Abstract
Accumulating evidence suggests that interferon (IFN) alpha/beta are involved in antitumor immunity and cancer immunoediting, but information on the antitumor effects of IFN alpha/beta in lung cancer is limited. In our study, we elucidated the IFN alpha/beta signature during both human fetal lung development and lung tumorigenesis. Our findings indicated gradual upregulation in the IFN alpha/beta signature during human fetal lung development. In addition, this signature was progressively downregulated in normal human airway epithelial cells from lung cancer patients, in immortalized human bronchial epithelial cell lines from later passages, in late-stage lung squamous cell carcinoma (LSCC) tissues, and in LSCC tissues exhibiting lymph node metastasis. Therefore, from its earliest stages, lung tumorigenesis may be associated with a decreased IFN alpha/beta signature. This association may provide insight to guide the detection of high-risk lung cancer patients.
Introduction
Interferon (IFN) alpha/beta directly influence tumor cell proliferation, apoptosis, angiogenesis, and immune function (Sidky and Borden 1987; Brierley and Fish 2002; Chawla-Sarkar and others 2003). IFN alpha/beta also induce tumor suppressor genes (Takaoka and others 2003) and affect host immune cells during the antitumor response (Dunn and others 2005). Moreover, similar to IFN gamma, IFN alpha/beta have been shown to be critical in the process of cancer immunoediting (Dunn and others 2005; Diamond and others 2011).
IFN alpha/beta are currently used in the treatment of several types of cancer, including chronic myeloid leukemia, malignant melanoma, and pancreatic cancer (Bekisz and others 2004; Kameshima and others 2013). However, information on the antitumor effects of IFN alpha/beta in lung cancer is limited (Ruotsalainen and others 2000; Trinchieri 2010; Sundar and others 2014). A more fundamental understanding of IFN alpha/beta is needed to provide further insights that may lead to the development of effective IFN-based cancer immunotherapies.
With the development of high-throughput methods, including gene expression analysis, a valuable and deeper biological understanding of lung cancer has rapidly developed. Furthermore, certain tumor initiation processes as well as genetic and genomic changes have been progressively revealed (Nacht and others 2001; Lawrence and others 2014). Our group previously reported gene expression profiles during human lung development, and a large number of genes involved in lung development were found to be related to the immune response (Feng and others 2014). Herein, we present alterations in the expression profile of the IFN alpha/beta signature in the fetal lung, in normal human airway epithelial cells (AECs), in an immortalized human bronchial epithelial cell line (Y-BE), and in lung squamous cell carcinoma (LSCC) tissues.
Materials and Methods
Clinical specimen collection and immortalized human bronchial epithelial cell lines
AECs of the large airway were collected from consecutive patients with suspected lung cancer using a disposable brush (Endoscopic Cytobrush; Micro-Tech). This collection was performed by a bronchoscopist during bronchoscopy before surgery in the endoscopy departments of 2 cancer centers (Cancer Hospital, Chinese Academy of Medical Sciences [CAMS], and the Beijing Cancer Hospital, Peking University). The inclusion criteria and the sampling location for the enrolled clinical samples are presented in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/jir). As soon as the brush was removed from the patient's airway, the assistant placed the brush head in a tube with RNAlater™ (Ambion), and the brush was sufficiently shaken to release all of the cells on the brush into the reagent. The immortalized human bronchial epithelial cell line Y-BE, derived from outgrowths of biopsy tissue from a female individual without cancer, was established and maintained in our laboratory as previously reported (Lu and others 1996).
LSCC tissue samples (69 cases) were collected from patients who had undergone surgical resection at the Cancer Hospital, CAMS. Fresh tissues were snap frozen in liquid nitrogen and stored at 80°C before RNA preparation. Histological and TNM (tumor, node, and metastasis) staging information was collected from electronic medical records. The use of human samples in this study was approved by the Institutional Review Boards of CAMS, and written informed consent was obtained from all patients.
