Abstract
Human induced pluripotent stem cell (hiPSC) technologies are powerful tools for modeling development and disease, drug screening, and regenerative medicine. Faithful gene targeting in hiPSCs greatly facilitates these applications. We have developed a fast and precise clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) technology-based method and obtained fluorescent protein and antibiotic resistance dual knockin reporters in hiPSC lines for neurogenin2 (NEUROG2), an important proneural transcription factor. Gene targeting efficiency was greatly improved in CRISPR/Cas9-mediated homology directed recombination (∼33% correctly targeted clones) compared to conventional targeting protocol (∼3%) at the same locus. No off-target events were detected. In addition, taking the advantage of the versatile applications of the CRISPR/Cas9 system, we designed transactivation components to transiently induce NEUROG2 expression, which helps identify transcription factor binding sites and trans-regulation regions of human NEUROG2. The strategy of using CRISPR/Cas9 genome editing coupled with fluorescence-activated cell sorting of neural progenitor cells in a knockin lineage hiPSC reporter platform might be broadly applicable in other stem cell derivatives and subpopulations.
Introduction
The generation of human induced pluripotent stem cells (hiPSCs) [1–3] has revolutionized the stem cell field in the past 9 years. hiPSCs have rapidly evolved to become a powerful tool in modeling human development and disease. Currently, hiPSCs, obtained from different somatic cell types via various induction methods, are an indispensable resource in the study of human gene functions and regulations, drug discovery and testing, and regenerative medicine. However, differentiation protocols of hiPSCs toward many somatic cell lineages are often lengthy and not efficient, preventing the full realization of the hiPSC application potential. Lineage specific knockin fluorescence reporters, which recapitulate the expression of important endogenous genes that mark the commitment to certain developmental pathways, will guide and facilitate the optimization of efficient differentiation protocol, allow for direct visualization and tracking of the in vitro and in vivo behavior of hiPSC derivatives, and provide a handle for purification of desired cell populations differentiated from hiPSCs [4–14].
Creation of knockin reporter hiPSC lines by conventional gene targeting, however, is very tedious, especially for genes that are lineage-specific and not expressed at the undifferentiated iPSC stage. Usually targeting efficiency is in the range of 1%–3% [4–14], requiring that over 100–200 hiPSC clones be maintained at the same time. Such hiPSC targeting experiments may take 6–12 months to complete, potentially posing a barrier for high-throughput generation of multigene and multi-reporter knockin hiPSC lines, which are useful tools in lineage tracing and characterization. To overcome this critical bottleneck, we have implemented the highly efficient clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR associated protein 9 (Cas9) system in the creation of hiPSC knockin reporters.
CRISPR/Cas9 system is a genome editing tool that has recently emerged to have dramatically improved the efficiency of genetic engineering in various types of organisms and cell types. Originally found in bacteria and archaea, CRISPR/Cas9 is part of the adaptive immune response against bacteriophage infection and invasion, during which the foreign DNAs from bacteriophages are converted by the bacterial immune system into CRISPR RNA (crRNA). crRNA possesses the specific sequence of the foreign attacker that the host targets to eliminate. crRNA works by coupling with another type of RNA in the bacteria, tracrRNA (for trans-activating crRNA), which binds to a Cas RNA-guided type II DNA endonuclease. The association of crRNA and tracrRNA brings Cas endonuclease to the bacteriophage's genome where a protospacer adjacent motif (PAM) and the sequence identical or similar to crRNA can be found. The Cas endonuclease (multiple types of Cas endonucleases have been identified, however, Cas9 derived from Streptococcus pyogenes has been most commonly employed in genome editing so far) then induces DNA double-stranded breaks (DSBs) at, or adjacent to, the PAM sequence, aiming to disintegrate the foreign genome. The increase of the frequency of DSB at predetermined sites allows a greater opportunity for the occurrence of nonhomologous end joining (NHEJ), or if exogenous targeting vectors are present, introduction of transgene sequences (eg, targeting vectors or tags) via homologous directed recombination (HDR). As the CRISPR/Cas9 system was developed to become an important genome engineering tool in the laboratory, crRNA and tracrRNA were assembled into a single guide RNA (sgRNA) [15] and were applied to a list of broad applications including generation of knockout mice of multiple genes at one-step and targeted gene corrections [16–23].
Although work on CRISPR/Cas9-mediated genome editing has exploded in the past 2 years, detailed reports on generation, verification, and characterization of neural lineage-specific knockin reporter hiPSC lines with CRISPR/Cas9 are scarce. This might be partially due to the observation that NHEJ tends to occur at a much higher rate than HDR, even if meticulously designed targeting vectors are present in abundance [24,25]. To overcome these hurdles, here, using a combinatorial strategy of CRISPR/Cas9 system and the hiPSC platform, we optimized targeting efficiency and generated hiPSC dual knockin reporter clones for the gene neurogenin2 (NEUROG2), an important proneural gene in central nervous system (CNS) development. The highly efficient and precise knockin strategy allows us to monitor NEUROG2 expression along the time course of neural differentiation by directly visualizing the expression of fluorescent protein mCherry, which faithfully recapitulates the expression of endogenous NEUROG2. Moreover, the truthful substitute expression of both mCherry and the hygromycin resistance offers flexibility in the purification of NEUROG2+ populations by either fluorescence-activated cell sorting (FACS) or drug selection. Further, using these reporter clones, we performed CRISPR/Cas9-mediated transcription activation at the potential promoter region of NEUROG2. These experiments provide an example for the versatile applications of CRISPR/Cas9 system in accelerating the characterization of the fine-tuning of transcriptional regulation in human neurogenesis using hiPSCs.
Materials and Methods
Construction of NEUROG2-IRES-mCherry-IRES-hygromycin knockin vector
The targeting vector is constructed in DH5α using recombineering and multisite gateway as described previously [7,8,26] with the following layout (Fig. 1A): 5′ homology arm-endogenous NEUROG2 genomic fragment-IRES-mCherry-IRES-hygromycin resistance cassette-LoxP-RNA polymerase II promoter driven neomycin resistance cassette-LoxP-3′ homology arm-HSV-TK promoter driven thymidine kinase cassette, where IRES is the internal ribosome entry site. A human bacterial artificial chromosome (BAC) clone containing the NEUROG2 genomic sequence (Clone No. RP11-433J13; Life Technologies) was verified by polymerase chain reaction (PCR) amplification of the NEUROG2 gene. To generate the targeting construct, pStartK (Cat. No. 20346; Addgene) plasmid was used as the template to amplify the fragment outside Gateway compatible cassettes attL1 and attL2. The primers contained two overhangs that were homologous to the flanking sequence of NEUROG2, so that when the PCR product was transformed into the electrocompetent NEUROG2 BAC, full-length NEUROG2 gene and ∼3.0 kb of its upstream and ∼4.6 kb of its downstream sequences were pulled out into pStartK as selected by kanamycin. An IRES-mCherry-IRES-hygromycin resistance cassette (abbreviated as ImCIH) was assembled using a four-way LR reaction of Multisite Gateway approach [27]. Negative selection site HSV-TK6 (Cat. No. 20350; Addgene) was ligated via LR recombination. The final construct was selected with ampicillin and named pWSTK6_Ngn2ImCIH. To identify homologous recombinants, genomic DNA of clones obtained from both positive and negative selection (see Generation of the NEUROG2-IRES-mCherry-IRES-hygromycin knockin reporter line in hiPSC ND2.0) were examined by Southern blot analysis using a nonradioactive digoxigenin detection protocol (Dig-high prime DNA labeling and detection kit; Roche) as described previously [26] using a 735 bp 5′ flanking probe and a 567 bp 3′ flanking probe (Fig. 1C and Supplementary Fig. S1 and Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). In addition, positive clones of NEUROG2-mCherry-hygromycin knockin hiPSCs were transiently transfected using a Cre construct to excise the floxed neo cassette. Single cell clones were manually isolated and further expanded. Genomic DNA of these clones was examined by PCR to demonstrate the removal of neo cassette (Supplementary Fig. S2).
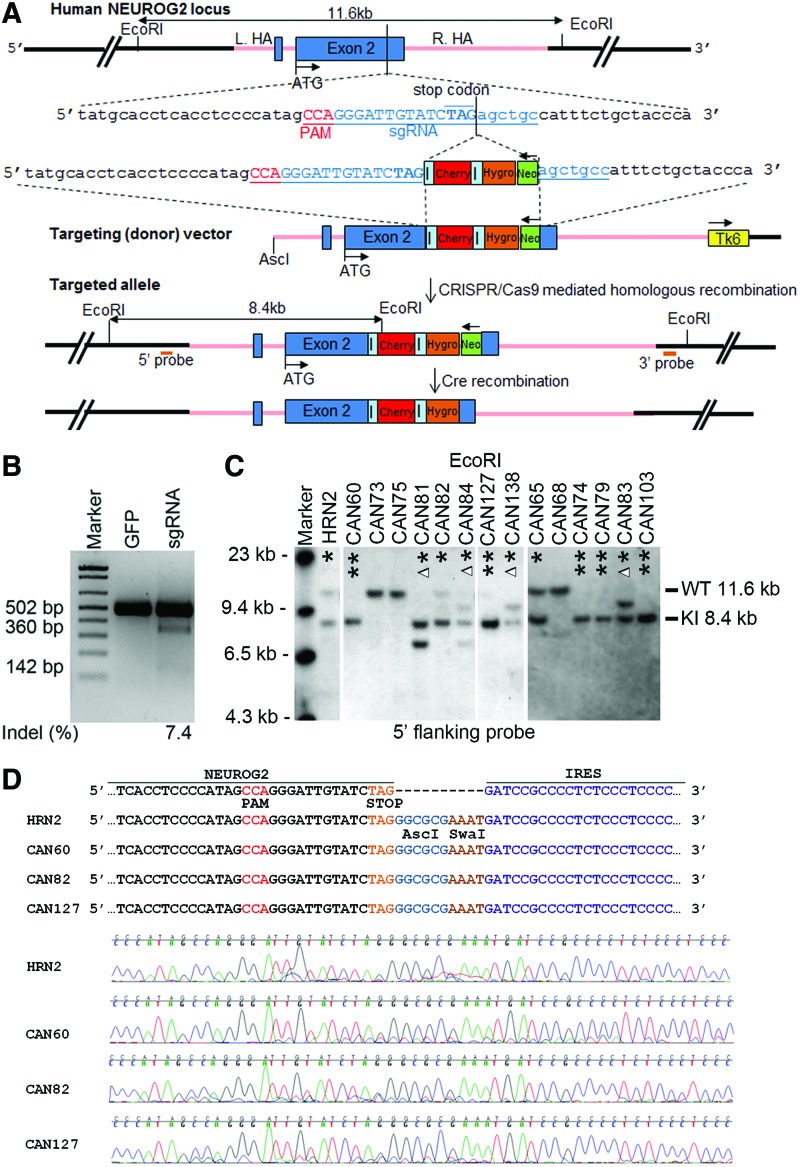
FIG. 1.
