Abstract
With both in vivo and in vitro experiments, the present study was conducted to investigate the effect of regulatory T cell (Treg) on promoting T-lymphocyte apoptosis and its regulatory mechanism through transforming growth factor-beta (TGF-β1) signaling in mice. A murine model of polymicrobial sepsis was reproduced by cecal ligation and puncture (CLP); PC61 and anti-TGF-β antibodies were used to decrease counts of CD4+CD25+ Tregs and inhibit TGF-β activity, respectively. Splenic CD4+CD25+ Tregs and CD4+CD25− T cells were isolated. Phenotypes, including cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), forkhead/winged helix transcription factor p3 (Foxp3), and TGFβ1m+, as well as the apoptotic rate of CD4+CD25− T cell, were analyzed by flow cytometry. Real-time reverse transcription-polymerase chain reaction was performed to determine mRNA expression of TGF-β1, and the expressions of Smad2/Smad3, Bcl-2 superfamily members of Bcl-2/Bim, cytochrome C, the mitochondrial membrane potential, and caspases in CD4+CD25− T cells were simultaneously determined. After treatment with PC61 or anti-TGF-β antibody, CTLA-4, Foxp3, and TGFβ1m+ expressions of CD4+CD25+ Tregs were markedly decreased in comparison to that of the CLP group and the apoptosis rate of CD4+CD25− T cells was significantly positively correlated with the expression of TGF-β1. Meanwhile, levels of P-Smad2/P-Smad3, proapoptotic protein Bim, cytochrome C, and activity of caspase-3, -8, -9 were downregulated, whereas the mitochondrial membrane potential and antiapoptotic protein Bcl-2 expression were restored. Taken together, our data indicated that the TGF-β1 signal could be partly involved in the apoptosis of CD4+CD25− T cells promoted by CD4+CD25+ Tregs, therefore inhibition of TGF-β1 expression may provide a novel strategy for the improvement of host immunosuppression following sepsis.
Introduction
Sepsis represents a complex clinical morbidity that results from a harmful or devastating host response to infection. Accumulating evidence has demonstrated that acute insults, including major burns, trauma, and hemorrhage, could lead to marked T-cell immune suppression. CD4+CD25+ Tregs, as a class of mature T-cell subsets with immune function, play important roles in the maintenance of immunologic self-tolerance and in the downregulation of various immune responses (Zhang and others 2011; Onyilagha and others 2014), such as induction of T-lymphocyte apoptosis, inhibition of the function of CD4+/CD8+ T lymphocytes, and mediation of shifting from the T-cell (Th) 1 to Th2 response.
Recently, there has been an increasing interest in investigating the biology of CD4+CD25+ Tregs and their role as well as regulatory mechanism in the apoptosis of T lymphocyte. Mechanisms underlying T-lymphocyte apoptosis as induced by CD4+CD25+ Tregs were complicated, including downregulation of expression of interleukin (IL)-2 and consumption of IL-2, resulting in a form of apoptosis dependent on cytokine deprivation, through the Fas/Fasl pathway as well as in a granzyme-dependent manner (Hesse and others 2004; Venet and others 2006; Luan and others 2012; Zhu and others 2012). However, the precise regulatory mechanism has yet to be fully elucidated.
Transforming growth factor (TGF)-β1 was proved to possess the distinct property in normal tissue homeostasis by regulating diverse functions such as cellular differentiation, apoptosis, cell cycle arrest, and cellular migration (Caraci and others 2008; Horbelt and others 2012; Goel and others 2013). The superfamily consists of structurally and functionally related cytokines that signal through a pair of transmembrane serine–threonine kinase receptors known as the type I and type II receptors. TGF-β1 can bind to its type I receptors and form a heteromeric complex with the type II receptor which, in turn, activates intracellular Smad transcription factors to mediate downstream signaling events (Schmierer and Hill 2007; Penn and others 2012), and then introduces the above complex into the nucleus, where it regulates its target gene expression (Chen and others 2014). In the respiratory system, the expression of TGF-β1 and Smad3-dependent signaling pathway were markedly activated in rats with sepsis-induced acute lung injury (Xu and others 2013). Other studies suggested that downregulation of TGF-β1 and cysteinyl aspartate-specific protease (caspase)-3 signaling pathways might improve survivability in septic rats (Maitra and others 2005a, 2005b). Moreover, the study in vitro showed that CD4+CD25+ Tregs would mediate suppression of T-cell function through cell surface presentation of TGF-β to TGF-βR on target cells, suggesting that TGF-β was crucial in the immunosuppressive response induced by CD4+CD25+ Tregs (Nakamura and others 2001).
However, it is not clear whether apoptosis of CD4+CD25− T cells is associated with the activation of CD4+CD25+ Tregs in the pathogenesis of sepsis, as well as the involvement of TGF-β1 signaling in this process. There are different molecules and pathways involved in the T-lymphocyte apoptosis; however, the present study is performed to determine whether CD4+CD25+ Tregs promote the apoptosis of CD4+CD25− T cells partly through the TGF-β1 signaling pathway in sepsis.
Materials and Methods
Ethics statement
All experimental manipulations were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Scientific Investigation Board of the Chinese PLA General Hospital (No. SYXK2012-0014), Beijing, China.
Medium and reagents
Thiazolyl blue (MTT) and Triton X-100 were purchased from Sigma. RPMI 1640, fetal calf serum (FCS), glutamine, penicillin, streptomycin, and HEPES were purchased from TianRunShanda Biotech Co. Ltd. Antibodies used for flow cytometry analysis, including fluorescein isothiocyanate (FITC)-conjugated anti-mouse Foxp3, FITC-conjugated anti-mouse cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), and anti-mouse IgG1-FITC, were purchased from eBioscience, Annexin V-FITC was purchased from BD, allophycocyanin (APC)-conjugated anti-mouse TGFβ1m+ was purchased from Sigma, and mouse IgG2b-APC was purchased from Santa Cruz Biotechnology. PC-61 and rat immunoglobulin G1 (HRPN) were purchased from BioXCell. Anti-TGF-β1 was purchased from R&D System, Inc. The total RNA isolation system and reverse transcription system were purchased from Invitrogen and Promega. Rabbit anti-mouse Smad2/3 and phosphorylation (P)-Smad2/3 monoclonal antibodies were purchased from Cell Signaling. Rat anti-mouse Bcl-2 and the Bim antibody were purchased from Santa Cruz Biotechnology. The Amersham ECL™ advance Western Blotting Detection Kit was purchased from Amersham Pharmacia Biotech. The CD4+CD25+ Regulatory T-Cell Isolation Kit was purchased from Miltenyi Biotec GmbH.
Animal cecal ligation and puncture model
Male BALB/C mice used in our experiments (weight range 18–22 g) were provided by the Institute of Laboratory Animals Sciences, Chinese Academy of Medical Sciences. All animals were housed in separate cages in a temperature-controlled room with a 12-h light/12-h dark cycle. Polymicrobial sepsis was induced by the cecal ligation and puncture (CLP) procedure described by Wichterman and others (1980). Briefly, after being anesthetized, the mice were placed in the supine position and their abdomens were shaved. An abdominal midline incision of 1 cm was made to expose the cecum. According to Rittirsch and others (2009), the cecum was ligated at the middle and punctured twice with a 21-gauge (0.723-mm) needle to induce a moderate severity sepsis. Sham-operated mice underwent the same laparotomy procedure with the exception of the ligation and perforation (Huang and others 2014).
