Abstract
Background:
Dexmedetomidine a new drug, which is alpha-two agonist, is recommended by manufacturers as an adjuvant in epidural analgesia and anesthesia.
Aims:
To study the effects of dexmedetomidine on quality and efficacy of the epidural bupivacaine 0.5% for vaginal hysterectomies, by studying the onset of action, duration of action, highest dermatomal level achieved, degree of motor blockade, intraoperative and postoperative anesthesia and analgesia achieved.
Setting and Design:
Prospective randomized study.
Materials and Methods:
In this study, 60 American Society of Anesthesiologists I and II patients requiring vaginal hysterectomy were enrolled. Patients were randomly divided into two groups - Group I: Control group receiving epidural bupivacaine 0.5% 15–20 ml only.
Group II:
Group receiving of epidural bupivacaine 0.5% 15–20 ml with dexmedetomidine 05 mcg/kg. Following parameters were noted: Time to onset of T10 dermatomal level, maximum sensory level achieved, time for complete motor block, time for two segmental dermatomes regression, regression to S1 dermatome, time for first rescue analgesic and total top ups required during study.
Statistical Analysis:
Mean and standard deviation was calculated. We used two independent sample t-test to find the P value. Software used STATA 13.0.
Results:
The demographic profile was comparable between the groups. There was significant difference between two groups (P < 0.001) regarding onset of analgesia to T10 (17.12 ± 2.44 vs. 10.14 ± 2.94), time to achieve complete motor block (27.16 ± 4.52 vs. 22.98 ± 4.78), which was earlier in dexmedetomidine with bupivacaine group. Prolonged postoperative analgesia, less rescue top ups and adequate sedation score was found with dexmedetomidine group. The intraoperative hemodynamic changes were comparable in both the groups. The incidence of dry mouth, shivering and nausea was more with the dexmedetomidine group.
Conclusion:
We conclude that epidural dexmedetomidine 0.5 µg/kg is a good adjuvant providing early onset of sensory and motor block, adequate sedation and prolonged postoperative analgesia with minimal side-effects.
Keywords: Bupivacaine, dexmedetomidine, epidural anesthesia, vaginal hysterectomy
INTRODUCTION
Epidural anesthesia is the most commonly used technique for providing not only surgical anesthesia but also postoperative analgesia in surgical patients.[1] Early postoperative mobilization, rehabilitation, minimal pain and discomfort are the most desirable features of the modern surgery.[2,3,4] Local anesthetic agents like bupivacaine, lignocaine with or without adrenaline are in use and are the gold standard drugs.[5,6] Opioids like fentanyl, morphine, buprenorphine are used traditionally as an adjuvant, but come with a side-effect such as pruritus, urinary retention, nausea, vomiting and respiratory depression.[7,8,9] Many new adjuvants to local anesthetics are being tried and alpha-two agonists are one of them. The anesthestic and analgesic requirement get reduced to a huge extent by use of these adjuvants because of their analgesic properties as well as augmentation of local anesthetic mediated by hyperpolariztion of nerve tissues by altering transmembrane potential and ion conductance at the locus coerulus in the brainstem.[10] In vaginal hysterectomies, after induction of neuroaxial blockade profound muscle relaxation that occurs causes inability to move legs. Also, prolonged duration of surgery, lithotomy position with trendelenberg tilt make patients uncomfortable. At this stage giving large doses of intravenous sedation defeats the very purpose of giving regional block.[10] Alpha-two agonists are the drugs, which have sedative and analgesic properties. The stable hemodynamic provided by these drugs makes them the desirable choice of adjuvant.
Dexmedetomidine is highly selective alpha-two adrenergic agonist with an affinity 8 times more than clonidine.[10] It acts on both pre- and post-synaptic sympathetic nerve terminals and central nervous system thereby decreasing the sympathetic outflow and nor epinephrine release causing sedative, anti-anxiety, analgesic, sympathetic and hemodynamic effects.[11,12,13] Dexmedetomidine causes dose-dependent bradycardia, hypotension so in this prospective study we have used minimum doses, that is, 0.5 µg/kg along with the local anesthetic agent bupivacaine as it is easily available and routinely practiced since many years so that we can study the properties of the new drug like dexmedetomidine.