RNA extraction and gene expression microarray
For the AEC samples, total RNA was extracted using the RNeasy Micro Kit (Qiagen), and for Y-BE samples and LSCC tissue samples, total RNA was extracted using the RNeasy Mini Kit (Qiagen). All of these kits were used according to product specifications. The RNA quantity was then measured using an ND-1000 UV-VIS Spectrophotometer (NanoDrop Technologies), and the RNA quality was determined using a 2100 Bioanalyzer (Agilent Technologies). All of the RNA samples used in this study exhibited OD260/280 ratios greater than 1.9 and RNA integrity numbers greater than 6.
AEC samples were analyzed using an Agilent SurePrint G3 Human GE v2 8x60K Microarray (G4851B), and Y-BE p115 and p25 (with analyses of each repeated 4 times) as well as LSCC tissue samples were analyzed using an Agilent Whole Human Genome Microarray 4x44K (G4112F). All of the sample labeling, hybridization, washing, and scanning steps were conducted following the manufacturer's specifications. For AEC samples, the slides were scanned using the Agilent SureScan Microarray Scanner (G2600D) and analyzed with Agilent Feature Extraction Software (v10.5.1.1), and for Y-BE and LSCC samples, the slides were scanned using the Agilent Microarray Scanner System (G2505B) and analyzed with Agilent Feature Extraction Software (v9.1). Data extraction and annotation were performed using GeneSpring GX v12.6.1 (Agilent). The raw data from the AEC samples were quantile normalized using GeneSpring, whereas the raw data from Y-BE and the LSCC tissues were normalized by the median scale method using the R package “limma” (www.r-project.org). All of the raw microarray data and the normalized data are available in the Gene Expression Omnibus (GSE67061).
Analysis of public microarray data sets
Two independent sets of airway epithelium-derived microarray data [“GSE4115” (Spira and others 2007) and “GSE19027” (Wang and others 2010)] as well as their corresponding clinical information were collected from existing publications for validation.
Statistical analyses
Gene set enrichment analysis (GSEA) is a well-established computational method that can be used to evaluate a query microarray data set using a collection of gene sets to identify related gene sets with common biological function, chromosomal location, or regulation (Subramanian and others 2005, 2007). GSEA is not focused on significantly changed genes, but rather on the common trends in the picked gene sets between 2 different phenotypes.
When performing GSEA, 1 or more functional gene subsets are first chosen from the Molecular Signatures Database (MSigDB), and then normalized signal intensity is used to rank genes according to their scores between 2 biological statuses. Eventually, the random distribution or a certain tendency of the gene set will be revealed. In our study, the GSEA was focused on “reactome IFN alpha/beta signaling” within MSigDB, which was calculated using the R statistical software package (www.r-project.org) for the enrolled expression profiles. “Reactome IFN alpha/beta signaling” is from the subcollection of “CP:REACTOME: Reactome gene sets” from “C2: curated gene sets,” including 64 genes involved in IFN alpha/beta signaling (Supplementary Table S1).
Results
Sample information and general results
In this study, comparisons among 8 paired groups were conducted to analyze the IFN alpha/beta signature (Table 1). In our previous study, the global gene expression profiles of mid-term and early term fetal lungs from the fetal tissues of spontaneous abortion cases as well as mature lungs from cancer-free peripheral lung tissues were presented; further data mining focused on the IFN alpha/beta signature among the human fetal lung samples was performed in the present study. Additionally, the expression profiles of AECs, Y-BE cells, and LSCC samples were obtained and provided the basis for the following data analysis.
Table 1.
Sample Information Among the 8 Paired Groups Investigated in the Study
| Group | Samples | GEO number | Comparison | Sample number |
|---|---|---|---|---|
| A | Fetal lung | GSE43767 | Mid-term fetal lung versus early-term fetal lung | 9 versus 10 |
| B | Fetal lung | GSE43767 | Mature lung versus mid-term fetal lung | 15 versus 9 |
| C | AECs | GSE67061 | AECs with lung cancer versus AECs without cancer | 56 versus 17 |
| D | AECs | GSE4115 | AECs with lung cancer versus AECs without cancer | 97 versus 90 |
| E | AECs | GSE19027 | AECs with lung cancer versus AECs without cancer | 21 versus 39 |
| F | Y-BE | GSE67061 | Y-BE p115 versus p25 | 4 versus 4 |
| G | LSCC | GSE67061 | Late stage versus early stage* | 22 versus 46 |
| H | LSCC | GSE67061 | With lymph node metastasis versus without lymph node metastasis | 33 versus 36 |
Late stage represents Stage III, whereas early stage represents Stages I and II.