Experimental design
One hundred eighty mice were used for both in vivo and in vitro experiments, respectively. In the in vivo study, 100 mice were randomly divided into 5 groups as follows: normal control group, sham group, CLP group, CLP with PC61 treatment group, and CLP with HRPN treatment group, and each of these groups consisted of 20 mice. PC61 (the specific antagonist for Tregs) or HRPN (rat immunoglobulin G1) at a dose of 0.1 mg was intraperitoneally injected to mice 3 days before CLP operation, respectively, in the CLP+ PC61 and CLP+HRPN groups. In the in vitro study, 80 mice were randomly divided into 4 groups, namely the normal control group (20 mice), sham group (20 mice), CLP-24-h group (20 mice), and CLP-48-h group (20 mice). Subsequently, these groups were further divided into 2 subgroups, were treated with or without the anti-TGF-β antibody, and finally, CD4+CD25+ Tregs were isolated in all mice. Animals of all groups were sacrificed at 48 h after CLP, and blood as well as spleen samples were harvested and stored immediately at −70°C for measurement of organ dysfunction-related variables. All spleen samples were used to procure CD4+CD25+ Tregs and CD4+CD25− T cells.
Isolation of splenic CD4+CD25+ Tregs
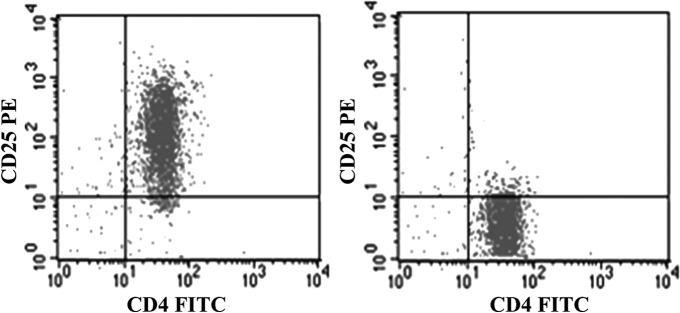
Spleens were obtained from healthy BALB/c mice and they were teased in 5 mL RPMI 1640. Mononuclear cells were separated using the Ficoll-Paque density gradient centrifugation, and CD4+CD25+ Tregs were then isolated from the mononuclear cells using the Mouse CD4+CD25+ Regulatory T-Cell Isolation Kit and a MiniMACS™ separator with a positive selection MS/LD column according to the manufacturer's instructions. The purity of isolated CD4+CD25+ Tregs was greater than 95% as assessed by flow cytometric analysis (Fig. 1), and the viability of CD4+CD25+ Tregs was about 98% as determined directly after the purification process by means of trypan blue exclusion.
FIG. 1.
The purity analysis of CD4+CD25+ Tregs and CD4+CD25− T cells.
Flow cytometric analysis
For flow cytometric characterization of surface CTLA-4, TGFβ1m+, and Foxp3, cultured CD4+CD25+ Tregs/CD4+ T cells (106) were prepared, and the isotype control was designed. One milliliter of freshly prepared fixation/permeabilization working solution was added to each sample and they were incubated with FITC-conjugated IgG specific for CTLA-4 as well as Foxp3/CD4, APC-conjugated IgG for TGFβ1m+, and FITC-conjugated IgG1 at 4°C for 1 h in the dark, and then followed by washing twice. Finally, cells were fixed in 1% formaldehyde/phosphate-buffered saline (PBS) and analyzed by FACSCalibur using CellQuest software (BD Biosciences). In addition, the apoptotic rate of CD4+CD25− T cells was measured by flow cytometry, 5 μL of freshly prepared 7-AAD and phycoerythrin Annexin V was added to each sample, and the negative control was designed. The correlation between the expression of TGFβ1m+ and the apoptotic rate of CD4+CD25− T cells was analyzed by statistical software.
In addition, flow cytometric analysis was performed to detect changes in mitochondrial membrane potential. An increased ratio of green fluorescent cells indicates mitochondrial damage. T cells were centrifuged, resuspended in PBS containing 0.5% FCS, incubated with DiOC6 in an incubator (37°C, 5% CO2) for 30 min, and then followed by washing twice. After being washed, cells were incubated with propidium iodide (1 mg/mL) at room temperature for 15 min in the dark, and finally, the results were obtained by flow cytometry.
Extraction of total RNA and reverse transcription-polymerase chain reaction
Total RNA was extracted from CD4+CD25+ Tregs (2×106) using TRIzol Kits as described previously (Zhang and others 2011; Onyilagha and others 2014). The concentration of purified total RNA was determined spectrophotometrically at 260 nm. TGF-β1 mRNA expression in cells was quantified by SYBR Green 2-step, real-time reverse transcription-polymerase chain reaction (RT-PCR). After removal of potentially contaminating DNA with DNase I, 1 μg of total RNA from each sample was used for RT with an oligo-dT and a SuperScript II to generate first-strand cDNA. The mRNA level of β-actin was also designed as an internal control for each sample. Primers for TGF-β1 were forward 5′-AACAATTCCTGGCGTTACCTT-3′ and reverse 5′-GAATCGAAAGCCCTGTA TTCC-3′. For β-actin, we used the following primers: forward 5′-ATTGGC AATGAGCGGTTCCG-3′ and reverse 5′-AGGGCAGTGATCTCCTTCTG-3′. The thermal cycling conditions were as follows: 94°C for 5 min, followed by 35 cycles of denaturation, annealing, and amplification (94°C for 30 s, 57°C for 45 s, 72°C for 1 min), and a final extension period of 7 min at 72°C. All samples were run in quadruplicate.
Western blot and confocal microscopy analysis
Western blot was performed to determine the expression of Smad2/Smad3, P-Smad2/Smad3, and Bcl-2 superfamily members of Bcl-2/Bim in CD4+CD25− T cell. The total protein concentration of mouse samples was measured using the Bicinchoninic Acid Protein Assay Kit (Pulilai Co.). Fifty micrograms of total proteins/sample was loaded on 10% Tris-HCl–sodium dodecyl sulfate–polyacrylamide gel, and the products were electro transferred to an Immobilon polyvinylidene difluoride membrane. After blocking with 10% skim milk overnight at 4°C, the membrane was incubated for 6 h at room temperature with anti-Smad2/Smad3 Ab or anti-P-Smad2/Smad3 Ab at a dilution of 1:500, followed by peroxidase-labeled affinity secondary Ab at a dilution of 1:5,000 for 1 h at room temperature. After 3 washings, the membrane was determined by using the ECL plus chemiluminescence kit.
CD4+CD25− T cell was activated for 48 h, washed with PBS thrice, and fixed with 4% paraformaldehyde in PBS for 20 min and permeabilized with 0.02% Triton X-100 for 20 min at room temperature. Sections were preblocked with 1% bovine serum albumin in PBS for 30 min and stained with anti-Cytochrome C Ab (1:10) for 1 h. After being washed in PBS thrice, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) for 5 min. Images were observed with a laser scanning confocal microscope (Leica). In addition, activities of caspase-3, caspase-8, as well as caspase-9 were measured by the chemical colorimetric assay according to the protocols provided by the manufacturer (RD Systems).
Statistical analysis
Data are represented as mean±standard deviation. Data sets were examined by 1-way ANOVA, and individual group means were then compared with Student's paired t test. Simple linear regression was used to determine correlation coefficients. All statistical tests were 2 sided, and a P value of 0.05 or less was considered to indicate statistical significance.
