neurosci. 2012;10:125–135. doi: 10.9758/cpn.2012.10.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A., Lindenmayer J.P. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br. J. Psychiatry Suppl. 1989:59–67. [PubMed] [Google Scholar]
- Koch K., Wagner G., Nenadic I., Schachtzabel C., Roebel M., Schultz C., Axer M., Reichenbach J.R., Sauer H., Schlosser R.G.M. Temporal modeling demonstrates preserved overlearning processes in schizophrenia: an fMRI study. Neuroscience. 2007;146:1474–1483. doi: 10.1016/j.neuroscience.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Koch K., Wagner G., Schachtzabel C., Schultz C., Sauer H., Schlosser R.G.M. Association between learning capabilities and practice-related activation changes in schizophrenia. Schizophr. Bull. 2010;36:486–495. doi: 10.1093/schbul/sbq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S., Kane J.M., Kissling W., Hamann J., Etschel E., Engel R.R. What does the PANSS mean? Schizophr. Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Li X., Branch C.A., DeLisi L.E. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr. Opin. Psychiatry. 2009;22:131–139. doi: 10.1097/YCO.0b013e328324bc43. [DOI] [PubMed] [Google Scholar]
- Lindquist M.A., Caffo B., Crainiceanu C. Ironing out the statistical wrinkles in “ten ironic rules”. Neuroimage. 2013;81:499–502. doi: 10.1016/j.neuroimage.2013.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann G., Hoehl S., Brauer J., Danielmeier C., Bornkessel-Schlesewsky I., Bahlmann J., Turner R., Friederici A. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb. Cortex. 2010;20:1286–1292. doi: 10.1093/cercor/bhp190. [DOI] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Doll A., Bauml J.G., Muhlau M., Schwerthoffer D., Scherr M., Zimmer C., Forstl H., Bauml J., Wohlschlager A.M., Koch K., Sorg C. Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Front. Hum. Neurosci. 2013;7:216. doi: 10.3389/fnhum.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Riedl V., Zherdin A., Muhlau M., Schwerthoffer D., Scherr M., Peters H., Zimmer C., Forstl H., Bauml J., Wohlschlager A.M., Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr. Bull. 2014;40:428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle D.J., Howseman A.M., Athwal B.S., Friston K.J., Frackowiak R.S., Holmes A.P. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11:708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- McIntosh A., Korostil M.l. Interpretation of neuroimaging data based on network concepts. Brain Imaging Behav. 2008;2:264–269. [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl. 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Chau W.K., Protzner A.B. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Meltzer J.A., Postman-Caucheteux W.A., McArdle J.J., Braun A.R. Strategies for longitudinal neuroimaging studies of overt language production. Neuroimage. 2009;47:745–755. doi: 10.1016/j.neuroimage.2009.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzak P.D., Riley J.D., Wang L., Whitman J.C., Ngan E.T., Woodward T.S. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr. Bull. 2012;38:803–813. doi: 10.1093/schbul/sbq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G., Wagner G., Langbein K., Maitra R., Smesny S., Dietzek M., Burmeister H.P., Reichenbach J.R., Schlosser R.G., Gaser C., Sauer H., Nenadic I. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr. Res. 2012;138:143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Carter C.S. Developing treatments for impaired cognition in schizophrenia. Trends Cogn. Sci. 2012;16:35–42. doi: 10.1016/j.tics.2011.11.017. [DOI] [PubMed] [Google Scholar]
- Murray G.K., Corlett P.R., Fletcher P.C. The neural underpinnings of associative learning in health and psychosis: how can performance be preserved when brain responses are abnormal? Schizophr. Bull. 2010;36:465–471. doi: 10.1093/schbul/sbq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad A.B., Ebdrup B.H., Siebner H.R., Rasmussen H., Aggernaes B., Glenthoj B.Y., Baare W.F. Impaired temporoparietal deactivation with working memory load in antipsychotic-naive patients with first-episode schizophrenia. World J. Biol. Psychiatry. 2011;12:271–281. doi: 10.3109/15622975.2010.556199. [DOI] [PubMed] [Google Scholar]