Gene targeting to the human neurogenin2 (NEUROG2) locus to create dual reporter human induced pluripotent stem cell (hiPSC) lines. (A) Design of targeting vector and single guide RNA (sgRNA) for making knockin NEUROG2-IRES-mCherry-IRES-hygromycin dual reporter hiPSC lines. The NEUROG2 genomic sequence, which consists of two exons (two blue boxes) and one intron, is tagged by mCherry (red box) and hygromycin resistance cassette (orange box) at the end of exon 2, connected in tandem by two internal ribosome entry site (IRES) sequences (light blue bar). The endogenous NEUROG2 gene is intact and NEUROG2 and the two tags mCherry and hygromycin resistance gene are driven by the endogenous NEUROG2 promoter. A floxed neomycin cassette (green box, driven by RNA pol II promoter) is used for positive selection. Tk6 cassette (yellow box, driven by TK promoter) outside of 3′ homology arm is designed for negative selection. The sgRNA sequence (underlined in blue) is designed so that it spans across the stop codon (TAG, in bold) of the endogenous NEUROG2. Protospacer adjacent motif (PAM) sequence is underlined in red. Pink line in the schematic represents the homology arms. Restriction enzyme EcoRI is used to digest the genomic DNA of hiPSC reporter clones for Southern blot analysis. AscI is used in the intermediate steps of creating the targeting (donor) vector. ATG designates the start codon of NEUROG2 gene. Both 5′ and 3′’ flanking probes are designed for Southern blot analysis to identify correctly targeted hiPSC clones. The correctly targeted knockin (KI) allele is 8.4 kb and the untargeted allele [wild-type (WT)] is 11.6 kb. (B) SURVEYOR assay of sgRNA-mediated cleavage in 293FT cells by the CRISPR/Cas9 system. SURVEYOR assay is used to determine whether a designed sgRNA will be able to mediate specific excision of Cas9 at desired location. In this case, the uncut band is 502 bp and the cut bands are ∼360 and 142 bp. The sgRNA illustrated in (A) results in an indel (insertion or deletion) rate of ∼7.4%. Green fluorescent protein (GFP) plasmid is used as a control. (C) Southern blot analysis for conventional (designated as HRN clones) and CRISPR/Cas9-mediated NEUROG2 targeting (designated as CAN clones). Genomic DNAs from selected clones are digested with EcoRI and hybridized with 5′ or 3′ flanking probes. Data from 5′ probe hybridization for selected clones are shown here in (C). Data from 3′ probe hybridization for selected clones are shown in Supplementary Fig. S1. For the 35 clones that are selected from the conventional straight gene targeting, only one clone (HRN2), is correctly targeted (targeting efficiency 1/35, 2.9%). For the 42 clones that are selected from the CRISPR/Cas9-mediated gene targeting, 14 clones are targeted (targeting efficiency 14/42, 33.3%). * And ** designate the targeting at one and two alleles respectively. Δ, altered band size at one allele. (D) Sequencing results of targeted clones at the junction of NEUROG2 and IRES. CCA is the PAM sequence, TAG is the stop codon. Clones obtained through conventional gene targeting (HRN2) and CRISPR/Cas9-mediated gene targeting (CAN60, 82, and 127) show identical sequence. Partial sequence of AscI and SwaI sites are introduced during the cloning of IRES to donor vectors. Color images available online at www.liebertpub.com/scd
Construction of sgRNA vector for the CRISPR/Cas9 system
Mammalian codon-optimized S. pyogenes Cas9-3X Flag vector JDS246 Cas9-003 and human-sgRNA-expression vector with U6 promoter MLM3636 were obtained from Addgene (Cat. Nos. 43861 and 43860). The sequence for making sgRNA for mediating NEUROG2 targeting was located so that it spanned across the stop codon TAG: 5′ GCAGCTCTAGATACAATCCCTGG 3′ (PAM is in bold, seed sequence is underlined, and nonseed sequence is in italic, Fig. 1A). In the targeting vector, the corresponding sgRNA sequence is split apart by ImCIH, therefore, only the endogenous genomic DNA but not the targeting (donor) vector will be cut by Cas9. To construct the sgRNA expression vector, a pair of oligos was designed using a web-based software ZiFiT at http://zifit.partners.org/ZiFiT/ [28–30], synthesized, annealed at 70°C for 20 min, and subcloned into MLM3636 sgRNA-expression vector with Golden gate assembly [31–33] using BsmBI restriction enzyme. The resultant plasmid was used to co-transfect hiPSCs ND2.0 (obtained from Center for Regenerative Medicine, National Institutes of Health) with Cas9 and the NEUROG2 targeting vector pWSTK6_Ngn2ImCIH.
SURVEYOR assay
To determine whether an sgRNA could induce DSBs at the desired genomic loci, SURVEYOR assay (Transgenomics) was performed as previously described [26,34]. SURVEYOR assays are mainly performed in easy-to-transfect cell lines such as 293FT cells. After sgRNAs are confirmed to elicit DSBs in 293FT cells, they will be further ranked and applied to the cell types of interest, such as hiPSCs. To quickly test whether the sgRNAs we designed could mediate the cutting of Cas9 at the NEUROG2 locus, we transfected 293FT cells with vectors that expressed the sgRNA and Cas9, and harvested cells for DNA extraction 72 h post-transfection. The DNA fragment of interest at the NEUROG2 region was PCR amplified using the following primers: forward primer: 5′ CGTCCTCCTCCGTGTCCTCCAATTCCACCT 3′; reverse primer: 5′ AAGAGAAAGGGGAGGAGCGTCAGTCCGCT C 3′. The PCR products were denatured and annealed, then treated with SURVEYOR nuclease, which would cut at the mismatches (Mm's) of the DNA double strands. If sgRNA and Cas9 caused DSBs at the NEUROG2 locus, NHEJ would subsequently occur, which resulted in mutations, insertions, or deletions (indels) at the immediately adjacent bases, which could be captured by PCR. During denature and annealing, the DNA strands with indels would have the chance to pair with the DNA strands that did not have any mutations or indels, therefore, forming “mismatches,” which could be recognized and cut by SURVEYOR nuclease. The digested PCR products were then separated in a 2.5% agarose gel. By these procedures we could prescreen and rank sgRNAs for subsequent experiments in hiPSCs. For the current work, 1.5 × 105 293FT cells (1-well of a 12-well plate) were transfected with 250 ng NEUROG2-sgRNA expression vector and 250 ng Cas9 expression vector using Lipofectamine 2000 (Life Technologies). Control cells were transfected with a green fluorescent protein (GFP) vector. Cells were harvested 72 h post-transfection, digested with 100 μL genomic DNA quick extraction solution (Epicentre Technologies), incubated at 68°C for 15 min, and then 95°C for 8 min. The concentration of the genomic DNA was adjusted to 200 ng/μL before it was PCR amplified using Herculase II (Stratagene). Approximately, 400 ng purified PCR product (PCR purification kit; Qiagen) was denatured by incubating at 95°C for 5 min and the temperature was slowly lowered to <30°C to allow the formation of heteroduplex. SURVEYOR assay reaction was setup as follows: 400 ng hybridized DNA, 1.5 μM MgCl2, 1 μL SURVEYOR Enhancer S, and 1 μL SURVEYOR nuclease. Reaction was incubated at 42°C for 60 min. Digested product was separated by a 2.5% agarose gel. Gel bands were quantified using ImageJ and the cleavage efficiency [percentage of insertion and deletion (indel%)] was calculated following a previously reported protocol [30,35] with the formula, Indel (%) = 100 × (1 − √(1 − fcut)). fcut = (b + c)/(a + b + c); a is the density of the undigested band, b and c are the density of the digested bands after cutting (Fig. 1B).
Off-target prediction and analysis
Off-target prediction and analysis were performed as described previously [30]. First, the sgRNA sequence in FASTA format was put into CasOT [36], a Perl-based software (http://eendb.zfgenetics.org/casot/download.php). For both seed and nonseed sequence, a value of 2 was chosen, which designated that fewer than or equal to 2 mismatches in nonseed and seed sequence would be tolerated in output sequence (potential off-targets site). Next, the chromosomal and genomic location, sequence of the site, Mm type, total number of mismatched bases, and exon information relating to the off-target site were provided by the CasOT program. Finally, PCR primers were designed to amplify the corresponding loci using the following conditions: 98°C for 2 min, 35 cycles of 98°C for 20 s, 59°C for 20 s, 72°C for 1 min, with a final extension at 72°C for 5 min. PCR products from multiple targeted NEUROG2 hiPSC reporter clones were subsequently sequenced and aligned to published sequences in National Center for Biotechnology Information (NCBI).
Generation of the NEUROG2-IRES-mCherry-IRES-hygromycin knockin reporter line in hiPSC ND2.0
The transgene-free, episomal vector reprogrammed ND2.0 hiPSC line was maintained on a layer of irradiation inactivated mouse embryonic fibroblast (MEF) cells in hiPSC medium consisting of DMEM:F12, 20% knockout serum replacement, 1% nonessential amino acid, 55 μM 2-mercaptoethanol, 2 mM l-glutamine, supplemented with 12 ng/mL basic fibroblast growth factor (bFGF). Cells were passaged using dispase (1 mg/mL) at a ratio of 1:4 every 4–5 days. Routine karyotyping examination was done every 10 passages. To generate feeder-free culture, cells were transferred to MEF conditioned medium and later adapted to Essential 8 medium (all above from Life Technologies). For reporter generation using conventional homologous recombination [7,8], a total of 1 × 107 ND2.0 hiPSCs were dissociated using accutase and incubated with 50 μg linearized targeting vector pWSTK6_Ngn2ImCIH. The mixture of DNA and cells was then transferred to a 4 mm cuvette and electroporated using a Bio-Rad Xcell Total system for a single pulse of 250 V, 250 μF. Electroporated cells were plated onto MEF layers for recovery. ROCK inhibitor (10 μm) was added for the first 24 h postelectroporation to enhance single cell survival. Seventy-two hours post-transfection, G418 (50 μg/mL; Life Technologies) and 2′-deoxy-2′-fluoro-β-d-arabinofuranosyl-5-iodouracil (FIAU, 125 nM; Moravek Biochemicals) were added to medium every day. Resistant clones were picked after 21 days of double selection from which genomic DNA was extracted. EcoRI-digested genomic DNA from each clone was hybridized to probes derived from sequences flanking 5′ and 3′ of the homology arms respectively by Southern blot analysis (Fig. 1C and Supplementary Fig. S1) [26]. For reporter generation using the CRISPR/Cas9 system-mediated homologous recombination, a total of 1 × 107 ND2.0 hiPSCs were electroporated with 25 μg sgRNA vector, 25 μg Cas9 expression vector, together with 50 μg linearized targeting vector. Similar to the conventional targeting approach, clones resistant to both positive and negative selection were isolated, validated by Southern blot analysis and individually expanded.