Results
Changes in CTLA-4, Foxp3, and TGFβ1m+ expressions after CLP induced sepsis
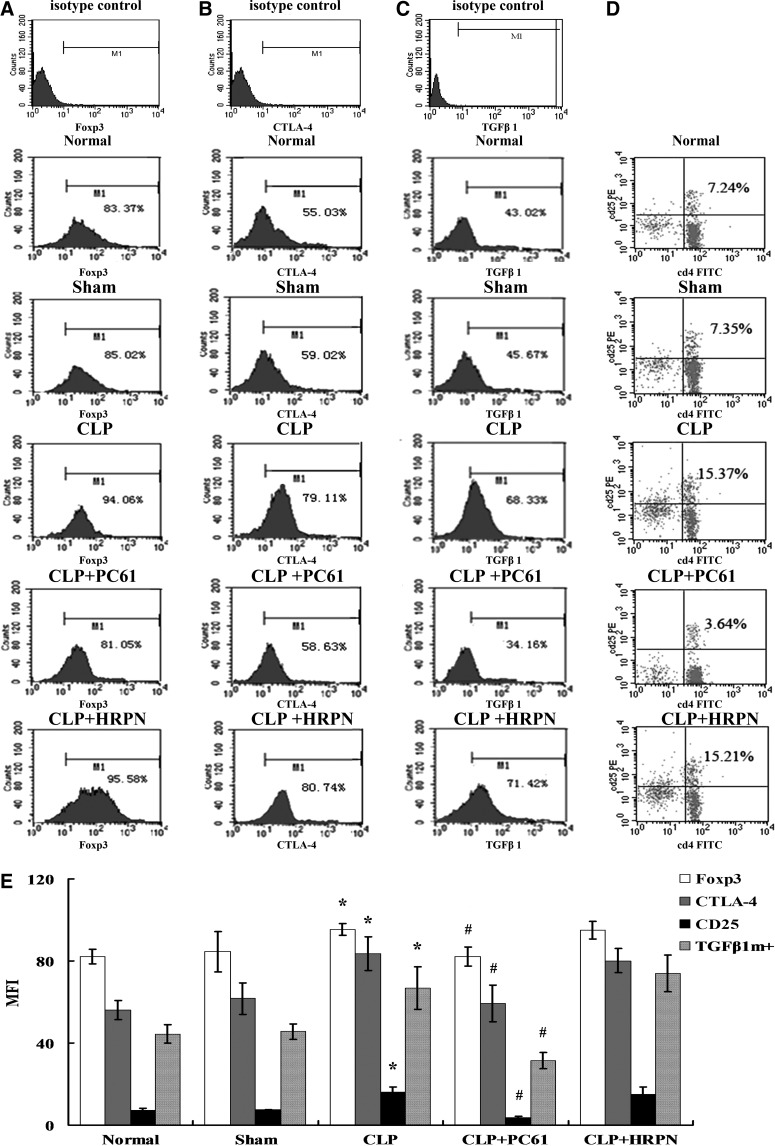
To investigate the effect of PC61 on splenic CD4+CD25+ Tregs, these cells were analyzed at designated time points in animals with normal, sham injury, CLP, CLP with PC61 treatment, and CLP with HRPN treatment, respectively. As shown in Fig. 2, expressions of Foxp3 and CTLA-4 in the CLP group were strongly enhanced in splenic CD4+CD25+ Tregs, while they were found to be at the normal level in the CLP+PC61 group (P<0.05 or P<0.01). Similarly, the expression of TGFβ1m+ on CD4+CD25+ Tregs was significantly intensified in the CLP group, but it was significantly lowered in the CLP+PC61 group, and it was even lower compared with the normal controls and sham group (P<0.05). The change in proportion of CD25 in CD4+ T cells was consistent with the expression level of TGFβ1m+ and lower compared with the sham group and the CLP+PC61 group (P<0.05).
FIG. 2.
Changes in phenotype and percentage of CD4+CD25+ Tregs (n=5, in each group). (A) Representative flow cytometric analysis of forkhead/winged helix transcription factor p3 (Foxp3) expression on CD4+CD25+ Tregs from normal, sham, cecal ligation and puncture (CLP), CLP+PC61, and CLP+rat immunoglobulin G1 (HRPN) groups. (B) Representative flow cytometric analysis of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) expression on CD4+CD25+ Tregs. (C) Representative flow cytometric analysis of transforming growth factor (TGF)-β1 expression on CD4+CD25+ Tregs. (D) Representative flow cytometric analysis and percentages for the marker of CD25 in CD4+CD25+ Tregs. (E) In the CLP group, levels of CD25, CTLA-4, Foxp3, and TGFβ1m+ on splenic CD4+CD25+ Tregs were strongly enhanced compared with those in the sham-injured group. Treatment with PC61 could markedly inhibit the expression of CD25, CTLA-4, Foxp3, and TGFβ1m+. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group.
Gene expressions of TGF-β1 in CD4+CD25+ Tregs following sepsis
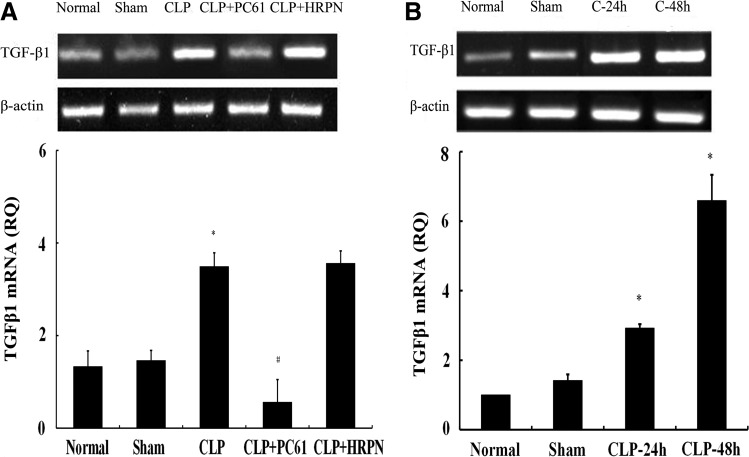
To determine the gene expression of TGF-β1, total RNA was extracted from CD4+CD25+ Tregs followed by RT-PCR. In the in vivo and in vitro experiments, changes in the mRNA level of TGF-β1 in both the normal group and sham group showed no statistically significant difference, while it was obviously upregulated (P<0.01) in the CLP group. In the in vivo study, level of TGF-β1 mRNA in CLP+PC61 group was lower than that in control group, sham group, and CLP group, respectively (P<0.01; Fig. 3A). In the in vitro study, mRNA level of TGF-β1 in the CLP 24-h or CLP 48-h group was markedly increased (P<0.01; Fig. 3B).
FIG. 3.
The mRNA expression of TGF-β1 in CD4+CD25+ Tregs from BALB/c mice. (A) In vivo, TGF-β1 mRNA expression shown by reverse transcription–polymerase chain reaction (RT-PCR) analysis in CD4+CD25+ Tregs was significantly upregulated after polymicrobial sepsis and it could be downregulated using PC61. (B) In vitro, mRNA level of TGF-β1 in CLP-24-h or CLP-48-h group was markedly increased. Expression of β-actin served as an internal control. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group.