- Nygard M., Eichele T., Loberg E.M., Jorgensen H.A., Johnsen E., Kroken R.A., Berle J.O., Hugdahl K. Patients with schizophrenia fail to up-regulate task-positive and down-regulate task-negative brain networks: an fMRI study using an ICA analysis approach. Front. Hum. Neurosci. 2012;6:149. doi: 10.3389/fnhum.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrmann P., Kugel H., Bauer J., Siegmund A., Kölkebeck, K., Suslow, T., Wiedl, K.H., Rothermundt, M., Arolt, V., Pedersen, A. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr. Res. 2008;106:156–163. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Liddle P.F. Dissociable morphometric differences of the inferior parietal lobule in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262:579–587. doi: 10.1007/s00406-012-0314-y. [DOI] [PubMed] [Google Scholar]
- Parsons B.D., Gandhi S., Aurbach E.L., Williams N., Williams M., Wassef A., Eagleman D.M. Lengthened temporal integration in schizophrenia. Neuropsychologia. 2013;51:372–376. doi: 10.1016/j.neuropsychologia.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Pedersen A., Wilmsmeier A., Wiedl K.H., Bauer J., Kueppers K., Koelkebeck K., Kohl W., Kugel H., Arolt V., Ohrmann P. Anterior cingulate cortex activation is related to learning potential on the WCST in schizophrenia patients. Brain Cogn. 2012;79:245–251. doi: 10.1016/j.bandc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Petersson K.M., Elfgren C., Ingvar M. Learning-related effects and functional neuroimaging. Hum. Brain Mapp. 1999;7:234–243. doi: 10.1002/(SICI)1097-0193(1999)7:4<234::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Imaging brain plasticity: conceptual and methodological issues: a theoretical review. Neuroimage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Raffard S., Gely-Nargeot M.C., Capdevielle D., Bayard S., Boulenger J.P. Learning potential and cognitive remediation in schizophrenia. Encéphale. 2009;35:353–360. doi: 10.1016/j.encep.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah M., Hussey D., Houle S., Kapur S., McIntosh A.R. Task-independent effect of time on rCBF. Neuroimage. 1998;7:314–325. doi: 10.1006/nimg.1998.0339. [DOI] [PubMed] [Google Scholar]
- Ross E.D. Cerebral localization of functions and the neurology of language: fact versus fiction or is it something else? Neuroscientist. 2010;16:222–243. doi: 10.1177/1073858409349899. [DOI] [PubMed] [Google Scholar]
- Rowland L.M., Griego J.A., Spieker E.A., Cortes C.R., Holcomb H.H. Neural changes associated with relational learning in schizophrenia. Schizophr. Bull. 2010;36:496–503. doi: 10.1093/schbul/sbq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser R., Koch K., Wagner G., Schultz C., Rbel M., Schachtzabel C., Reichenbach J.R., Sauer H. Intensive practice of a cognitive task is associated with enhanced functional integration in schizophrenia. Psychol. Med. 2009;39:1809–1819. doi: 10.1017/S0033291709005820. [DOI] [PubMed] [Google Scholar]
- Segaert K., Weber K., de Lange F.P., Petersson K.M., Hagoort P. The suppression of repetition enhancement: a review of fMRI studies. Neuropsychologia. 2013;51:59–66. doi: 10.1016/j.neuropsychologia.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. (quiz 34-57) [PubMed] [Google Scholar]
- Snodgrass J.G., Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. Hum. Learn. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Tandon R., Nasrallah H.A., Keshavan M.S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- van Raalten T.R., Ramsey N.F., Jansma J.M., Jager G., Kahn R.S. Automatization and working memory capacity in schizophrenia. Schizophr. Res. 2008;100:161–171. doi: 10.1016/j.schres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Wadehra, S., Pruitt, P., Murphy, E.R., Diwadkar, V.A., Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: an fMRI study. Schizophr Res 148, 38-49. [DOI] [PubMed]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.E., Blackford J.U., Luksik A., Gauthier I., Heckers S. Reduced habituation in patients with schizophrenia. Schizophr. Res. 2013:9964. doi: 10.1016/j.schres.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G., Musso F., Beckmann C., Mattay V., Egan M.F., Jones D.W., Callicott J.H., Coppola R., Weinberger D.R. Instability of prefrontal signal processing in schizophrenia. Am. J. Psychiatry. 2006;163:1960–1968. doi: 10.1176/ajp.2006.163.11.1960. [DOI] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]