Differentiation of the knockin reporter into NEUROG2(+)/mCherry(+) cells
Neural and neuronal differentiation was performed using previously described protocols with some modifications [7,8,30,37,38]. Briefly, to induce NEUROG2/mCherry expression, NEUROG2 hiPSC reporter cells were digested into small clumps using 0.5 mM EDTA, transferred to 10 cm Petri dishes (Corning) and suspended in hiPSC medium (without bFGF), and supplemented with 10 μM SB-431542, 1 μM dorsomorphin, 3 μM CHIR 99021 (all from Tocris), and 0.5 μM purmorphamine (EMD Millipore) for neural induction. Medium was replaced on day 2 with N2B27 medium consisting of DMEM:F12, 1% N2, 2% B27, and 1% antibiotic-antimycotic (all above from Life Technologies), supplemented with the same small molecules described above. On day 4, spheres were further induced to express NEUROG2/mCherry by changing the medium to N2B27 medium supplemented with 1 μM purmorphamine and 1 μM all-trans retinoic acid (Sigma) every other day. Spheres were then examined under fluorescence microscopy for the expression of mCherry or fixed and co-labeled with NEUROG2 antibody to corroborate endogenous expression. For further induction toward the neuronal lineage, day 12 differentiated cells or mCherry(+) postsorted cells were cultured 7–10 days in neuronal differentiation medium, consisting of N2B27 medium, supplemented with brain-derived neurotrophic factor (10 ng/mL), glial cell-derived neurotrophic factor (10 ng/mL), ascorbic acid (100 nM; Sigma), and cyclic adenosine monophosphate (1 μM; Sigma).
Purification of NEUROG2(+)/mCherry(+) cells by FACS
Purification of NEUROG2(+)/mCherry(+) cells was carried out as described previously [7,39]. Briefly, differentiated NEUROG2-mCherry reporter cells were harvested using accutase and resuspended in 1% fetal bovine serum in 1× phosphate-buffered saline at a concentration of 5 × 106 to 1 × 107 cells/mL. Cell purification was performed using a FACSAria II cell sorter system (BD) at 4°C at a rate of 2,500 cells/s. The mCherry positively sorted cells were reexamined by FACS and found to have a purity of 95%–99% (Fig. 5A).
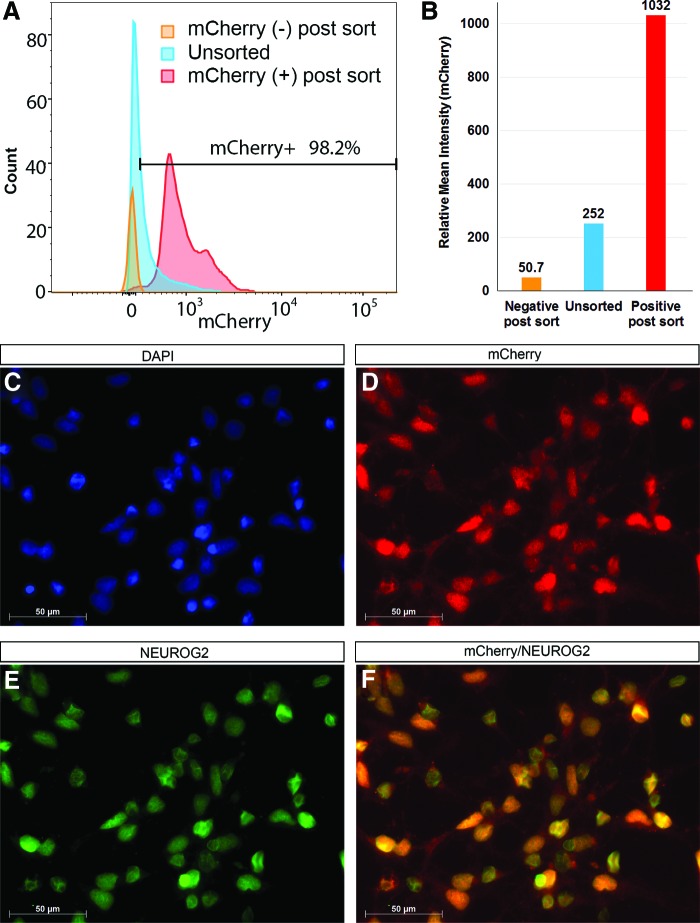
FIG. 5.
mCherry(+) cells can be purified by fluorescence-activated cell sorting (FACS). NEUROG2 knockin hiPSC clones are induced along the neural differentiation pathways for 6 days as EBs, then seeded onto Matrigel-coated plates and grown in neural induction medium. After an additional 2 days of differentiation, cells are dissociated for FACS purification using mCherry fluorescence. (A) Before sorting, about 30% of the cells are mCherry(+) (blue curve in A). Appropriate gate is set to collect both mCherry(+) (red curve in A) and mCherry(−) (orange curve in A) populations. Postsort examinations of mCherry(+) populations show that 95%–99% of cells are mCherry(+). (B) Relative mean intensity of mCherry for unsorted (blue column, relative mean intensity = 252), mCherry(+) sorted (red column, relative mean intensity = 1,032), and mCherry(−) sorted (orange column, relative mean intensity = 50.7) populations. (C–F) Immunocytochemistry shows that every mCherry(+) cell (red) co-expresses NEUROG2 (green, C–F, DAPI is shown in blue). Scale bar: 50 μm. FACS purification experiments are done for four clones HRN2, CAN60, CAN82, and CAN127. Data shown are from Clone CAN60. Color images available online at www.liebertpub.com/scd
Purification of NEUROG2(+)/mCherry(+) cells by drug selection using hygromycin B
Differentiated reporter cells were maintained in N2B27 neural differentiation medium and treated with hygromycin B (Sigma) at 10 or 50 μg/mL for 5 days. mCherry expression was monitored under the fluorescence microscopy. At the end of the 5-day treatment, mCherry(+) cells were examined by FACS and fluorescence microscopy.
CRISPR/Cas9-mediated transactivation of endogenous NEUROG2 expression
The dual expression construct pAC154-dual-dCas9VP160-sgExpression (abbreviated as dCas9VP160 below) was obtained from Addgene (Cat. No. 48240). The vector encodes a catalytically inactive Cas9 protein (dCas9) that is fused to 10 copies of VP16 transcriptional activation motifs. Once guided to the appropriate transcription factor binding site(s) (TFBSs), the activation motif could transiently turn on the endogenous gene. Five truncated (18-base) sgRNAs [40] were designed by targeting the potential NEUROG2 TFBSs between −500 bp and the transcription start site (TSS), and cloned into dCas9VP160 to guide the binding of dCas9 and VP160. The resultant vectors were named as sgRNA A1, A2, A3, A4, and A5 (Fig. 8A, B). To activate the endogenous NEUROG2 expression, NEUROG2 reporter line was cultured in hiPSC medium (without bFGF) supplemented with 10 μM SB-431542 and 1 μM dorsomorphin for 2 days. On day 3, NEUROG2 reporter hiPSC was transfected with 1 μg dCas9-activator vector per well of a 24-well plate using Lipofectamine 2000 (Life Technologies). Neural stem cells (NSCs) derived from the reporter clone were cultured in N2B27 medium with 20 ng/mL bFGF and transfected using the same protocol as for iPSCs. To evaluate transactivation efficiency, 48 h post-transfection, the percentage of live mCherry-positive cells was visualized and quantified under fluorescence microscopy (Fig. 8D). In addition, the transfected cells were also harvested for quantitative RT-PCR (qRT-PCR) to assess the NEUROG2 mRNA expression level (Fig. 8C, primer sequences for quantitative RT-PCR (qPCR) are listed in Supplementary Table S2).
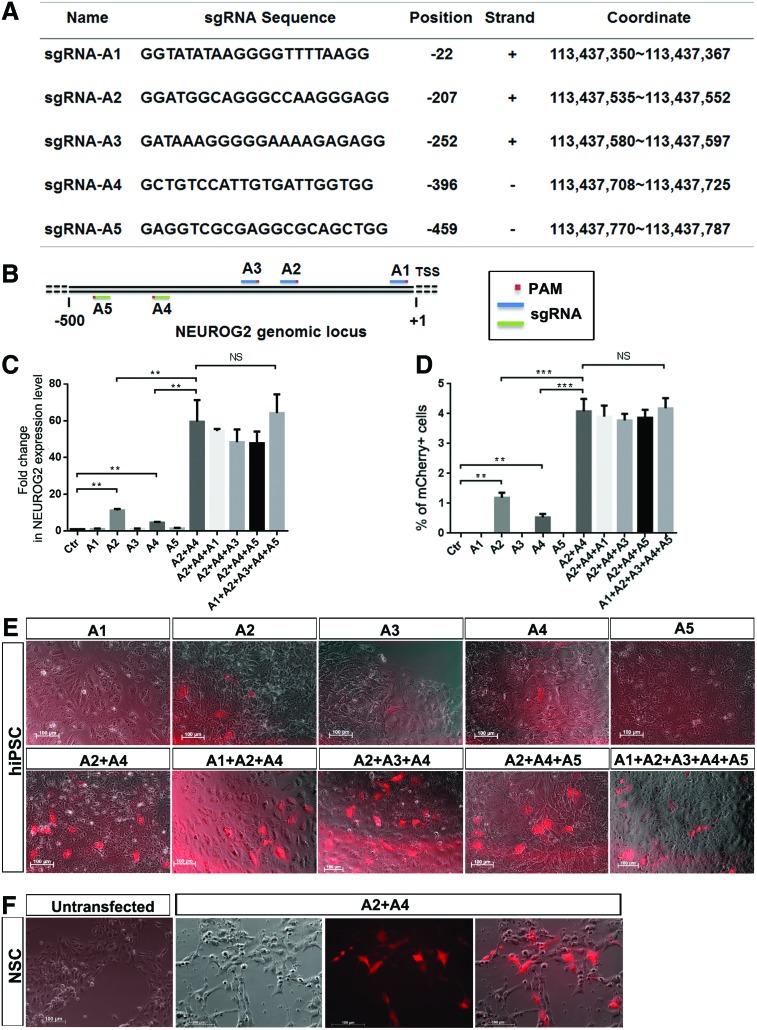
FIG. 8.
