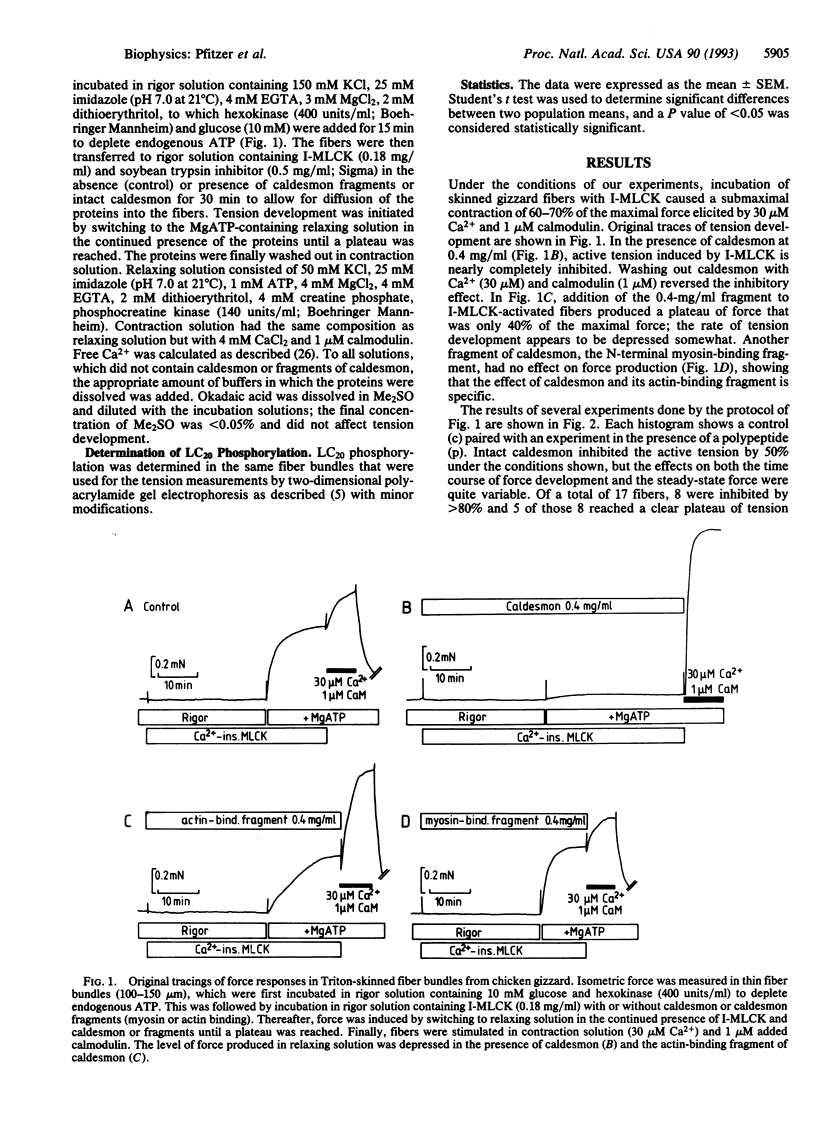
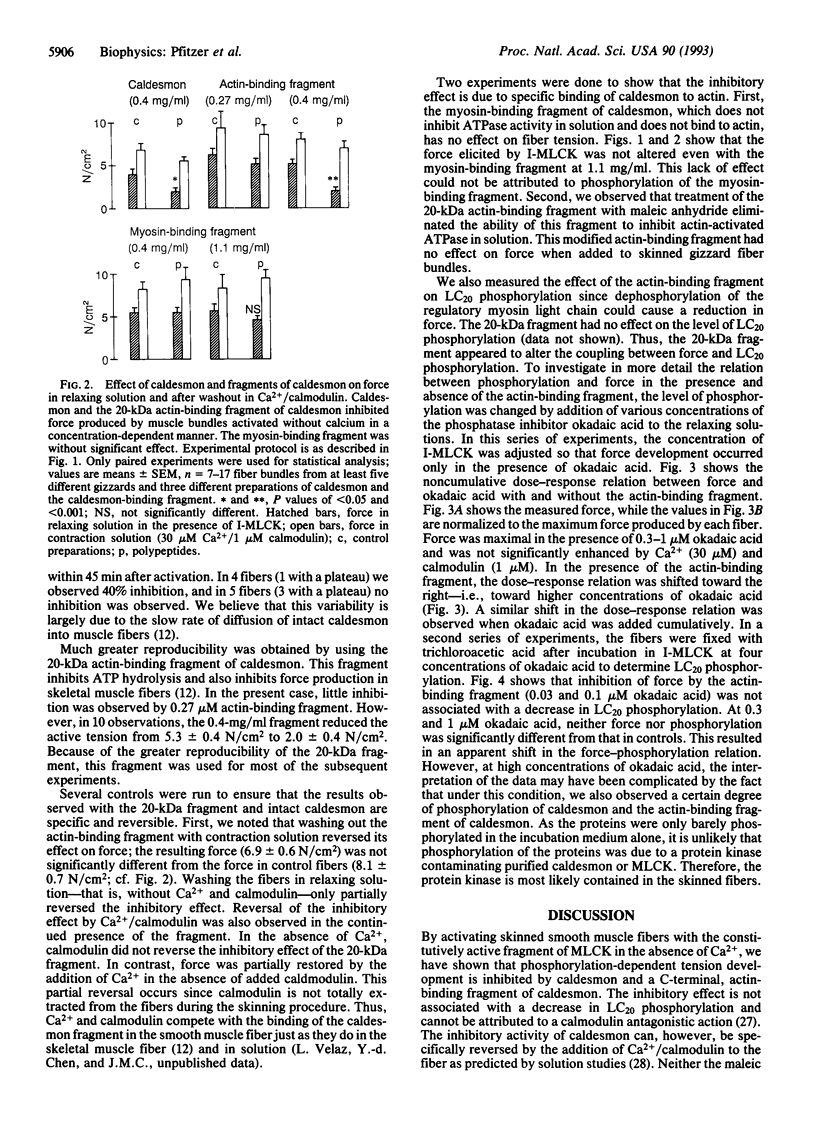
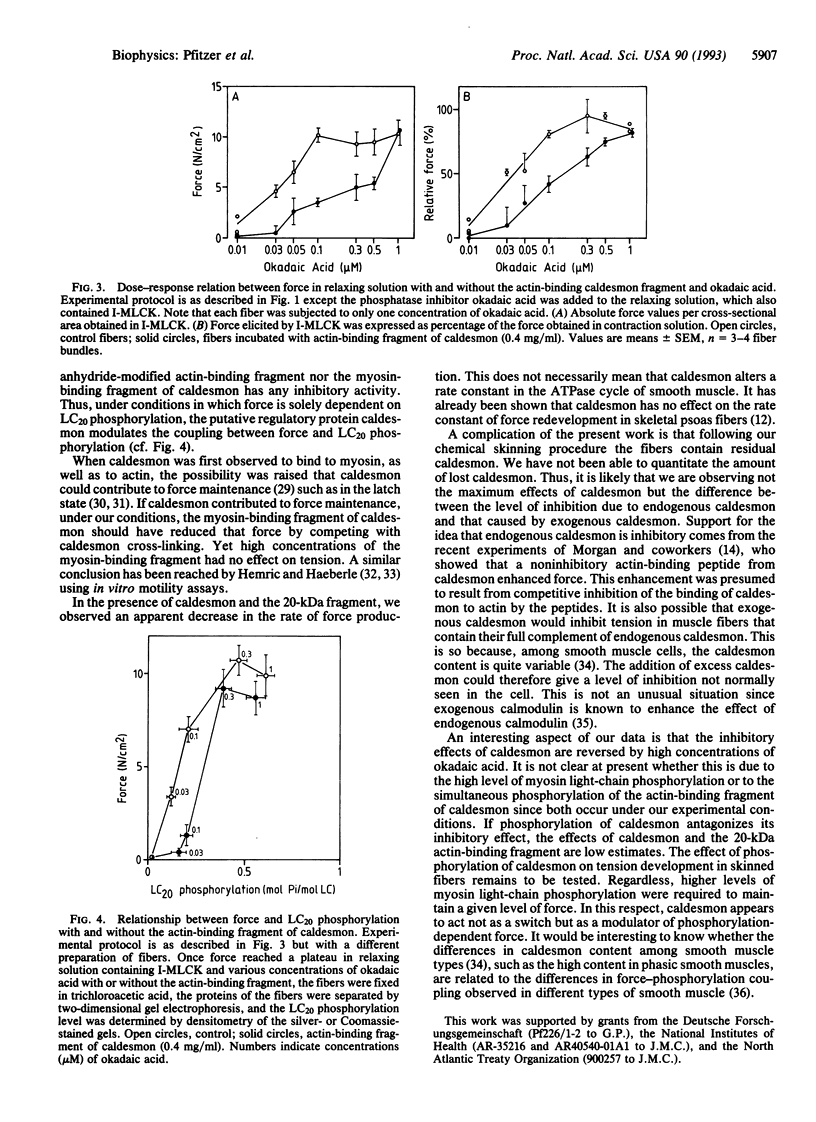
Abstract
Caldesmon is known to inhibit actin-activated myosin ATPase activity in solution, to inhibit force production when added to skeletal muscle fibers, and to alter actin movement in the in vitro cell motility assay. It is less clear that caldesmon can inhibit contraction in smooth muscle cells in which caldesmon is abundant. We now show that caldesmon and its 20-kDa actin-binding fragment are able to inhibit force in chemically skinned gizzard fiber bundles, which are activated by a constitutively active myosin light-chain kinase in the presence and absence of okadaic acid. This inhibitory effect is reversed by high concentrations of Ca2+ and calmodulin. Therefore, caldesmon may act by increasing the level of myosin phosphorylation required to obtain full activation. Our results also suggest that caldesmon does not act to maintain force in smooth muscle by cross-linking myosin with actin since competition of binding of caldesmon with myosin does not cause a reduction in tension.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews M. A., Maughan D. W., Nosek T. M., Godt R. E. Ion-specific and general ionic effects on contraction of skinned fast-twitch skeletal muscle from the rabbit. J Gen Physiol. 1991 Dec;98(6):1105–1125. doi: 10.1085/jgp.98.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal K. M., Kem W. R. Structure and action of heteronemertine polypeptide toxins: disulfide bonds of Cerebratulus lacteus toxin B-IV. J Biol Chem. 1977 May 25;252(10):3328–3331. [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Chalovich J. M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Chalovich J. M., Bryan J., Benson C. E., Velaz L. Localization and characterization of a 7.3-kDa region of caldesmon which reversibly inhibits actomyosin ATPase activity. J Biol Chem. 1992 Aug 15;267(23):16644–16650. [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Pfitzer G. Rapid myosin phosphorylation transients in phasic contractions in chicken gizzard smooth muscle. FEBS Lett. 1989 Nov 20;258(1):59–62. doi: 10.1016/0014-5793(89)81615-1. [DOI] [PubMed] [Google Scholar]