The correlation between expression of TGF-β1 and apoptosis of T cells
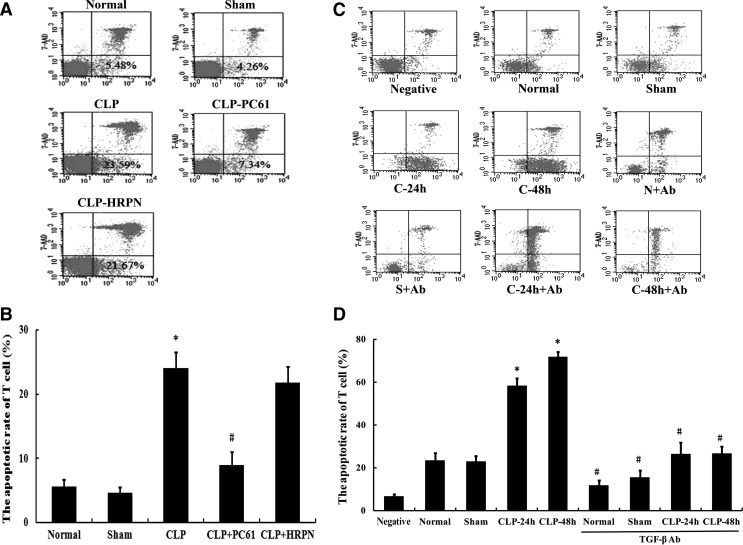
To evaluate the correlation between expression of TGF-β1 and apoptosis of CD4+CD25− T cells, the apoptosis rate of T cells was determined with flow cytometry at 48 h after CLP induced sepsis. As shown in Fig. 4, compared with the CLP group, treatment with PC61 could significantly decrease the apoptotic rate of CD4+CD25− T cells after sepsis (P<0.01). In the in vitro study, treatment with the anti-TGF-β antibody showed a similar effect on apoptosis of CD4+CD25− T cells. Moreover, TGF-β1 expression was significantly positively correlated with the apoptosis rate of CD4+CD25− T cells (Pearson correlation coefficient r=0.791, P<0.01; Table 1).
FIG. 4.
The apoptotic rate of CD4+CD25− T cells by flow cytometric analysis. (A) Using flow cytometric technique, the apoptotic rate of CD4+CD25− T cells was measured in normal, sham, CLP, CLP+PC61, and CLP+HRPN groups. (B) The apoptosis of CD4+CD25− T cells after sepsis was reduced after treatment with PC61. (C) Representative flow cytometric analysis for the apoptotic rate of CD4+CD25− T cells in negative control, normal, sham, CLP-24 h, CLP-48 h, normal+anti-TGF-β antibody, sham+anti-TGF-β antibody, CLP-24 h+anti-TGF-β antibody, and CLP-48 h+anti-TGF-β antibody groups. (D) Markedly increased apoptotic rate of CD4+CD25− T cells was found in the CLP group compared with the sham-injured group, which was blocked by treatment with anti-TGF-β antibody. Statistical significance: *P<0.05 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group, or anti-TGF-β antibody group versus anti-TGF-β antibody treatment void group.
Table 1.
Correlation Analysis Between the Expression of TGFβ1m+ on CD4+CD25+ Tregs and the Apoptotic Rate of T Cells
| Correlation | |
|---|---|
| TGFβ1m+ expressive rate (%) | 52.45±17.17 |
| T-cell apoptotic rate (%) | 12.96±8.84 |
| Pearson correlation coefficient r | 0.791 |
| P | 0.000 |
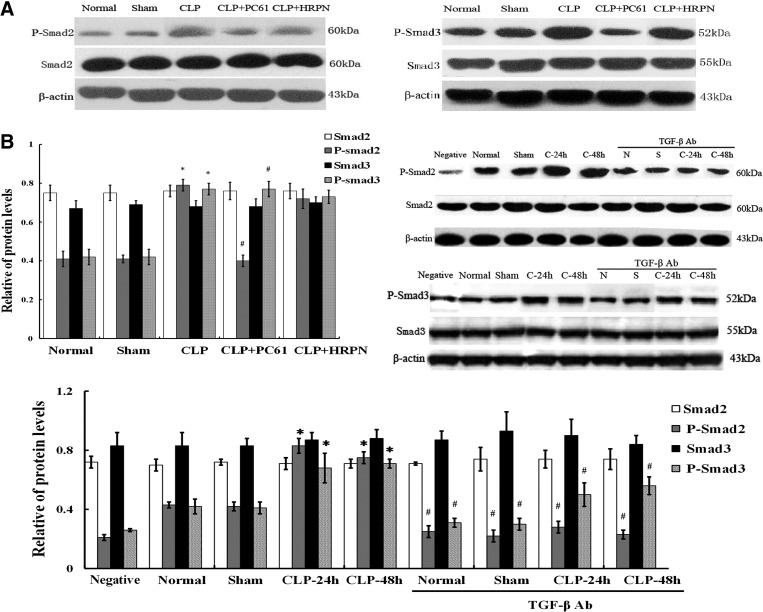
Levels of Smad2/Smad3 and P-Smad2/P-Smad3 in T cells after polymicrobial sepsis
TGF-β1 signaling was regulated through downstream signal transducers Smad2 and Smad3, which can be phosphorylated and then activated. As shown in Fig. 5, after CLP induced sepsis, the expression of total Smad2/Smad3 showed no significant differences in CD4+CD25− T cells among various groups, whereas P-Smad2/P-Smad3 expressions were significantly higher than that in the sham group (all P<0.01). Treatment with PC61 or anti-TGF-β antibody significantly inhibited expression levels of P-Smad2/P-Smad3 (all P<0.01), indicating that the TGF-β1 signaling pathway was activated after septic challenge.
FIG. 5.
The protein expression of Smad2/Smad and P-Smad2/P-Smad3 in the CD4+CD25− T cells after polymicrobial sepsis. (A) Levels of Smad2/Smad3, P-Smad2/Smad3, and Bcl-2 superfamily members of Bcl-2/Bim in CD4+CD25− T cells were determined by Western blot analysis. (B) There were no significant differences of Smad2/Smad3 expression in CD4+CD25− T cells among various groups; the expressions of P-smad2/P-Smad3 in the CLP group were significantly higher than that in sham group, while P-smad2/P-Smad3 expression in CLP+(PC61 or anti-TGF-β antibody) group was significantly lower than that in the CLP group (P<0.01). Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group, or anti-TGF-β antibody group versus anti-TGF-β antibody treatment void group.