CRISPR/Cas9-mediated transactivation of endogenous NEUROG2 expression at both iPSC and neural stem cell (NSC) stage. (A, B) Sequence and location of five truncated sgRNAs upstream of the transcription start site (TSS) of the NEUROG2 gene, based on Human Genome Browser–hg19 assembly (sequences) human reference sequence (GRCh37). (C) Quantitative RT-PCR result of fold change of NEUROG2 gene expression after endogenous promoter activation by sgRNA in reporter clones at the iPSC stage. (D) The data obtained by direct visualization and quantification of mCherry(+) cells under the fluorescence microscope are consistent with the trend observed from NEUROG2 quantitative RT-PCR (qPCR) result shown in (C). (E) No mCherry-expressing cells are observed 48 h post-transfection with sgRNA-A1, A3 or A5, while about 1% and 0.5% of cells expressed mCherry in sgRNA-A2 or A4 transfected wells (n = 3 for each transfection, P < 0.05). When sgRNA-A2 and A4 are combined and co-transfected to the same wells, the mCherry expression is significantly elevated. Addition of A1, A3 or A5 does not increase NEUROG2/mCherry expression at a detectable level. (F) sgRNA A2 and A4 in combination is able to transactivate NEUROG2 promoter in NSCs derived from reporter clones. The untransfected NSCs do not express mCherry, while the NSCs transfected with sgRNA A2+A4 showed increased number of mCherry(+) cells. The experiments have been repeated in clones HRN2, CAN60, CAN82, and CAN127. Color images available online at www.liebertpub.com/scd
Quantitative RT-PCR
Total RNAs were extracted using Quick-RNA miniPrep kit (Zymo Research). One microgram of RNA was converted to cDNA using the SuperScript III First-Strand Synthesis System (Life Technologies). qRT-PCR was performed to determine NEUROG2 mRNA levels using the iQ SYBR Green Supermix kit (Bio-Rad). GAPDH was used as an internal control. The relative change in NEUROG2 mRNA expression was evaluated using the comparative threshold cycle ΔΔCt method.
Immunocytochemistry
Immunocytochemistry and histology were performed as described [7,41]. Briefly, cells grown on glass coverslips were fixed using 4% paraformaldehyde and incubated in blocking buffer (5% goat serum, 1% bovine serum albumin, and 0.1% Triton X-100) for 30 min. Cells were then incubated in primary antibodies diluted in blocking buffer at 4°C overnight. Appropriate secondary antibodies were used for single and double labeling. All secondary antibodies were tested for cross-reactivity and nonspecific immunoreactivity. The following primary antibodies were used: NEUROG2 (1:500; EMD Millipore), OCT4 (1:500; Abcam), SOX2 (1:200, MAB2018; R&D Systems), SSEA4 [1:10, MC-813-70; Developmental Studies Hybridoma Bank (DSHB)], TRA1-81 (1:100, MAB4381; EMD Millipore), β3 tubulin (1:4,000; Sigma), and GABA (1:200; Sigma). Bis-benzamide (Sigma) was used to identify the nuclei. Images were captured using a Zeiss Axiovision microscope with z-stack split view function. Note that all images involved mCherry were captured directly under fluorescence or confocal microscope without mCherry antibody immunostaining unless indicated otherwise.
Results
Improved gene targeting efficiency in CRISPR/Cas9 mediated homologous recombination
Two strategies were employed to generate the NEUROG2-IRES-mCherry-IRES-hygromycin knockin reporter line. First, a conventional homologous recombination was used. ND2.0 hiPSCs were electroporated with targeting vector pWSTK6_Ngn2ImCIH (Fig. 1A). Among the 35 clones selected through both positive (G418) and negative (FIAU) selection, 1 clone (named HRN2) was identified to be correctly targeted in one allele while the other allele remained intact as confirmed by Southern blot analysis (Fig. 1C and Supplementary Fig. S1). The efficiency was 2.9% (1/35 clones). In contrast, when experiment was performed with co-electroporation of CRISPR/Cas9 system components, that is, sgRNA for NEUROG2 and Cas9 expression vectors, the efficiency increased to 33.3% (14/42 clones, Fig. 1C and Supplementary Fig. S1). Interestingly, 7 of the 14 positive clones (clone names CAN60, 74, 79, 103, 127, 131, and 134) were correctly targeted at both alleles in CRISPR/Cas9-mediated gene targeting. In contrast, it was extremely difficult to target to both alleles if conventional gene targeting approach was used (Fig. 1C and Supplementary Fig. S1 and Supplementary Table S3). To ensure that the gene targeting process did not introduce any unwanted mutations to the NEUROG2 locus, we sequenced the knockin clones at the junction of NEUROG2 and IRES cassette. As shown in Fig. 1D, no mutations were found in any of the clones that were generated via conventional or CRISPR/Cas9-mediated gene targeting.
sgRNA is specific for the NEUROG2 genomic locus
A 20-nt long sgRNA was designed to guide the Cas9 endonuclease to cut at the very 3′ end (stop codon) of the NEUROG2 gene. As shown in Fig. 1B, SURVEYOR assay performed in 293FT cells confirmed that the sgRNA was able to cut specifically at the NEUROG2 stop codon region, as compared to the GFP control. Based on SURVEYOR assay and the measurements of the density of bands on agarose gel, the indel% of the sgRNA was 7.4% (Fig. 1B), which was predicted to be able to elicit sufficient DSBs in hiPSCs at the NEUROG2 locus, so that the rate for DNA repair and homologous recombination could increase.
One thing worth mentioning is that, the entire sequence of sgRNA as a whole would not be found in the targeting vector, which had additional reporter cassettes inserted between sgRNA's seed and nonseed sequence (Fig. 1A). Therefore, the sgRNA would only be able to target the endogenous NEUROG2 locus where Cas9 (co-introduced into the cells during electroporation) will then cut and elicit DSBs. The sgRNA and Cas9 would not make undesired excision at targeting vectors.
No off-target mutations were detected in knockin reporter clones
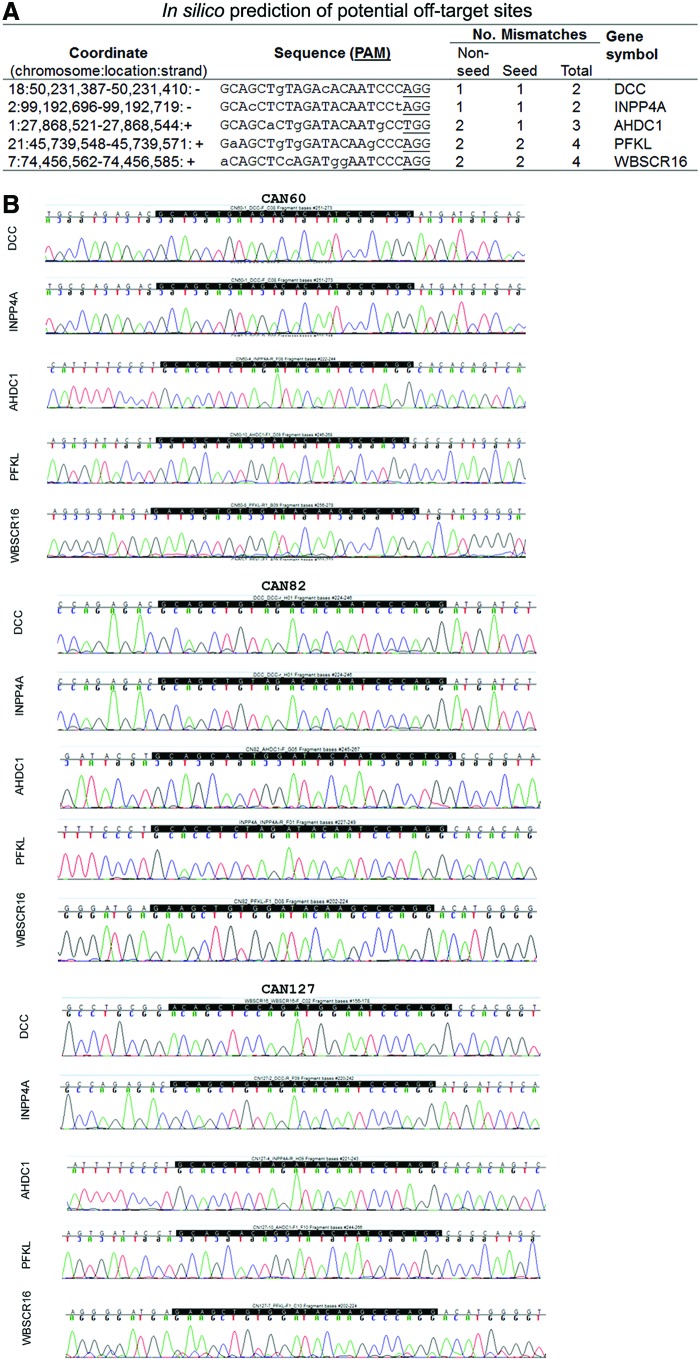
Although every effort was made to ensure the specificity of the sgRNA, there was a possibility for the sgRNA to target genomic locations that share homology with the 3′ end of the NEUROG2 sequence, where the CRISPR/Cas9 system was designed to cleave. To determine whether any off-target events had occurred, we first used CasOT for genome-wide in silico prediction to narrow down the potential sites [36]. CasOT results indicated that 50 genomic loci (Fig. 2A and Supplementary Table S4) contained a combined number of mismatched bases of ≤4, when compared with the seed and/or the nonseed sequence of the sgRNA. Based on previous reports [26,30,36], if the sum of the number of mismatched bases for a potential site exceeds 4, then the likelihood for the site to be targeted by a defined CRISPR/Cas9 sgRNA is close to 0. On the other hand, if the sum is less than or equal to 4, additional examination to the locus is warranted. Of the 50 loci, 23 were from the unannotated areas of the genome, and 27 loci were located in annotated regions, of which, 2 were located in exons (of genes PFKL and WBSCR16, Fig. 2A), 23 in introns, and 2 in potentially important intergenic locations such as long noncoding RNA regions (LINC01135 and LINC00879, Supplementary Table S4). Next, we sequenced all of the 27 loci for the four clones that we intended to characterize further, HRN2, CAN60, CAN82, and CAN127. Our sequencing results (Fig. 2B and Supplementary Tables S4 and S5, and data not shown) showed that, based on the alignment in UCSC BLAT and NCBI database, no mutations were found at 26 out of the 27 loci examined. At one locus, intron 8 of the gene TTLL11, a point mutation was found in all clones and the parental hiPSC line ND2.0, indicating that this was probably a single nucleotide polymorphism or a preexisting mutation, which was not introduced via the process of CRISPR-mediated gene targeting. Therefore, we concluded that no off-target events were detected at any of the 27 sites in any clones tested (Supplementary Table S5).
FIG. 2.
