Abstract
Expanding cryopreservation methods to include a wider range of cell types, such as those sensitive to freezing, is needed for maintaining the viability of cell-based regenerative medicine products. Conventional cryopreservation protocols, which include use of cryoprotectants such as dimethylsulfoxide (Me2SO), have not prevented ice-induced damage to cell and tissue matrices during freezing. A family of antifreeze proteins (AFPs) produced in the larvae of the beetle, Dendroides canadensis allow this insect to survive subzero temperatures as low as −26°C. This study is an assessment of the effect of the four hemolymph D. canadensis AFPs (DAFPs) on the supercooling (nucleating) temperature, ice structure patterns and viability of the A10 cell line derived from the thoracic aorta of embryonic rat. Cryoprotectant solution cocktails containing combinations of DAFPs in concentrations ranging from 0–3mg/mL in Unisol base mixed with 1M Me2SO were first evaluated by cryomicroscopy. Combining multiple DAFPs demonstrated significant supercooling point depressing activity (~9°C) when compared to single DAFPs and/or conventional 1M Me2SO control solutions. Concentrations of DAFPs as low as 1μg/mL were sufficient to trigger this effect. In addition, significantly improved A10 smooth muscle cell viability was observed in cryopreservation experiments with low DAFP-6 and DAFP-2 concentrations in combination with Me2SO. No significant improvement in viability was observed with either DAFP-1 or DAFP-4. Low and effective DAFP concentrations are advantageous because they minimize concerns regarding cell cytotoxicity and manufacturing cost. These findings support the potential of incorporating DAFPs in solutions used to cryopreserve cells and tissues.
Keywords: antifreeze proteins, cryoprotectants, recrystallization, Dendroides canadensis, supercooling, thermal hysteresis, cryopreservation, subzero storage, ice-free cryopreservation, cryomicroscopy, cell viability
Introduction
There are many lessons to be learned from the adaptation mechanisms of some organisms to survive extreme temperatures [3] for the cryopreservation of cell based regenerative medicine products. One of the most interesting freeze avoidance methods involves the production of antifreeze proteins (AFPs), first discovered in polar marine fishes [8, 10] and later in a variety of other organisms (both freeze avoiding and freeze tolerant), including insects [12, 18] and other arthropods [26,11,15,43,2, 20], freeze tolerant plants [44, 21, 16, 22], fungi and bacteria [16,4]. These proteins inhibit ice crystal growth by lowering the non-equilibrium freezing point of water, without significantly impacting the melting point, thereby producing a phenomenon called thermal hysteresis (TH) [9] that allows these organisms to survive at subzero temperatures. The mechanism by which AFPs depress the freezing temperature has been suggested to occur via binding of these proteins to specific planes on ice crystals, thereby halting or slowing subsequent crystal growth [45, 27]. More recent work demonstrated that AFP activity can also be due to long range changes in water dynamics resulting from AFP-water interactions up to 27Å (Angstrom) from the protein surface [19, 31]. In addition to inhibiting intrinsic ice crystallization, certain insect AFPs have been shown to promote supercooling by inhibiting ice nucleators [13]. Both thermal hysteresis and inhibition of ice nucleators can be significantly enhanced when certain DAFPs are combined, especially in the presence of low molecular mass solutes such as glycerol [12, 13, 47]. Also, some AFPs, including those from Dendroides canadensis (29, 44), fish (30), and from freeze tolerant organisms such as plants (22), inhibit ice recrystallization and affect ice crystal morphology, thereby assisting freeze tolerance. Based on these inherent properties, significant interest arose in the potential of utilizing these proteins in protecting cells, tissues, and organs during storage at subzero or cryogenic temperatures in order to enhance their recovery upon rewarming. Cryopreservation by freezing is hindered by ice-induced damage which can be minimized through the process of vitrification; an ice-free cryopreservation method that currently employs high, relatively toxic concentrations of cryoprotectants (CPAs) in combination with relatively rapid cooling rates. Limiting or inhibiting ice damage to cells and tissues while avoiding high cryoprotectant concentrations may be possible by mimicking the strategy of AFP production in overwintering organisms. This can be simulated by creating cryoprotectant cocktail formulations consisting of non-toxic concentrations of CPAs such as dimethylsulfoxide (Me2SO) combined with AFPs.