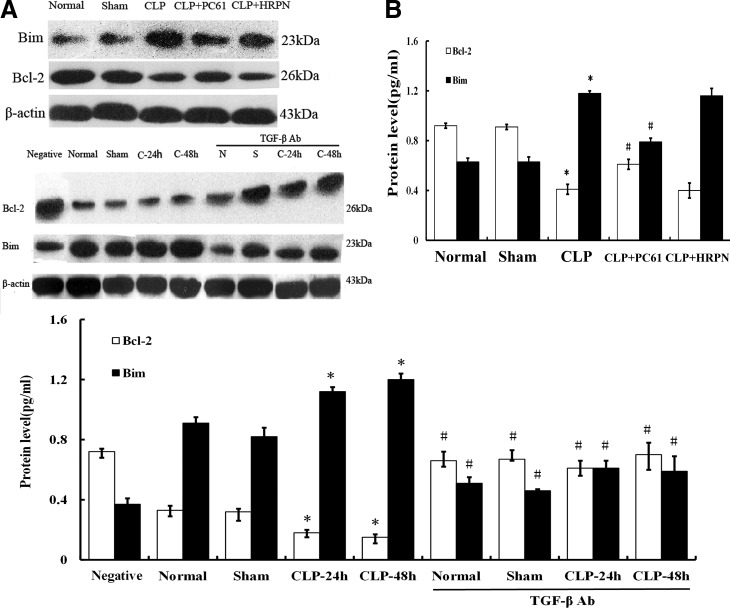
Analysis of apoptotic protein Bcl-2 and Bim in T cells after sepsis
It is well known that the intrinsic pathways of apoptosis are regulated by members of the Bcl-2 family, which is composed of proapoptotic proteins (Bim, etc.) and antiapoptotic proteins (Bcl-2, etc.). In the present study, expression of antiapoptotic protein Bcl-2 in CD4+CD25− T cells in the CLP group was shown to have decreased significantly (P<0.01) compared with the sham group, while the expression of proapoptotic protein Bim increased (all P<0.01; Fig. 6). With the treatment of PC61 or anti-TGF-β antibody, the expression of the antiapoptotic protein Bcl-2 in splenic T cells after sepsis was significantly lower compared with that of the sham group (P<0.01), whereas it was markedly higher than that in the CLP group (P<0.01; Fig. 6). Conversely, proapoptotic protein Bim expressions in the CLP+(PC61/TGF-β antibody) groups were lower than that in the CLP group after sepsis (all P<0.01).
FIG. 6.
Protein levels of Bcl-2/Bim in CD4+CD25− T cells after CLP induced sepsis. (A) Using Western blot, Bcl-2 and Bim protein expressions were determined both in vivo and in vitro. (B) Expression of antiapoptotic protein Bcl-2 in CD4+CD25− T cells was significantly decreased after septic challenge (P<0.01), while expression of proapoptotic protein Bim was enhanced (P<0.01). Conversely, Bcl-2 expression was increased and Bim expression was decreased by use of PC61 or anti-TGF-β antibody. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group, or anti-TGF-β antibody group versus anti-TGF-β antibody treatment void group.
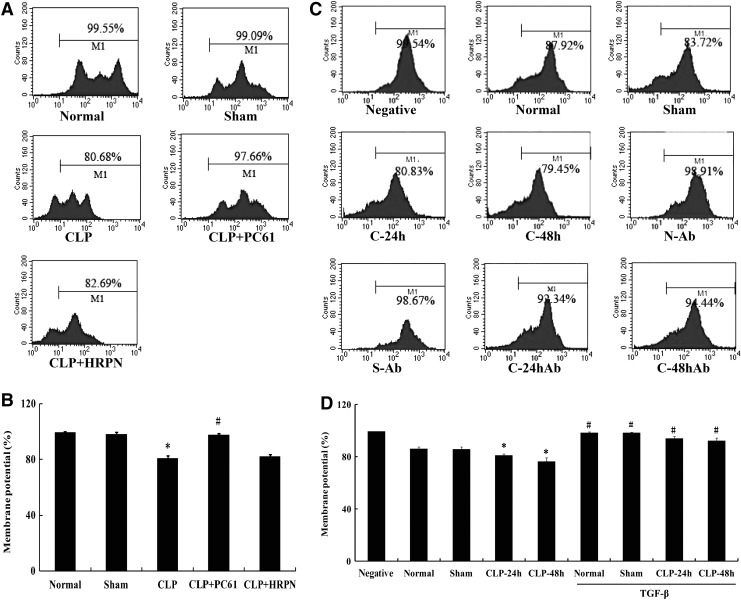
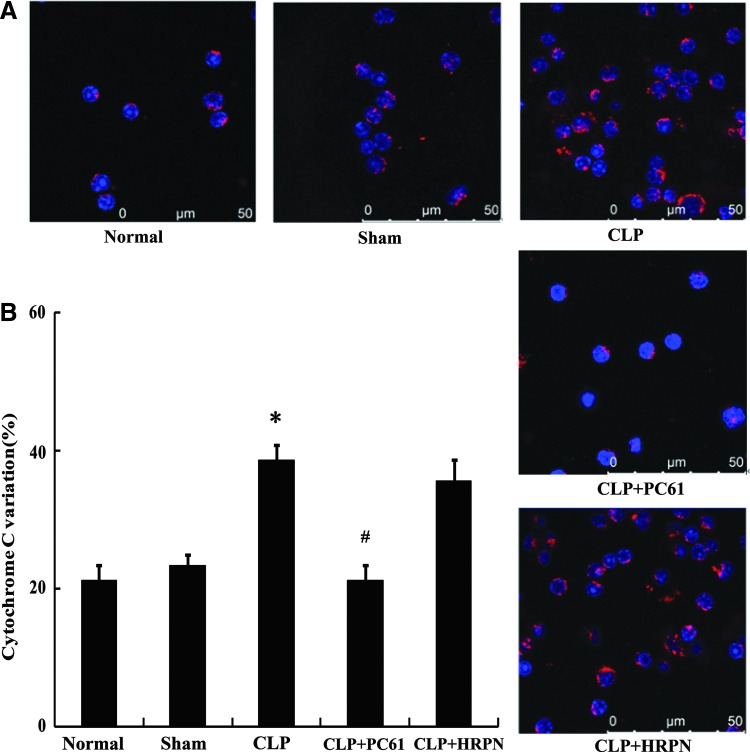
Alterations in cytochrome C and mitochondrial membrane potential in T cells after sepsis
To further elucidate the mechanism concerning the involvement of TGF-β1 in T-lymphocyte apoptosis mediated by CD4+CD25+ Tregs, alterations in both cytochrome C and mitochondrial membrane potential in T cells were analyzed. The mitochondrial membrane potential in CD4+CD25− T cells in the CLP-induced sepsis group was significantly reduced (P<0.01; Fig. 7) compared with that of the sham-injured group, whereas it could be restored by treatment with PC61 or anti-TGF-β antibody (all P<0.01). As shown in Fig. 8, the change trend of cytochrome C was adverse to that of mitochondrial membrane potential, and expression of cytochrome C in the CLP group was markedly higher than that in the sham group (P<0.01). In comparison to the sham group, expressions of cytochrome C in the CLP+(PC61/TGF-β antibody) groups were not statistically significant, but were lower than that in the CLP group (all P<0.01).
FIG. 7.
The alterations of mitochondrial membrane potential in CD4+CD25− T cells. (A) Representative flow cytometric analysis of mitochondrial membrane potential in normal, sham, CLP, CLP+PC61, and CLP+HRPN groups. (B) The activity of mitochondrial membrane potential of splenic T cells was significantly downregulated in the CLP group. Treatment with PC61 completely restored mitochondrial membrane potential activity after sepsis. (C) Representative flow cytometric analysis of mitochondrial membrane potential in negative control, normal, sham, CLP-24 h, CLP-48 h, normal+anti-TGF-β antibody, sham+anti-TGF-β antibody, CLP-24 h+anti-TGF-β antibody, and CLP-48 h+anti-TGF-β antibody groups. (D) The mitochondrial membrane potential activity of T cells was elevated in anti-TGF-β antibody group. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group, or anti-TGF-β antibody group versus anti-TGF-β antibody treatment void group.
FIG. 8.