Potential off-target genomic sites for the NEUROG2 sgRNA. (A) In silico prediction of potential off-target sites by CasOT program. Generally, if the combined number of mismatches (Mm's) within seed and nonseed sequence is >4, off-target activities are unlikely. If the number of Mm's within the seed sequence is <2, off-target events need to be monitored and examined. A complete list of off-target prediction is shown in Supplementary Table S4. Two sites in exons and three sites in introns are shown here. (B) No mutation is detected in any locus in CRISPR/Cas9 clones as shown by sequencing. Color images available online at www.liebertpub.com/scd
CRISPR/Cas9-mediated NEUROG2 knockin reporter maintained pluripotency after long-term passage
As with the parental hiPSC line ND2.0, NEUROG2 reporter clones generated by conventional or CRISPR/Cas9-mediated gene targeting, irrespective of being targeted at one or two alleles of NEUROG2, expressed pluripotency markers OCT4, SSEA4, SOX2, and TRA1-81 (Supplementary Fig. S3A, B, D, E, G, H, J, K), as well as maintained a normal karyotype (Supplementary Fig. S3C, F, I, L). The reporter clones were passaged every 4–5 days at a ratio of 1:4, similar to their parental hiPSC line ND2.0. Such profile remained after long-term culture for as long as 30 passages (the last time point examined). These results indicated that the dual reporter clones maintained similar proliferation and differentiation properties to the nonengineered parental line, and that CRISPR/Cas9-mediated HDR did not adversely alter the overall hiPSC properties.
Induction of mCherry expression in NEUROG2 reporter line
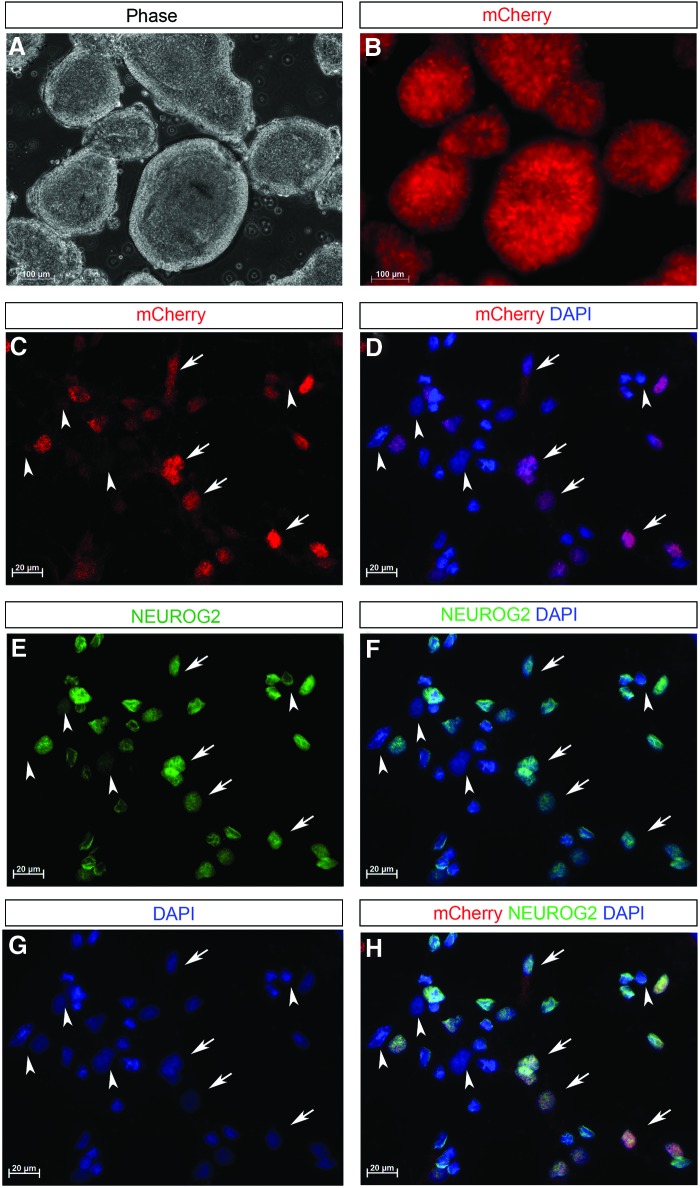
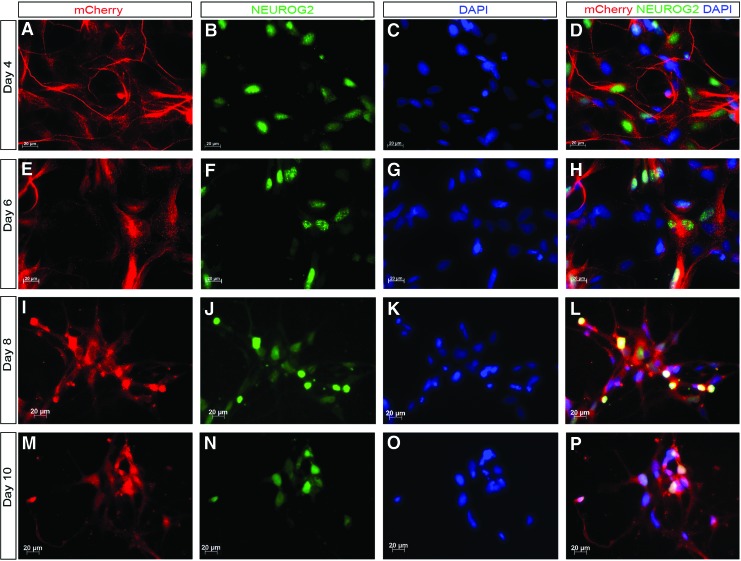
One major goal of this work was to generate a tool which could be used to purify NEUROG2+ precursors. We therefore differentiated clones HRN2, CAN60, CAN82, and CAN127 into NEUROG2-expressing cells using NSC differentiation protocol. Along the differentiation process, as early as day 5 (1 day after the addition of purmorphamine and retinoic acid to the culture medium), faint mCherry(+) cells could be detected scattering in the culture (data not shown). By day 7 of neural induction, more cells had started to express mCherry that could be detected by direct visualization under the fluorescence microscopy (Fig. 3A, B). To examine whether mCherry expression mimicked the expression of NEUROG2, we performed immunocytochemistry staining, which showed that almost every NEUROG2+ cell expressed mCherry, and vice versa (Fig. 3C–H). To further monitor whether mCherry expression reflected endogenous NEUROG2 expression during the entire neuronal differentiation process, we performed a time course along neuronal differentiation of the knockin reporter clones. After the initial neural induction, cells were further differentiated for 4, 6, 8, and 10 days, respectively. As shown in Fig. 4, mCherry expression (Fig. 4A, E, I, M) not only colocalized with NEUROG2's typical nuclear staining (Fig. 4B, F, J, N), but it also finely outlined the morphology of the reporter clone-derived neurons, including the cell bodies and the extensive processes (Fig. 4). Taken together, these data indicated that mCherry expression faithfully recapitulated the expression of the transcription factor NEUROG2 during neural differentiation, and that mCherry expression might be used as a surrogate marker for monitoring NEUROG2 expression, tracing the oscillation of NEUROG2 expression along a variety of neural induction protocols.
FIG. 3.
mCherry expression reflects endogenous NEUROG2 expression during neural differentiation. (A, B) NEUROG2 knockin hiPSC clones are induced as embryoid bodies (EBs). After 7 days of differentiation, many EBs express mCherry that is directly visible under fluorescence microscope. (C–H) When cells are dissociated and replated as monolayer adherent culture, mCherry (red) and NEUROG2 immunostaining (green) clearly show colocalization with each other (arrows in C–H, DAPI is shown in blue). Consistently, NEUROG2 negative cells do not express mCherry (arrowheads in C–H). Scale bar: 100 μm in (A, B); 20 μm in (C–H). Similar expression pattern is obtained from all four clones tested including HRN2, CAN60, CAN82, and CAN127. Images shown are from Clone CAN82. Color images available online at www.liebertpub.com/scd
FIG. 4.
mCherry expression reflects endogenous NEUROG2 expression during neural differentiation. NEUROG2 knockin hiPSC clones are induced along the neural differentiation pathways. After 6 days of differentiation as EBs in suspension culture, cells are plated onto Matrigel-coated dishes and grown in neural induction medium (see Materials and Methods section for details) for an additional 6 days before they are switched to be grown in neuronal differentiation medium. Cells are then cultured for 4 (A–D), 6 (E–H), 8 (I–L), and 10 (M–P) days before they are fixed for NEUROG2 immunostaining. mCherry expression (red) colocalizes with NEUROG2 (green) expression along the entire neuronal differentiation time course. DAPI is shown in blue. mCherry expression also delineates the neuronal cytoplasm and processes (A, E, I, M). Nuclei are revealed by DAPI staining. Scale bar: 20 μm. Similar differentiation pattern is obtained from all four clones tested (HRN2, CAN60, CAN82, and CAN127). Data shown are from Clone CAN60. Color images available online at www.liebertpub.com/scd
FACS purification of mCherry(+) cells
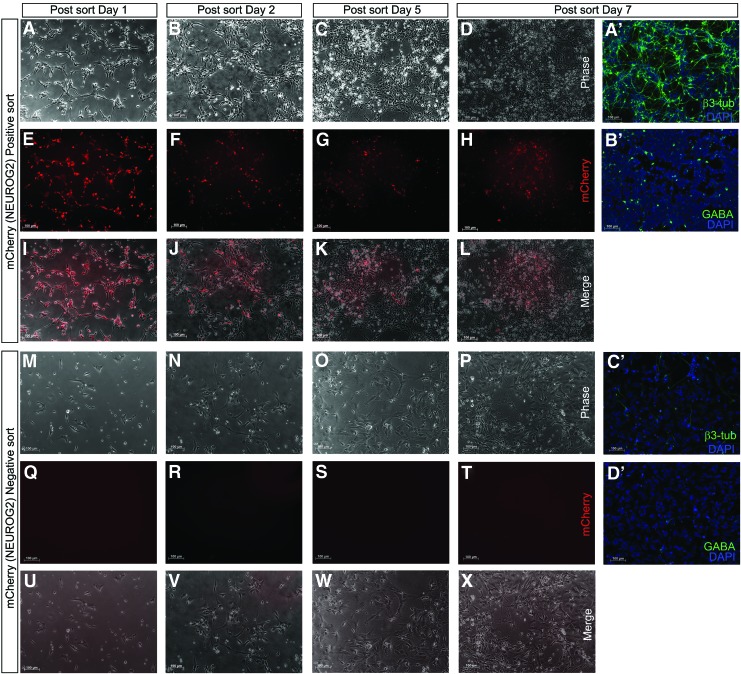
After a total of 8 days of neural induction, cells were dissociated for FACS purification using mCherry fluorescence. About 30% of the cells were mCherry(+) (Fig. 5A), which were also confirmed to be NEUROG2 immunoreactive (Fig. 5C–F). To compare the expression profile of NEUROG2 in mCherry(+) and mCherry(−) cell populations, we performed neuronal differentiation of both populations in parallel and collected data along the time course of day 1, 2, 5, and 7 (Fig. 6). Both mCherry(+) and mCherry(−) cells were able to differentiate and continued to proliferate under these culture conditions. As shown in Fig. 6A–L, mCherry expression gradually decreased in mCherry(+) sorted population along the time course, as monitored under fluorescence microscopy, indicating that the expression of NEUROG2 decreased as cells matured toward more terminal cell types, consistent with previous reports showing that NEUROG2 expression marked the terminal differentiation of neuronal progenitors [42]. As expected, with further differentiation, mCherry(+) sorted cells gave rise to numerous neurons with extensive processes as delineated by β3 tubulin immunocytochemistry (Fig. 6A′). A portion of these β3 tubulin+ cells might be GABAergic interneurons as shown by the positive GABA staining (Fig. 6B′). mCherry(−) sorted cells, on the other hand, despite having the capacity to proliferate (Fig. 6M–P), remained mCherry/NEUROG2 negative (Fig. 6Q–T), as indicated by the absence of mCherry expression throughout the period of the time course. In addition, compared to mCherry(+) sorted cells, mCherry(−) sorted population generated significantly lower number of β3 tubulin expressing neurons (compare Fig. 6A′ and C′) or GABA+ cells (compare Fig. 6B′ and D′), implicating the importance of NEUROG2 in neuronal differentiation. FACS purification and time course experiments were performed for clones HRN2, CAN60, CAN82, and CAN127 and similar results were obtained.