Fish AFPs are known to express a relatively low thermal hysteresis activity (1–2°C) and their utilization in preserving mammalian cells was investigated with varied results. Cryopreservation of red blood cells through the incorporation of winter flounder type I AFP to the extracellular cryoprotectant hydroxyethyl starch was successfully improved at low AFP concentrations (5–160μg/mL) and contraindicated at higher concentrations. The improvement in cell viability was associated with partial inhibition of ice recrystallization in the extracellular region during late stages of the warming cycle [6,7]. On the other hand, Carpenter and Hansen (6) reported that higher AFP concentrations almost completely inhibited ice recrystallization in regions devoid of cells, but led to massive growth of destructive ice crystals in association with cells.
Another study [23] showed that the addition of type I fish AFP to Me2SO in the cryopreservation of myelogenous leukemia cells resulted in statistically significant decreases in cell recovery at all concentrations up to 1000 μg/mL with the more deleterious effects observed at higher concentrations (>100μg/mL). In contrast, other studies have reported a positive impact of fish AFPs in enhancing the viability of mammalian cells following hypothermic storage at 4°C for 24–72 hrs [37,31] and of mammalian organs following subzero storage at −1°C to −4°C [41,1]. This better performance at the hypothermic/high-subzero temperature range relative to cryogenic temperatures is consistent with the function of fish AFPs within their environment in cold seawater.
Many insect-derived AFPs, such as those derived from the overwintering larvae of the beetle Dendroides canadensis (DAFPs), express higher thermal hysteresis activity (3–6°C) than those of fish (1–2°C) in their hemolymph and gut fluid and survive much lower temperatures (−26°C in the case of D. canadensis) [12]. While in vitro studies showed that DAFPs inhibit both hemolymph protein ice nucleators and ice nucleating bacteria isolated from the gut, the magnitude of this inhibition was not large enough to account for the extensive supercooling necessary for successful overwintering [33,34]. However, D. canadensis accumulates glycerol at concentrations of 1M or more. The colligative effect of the 1M glycerol directly promotes supercooling by approximately 2–6°C [14], but glycerol also enhances DAFP activity such that the DAFP-glycerol combination produced a higher inhibition activity of hemolymph ice nucleators and ice nucleating bacteria in distilled water than DAFPs alone [11].
The purpose of this study was to experimentally determine whether DAFPs enhance the supercooling and cryopreservation capabilities of other CPA solutions, such as Me2SO, which generally produces higher mammalian cell viability than glycerol following cryopreservation. This was done by evaluating the ice nucleating temperature (also known as the supercooling point temperature), ice growth and crystal morphology of various DAFP-cell permeating cryoprotectant solution cocktails as well as post-thaw cell viability following cryopreservation with these new formulations.