AECs, airway epithelial cells; LSCC, lung squamous cell carcinoma.
GSEA for “reactome IFN alpha/beta signaling”
Upregulation of the IFN alpha/beta signature in the fetal lung development
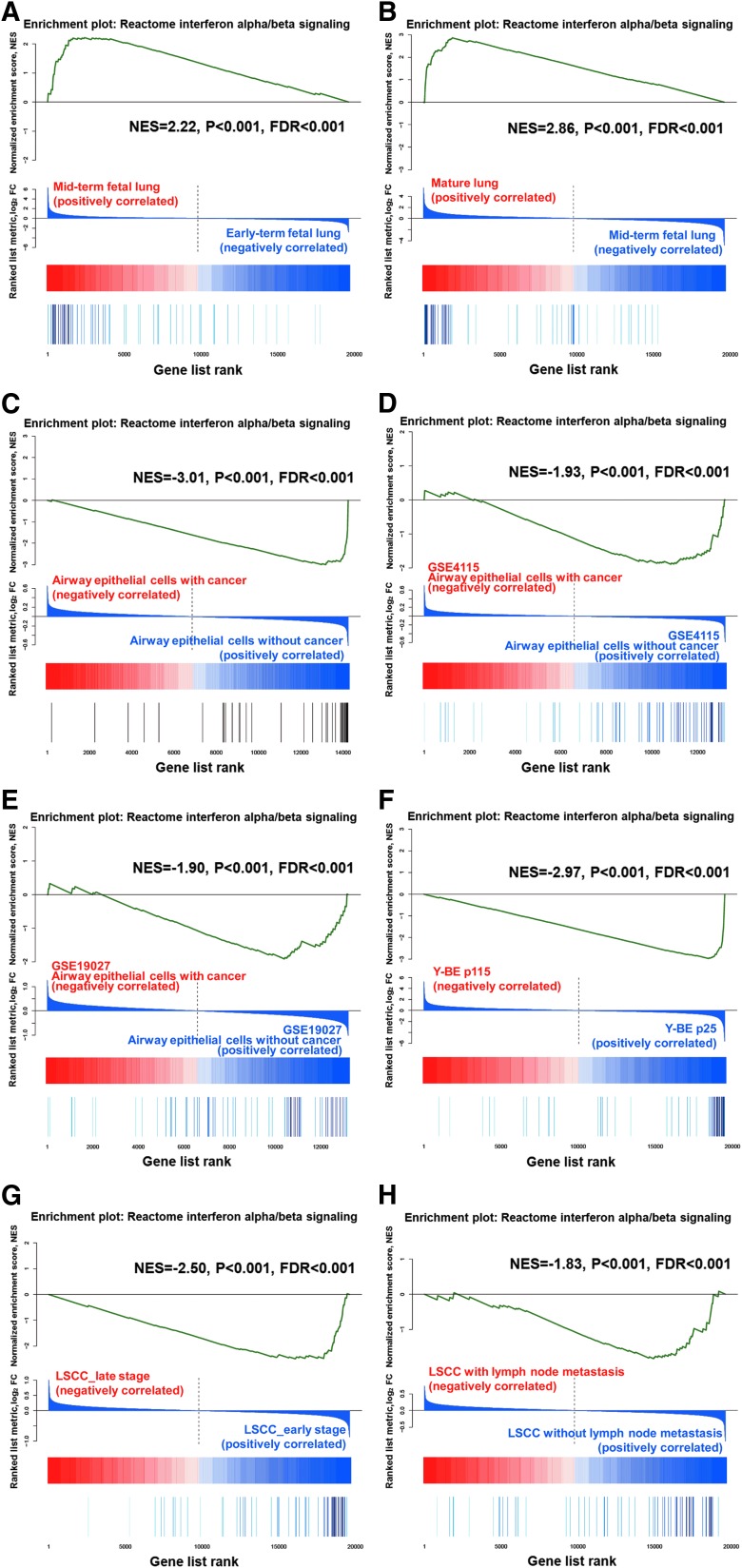
We applied GSEA to the fetal lung data sets (from previous work by our group, GSE43767). The IFN alpha/beta signature was enriched in the mid-term fetal lung compared with the early term fetal lung, with a normalized enrichment score (NES) of 2.22 (P value <0.001, false discovery rate (FDR) <0.001, (Fig. 1A). The same trend was also observed for the mature lung compared with the mid-term fetal lung, with an NES of 2.86 (P value <0.001, FDR <0.001, (Fig. 1B). These results demonstrate progressive upregulation of the IFN alpha/beta signature in the fetal lung development.