FIG. 6.
Expression of mCherry in positively and negatively sorted cell populations. After sorting, both mCherry(+) (A–L) and mCherry(−) (M–X) cells are cultured in neuronal differentiation medium for 1, 2, 5, and 7 days, during which time both populations of cells continue to grow. mCherry expression gradually decreases in mCherry(+) cells, which is visible under fluorescence microscope (A–L). Although mCherry(−) cells have the capacity to proliferate (compare M–P), they do not give rise to mCherry(+) cells, as indicated by the absence of mCherry expression throughout the time course period. After 7 days of differentiation, mCherry(+) sorted cells are able to give rise to numerous β3 tubulin-expressing neurons (A′), some of which are interneurons marked by GABA+ staining (B′). In contrast, mCherry(−) sorted populations give rise to significantly lower number of β3 tubulin-expressing neurons (C′), and GABA+ interneurons (D′). Scale bar: 100 μm. FACS purification and time course experiments are performed for clones HRN2, CAN60, CAN82, and CAN127. Data shown are from Clone CAN60. Color images available online at www.liebertpub.com/scd
Dual reporter design for NEUROG2
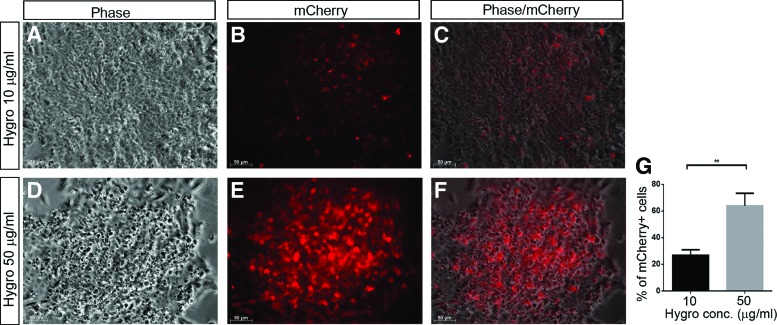
To enhance the application versatility of the NEUROG2 hiPSC knockin reporter, a hygromycin resistance cassette, which encodes a kinase that phosphorylates hygromycin and subsequently confers hygromycin resistance for the cells, was designed as a second reporter downstream of the fluorescent protein mCherry, connected by IRES (Fig. 1A). Both reporters and the NEUROG2 gene itself would be expressed under the control of the endogenous NEUROG2 promoter. Both alleles of NEUROG2 were preserved to avoid potential problems that might be caused by haploinsufficiency. If our dual reporter was successfully made, NEUROG2+ cells could then be purified by either mCherry FACS or hygromycin drug selection. A dosage curve was generated and 50 μg/mL of hygromycin was determined to be the optimal concentration for selecting mCherry(+) cells after differentiation, as lower concentrations (eg, 10 μg/mL) was not sufficient to kill all mCherry(−) cells while higher concentrations (eg, 100 μg/mL) caused cell death including mCherry(+) cells (Fig. 7). Cells treated with 50 μg/mL hygromycin showed a significantly higher proportion of mCherry expression than the cells treated with 10 μg/mL hygromycin (Fig. 7, quantification is shown in Fig. 7G). The availability of hygromycin drug selection as a second reporter in addition to mCherry offered an alternative enrichment approach for NEUROG2+ cells.
FIG. 7.
The hygromycin resistance gene offers an additional selection method. The NEUROG2-mCherry-hygromycin resistance knockin reporter is designed for purification by either FACS or drug selection after differentiation. NEUROG2 reporter cells are differentiated toward neural lineage for 7 days, then treated with hygromycin. In 10 μg/mL hygromycin treated dishes (A–C), about 30% cells are mCherry+ as detected under fluorescence microscope, while in 50 μg/mL hygromycin treated dishes (D–F) and 66%–70% of total cell population is mCherry(+). Cell counting is done with ImageJ software. Quantification is done using Student's t-test (G). The experiments have been repeated in clones HRN2, CAN60, CAN82, and CAN127, for three times per group. **P < 0.001. Color images available online at www.liebertpub.com/scd
CRISPR/Cas9-mediated transactivation of endogenous NEUROG2 expression
To fully realize the potential applications of our NEUROG2 knockin dual reporter, we attempted to use CRISPR/Cas9-mediated transactivation to transiently switch on endogenous NEUROG2 promoter and to observe the reporter expression. Based on human–mouse–rat (HMR) conserved analysis available at the UCSC genome web browser, a total of 11 potential TFBSs (named TFBS1 through TFBS11 herein for convenience) were shown within 500 bp upstream of the TSS of NEUROG2 (Fig. 8), Of these TFBSs, TFBS2 contains a TATA box, a classic core promoter sequence for transcription factor binding. In addition, in the sequence between TFBS7 and TFBS9, there are two CAAT box sequences, another classic consensus sequence for transcription factor binding, although this locus is not labeled as a “TFBS” in HMR conserved analysis. Based on these information and the rules for designing sgRNA, we located PAM sequences and designed five truncated sgRNAs [43] (sgRNA A1 through A5, Fig. 8A, B) from ZiFit. sgRNA A1 was designed to target TFBS1, 2 (where a TATA box was located), and 3, A2 to target TFBS4 and 5, A3 to target TFBS6 and 7, A4 to target the two CAAT boxes and adjacent sequences, and A5 to target TFBS9, 10, and 11. Because no suitable PAM sequence was found within or adjacent to TFBS8, no sgRNA was designed for this site.
Our results showed that, no mCherry-expressing cells were observed 48 h post-transfection with sgRNA A1, A3, or A5, while about 1% and 0.5% of cells expressed mCherry in sgRNA A2 or A4 transfected wells (Fig. 8D, E, n = 3 for each transfection), which corresponded to elevated levels of NEUROG2 mRNA expression of 11-folds (A2) and 4.6-folds (A4) respectively, as evaluated by qRT-PCR (Fig. 8C). When cells were co-transfected with A2 and A4, mCherry expression was boosted and 5% of the cells expressed mCherry (Fig. 8D, E), resulting in a more than 60-fold increase in NEUROG2 mRNA expression as detected by qRT-PCR (Fig. 8C). Addition of any other three sgRNAs, either individually or combined, to A2/A4 cocktail did not further increase mCherry/NEUROG2 expression (Fig. 8C–E). These results indicated that sgRNA A2 and A4 had synergistic effect on activating NEUROG2 expression. In addition, the synergistic action of sgRNA A2 and A4 appeared to have similar transactivation effect in NSCs, as co-transfection of A2 and A4 turned on NEUROG2 expression in reporter derived NSCs as well (Fig. 8F).
Discussion
CRISPR/Cas9 genetic editing tools and iPSC generation are two of the fastest-developing biomedical technologies in recent years. In this work, using CRISPR/Cas9-mediated gene targeting approach, we have created a dual knockin hiPSC reporter for NEUROG2, a transcription factor important in neural development and maturation. These reporter clones and the CRISPR-mediated approach of transcriptional regulation offer a useful avenue to characterize the role of NEUROG2 in human CNS.
Targeting efficiency
The targeting efficiency at the NEUROG2 locus is greatly improved in CRISPR/Cas9-mediated system compared to the conventional straight homologous recombination (33% vs. 3%). However, it is also worth noting that several clones we obtained from the CRISPR/Cas9-mediated targeting showed altered band sizes in Southern blot analysis (Fig. 1C). This is a complex situation and several possibilities might exist. One is that the donor vectors might have formed head-to-tail concatemers [44,45] before integrating into the NEUROG2 site by HDR. Another possibility is that, despite the presence of sufficient amount of donor vectors, the error-prone NHEJ still might occur at a much higher rate than HDR [24,25], causing unwanted insertions or deletions. This phenomenon entails the optimization of the dosage of targeting vectors and the sgRNA/Cas9 vectors. A recent report has identified two small molecules, L755507 and Brefeldin A, to be able to increase the efficiency of HDR [24] in several somatic cell lines, tumor cell lines, and human embryonic stem cell-derived NSCs. Similarly, another report enhances HDR rate by introducing shRNA to specifically inhibit essential proteins/enzymes for NHEJ [25]. It would be interesting to test whether these approaches would also improve HDR efficiency in hiPSCs, provided that their use does not alter basic and critical properties of hiPSCs, including genetic and epigenetic stability in long-term culture, proliferation, and differentiation profiles.
sgRNA design for targeting the NEUROG2 locus with CRISPR/Cas9
Our goal is to generate a knockin and add-on reporter at the NEUROG2 locus in hiPSCs, which requires a precise insertion of the IRES-mCherry-IRES-hygromycin resistance reporter cassettes right downstream of the stop codon of NEUROG2. In contrast to CRISPR/Cas9-mediated insertion-deletion experiments, where mutations or small indels are generated at the designated genomic loci via NHEJ, the knockin/add-on experiments will require the insertion of the add-on sequence (a reporter, a tag or a cDNA expression cassette), at the designated loci, via HDR. To avoid unintended binding and cutting of sgRNA/Cas9 to the targeting vector, the sgRNA sequence has to be very specific to the genomic locus of NEUROG2. Therefore, when designing sgRNA, we targeted the NEUROG2 stop codon area, where the insertion of IRES-mCherry-IRES-hygromycin will occur. We made sure that the entirety of sgRNA's seed sequence (5′ GGGATTGTATCT 3′) and nonseed sequence (5′ AGAGCTGC 3′) only exist in the genomic locus while these two parts are split by the insertion of the IRES-reporter cassettes in the targeting vector, therefore the targeting vector will be able to successfully evade the sgRNA-guided Cas9 excision.