The change in cytochrome C in CD4+CD25− T cells after sepsis. (A) Representative confocal analysis of cytochrome C in CD4+CD25− T cells from normal, sham, CLP, CLP+PC61, and CLP+HRPN groups. Cytochrome C protein was shown by antibodies directly labeled with phycoerythrin (red). (B) Expression of cytochrome C was significantly enhanced in mice subjected to CLP compared with sham-injured group. Treatment with PC61 markedly inhibited cytochrome C protein expression in CD4+CD25− T cells. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group.
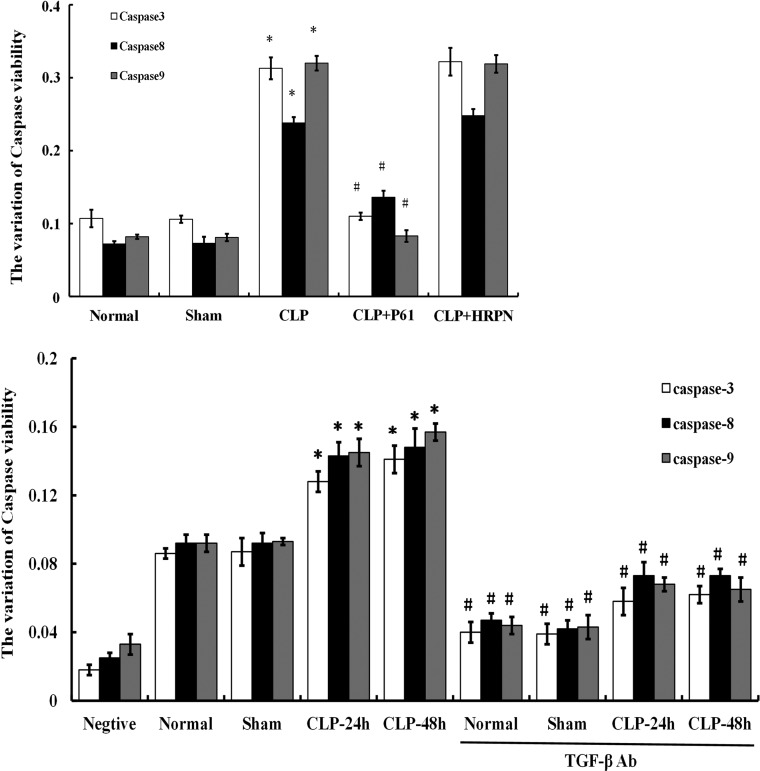
Activities of caspases in T cells after CLP induced sepsis
The major executioners in the apoptotic program are proteases known as caspases. Using the chemical colorimetric assay, we measured the activation of caspase-3, caspase-8, as well as caspase-9 in CD4+CD25− T cells. As shown in Fig. 9, after CLP induced sepsis, activities of caspase-3, caspase-8, and caspase-9 were significantly elevated (P<0.01). Treatment with PC61 or anti-TGF-β antibody could markedly downregulate the activity of caspase-3, caspase-8, and caspase-9 in the CLP group (all P<0.01).
FIG. 9.
The activities of caspases in CD4+CD25− T cells by chemical colorimetric assay. Activities of caspase-3, caspase-8, and caspase-9 in CD4+CD25− T cells in the CLP group were significantly increased compared with that of the sham group. Whereas in CLP+PC61/anti-TGF-β antibody group, activities of caspase-3, caspase-8, and caspase-9 were lower than that in the CLP group. Caspase-8 activity was markedly higher than normal group and sham group, but still lower compared with the CLP group. Statistical significance: *P<0.01 as CLP group versus sham group; #P<0.01 as PC61 group versus CLP group, or anti-TGF-β antibody group versus anti-TGF-β antibody treatment void group.
Discussion
To date, it is known that the vexed sepsis syndrome may lead to both widespread activation as well as dysfunction of the innate immune system (Souza and others 2010). It has been proposed that Tregs play a central role in the maintenance of immune tolerance and immune balance in the peripheral lymphatic system (Luan and others 2014), and studies in many animal models have demonstrated their capacity to inhibit inflammatory pathologies in vivo (Hanschen and others 2012; Luan and others 2014). It is likely that Tregs might also be essential elements in the process of effective immune suppression response to trauma or sepsis, and they induce apoptosis of T lymphocytes (Maizels 2005; Miller, and others 2013). The cell death in the adaptive immune system is beneficial to the host by downregulating the inflammatory response to septic challenge, however, an extensive loss of immune cells may compromise the ability of the host to eliminate the invading pathogens, and it finally leads to septic death. This phenomenon suggests that increased apoptosis in T lymphocytes plays a critical role in the production of an adverse outcome to patients suffering from severe sepsis (Kumar and others 2011; Hotchkiss and others 2013; Jones and Puskarich 2014; Luan and others 2015).
Apoptosis of T cell induced by Tregs was proved to contribute to the immune dysfunction and multiple organ failure observed in septic complications. Nevertheless, the definite signaling mechanism is not clear. TGF-β1, a pleiotropic cytokine secreted by Th2 or Tregs, produces both membrane-bound (TGFβ1m+) and secreted TGF-β1 under certain conditions (Luan and others 2014). Experiments in vitro showed that CD4+CD25+ Tregs could suppress the proliferation of CD4+CD25− T cell through the cell surface TGFβ1m+ in a cell contact-dependent manner (Miller and others 2014). Therefore, we would expect that CD4+CD25+ Tregs might promote CD4+CD25− T-cell apoptosis in the setting of polymicrobial sepsis through TGF-β1 signaling.
Both in experimental and clinical sepsis, CD4+CD25+ Tregs mediate immune suppression through cell–cell contact with surface molecules. Investigations of the mechanisms of Treg function have identified growing expressions of constitutive and high surface molecules, particularly CTLA-4 and TGF-β (Zhang and others 2008), and they correlate with the immunosuppressive properties of Tregs (Olson and others 2012; Deppong and others 2013). In the current study in vivo, it was found that expression levels of CTLA-4, Foxp3, and TGF-β1 were strongly decreased on splenic CD4+CD25+ Tregs treated with PC61 in comparison to Tregs from sham-injured mice. Consistent with what has been reported, the results demonstrated that TGF-β1 expression was related to immunosuppressive function of CD4+CD25+ Tregs after sepsis. Consequently, the relationship between expression of TGF-β1 and apoptosis rate of CD4+CD25− T cell was analyzed in the study with significantly positive correlation, indicating that TGF-β1 appeared to be involved in CD4+CD25+ Treg in promoting apoptosis of T lymphocytes following polymicrobial sepsis.
With this in mind, TGF-β-triggered signals are known to be transduced by Smads, which are known as a family of proteins that serve as substrates for TGF-β receptors, we sought to identify major molecules that may be involved in the TGF-β signaling. In addition, with phosphorylation of Smad2 and Smad3, the phosphorylated intermediate is associated with a co-Smad in the cytoplasm (Yang and others 1999; Gao and others 2006), which moves in turn to the nucleus, where transcriptional regulation occurs through direct DNA binding by the Smad complex (Gaarenstroom and Hill 2014; Zhao and others 2014). In this study, expressions of Smad2/Smad3 and P-Smad2/P-Smad3 in the T cell were determined, and it was shown that P-Smad2/P-Smad3 expression was markedly enhanced in CLP-induced sepsis, while inhibition of TGF-β1 expression in CD4+CD25+ Treg resulted in a decreased P-Smad2/P-Smad3 expression both in vitro and in vivo. The data indicated that the combination of TGF-β1 on the surface of CD4+CD25+ Tregs could activate the downstream signal transducers of TGF-β, including Smad2/Smad3.