- Fürst D. O., Cross R. A., De Mey J., Small J. V. Caldesmon is an elongated, flexible molecule localized in the actomyosin domains of smooth muscle. EMBO J. 1986 Feb;5(2):251–257. doi: 10.1002/j.1460-2075.1986.tb04206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerthoffer W. T. Dissociation of myosin phosphorylation and active tension during muscarinic stimulation of tracheal smooth muscle. J Pharmacol Exp Ther. 1987 Jan;240(1):8–15. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Haeberle J. R., Hathaway D. R., Smith C. L. Caldesmon content of mammalian smooth muscles. J Muscle Res Cell Motil. 1992 Feb;13(1):81–89. doi: 10.1007/BF01738431. [DOI] [PubMed] [Google Scholar]
- Haeberle J. R., Trybus K. M., Hemric M. E., Warshaw D. M. The effects of smooth muscle caldesmon on actin filament motility. J Biol Chem. 1992 Nov 15;267(32):23001–23006. [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Regulation of shortening velocity by cross-bridge phosphorylation in smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 1):C86–C94. doi: 10.1152/ajpcell.1988.255.1.C86. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Ikebe M., Stepinska M., Kemp B. E., Means A. R., Hartshorne D. J. Proteolysis of smooth muscle myosin light chain kinase. Formation of inactive and calmodulin-independent fragments. J Biol Chem. 1987 Oct 5;262(28):13828–13834. [PubMed] [Google Scholar]
- Itoh T., Ikebe M., Kargacin G. J., Hartshorne D. J., Kemp B. E., Fay F. S. Effects of modulators of myosin light-chain kinase activity in single smooth muscle cells. Nature. 1989 Mar 9;338(6211):164–167. doi: 10.1038/338164a0. [DOI] [PubMed] [Google Scholar]