FIG. 1.
Gene set enrichment analysis (GSEA) for “reactome IFN alpha/beta signaling” expression profiles for Groups A, B, C, D, E, F, G, and H. Each of the vertical bars at the bottom represents a gene, and the green curve expresses the normalized enrichment score of each gene. (A) Depicts the results for the fetal lung data sets of Group A, which correlate with the phenotype of the mid-term fetal lung compared with the early term fetal lung. (B) Depicts the results for the fetal lung data sets of Group B, which correlate with the phenotype of the mature lung compared with the mid-term fetal lung. (C) Presents the results for the airway epithelial cell (AEC) data sets of Group C, which correlate with the phenotype with lung cancer compared with that without cancer. (D) Presents the results for the AEC data sets (GSE4115) of Group D, which correlate with the phenotype with lung cancer compared with that without cancer. (E) Depicts the results for the AEC data sets (GSE19027) of Group E, which correlate with the phenotype with lung cancer compared with that without cancer. (F) Depicts the results for the Y-BE data sets of Group F, which correlate with the phenotype of p115 compared with p25. (G) Depicts the results for the lung squamous cell carcinoma (LSCC) data sets of Group G, which correlate with the phenotype at the late stage compared with the early stage. (H) Presents the results for the LSCC data sets of Group H, which correlate with the phenotype with lymph node metastasis compared with that without lymph node metastasis.
Downregulation of the IFN alpha/beta signature in AECs from lung cancer patients
The basic characteristics of the enrolled AEC samples are presented in Supplementary Table S2. GSEA revealed that the IFN alpha/beta signature was significantly enriched in AECs from the noncancer group, with an NES of −3.01 [P value <0.001, FDR <0.001, (Fig. 1C)]. The additional 2 independent public sets of airway epithelium-derived microarray data (GSE4115 and GSE19027) were analyzed using the same method, and the IFN alpha/beta signature was also found to be enriched in noncancer patients (Fig. 1D, E). The results demonstrate downregulation of the IFN alpha/beta signature in AECs from lung cancer patients.
Downregulation of the IFN alpha/beta signature in Y-BE cells at a later passage (p115 versus p25)
Based on a comparison of the expression profiles of p115 and p25, the GSEA results indicate that IFN alpha/beta signature-related gene sets were enriched in Y-BE cells at p25, with an NES of −2.97 [P value<0.001, FDR <0.001, (Fig. 1F)]. These results suggest that downregulation of the IFN alpha/beta signature is significantly associated with later passages.
Downregulation of the IFN alpha/beta signature in late-stage LSCC tissue with or without lymph node metastasis
The basic characteristics of the enrolled LSCC tissue samples are presented in Supplementary Table S3. Based on a comparison of the expression profiles of late-stage (stage III) and early stage (stage I and stage II) LSCC tissues, the IFN alpha/beta signature was significantly upregulated in early stage LSCC tissues [NES=−2.50, P value <0.001, FDR <0.001, (Fig. 1G)]. In addition, a comparison of the expression profile of LSCC tissues with lymph node metastasis with that of LSCC tissues without lymph node metastasis revealed that this signature was significantly upregulated in the tissues without lymph node metastasis [NES=−1.83, P value <0.001, FDR <0.001, (Fig. 1H)]. Thus, IFN alpha/beta signature downregulation was associated with late-stage LSCC and lymph node metastasis.