Materials and Methods
Preparation of antifreeze proteins and cryoprotectant solutions
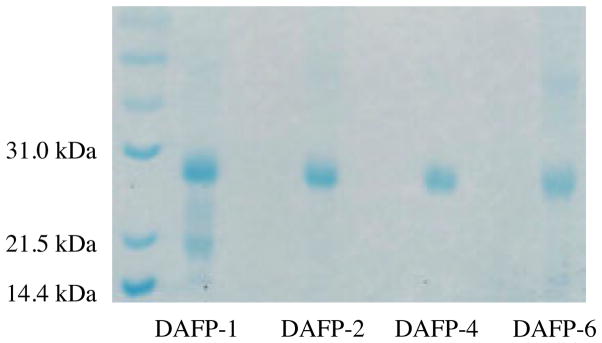
Recombinant D. canadensis DAFPs-1, 2, 4 and 6 were expressed in transformed E. coli as described previously [47], except that a bio-fermentor was used to grow the transformed bacterial cells instead of a 1L shaker flask. Conditions were as follows. Fed batch fermentation was carried out in a 7.5L fermentor (BioFlo 115, New Brunswick Scientific, Edison NJ). All chemicals were purchased from Fisher Scientific, Hanover Park, IL unless otherwise indicated. The chemicals in the K12 trace solution were purchased from Sigma, St. Louis MO. Fermentation medium was composed of (per L): 5.0g KH2PO4, 3.0g K2HPO4, 1.7g (NH4)2SO4, and 10g yeast extract. After autoclaving, 0.5g MgSO4·7 H20, 50mL/L 50% glucose solution and 5ml/L of K12 trace metals solution composed of (per L) 1.6g FeCl3, 0.2g CoCl2·6H2O, 0.1g CuCl2, 0.2g ZnCl2·4H20, 0.2g NaMoO4, 0.05g H3BO4, 10mL HCl was added. Carbenicillin (Promega, Madison WI) and chloramphenicol (Sigma, St. Louis MO) were added at final concentrations of 100 μg/mL and 34 μg/mL respectively. Antifoam A (Sigma, St. Louis, MO) was used to suppress foaming. Fermentation conditions were: 37°C, pH7.0 (maintained by automatic addition of 3N NaOH or 1N H3PO4), 1.0–1.5vvm air. A setting of 40% dissolved oxygen was controlled by an agitation cascade (300–900 rpm). An overnight culture of transformed E. coli BL2(DE3) pLysS competent cells [47] in LB medium was used as inoculum. After the initial glucose was consumed, feeding medium (per L), 100g glucose, 27.5g (NH4)2SO4, 1.5g MgSO4 ·7 H20 and 100g yeast extract was continuously added by a feed pump. When the OD600 reached 10, expression was induced by the addition of 1.0 mM isopropyl D-1-thiogalactopyranoside (IPTG) (Agilent, Santa Clara CA). The run was stopped 12–16 hours after induction. Cells were collected by centrifugation at 6,000 rpm for 20 min. The extracted crude protein was then purified using cobalt resin (Clontech, Mountain View, CA), dialyzed and freeze-dried. The purified DAFPs were run on a 10% SDS gel. Figure 1 shows the purity of each of the resulting DAFPs.
Figure 1.
A 10% polyacrylamide gel showing the purity of recombinant DAFPs 1, 2, 4, and 6. The recombinant proteins run at a molecular mass of ~28–29KDa. DAFPs 1 and 6 show slight contamination and/or breakdown of DAFP, perhaps due to proteolysis. Molecular markers are shown in the left lane.
All DAFPs were reconstituted in Unisol solution; composition listed in Table I [43], to obtain formulations containing 1M Me2SO with single and multiple combinations of DAFPs at concentrations of 0–3mg/mL and 0–300μg/mL respectively.
Table I.
Unisol solution formulation
| Components (mm) | Unisol I a |
|---|---|
| Na+ | 62.5 |
| K+ | 70.0 |
| Ca++ | 0.05 |
| Mg++ | 15.0 |
| Cl− | 30.1 |
| H2PO4− | 2.5 |
| HCO3− | 5.0 |
| HEPES | 35.0 |
| Lactobionate− | 30.0 |
| Sucrose | 25.0 |
| Mannitol | 25.0 |
| Glucose | 5.0 |
| Gluconate | 70.0 |
| Adenosine | 2.0 |
| Glutathione | 3.0 |
| pH | 7.6 |
Unisol Intracellular-base solution without Dextran [29].
Cryomicroscopic analysis of cryoprotectant solutions
Ice nucleation
Several CPA solutions were prepared in the Unisol base; DAFPs in Me2SO, Me2SO without DAFPs, and Unisol-only controls. Solutions were then analyzed using a cryomicroscope (Linkam MDS 600 Cryostage) coupled with a digital camera (Olympus). Samples of each solution in 5 μL volumes were loaded between glass coverslips and rapidly cooled at a rate of 40°C/minute until the onset of ice nucleation followed by rewarming to the melting point of the solution. Digital images of ice were acquired. When processing the images, the ice nucleation temperature (Tn) (supercooling point temperature) was determined.
Growth and morphology of seed ice crystals
Samples, containing either IM Me2SO or 1μg/ml DAFP-1,2,4 and 6 (total DAFP concentration) in 1M Me2SO, were flash frozen followed by melting until a single seed ice crystal remained. The stage was slowly cooled at 0.2°C/min to observe the growth rate and pattern of ice crystals at different time points.