- Katsuyama H., Wang C. L., Morgan K. G. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992 Jul 25;267(21):14555–14558. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Inhibition of smooth muscle actin-activated myosin Mg2+-ATPase activity by caldesmon. J Biol Chem. 1984 Nov 25;259(22):13656–13659. [PubMed] [Google Scholar]
- Pfitzer G., Merkel L., Rüegg J. C., Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflugers Arch. 1986 Jul;407(1):87–91. doi: 10.1007/BF00580726. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Takahashi K., Wakabayashi I. Caldesmon150 regulates the tropomyosin-enhanced actin-myosin interaction in gizzard smooth muscle. Biochem Biophys Res Commun. 1985 Oct 30;132(2):645–651. doi: 10.1016/0006-291x(85)91181-7. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Mrwa U., Hofmann F., Rüegg J. C. Calmodulin is essential for smooth muscle contraction. FEBS Lett. 1981 Mar 23;125(2):141–145. doi: 10.1016/0014-5793(81)80704-1. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Resnick M., Morgan K. G. Change of Ca2+ requirement for myosin phosphorylation by prostaglandin F2 alpha. Am J Physiol. 1991 Aug;261(2 Pt 1):C253–C258. doi: 10.1152/ajpcell.1991.261.2.C253. [DOI] [PubMed] [Google Scholar]
- Szpacenko A., Wagner J., Dabrowska R., Rüegg J. C. Caldesmon-induced inhibition of ATPase activity of actomyosin and contraction of skinned fibres of chicken gizzard smooth muscle. FEBS Lett. 1985 Nov 11;192(1):9–12. doi: 10.1016/0014-5793(85)80032-6. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Hiwada K., Kokubu T. Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986 Nov 26;141(1):20–26. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- Tansey M. G., Hori M., Karaki H., Kamm K. E., Stull J. T. Okadaic acid uncouples myosin light chain phosphorylation and tension in smooth muscle. FEBS Lett. 1990 Sep 17;270(1-2):219–221. doi: 10.1016/0014-5793(90)81272-p. [DOI] [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Winder S. J., Walsh M. P. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990 Jun 15;265(17):10148–10155. [PubMed] [Google Scholar]