Detection of off-target events
Because sgRNA is a 20-base short fragment, potential off-target activities are a major concern for the application of the CRISPR/Cas9 technology. Off-target events can cause genome instability such as mutations, insertions, deletions, even translocations, which may disrupt normal gene functions. Several strategies have been developed to detect potential off-target activity, including in silico prediction [36,46], whole genome sequencing, nuclease-digested genomes sequencing (Digenome-seq) [47] and integrase-defective lentiviral vectors [48]. In this work, to comprehensively evaluate off-target events, we used a two-step strategy, which is to first narrow down potential off-target sites by in silico prediction, and then sequence the predicted potential off-target sites in multiple clones. According to published reports, genomic loci with Mm >5 bases almost never are mistargeted by a designated sgRNA, we therefore set the threshold of Mm to 2 bases for seed sequence and 2 bases for nonseed sequence (total Mm ≤4) in CasOT, the in silico prediction software. Based on the sequencing results of 27 sites from all clones tested (Fig. 2 and Supplementary Tables S4 and S5), our CRISPR/Cas9-mediated dual reporter generation did not introduce off-target events in any of the clones tested (Supplementary Tables S4 and S5). Therefore, these clones are suitable to be used as a NEUROG2 reporter. It is possible that the two-step off-target identification protocol needs to be more stringent to exhaust all possibilities as described in a recent report where off-target sites were identified by a genetic cassette inserted to the target site [48]. Based on our observation, NEUROG2 reporter clones maintained typical iPSC morphology and marker expression after long-term culture when they were grown on MEF feeders or in feeder-free conditions in Essential 8 medium (Supplementary Fig. S3). These data further support the conclusion that the CRISPR/Cas9-mediated targeting to the NEUROG2 locus was successful and that the resultant clones can be applied to downstream investigations for differentiation, lineage specification and transplantation analysis. For future practice, we are implementing a combinatorial approaches that have been reported to reduce off-target activities, including paired Cas9 nickases [26,30,49,50], RNA-guided FokI nucleases [51,52], and truncated sgRNAs [40], to further increase on-target efficiency and decrease off-target events.
Choice of reporters and design of a dual reporter for versatile applications
NEUROG2 is a transcription factor and it cannot be used as a marker for cell sorting or direct monitoring. In this work, we knocked in two reporters, mCherry and hygromycin resistance gene to the NEUROG2 locus. The first reporter, mCherry fluorescent protein, originally created by Roger Tsien lab, belongs to a group of improved red fluorescent proteins [23], with peak excitation at 587 nm and maximum emission at 610 nm. As a monomer, mCherry is much less toxic to cells or live animals than earlier versions of red fluorescent proteins that are tetramer or dimer in configuration [53,54]. In addition, it also has enhanced tolerance for fusion proteins at both N- and C-terminus [23], which suits better in our current scenario, as based on our design, mCherry would be connected downstream of NEUROG2 and upstream to hygromycin resistance cassette, although not directly fused at either terminus with these proteins. Consistent with previous reports that the production of mCherry protein does not interfere with normal function of a cell or a tissue that expresses it, we have confirmed that the expression of mCherry does not interfere with the regular differentiation and function of the hiPSC culture, as our results clearly showed that mCherry expression followed an identical gene expression pattern of NEUROG2, and mCherry(+) cells were able to be give rise to mature neurons (Figs. 3–6). To provide versatile applications to the NEUROG2 reporter, we included hygromycin-resistance cassette as a second reporter downstream of mCherry, connected by an IRES. The ability to use hygromycin to select for NEUROG2+ cells has added convenience to daily practice and offers an alternative purification approach to cater to different experimental designs and purposes (Fig. 7).
Although it has been reported that the expression level of the gene fragment downstream of an IRES might be diminished, our NEUROG2 clones showed robust expression for both reporters mCherry and hygromycin resistance, which were connected by two sets of IRES in tandem. Both reporters recapitulated the endogenous NEUROG2 gene expression temporally and spatially (Figs. 3–7), indicating that the endogenous NEUROG2 promoter was able to drive the expression of all three genes (endogenous NEUROG2, mCherry, and the hygromycin resistance gene) at the same time. The result also supports that IRES is a viable choice for multigenic reporter design, although other options such as the self-cleaving 2A sequences are available as well.
Transient expression by CRISPR-mediated transactivation
To quickly validate our dual reporter iPSC lines, and to realize the broad applications of the CRISPR/Cas9 system, we designed CRISPR/Cas9 transactivator components [55] for NEUROG2 gene. The potential sites for sgRNA to target were chosen from a list of TFBSs within 500 bases upstream of TSS of NEUROG2 based on HMR conserved analysis. The two sgRNAs A2 and A4, which were able to transactivate NEUROG2 expression and have synergistic effects with each other (Fig. 8), were designed to target sequences located at 207- and 396-base upstream of TSS, respectively. It is worth noting that although two CAAT boxes were located at A4's targeting region, no typical transcription activation elements were found in A2's targeting region. A very recent publication using an enhanced version of sgRNA demonstrates that sequences within −200 bases of NEUROG2 TSS also contain fragments that confer transactivation, although they need to be activated by a more robust system [56]. Nevertheless, by identifying sgRNAs that transactivate NEUROG2, one could not only locate potential promoter regions, but test whether the downstream reporters recapitulate the expression of the endogenous NEUROG2, circumventing the potentially lengthy differentiation process. This is of particular importance for difficult-to-differentiate lineages for which defined protocols are lacking. Taking the advantages of both CRISPR-mediated transactivation approach and a battery of neural lineage reporters generated in the lab, including NEUROG2-mCherry-hygromycin (this article), OLIG2-GFP, and MNX1 (HB9)-GFP hiPSC reporters (article in preparation), we have been able to test and validate multiple sgRNAs that could transactivate these transcription factors. These sgRNAs will be further applied to experiments such as promoter hunting, direct lineage conversion by overexpression of specific transcription factors [57], and transcription binding site identification, among other purposes.
Significance of NEUROG2 reporter for application in neural differentiation
NEUROG2 belongs to the neurogenin family of the class II basic helix-loop-helix transcription factors, which orchestrates neural cell type fate choices during development. Rodent Neurog1 and 2 have been shown to promote neuronal cell type determination and regional patterning specification, in both CNS and periperal nervous system [58–60]. Specifically, Neurog1 and 2 are required for differentiation and specification of spinal motor neurons [61–63], telencephalic neurons and thalamic neurons [60], olfactory cortical neurons [64,65], hippocampal granule neurons [66], and cranial sensory ganglia [67,68]. Neurog1 and 2 also control the maturation of neurons by maintaining the balance of neuronal progenitors and terminally differentiated neurons [69]. Neurog1 and 2 double knockout mice fail to generate motor neurons and glutamatergic neurons [59], and they cannot form dorsal ventral neuronal identify in the forebrain [60]. In addition, Neurog1 and 2 cross-regulate each other and cooperate with other transcription factors. For instance, Neurog2 interacts with Ascl1 (Mash1) [60], which is expressed in the complementary ventral regions of telecephalon and is downregulated by neurogenins in the dorsal cortical regions. Neurog2 also counteracts the transcriptional repressor activities of Olig2 in motor neuron determination, where Neurog2 competes with Olig2 for DNA binding targets (such as E-box elements) and squelches Olig2 from its binding partners at the protein level [70]. Moreover, Neurog1 and 2 cooperate with Fezf2, and Hes1 and 5 [71] for cortical neuron specification during cortical development through Notch and Wnt pathways. It has been shown that phosphorylation of Neurog2 by glycogen synthase kinase 3 (GSK3) is necessary and sufficient to specify motor neuron fate in the spinal cord [42] and important in neocortical neurogenesis [42]. Considering that GSK3 can be regulated by multiple pathways such as Wnt and BMP, Neurog2 might play a broad even decisive role in neurogenesis [72]. Indeed, Neurog2 has been used as a single or major transcritption factor that forces reprogramming of other somatic cell types into neurons both in vivo in the mouse neonatal cerebral cortex [73,74] and in vitro [75,76].
Most of these experiments, however, only reveal the function of mouse neurogenins. The precise function of human NEUROG1 and 2 in development and disease remains largely unknown, mainly due to the limited availability of research materials such as human fetal and postmortem tissues [77]. The dual NEUROG2 knockin reporter will facilitate in exploring the potentially dynamic roles of neurogenins in human nervous system, offer novel insights on the complex transcriptional control of human neural development, and provide a tool for future study on gene functions and neural circuit regulation.
In summary, we have generated dual reporter for NEUROG2 using the highly efficient yet precise CRISPR/Cas9-mediated gene targeting in hiPSCs. The dual reporter enables direct monitoring of NEUROG2 expression and obtaining purified NEUROG2 populations derived from hiPSCs, serving as an invaluable tool for subsequent in-depth characterization of hiPSC differentiation along the neural lineage.