Cryomicroscopic analysis of cells treated with cryoprotectant solutions
Cells derived from embryonic rat thoracic aorta smooth muscle (A10 cell line) were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM) growth medium supplemented with 10% inactivated fetal bovine serum (FBS) and penicillin/streptomycin. Cell suspensions were then seeded onto autoclaved 12 mm glass coverslips placed in a 24-well plate at a density of 15×103 cells/well and incubated for 24 hrs at 37°C and 95% humidity to promote cell adhesion onto the glass surface. Cell-seeded coverslips were then carefully transferred to a new 24-well plate and treated with 500 μL of CPA test solutions for 15 minutes on ice. The following cryoprotectant solutions were compared:
1M Me2SO in Unisol
1μg/mL DAFP-2 plus 1M Me2SO in Unisol
3μg/mL DAFP-1,2 (total DAFP concentration, equal parts DAFP-1 and 2) plus 1M Me2SO in Unisol
1μg/mL DAFP-1,2,4,6 (total DAFP concentration, equal parts DAFP-1, 2, 4 and 6) plus 1M Me2SO in Unisol
Once the cell-seeded coverslip was treated by the CPA solutions, it was removed from the plate, loaded with an additional 5μL of the CPA solution, sealed with a second coverslip and placed on the cryostage. Samples were cooled at two rates, 50°C/min and 1°C/min until freezing occurred, followed by rewarming to the melting point. Digital images of the ice morphology and records of nucleation temperatures were obtained as described above.
Evaluation of cell viability after cryopreservation
Rat smooth muscle cells (A10) were plated at 20,000 cells/well in a 96-well culture plate. The next day, the cells were exposed to cryoprotectant solutions containing 1M Me2SO with varying concentrations of DAFPs 1, 2, 4, or 6 in Unisol. Plates were then cooled to −80°C using a controlled rate freezer (Planar). The cooling profile consisted of a constant rate of 1.0°C/minute with a nucleation step at −4.0°C. Plates were stored at least overnight at −135°C. Rewarming was performed in two steps, a 30 minute incubation at −20°C followed by rapid warming using a 37°C water bath [5]. The cryoprotectant solutions were removed by replacement with culture media on ice before being incubated for 60 minutes at 37°C in an incubator. Resazurin dye (Biotium), 200 μL/well, was added and the plates were incubated for 3 hours at 37°C before being read on a fluorescent microplate reader (Molecular Dynamics) at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. Percent viability presented on the graph in Figure 7 was normalized in terms of viability values of control cell samples which were conventionally cryopreserved with 1M Me2SO alone in Unisol (bar across graph). For comparison purposes another control group labeled as “None” was frozen with no DAFP or 1M Me2SO and was included as a reference.
Figure 7.
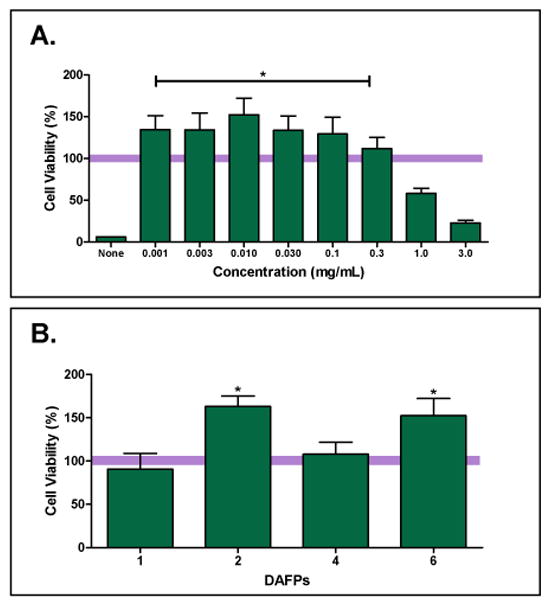
A10 cell viability after cryopreservation with varying concentrations of DAFPs combined with 1M Me2SO in Unisol normalized to viability of cells treated with 1M Me2SO-only in Unisol. A: A10 cells cryopreserved in the indicated concentrations of DAFP-6. “None” label represents viability of cells frozen without Me2SO or DAFPs. (Bar across graph at 100% corresponds to cell viability after cryopreservation with 1M Me2SO-only, for demonstration purposes). B: Peak cell viability observed in DAFP cryopreservation experiments; DAFP-1 & DAFP-2 at 1 μg/mL, and DAFP-4 & DAFP-6 at 10 μg/mL. Data is presented as the mean (±SE) of 12 replicates in percent of 1M Me2SO controls, * P<0.05.