Objectives of the study
To study the effects of dexmedetomidine on quality and efficacy of the epidural bupivacaine 0.5% for vaginal hysterectomies, by studying the onset of action, duration of action, highest dermatomal level achieved, degree of motor blockade, preoperative and postoperative analgesia achieved
To study intraoperative and postoperative anesthesia and analgesia, hemodynamic effects, sedative effects, and any other side-effects of the dexmedetomidine.
MATERIALS AND METHODS
After obtaining the approval of the research ethical committee; and after informed consent 60 American Society of Anesthesiologists (ASA) I and II patients for vaginal hysterectomy included in this prospective randomized study.
Inclusion criteria
ASA I and II
Age between 30 and 60 years
Surgery not exceeding more than 120 min.
Exclusion criteria
Patients with diabetes mellitus, ischemic heart disease, hypertension, rhythm disturbances, chronic obstructive pulmonary disease, coagulation abnormalities, spinal deformities, and patients allergic to amide group of local anesthetic agents were excluded from the study.
Patients were randomly divided into two groups:
Group I: Control group receiving epidural 0.5% bupivacaine 15–20 ml only
Group II: Group receiving epidural 0.5% bupivacaine 15–20 ml with dexmedetomidine 0.5 µg/kg.
In the preoperative room, venous access was achieved with the 18 gauge vasofix. All the patients were preloaded with 500 ml of the lactated Ringer solution.
After taking the patient into the operative room, multipara monitor was attached to and baseline value of pulse rate (PR), noninvasive blood pressure (NIBP), pulse oximetry (SpO2), respiratory rate was noted. After taking all aseptic precautions, the lumbar epidural block was induced using 18 gauge touhy needle, in sitting position. After skin infiltration with 2% lignocaine in L2-L3 inter-space, the epidural needle was inserted, and epidural space was identified by loss of resistance to air technique. An epidural catheter was inserted and kept 4 cm in the epidural space and then fixed on the back of the patient. Test dose of 3 ml 2% lignocaine adrenaline was given through the catheter after giving supine position. After ruling out intradural and intravascular placement of the catheter, the study drug was given epidurally, which was prepared by anesthesia technician who was unaware of the study design.
The following solutions were randomly administered:
Group I: Received 15 ml of 0.5% bupivacaine
Group II: Received 15 ml of 0.5% bupivacaine with 0.5 µg/kg of the dexmedetomidine.
Bilateral pin prick method was used to evaluate and check sensory level. Motor blockade was assessed by modified Bromage scale (0 = no block, 1 = inability to raise extended leg; 2 = inability to flex knee; 3 = inability to flex ankle and foot). Sensory and motor block was assessed every 5 min after giving the study drug for 30 min. Following parameters were also observed after the epidural block: Time of onset of the analgesia to T10, maximum sensory level achieved, time for complete motor block (Bromage scale 3), time for two segmental dermatomal regression, regression to S1 dermatome, time for first rescue analgesic and total top ups required during the study. Lithotomy position was given after 25–30 min of epidural injection. Hemodynamic parameters -NIBP, PR, SpO2, electrocardiography were measured continuously and recordings were made every 5 min till the completion of the surgery and every 15 min after the patients were shifted to the recovery unit.
Hypotension was defined as decrease in the systolic blood pressure more than 30% of the baseline value and treated with the 3–6 mg of the mephenteramine and bradycardia was defined as PR < 50/min and was treated with the atropine 0.6 mg.
Grading of sedation was evaluated by the Ramsey five point scale: (1) Alert and wide awake, (2) arousable to verbal command, (3) arousable to gentle tactile stimuli (4) arousable to vigorous stimulation and (5) unarousable. Sedation scores were recorded just before the initiation of surgery and thereafter every 20 min during the surgical procedure. Any untoward incident and side effects during the study period were carefully observed and managed symptomatically. Patient satisfaction score was noted.
Statistical analysis
Mean and standard deviation was calculated, and we used two independent sample t-test to compare both the groups and to find the P value. P < 0.05 considered to be significant. Statistical software used was STATA 13.0. (Statacorp LP, 4905 Lakeway Drive, College Station, TX 77845, USA).