Supplementary Material
Acknowledgments
This work was supported by the Department of Neurosurgery, University of Texas Health Science Center at Houston, Memorial Hermann Foundation-Staman Ogilvie Fund (to Y.L.), the Bentsen Stroke Center Fund (to Y.L.), Mission Connect-TIRR Foundation (to Y.L.), NIH/NIAMS subcontract (to Y.L.), NIH R01NS061983 and R01ES015988 (to W.D.), the National Multiple Sclerosis Society (to W.D.), and Shriners Hospitals for Children (to W.D.). We wish to thank Dr. J. Zhang for technical assistance and Dr. A. Hazen at the Flow Cytometry Core for assistance in cell sorting.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- 4.Zwaka TP. and Thomson JA. (2003). Homologous recombination in human embryonic stem cells. Nat Biotechnol 21:319–321 [DOI] [PubMed] [Google Scholar]
- 5.Davis RP, Ng ES, Costa M, Mossman AK, Sourris K, Elefanty AG. and Stanley EG. (2008). Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111:1876–1884 [DOI] [PubMed] [Google Scholar]
- 6.Ruby KM. and Zheng B. (2009). Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells 27:1496–1506 [DOI] [PubMed] [Google Scholar]
- 7.Xue H, Wu S, Papadeas ST, Spusta S, Swistowska AM, MacArthur CC, Mattson MP, Maragakis NJ, Capecchi MR, et al. (2009). A targeted neuroglial reporter line generated by homologous recombination in human embryonic stem cells. Stem Cells 27:1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Jiang P. and Deng W. (2011). OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nat Protoc 6:640–655 [DOI] [PubMed] [Google Scholar]
- 9.Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, et al. (2009). Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol 27:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schinzel RT, Ahfeldt T, Lau FH, Lee YK, Cowley A, Shen T, Peters D, Lum DH. and Cowan CA. (2011). Efficient culturing and genetic manipulation of human pluripotent stem cells. PLoS One 6:e27495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P, Rodriguez RT, Wang J, Ghodasara A. and Kim SK. (2011). Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 8:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulburn AL, Alden D, Davis RP, Micallef SJ, Ng ES, Yu QC, Lim SM, Soh CL, Elliott DA, et al. (2011). A targeted NKX2.1 human embryonic stem cell reporter line enables identification of human basal forebrain derivatives. Stem Cells 29:462–473 [DOI] [PubMed] [Google Scholar]
- 13.Aizawa E, Hirabayashi Y, Iwanaga Y, Suzuki K, Sakurai K, Shimoji M, Aiba K, Wada T, Tooi N, et al. (2012). Efficient and accurate homologous recombination in hESCs and hiPSCs using helper-dependent adenoviral vectors. Mol Ther 20:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brafman DA, Moya N, Allen-Soltero S, Fellner T, Robinson M, McMillen ZL, Gaasterland T. and Willert K. (2013). Analysis of SOX2-expressing cell populations derived from human pluripotent stem cells. Stem Cell Reports 1:464–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA. and Charpentier E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F. and Jaenisch R. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE. and Church GM. (2013). RNA-guided human genome engineering via Cas9. Science 339:823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR. and Joung JK. (2013). Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedland AE, Tzur YB, Esvelt KM, Colaiacovo MP, Church GM. and Calarco JA. (2013). Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods 10:741–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiCarlo JE, Norville JE, Mali P, Rios X, Aach J. and Church GM. (2013). Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res 41:4336–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA. and Zhang F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, et al. (2014). Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156:836–843 [DOI] [PubMed] [Google Scholar]
- 23.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE. and Tsien RY. (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572 [DOI] [PubMed] [Google Scholar]
- 24.Yu C, Liu Y, Ma T, Liu K, Xu S, Zhang Y, Liu H, La Russa M, Xie M, Ding S. and Qi LS. (2015). Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell 16:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu VT, Weber T, Wefers B, Wurst W, Sander S, Rajewsky K. and Kuhn R. (2015). Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol 33:543–548 [DOI] [PubMed] [Google Scholar]
- 26.Xue H, Wu J, Li S, Rao MS. and Liu Y. (2016). Genetic modification in human pluripotent stem cells by homologous recombination and CRISPR/Cas9 system. Methods Mol Biol 1307:173–190 [DOI] [PubMed] [Google Scholar]
- 27.Macarthur CC, Xue H, Van Hoof D, Lieu PT, Dudas M, Fontes A, Swistowski A, Touboul T, Seerke R, et al. (2012). Chromatin insulator elements block transgene silencing in engineered human embryonic stem cell lines at a defined chromosome 13 locus. Stem Cells Dev 21:191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sander JD, Zaback P, Joung JK, Voytas DF. and Dobbs D. (2007). Zinc finger targeter (ZiFiT): an engineered zinc finger/target site design tool. Nucleic Acids Res 35:W599–W605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander JD, Maeder ML, Reyon D, Voytas DF, Joung JK. and Dobbs D. (2010). ZiFiT (zinc finger targeter): an updated zinc finger engineering tool. Nucleic Acids Res 38:W462–W468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Xue H, Long B, Sun L, Truong T. and Liu Y. (2015). Efficient generation of hiPSC neural lineage specific knockin reporters using the CRISPR/Cas9 and Cas9 double nickase system. J Vis Exp e52539; DOI: 10.3791/52539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber E, Gruetzner R, Werner S, Engler C. and Marillonnet S. (2011). Assembly of designer TAL effectors by Golden Gate cloning. PLoS One 6:e19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber E, Engler C, Gruetzner R, Werner S. and Marillonnet S. (2011). A modular cloning system for standardized assembly of multigene constructs. PLoS One 6:e16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler C, Gruetzner R, Kandzia R. and Marillonnet S. (2009). Golden gate shuffling: a one-pot DNA shuffling method based on type IIs restriction enzymes. PLoS One 4:e5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guschin DY, Waite AJ, Katibah GE, Miller JC, Holmes MC. and Rebar EJ. (2010). A rapid and general assay for monitoring endogenous gene modification. Methods Mol Biol 649:247–256 [DOI] [PubMed] [Google Scholar]
- 35.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA. and Zhang F. (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8:2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao A, Cheng Z, Kong L, Zhu Z, Lin S, Gao G. and Zhang B. (2014). CasOT: a genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 30:1180–1182 [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Hoing S, Moritz S, Parga JA, Wagner L, et al. (2013). Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One 8:e59252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swistowski A, Peng J, Han Y, Swistowska AM, Rao MS. and Zeng X. (2009). Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One 4:e6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I. and Rao MS. (2004). CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol 276:31–46 [DOI] [PubMed] [Google Scholar]
- 40.Fu Y, Sander JD, Reyon D, Cascio VM. and Joung JK. (2014). Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32:279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Wu Y, Lee JC, Xue H, Pevny LH, Kaprielian Z. and Rao MS. (2002). Oligodendrocyte and astrocyte development in rodents: an in situ and immunohistological analysis during embryonic development. Glia 40:25–43 [DOI] [PubMed] [Google Scholar]
- 42.Ma YC, Song MR, Park JP, Henry Ho HY, Hu L, Kurtev MV, Zieg J, Ma Q, Pfaff SL. and Greenberg ME. (2008). Regulation of motor neuron specification by phosphorylation of neurogenin 2. Neuron 58:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu Y, Reyon D. and Joung JK. (2014). Targeted genome editing in human cells using CRISPR/Cas nucleases and truncated guide RNAs. Methods Enzymol 546:21–45 [DOI] [PubMed] [Google Scholar]
- 44.Capecchi MR. (2005). Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet 6:507–512 [DOI] [PubMed] [Google Scholar]
- 45.Folger KR, Wong EA, Wahl G. and Capecchi MR. (1982). Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol 2:1372–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bae S, Park J. and Kim JS. (2014). Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30:1473–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Bae S, Park J, Kim E, Kim S, Yu HR, Hwang J, Kim JI. and Kim JS. (2015). Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods 12:237–243 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Wang Y, Wu X, Wang J, Wang Y, Qiu Z, Chang T, Huang H, Lin RJ. and Yee JK. (2015). Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol 33:175–178 [DOI] [PubMed] [Google Scholar]
- 49.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, Scott DA, Inoue A, Matoba S, Zhang Y. and Zhang F. (2013). Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154:1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L. and Church GM. (2013). CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31:833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ. and Joung JK. (2014). Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilinger JP, Thompson DB. and Liu DR. (2014). Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification. Nat Biotechnol 32:577–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiler C, Davuluri G, Abrams J, Byfield FJ, Janmey PA. and Pack M. (2012). Smooth muscle tension induces invasive remodeling of the zebrafish intestine. PLoS Biol 10:e1001386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baird GS, Zacharias DA. and Tsien RY. (2000). Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci U S A 97:11984–11989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, et al. (2014). Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, et al. (2015). Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature 517:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, Iyer PRE, Lin S, Kiani S, Guzman CD, et al. (2015). Highly efficient Cas9-mediated transcriptional programming. Nat Methods 12:326–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Q, Kintner C. and Anderson DJ. (1996). Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87:43–52 [DOI] [PubMed] [Google Scholar]
- 59.Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C. and Guillemot F. (1998). The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron 20:483–494 [DOI] [PubMed] [Google Scholar]
- 60.Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ. and Guillemot F. (2000). A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev 14:67–80 [PMC free article] [PubMed] [Google Scholar]
- 61.Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K. and Nakafuku M. (2001). Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron 31:757–771 [DOI] [PubMed] [Google Scholar]
- 62.Novitch BG, Chen AI. and Jessell TM. (2001). Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31:773–789 [DOI] [PubMed] [Google Scholar]
- 63.Scardigli R, Schuurmans C, Gradwohl G. and Guillemot F. (2001). Crossregulation between Neurogenin2 and pathways specifying neuronal identity in the spinal cord. Neuron 31:203–217 [DOI] [PubMed] [Google Scholar]
- 64.Dixit R, Wilkinson G, Cancino GI, Shaker T, Adnani L, Li S, Dennis D, Kurrasch D, Chan JA, et al. (2014). Neurog1 and neurog2 control two waves of neuronal differentiation in the piriform cortex. J Neurosci 34:539–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roybon L, Deierborg T, Brundin P. and Li JY. (2009). Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci 29:232–243 [DOI] [PubMed] [Google Scholar]
- 66.Roybon L, Hjalt T, Stott S, Guillemot F, Li JY. and Brundin P. (2009). Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One 4:e4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL. and Anderson DJ. (1998). neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron 20:469–482 [DOI] [PubMed] [Google Scholar]
- 68.Zirlinger M, Lo L, McMahon J, McMahon AP. and Anderson DJ. (2002). Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci U S A 99:8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hindley C, Ali F, McDowell G, Cheng K, Jones A, Guillemot F. and Philpott A. (2012). Post-translational modification of Ngn2 differentially affects transcription of distinct targets to regulate the balance between progenitor maintenance and differentiation. Development 139:1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SK, Lee B, Ruiz EC. and Pfaff SL. (2005). Olig2 and Ngn2 function in opposition to modulate gene expression in motor neuron progenitor cells. Genes Dev 19:282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu T, Nakazawa M, Kani S, Bae YK, Shimizu T, Kageyama R. and Hibi M. (2010). Zinc finger genes Fezf1 and Fezf2 control neuronal differentiation by repressing Hes5 expression in the forebrain. Development 137:1875–1885 [DOI] [PubMed] [Google Scholar]
- 72.Busskamp V, Lewis NE, Guye P, Ng AH, Shipman SL, Byrne SM, Sanjana NE, Murn J, Li Y, et al. (2014). Rapid neurogenesis through transcriptional activation in human stem cells. Mol Syst Biol 10:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B. and Gotz M. (2007). Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci 27:8654–8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mazzoni EO, Mahony S, Closser M, Morrison CA, Nedelec S, Williams DJ, An D, Gifford DK. and Wichterle H. (2013). Synergistic binding of transcription factors to cell-specific enhancers programs motor neuron identity. Nat Neurosci 16:1219–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM. and Zhang CL. (2013). Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun 4:2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, et al. (2013). Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78:785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perrin FE, Boniface G, Serguera C, Lonjon N, Serre A, Prieto M, Mallet J. and Privat A. (2010). Grafted human embryonic progenitors expressing neurogenin-2 stimulate axonal sprouting and improve motor recovery after severe spinal cord injury. PLoS One 5:e15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krentz NA, Nian C. and Lynn FC. (2014). TALEN/CRISPR-mediated eGFP knock-in add-on at the OCT4 locus does not impact differentiation of human embryonic stem cells towards endoderm. PLoS One 9:e114275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.