Fold change in the expression of IFN alpha/beta signature-related genes between different developmental stages
The fold change in the expression of IFN alpha/beta signature-related genes was calculated between fetal lung data, our AEC data, Y-BE data, and LSCC data. The corresponding numbers of genes detected in these 4 data sets among the 64 genes involved in the “reactome IFN alpha/beta signaling” were 58 genes, 51 genes, 56 genes, and 58 genes, respectively, which were termed “IFN alpha/beta signature genes.”
For the fetal lung data, 45 genes among the 58 detected with IFN alpha/beta signature genes were upregulated as part of the phenotype of the mid-term fetal lung compared with the early term fetal lung, whereas 49 genes among the 58 detected with IFN alpha/beta signature genes were upregulated as part of the phenotype of the mature lung compared with the mid-term fetal lung. The gene expression values showed a mainly upward trend during human fetal lung development.
For our AEC samples, 46 genes among the 51 detected IFN alpha/beta signature genes were downregulated as part of the phenotype of the AECs with lung cancer compared with the AECs without cancer. For the Y-BE samples, 47 genes of the 56 detected IFN alpha/beta signature genes were downregulated as part of the phenotype of Y-BE p115 compared with Y-BE p25. Finally, for the LSCC samples, of the 58 detected IFN alpha/beta signature genes, 33 genes were downregulated as part of the phenotype of late stage LSCC compared with early stage LSCC, whereas 42 genes were downregulated as part of the phenotype of LSCC with lymph node metastasis compared with LSCC without lymph node metastasis (Supplementary Fig. S2). Additionally, the gene expression values showed a mainly downward trend during human lung tumorigenesis.
Discussion
In this study, progressive upregulation of the IFN alpha/beta signature was revealed during the progression from the early term fetal lung to the mid-term fetal lung as well as from the mid-term fetal lung to the mature lung. Downregulation of this signature was also observed in the AECs from lung cancer patients, the Y-BE cells at a later passage, the late-stage LSCC tissues, and the LSCC tissues exhibiting lymph node metastasis. The fold change in the expression of the IFN alpha/beta signature genes between these groups also followed the same trend.
AECs, Y-BE cells, and LSCC samples are characterized as normal cells in the presence or absence of nearby cancerous lesions, as an immortalized cell line that exhibits altered proliferation, but is nontumorigenic in nude mice (An and others 2003), and as cancerous cells, respectively. Collectively, these cells reflect a progressively increasing degree of malignancy. Our study results indicate that the IFN alpha/beta signature gradually increases during human fetal lung development. Conversely, the IFN alpha/beta signature was downregulated during lung tumorigenesis. Furthermore, repression of this signature was associated with the lymph node metastasis of lung cancer, reflecting the signature's involvement in late-stage tumorigenesis.
The similarity and relationship between embryo development and tumorigenesis have been investigated and recognized in regard to invasive cellular behaviors, gene expression, and other important biological behaviors, including immune escape mechanisms (Wilczynski 2006; Naxerova and others 2008; Ma and others 2010). Recently published findings have also demonstrated the timely and regulated maturation of the T cell repertoire during human fetal development (Rechavi and others 2015). Consistent with these findings, upregulation of the IFN alpha/beta signature during fetal lung development as well as downregulation during lung tumorigenesis was observed in our study. Regarding AECs, which are normal airway cells, downregulation of the IFN alpha/beta signature was observed in the AECs from lung cancer patients, supporting the theory that alterations in the airway epithelium mirror several of the alterations observed in lung cancer itself (Steiling and others 2008; Gomperts and others 2013).
Beyond the airway epithelium, Y-BE cells, an immortalized human bronchial epithelial cell line established and preserved by our laboratory, are probably at the premalignant stage and may serve as a useful in vitro model for investigating the multiple events involved in carcinogenesis (Lu and others 1996; An and others 2003). Our previous work demonstrated faster cell growth, reduced dependence on epidermal growth factor, resistance to serum-induced differentiation, and a decrease in cisplatin-induced apoptosis in the later passages of these cells, although both early and late passages were not oncogenic when applied in tumor transplantation into nude mice (Cheng and others 2001). These results indicated that Y-BE cells at later passages approach malignant transformation more often compared with earlier passages. Consistent with these previous findings, IFN alpha/beta signature downregulation was detected in the later passage of Y-BE cells (p115) in the present study.