Apoptotic cell death can be initiated by a plethora of stimuli that generally feed into 1 of 2 known signaling pathways, including the intrinsic (mitochondrial) pathway and the extrinsic death receptor pathway (Hattori and others 2010). The intrinsic apoptosis pathway is highly dependent on the balance of the Bcl-2 family members; a dominant member of antiapoptotic family, such as Bcl-2 and Bcl-xL, can function as an antiapoptotic protein, whereas Bcl-xS, as well as a related protein called Bim, can promote cell death. Weber and others (2008) found that there was an increase in the proapoptotic protein Bim and a decrease in the level of antiapoptotic molecules (Bcl-2 and Bcl-xL) in the lymphocytes of patients with severe sepsis. Studies using transgenic mice that selectively overexpress Bcl-2 and Bcl-xL in T lymphocytes suggested that both the antiapoptotic molecules could appreciably protect against lymphocyte apoptosis and significantly improve survival in CLP-induced sepsis (Hotchkiss and others 1999; Schwulst and others 2008). In agreement with this finding, our studies in vitro and in vivo also showed decreased antiapoptotic protein Bcl-2 and increased proapoptotic protein Bim expression in T lymphocytes in mice after CLP. However, through treatment with the PC61 or anti-TGF-β antibody, the expression of Bim was obviously downregulated and Bcl-2 expression was significantly upregulated. It was revealed that blockade of TGF-β could markedly protect against T-lymphocyte apoptosis after CLP induced sepsis. Therefore, it was indicated that TGF-β1 could be involved in T-lymphocyte apoptosis through activating the intrinsic cell death signaling pathway in the development of sepsis.
It has been reported that the intrinsic pathway also involves the release of cytochrome C and the activation of caspase-9 (Wen and others 2014; Winter and others 2014). The present study showed that inhibition of TGF-β1 expression in CLP-induced sepsis resulted in a remarkable decrease in cytochrome C. Previous reports have shown that mitochondria sense the metabolic catastrophic signals and commit cells to apoptosis by releasing death-inducing factors such as cytochrome C into the cytosol due to the permeabilization of mitochondrial outer membrane (Yang and others 1997). In our in vivo and in vitro experiments, the release of ctochrome C could result in mitochondrial respiration failure and the loss of mitochondrial functions. In our current study, it was also shown that the mitochondrial membrane potential in CD4+CD25− T cell in the CLP group was enhanced by treatment with PC61 or anti-TGF-β antibody. It has been demonstrated that cytochrome C can activate caspase-9, and activation of caspase-9 initiates the so-called caspase cascade (caspase-3 and other caspases) that induces cell apoptosis (Wurstle and others 2012; Bourguet and others 2014; Floyd and others 2014). In this study, our data revealed that the alteration trend in cytochrome C was adverse to that of mitochondrial membrane potential, and activities of caspase-3, caspase-8, and caspase-9 in the CD4+CD25− T-cell response could be downregulated by treatment with PC61 or anti-TGF-β antibody both in vivo and in vitro. Nevertheless, this study has limitations. Systemic administration of anti-TGF-β antibody does not specifically block Treg-produced TGF-β1 only but it also abrogates dendritic cell- and macrophage-secreted TGF-β1.Therefore, in a further study, the FoxP3YFP-Cre mice crossed with TGFb1flox ex6 mice should be used to confirm that CD4+CD25+ Tregs promote the apoptosis of CD4+CD25− T cells through the TGF-β1 signaling pathway in the setting of sepsis.
In conclusion, our study suggested that CD4+CD25− T-cell apoptosis was associated with the TGF-β1 signal in CD4+CD25+ Tregs. CD4+CD25+ Tregs could activate the downstream signaling molecules, including Smad2/Smad3 in the CD4+CD25− T cells through its surface TGF-β1. Subsequently, Bcl-2 superfamily members would be activated through increasing the expression of proapoptotic protein Bim and reducing the expression of antiapoptotic protein Bcl-2. Then, the apoptotic protein might be freed and translocated to the mitochondria outer membrane, leading to mitochondrial dysfunction, followed by increased cytochrome C release and caspase-3/9 activation and completed by DNA fragmentation. Taken together, these findings further suggest that sepsis-activated CD4+CD25+ Tregs might promote the apoptosis of CD4+CD25− T cells, and signals mediated by TGF-β1 might be partly involved in this process, thereby contributing to the development of the immunosuppressive state following septic complications.
Acknowledgments
This study was supported, in part, by grants from the National Natural Science Foundation (Nos. 81130035, 81372054, 81272090, 81121004), the National Basic Research Program of China (No. 2012CB518102), the Medical Research Foundation of Chinese PLA (Nos. AWS11J008, BWS12J050), and the Project of State Key Laboratory of Trauma, Burns, and Combined Injury (SKLKF201304).
Author Disclosure Statement
The authors have no financial conflicts of interest.
References
- Bourguet CB, Boulay PL, Claing A, Lubell WD. 2014. Design and synthesis of novel azapeptide activators of apoptosis mediated by caspase-9 in cancer cells. Bioorg Med Chem Lett 24(15):3361–3365 [DOI] [PubMed] [Google Scholar]
- Caraci F, Battaglia G, Busceti C, Biagioni F, Mastroiacovo F, Bosco P, Drago F, Nicoletti F, Sortino MA, Copani A. 2008. TGF-beta 1 protects against Abeta-neurotoxicity via the phosphatidylinositol-3-kinase pathway. Neurobiol Dis 30(2):234–242 [DOI] [PubMed] [Google Scholar]
- Chen C, Lei W, Chen W, Zhong J, Gao X, Li B, Wang H, Huang C. 2014. Serum TGF-beta1 and SMAD3 levels are closely associated with coronary artery disease. BMC Cardiovasc Disord 14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppong CM, Bricker TL, Rannals BD, Van Rooijen N, Hsieh CS, Green JM. 2013. CTLA4Ig inhibits effector T cells through regulatory T cells and TGF-beta. J Immunol 191(6):3082–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd DH, Zhang Y, Dey BK, et al. . 2014. Novel anti-apoptotic microRNAs 582-5p and 363 promote human glioblastoma stem cell survival via direct inhibition of caspase 3, caspase 9, and Bim. PLoS One 9(5):e96239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarenstroom T, Hill CS. 2014. TGF-beta signaling to chromatin: how Smads regulate transcription during self-renewal and differentiation. Semin Cell Dev Biol 32:107–118 [DOI] [PubMed] [Google Scholar]
- Gao R, Lu Y, Xin YP, Zhang XH, Wang J, Li YP. 2006. The effects of different immunosuppressants on chronic allograft nephropathy by affecting the transforming growth factor-beta and Smads signal pathways. Transplant Proc 38(7):2154–2157 [DOI] [PubMed] [Google Scholar]
- Goel SA, Guo LW, Shi XD, Kundi R, Sovinski G, Seedial S, Liu B, Kent KC. 2013. Preferential secretion of collagen type 3 versus type 1 from adventitial fibroblasts stimulated by TGF-beta/Smad3-treated medial smooth muscle cells. Cell Signal 25(4):955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanschen M, Tajima G, O'Leary F, Hoang K, Ikeda K, Lederer JA. 2012. Phospho-flow cytometry based analysis of differences in T cell receptor signaling between regulatory T cells and CD4+ T cells. J Immunol Methods 376(1–2):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Takano K, Teramae H, Yamamoto S, Yokoo H, Matsuda N. 2010. Insights into sepsis therapeutic design based on the apoptotic death pathway. J Pharmacol Sci 114(4):354–365 [DOI] [PubMed] [Google Scholar]
- Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172(5):3157–3166 [DOI] [PubMed] [Google Scholar]
- Horbelt D, Denkis A, Knaus P. 2012. A portrait of transforming growth factor beta superfamily signalling: background matters. Int J Biochem Cell Biol 44(3):469–474 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, Payen D. 2013. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13(12):862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Swanson PE, Knudson CM, et al. . 1999. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol 162(7):4148–4156 [PubMed] [Google Scholar]
- Huang M, Liu C, Hu Y, Wang P, Ding M. 2014. Gamma-secretase inhibitor DAPT prevents neuronal death and memory impairment in sepsis associated encephalopathy in septic rats. Chin Med J (Engl) 127(5):924–928 [PubMed] [Google Scholar]
- Jones AE, Puskarich MA. 2014. The Surviving Sepsis Campaign guidelines 2012: update for emergency physicians. Ann Emerg Med 63(1):35–47 [DOI] [PubMed] [Google Scholar]
- Kumar G, Kumar N, Taneja A, et al. . 2011. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 140(5):1223–1231 [DOI] [PubMed] [Google Scholar]
- Luan YY, Dong N, Xie M, Xiao XZ, Yao YM. 2014. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interferon Cytokine Res 34(1):2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan YY, Yao YM, Sheng ZY. 2012. Update on the immunological pathway of negative regulation in acute insults and sepsis. J Interferon Cytokine Res 32:288–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan YY, Yao YM, Xiao XZ, Sheng ZY. 2015. Insights into the apoptotic death of immune cells in sepsis. J Interferon Cytokine Res 35(1):17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra SR, Bhaduri S, El-Maghrabi MR, Shapiro MJ. 2005a. Inhibition of matrix metalloproteinase on hepatic transforming growth factor beta1 and caspase-3 activation in hemorrhage. Acad Emerg Med 12(9):797–803 [DOI] [PubMed] [Google Scholar]
- Maitra SR, Shapiro MJ, Bhaduri S, El-Maghrabi MR. 2005b. Effect of chemically modified tetracycline on transforming growth factor-beta1 and caspase-3 activation in liver of septic rats. Crit Care Med 33(7):1577–1581 [DOI] [PubMed] [Google Scholar]
- Maizels RM. 2005. Infections and allergy—helminths, hygiene and host immune regulation. Curr Opin Immunol 17(6):656–661 [DOI] [PubMed] [Google Scholar]
- Miller MM, Fogle JE, Ross P, Tompkins MB. 2013. Feline glycoprotein A repetitions predominant anchors transforming growth factor beta on the surface of activated CD4(+)CD25(+) regulatory T cells and mediates AIDS lentivirus-induced T cell immunodeficiency. AIDS Res Hum Retroviruses 29(4):641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Petty CS, Tompkins MB, Fogle JE. 2014. CD4+CD25+ T regulatory cells activated during feline immunodeficiency virus infection convert T helper cells into functional suppressors through a membrane-bound TGFbeta/GARP-mediated mechanism. Virol J 11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. 2001. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194(5):629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. 2012. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol 189(12):5590–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyilagha C, Okwor I, Kuriakose S, Singh R, Uzonna J. 2014. Low-dose intradermal infection with trypanosoma congolense leads to expansion of regulatory T cells and enhanced susceptibility to reinfection. Infect Immun 82(3):1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn JW, Grobbelaar AO, Rolfe KJ. 2012. The role of the TGF-beta family in wound healing, burns and scarring: a review. Int J Burns Trauma 2(1):18–28 [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4(1):31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmierer B, Hill CS. 2007. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol 8(12):970–982 [DOI] [PubMed] [Google Scholar]
- Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS. 2008. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock 30(2):127–134 [DOI] [PubMed] [Google Scholar]
- Souza HP, Lima-Salgado T, da Cruz Neto LM. 2010. Toll-like receptors in sepsis: a tale still being told. Endocr Metab Immune Disord Drug Targets 10(3):285–291 [DOI] [PubMed] [Google Scholar]
- Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. 2006. Human CD4+CD25+ regulatory T lymphocytes inhibit lipopolysaccharide-induced monocyte survival through a Fas/Fas ligand-dependent mechanism. J Immunol 177(9):6540–6547 [DOI] [PubMed] [Google Scholar]
- Weber SU, Schewe JC, Lehmann LE, Müller S, Book M, Klaschik S, Hoeft A, Stüber F. 2008. Induction of Bim and Bid gene expression during accelerated apoptosis in severe sepsis. Crit Care 12(5):R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Zhang X, Cai J, Yang PH. 2014. A novel strategy for real-time and in situ detection of cytochrome c and caspase-9 in Hela cells during apoptosis. Analyst 139(10):2499–2506 [DOI] [PubMed] [Google Scholar]
- Wichterman KA, Baue AE, Chaudry IH. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J Surg Res 29(2):189–201 [DOI] [PubMed] [Google Scholar]
- Winter E, Dal Pizzol C, Filippin-Monteiro FB, Brondani P, Silva AM, Silva AH, Bonacorso HG, Martins MA, Zanatta N, Creczynski-Pasa TB. 2014. Antitumoral activity of a trichloromethyl pyrimidine analogue: molecular cross-talk between intrinsic and extrinsic apoptosis. Chem Res Toxicol 27(6):1040–1049 [DOI] [PubMed] [Google Scholar]
- Wurstle ML, Laussmann MA, Rehm M. 2012. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318(11):1213–1220 [DOI] [PubMed] [Google Scholar]
- Xu F, Lin SH, Yang YZ, Guo R, Cao J, Liu Q. 2013. The effect of curcumin on sepsis-induced acute lung injury in a rat model through the inhibition of the TGF-beta1/SMAD3 pathway. Int Immunopharmacol 16(1):1–6 [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275(5303):1129–1132 [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 18(5):1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Qian FH, Liu H, Zhou LF, Huang M, Zhang XL, Yin KS. 2008. Expression of surface markers on peripheral CD4+CD25high T cells in patients with atopic asthma: role of inhaled corticosteroid. Chin Med J (Engl) 121(3):205–212 [PubMed] [Google Scholar]
- Zhang Y, Yao YM, Huang LF, Dong N, Yu Y, Sheng ZY. 2011. The potential effect and mechanism of high-mobility group box 1 protein on regulatory T cell-mediated immunosuppression. J Interferon Cytokine Res 31(2):249–257 [DOI] [PubMed] [Google Scholar]
- Zhao TT, Zhang HJ1, Lu XG, Huang XR, Zhang WK, Wang H, Lan HY, Li P. 2014. Chaihuang-Yishen granule inhibits diabetic kidney disease in rats through blocking TGF-beta/Smad3 signaling. PLoS One 9(3):e90807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang F, Lou J, Fan X, Bao R, Wu Y, Chen F, Deng X, Li J. 2012. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One 7(5):e35523. [DOI] [PMC free article] [PubMed] [Google Scholar]