RESULTS
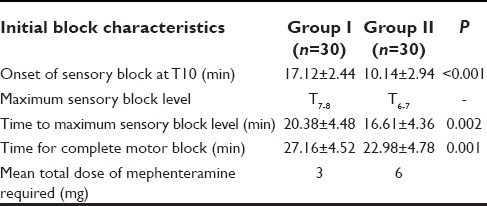
Totally 60 patients were enrolled for the study and were randomly divided into two groups. The demographic characteristics in both groups in terms of age, weight, height, and mean duration of the surgery were comparable [Table 1].
Table 1.
Demographic profile of the patients

The onset of analgesia at T10 dermatomal level was significantly earlier with bupivacaine with dexmedetomedine group as compared to plain bupivacaine group (P < 0.001). Similarly, group II achieved higher dermatomal spread in shorter time period (16.61 ± 4.36) as compared to group I. Complete motor block was achieved quite early (22.98 ± 4.78) in group II as compared to group I. In this study seven patients from group II were required to be given mephenteramine 6 mg for hypotension after 1 h of the block while in group I only two patients required to be given the vasopressor [Table 2].
Table 2.
Sensory and motor blockade profile
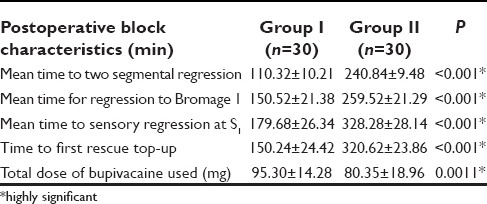
The findings of Table 3 reveal the statistically significant values on a comparison of the post-operative block characteristics in between two groups. The adjuvant group provided the mean time to two segmental regression was (240.84 ± 9.48) versus (110.32 ± 10.21) with bupivacaine alone, which was significantly prolonged. Similarly early return of the motor power to Bromage 1 in group I was (150.52 ± 21.38) as compared to in group II (259.52 ± 21.29). As a result, the time for rescue analgesia was shorter with group I (150.24 ± 24.42) as compared to group II (320.62 ± 23.86). The group II experienced the prolonged pain free period as compared to group I. The superior block characteristics by addition of the dexmedetomidine were clearly evident from lesser dose of local anesthetic consumption (80.35 ± 18.96) as compared to group I (95.30 ± 14.28).
Table 3.
Postoperative block characteristics
Sedation score was 2 on Ramsey sedation scale in all patients in group II. All the patients were calm and tranquil in group II. In group I patients were given midazolam for sedation.
Side effects like dry mouth, shivering, dizziness were more with dexmedetomidine group as compared to the plain bupivacaine group.
Hemodynamic changes in terms of PR, blood pressure, were comparable in between groups.
DISCUSSION
Selection of the exclusive epidural route during this study was done deliberately to avoid the spinal anesthesia induced sudden hypotension, to provide the post-operative pain relief and to study the analgesic, anesthetic potency, safety of the dexmedetomidine. This study directly shows the effects of the epidural dexmedetomidine.
To provide sedation, stable hemodynamics and prolonged postoperative analgesia are the main desirable qualities of an adjuvant used in epidural anesthesia.[14] The demographic profile in the present study was comparable to other studies and did not show any statistical difference.
In the present study, the dexmedetomidine showed an earlier onset of sensory and motor blockade. Postoperatively number of the top ups were less with the bupivacaine dexmedetomidine group as compared to bupivacaine alone.
Sukhminder Bajwa et al.[10] also found the early onset of analgesia and motor blockade in epidural dexmedetomidine when used with ropivacaine. Gupta et al.[15] found similar results with epidural dexmedetomidine when used with levobupivacaine in doses comparable to our study.
Sedation score was 2 on Ramsey sedation scale throughout the surgery and up to 2 h in post-operative room with the dexmedetomidine group, whereas in group I patients were given midazolam for the same effects. All these results show the analgesic, anesthetic and sedative properties of the dexmedetomidine.