For the LSCC groups, a late stage and lymph node metastasis were correlated with IFN alpha/beta signature downregulation, which is consistent with the hypothesis that IFN alpha/beta play an essential role in enabling the host to restrict tumor growth (Gresser and Belardelli 2002).
Our study has shed light on the alterations in the IFN alpha/beta signature during the early events of lung tumorigenesis. Because AECs and Y-BE cells are normal and premalignant cells, respectively, the expression profiles of these cells may represent several of the early events that occur during the course of lung tumorigenesis. The common downregulation of the IFN alpha/beta signature may be indicative of an early event in lung tumorigenesis.
The rate of lung cancer in never smokers is expected to increase due to lung cancer screening projects, especially with the revelation of the mortality benefits (National Lung Screening Trial Research and others 2013) and with increasing public awareness of cancer (Ironmonger and others 2015). All of the AEC samples used in the present study were obtained from nonsmoking patients; thus, fundamental data on nonsmoker airway expression profiles are available for future research. In addition, the decreased IFN alpha/beta signature during this early event may be useful for the detection of high-risk lung cancer patients among nonsmokers. Certainly, this possibility merits further study.
In summary, the IFN alpha/beta signature is increased during fetal lung development and decreased during lung tumorigenesis. Knowledge of the regulation of the IFN alpha/beta signature may provide certain indicators for the detection of high-risk lung cancer patients.
Supplementary Material
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 Program, No. 2014AA020602) and the Beijing Science and Technology Plan Projects (Z111107067311018). Accession numbers: All new raw microarray data and normalized data have been submitted to the Gene Expression Omnibus under accession number GSE67061 (this remains private, so the data can be accessed using the following link: www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=cfuziwsabjqbbwr&acc=GSE67061).
Author Disclosure Statement
No competing financial interests exist.
References
- An Q, Pacyna-Gengelbach M, Schluns K, Deutschmann N, Guo S, Gao Y, Zhang J, Cheng S, Petersen I. 2003. Identification of differentially expressed genes in immortalized human bronchial epithelial cell line as a model for in vitro study of lung carcinogenesis. Int J Cancer 103(2):194–204 [DOI] [PubMed] [Google Scholar]
- Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. 2004. Human interferons alpha, beta and omega. Growth Factors 22(4):243–251 [DOI] [PubMed] [Google Scholar]
- Brierley MM, Fish EN. 2002. Review: IFN-alpha/beta receptor interactions to biologic outcomes: understanding the circuitry. J Interferon Cytokine Res 22(8):835–845 [DOI] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. 2003. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8(3):237–249 [DOI] [PubMed] [Google Scholar]
- Cheng S, Gao Y, Dong X, Lu Y, An Q, Tong T, Wang Y. 2001. Molecular and cytogenetic alterations in early stage of carcinogenesis of human lung. Cancer Lett 162 (Suppl):S5–S10 [DOI] [PubMed] [Google Scholar]
- Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD. 2011. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208(10):1989–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM, Schreiber RD. 2005. A critical function for type I interferons in cancer immunoediting. Nat Immunol 6(7):722–729 [DOI] [PubMed] [Google Scholar]
- Feng L, Wang J, Cao B, Zhang Y, Wu B, Di X, Jiang W, An N, Lu D, Gao S, Zhao Y, Chen Z, Mao Y, Gao Y, Zhou D, Jen J, Liu X, Zhang Y, Li X, Zhang K, He J, Cheng S. 2014. Gene expression profiling in human lung development: an abundant resource for lung adenocarcinoma prognosis. PLoS One 9(8):e105639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts BN, Walser TC, Spira A, Dubinett SM. 2013. Enriching the molecular definition of the airway “field of cancerization”: establishing new paradigms for the patient at risk for lung cancer. Cancer Prev Res (Phila) 6(1):4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I, Belardelli F. 2002. Endogenous type I interferons as a defense against tumors. Cytokine Growth Factor Rev 13(2):111–118 [DOI] [PubMed] [Google Scholar]
- Ironmonger L, Ohuma E, Ormiston-Smith N, Gildea C, Thomson CS, Peake MD. 2015. An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br J Cancer 112(1):207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameshima H, Tsuruma T, Kutomi G, Shima H, Iwayama Y, Kimura Y, Imamura M, Torigoe T, Takahashi A, Hirohashi Y, Tamura Y, Tsukahara T, Kanaseki T, Sato N, Hirata K. 2013. Immunotherapeutic benefit of alpha-interferon (IFNalpha) in survivin2B-derived peptide vaccination for advanced pancreatic cancer patients. Cancer Sci 104(1):124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, Meyerson M, Gabriel SB, Lander ES, Getz G. 2014. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505(7484):495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YJ, Dong XY, Guo SP, Ke Y, Cheng SJ. 1996. Integration of SV40 at 12q23 in SV40-immortalized human bronchial epithelial cells. Carcinogenesis 17(9):2089–2091 [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang P, Wang F, Yang J, Yang Z, Qin H. 2010. The relationship between early embryo development and tumourigenesis. J Cell Mol Med 14(12):2697–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht M, Dracheva T, Gao Y, Fujii T, Chen Y, Player A, Akmaev V, Cook B, Dufault M, Zhang M, Zhang W, Guo M, Curran J, Han S, Sidransky D, Buetow K, Madden SL, Jen J. 2001. Molecular characteristics of non-small cell lung cancer. Proc Natl Acad Sci U S A 98(26):15203–15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Lung Screening Trial Research Team, Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC, Mahon I, Marcus PM, Sicks JD, Jain A, Baum S. 2013. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368(21):1980–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naxerova K, Bult CJ, Peaston A, Fancher K, Knowles BB, Kasif S, Kohane IS. 2008. Analysis of gene expression in a developmental context emphasizes distinct biological leitmotifs in human cancers. Genome Biol 9(7):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi E, Lev A, Lee YN, Simon AJ, Yinon Y, Lipitz S, Amariglio N, Weisz B, Notarangelo LD, Somech R. 2015. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med 7(276):276ra25. [DOI] [PubMed] [Google Scholar]
- Ruotsalainen T, Halme M, Isokangas OP, Pyrhonen S, Mantyla M, Pekonen M, Sarna S, Joensuu H, Mattson K. 2000. Interferon-alpha and 13-cis-retinoic acid as maintenance therapy after high-dose combination chemotherapy with growth factor support for small cell lung cancer—a feasibility study. Anticancer Drugs 11(2):101–108 [DOI] [PubMed] [Google Scholar]
- Sidky YA, Borden EC. 1987. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses. Cancer Res 47(19):5155–5161 [PubMed] [Google Scholar]
- Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, Sridhar S, Beamis J, Lamb C, Anderson T, Gerry N, Keane J, Lenburg ME, Brody JS. 2007. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med 13(3):361–366 [DOI] [PubMed] [Google Scholar]
- Steiling K, Ryan J, Brody JS, Spira A. 2008. The field of tissue injury in the lung and airway. Cancer Prev Res (Phila) 1(6):396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. 2007. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics 23(23):3251–3253 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102(43):15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar R, Soong R, Cho BC, Brahmer JR, Soo RA. 2014. Immunotherapy in the treatment of non-small cell lung cancer. Lung Cancer 85(2):101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H, Sasaki S, Imai K, Shibue T, Honda K, Taniguchi T. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 424(6948):516–523 [DOI] [PubMed] [Google Scholar]
- Trinchieri G. 2010. Type I interferon: friend or foe? J Exp Med 207(10):2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chorley BN, Pittman GS, Kleeberger SR, Brothers J, 2nd, Liu G, Spira A, Bell DA. 2010. Genetic variation and antioxidant response gene expression in the bronchial airway epithelium of smokers at risk for lung cancer. PLoS One 5(8):e11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczynski JR. 2006. Cancer and pregnancy share similar mechanisms of immunological escape. Chemotherapy 52(3):107–110 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.