In the present study, baseline heart rate was between 80 and 90/min. Heart rate dropped down to 56 and 70/min in six patients in epidural dexmedetomidine group. None of the patients needed atropine. Similarly, mean arterial pressure decreased from baseline in both the groups and comparable, but it never went below 70 mm of Hg. The decrease in the heart rate caused by alpha-two agonist can be explained on the basis of their central action where they decrease the sympathetic outflow and nor epinephrine release.[11,12,13] The stable hemodynamics can possibly be explained on the basis of lower volumes of the local anesthetic agent used, the lower doses of the adjuvant used.
Dry mouth, shivering was observed in five patients from dexmedetomidine group but was mildly discomforting to the patients and did not need any treatment. None of the patients in the present study had episodes of the respiratory depression.
CONCLUSION
Faster onset of action of local anesthetic agents, rapid establishment of both sensory and motor block, prolonged duration of analgesia in postoperative period, stable hemodynamics and minimal dose requirement make dexmedetomidine very effective adjuvant in epidural anesthesia. Dexmedetomidine reduces the dose of epidural bupivacaine, potentiates its action, provides adequate surgical anesthesia and postoperative analgesia with a desirable level of sedation and minimal side-effects
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schultz AM, Werba A, Ulbing S, Gollmann G, Lehofer F. Peri-operative thoracic epidural analgesia for thoracotomy. Eur J Anaesthesiol. 1997;14:600–3. doi: 10.1046/j.1365-2346.1994.00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H. Acute pain control and accelerated postoperative surgical recovery. Surg Clin North Am. 1999;79:431–43. doi: 10.1016/s0039-6109(05)70390-x. [DOI] [PubMed] [Google Scholar]
- 3.Bradshaw BG, Liu SS, Thirlby RC. Standardized perioperative care protocols and reduced length of stay after colon surgery. J Am Coll Surg. 1998;186:501–6. doi: 10.1016/s1072-7515(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227–30. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Zaric D, Nydahl PA, Philipson L, Samuelsson L, Heierson A, Axelsson K. The effect of continuous lumbar epidural infusion of ropivacaine (0.1%, 0.2%, and 0.3%) and 0.25% bupivacaine on sensory and motor block in volunteers: A double-blind study. Reg Anesth. 1996;21:14–25. [PubMed] [Google Scholar]
- 6.McClellan KJ, Faulds D. Ropivacaine: An update of its use in regional anaesthesia. Drugs. 2000;60:1065–93. doi: 10.2165/00003495-200060050-00007. [DOI] [PubMed] [Google Scholar]
- 7.Benzon HT, Wong HY, Belavic AM, Jr, Goodman I, Mitchell D, Lefheit T, et al. A randomized double-blind comparison of epidural fentanyl infusion versus patient-controlled analgesia with morphine for postthoracotomy pain. Anesth Analg. 1993;76:316–22. [PubMed] [Google Scholar]
- 8.Salomäki TE, Laitinen JO, Nuutinen LS. A randomized double-blind comparison of epidural versus intravenous fentanyl infusion for analgesia after thoracotomy. Anesthesiology. 1991;75:790–5. doi: 10.1097/00000542-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Lorenzini C, Moreira LB, Ferreira MB. Efficacy of ropivacaine compared with ropivacaine plus sufentanil for postoperative analgesia after major knee surgery. Anaesthesia. 2002;57:424–8. doi: 10.1046/j.0003-2409.2001.02393.x. [DOI] [PubMed] [Google Scholar]
- 10.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–70. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 12.Jaakola ML, Salonen M, Lehtinen R, Scheinin H. The analgesic action of dexmedetomidine: A novel alpha2-adrenoceptor agonist—in healthy volunteers. Pain. 1991;46:281–5. doi: 10.1016/0304-3959(91)90111-A. [DOI] [PubMed] [Google Scholar]
- 13.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Hohener D, Blumenthal S, Borgeat A. Sedation and regional anesthesia in the adult patients. Br J Anaesth. 2008;100:8–16. doi: 10.1093/bja/aem342. [DOI] [PubMed] [Google Scholar]
- 15.Kumkum Gupta, Bhawna Rastogi, Prashant K Gupta, Manish Jain, Suneeta Gupta, Deepti Mangla. Epidural 0.5% levobupivacaine with dexmedetomidine versus fentanyl for vaginal hysterectomy: A prospective study. Indian Journal of pain. 2014;28(3):149–154. [Google Scholar]