Statistical analysis
All data are expressed as mean ± standard of error (SE). All experiments were conducted a minimum of three times. To compare data among multiple groups, analyses of variances (ANOVA, with multiple comparisons using Tukey or Bonferroni post hoc test) was used employing 95% confidence interval, where p <0.05 were considered statistically significant.
Results
Cryomicroscopic analysis of cryoprotectant solutions
Ice nucleation
Ice formation in all solutions was uncontrolled and occurred spontaneously; however, the ice morphology observed was strongly dependent on the type and concentration of CPA as shown in Figure 2. Freezing was irregular in Unisol (Fig. 2A) and Me2SO (Fig. 2B) solutions while it exhibited a more uniform and less granular structure in solutions containing DAFP combinations and higher concentrations of single DAFPs in Me2SO and Unisol (Fig. 2C–E). Among single DAFP solutions, DAFP 6 was the most efficient in lowering the nucleation temperature (range: −24.1 °C to −29.6 °C) at concentrations higher than 300 μg/mL (Fig. 3A). Nucleation temperatures of solutions containing various combinations of DAFPs (range: −24.1 °C to −30.8 °C) were significantly lower (p<0.05) than those of Me2SO-alone (range: −17.7 °C to −24.4 °C) and single DAFP solutions (range: −16.5 °C to −22.64 °C) as shown in Figure 3B. All DAFP combinations, with the exception of DAFP 4,6 and DAFP 1,4,6 had nucleating temperatures ranging from −24.1 °C to −30.8°C. This decrease in the nucleation temperature of most DAFP combinations was not concentration dependent, at least within the range of concentrations tested, as dilutions as low as 1μg/mL were sufficient to prolong the supercooled state.
Figure 2.
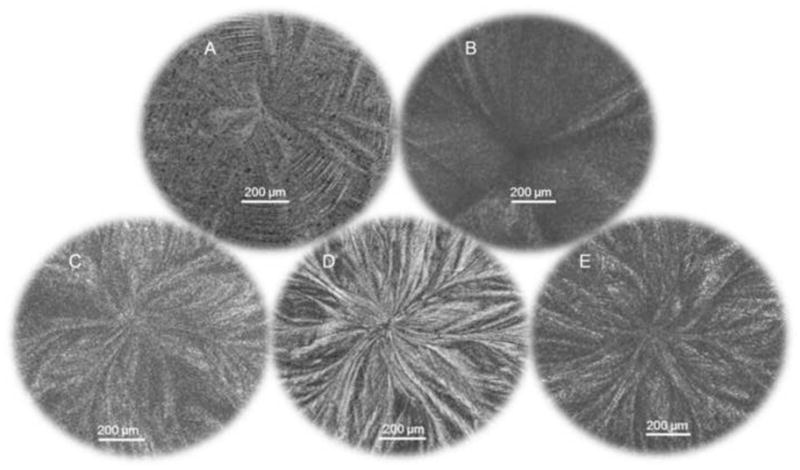
Cryomicroscopic images showing ice structure patterns of Unisol solutions containing A: Unisol only, B: 1M Me2SO only, C: 300 μg/mL DAFP 6 +1M Me2SO, D: 3 mg/mL DAFP 6 +1M Me2SO, and E: 300 μg/mL DAFP 1,2,4,6 +1M Me2SO. Ice patterns had no distinct features when DAFPs were absent indicating circular ice growth along low surface energy planes. Ice growth was more uniform when in presence of DAFPs indicating that this growth was diverted to the high surface energy planes.
Figure 3.
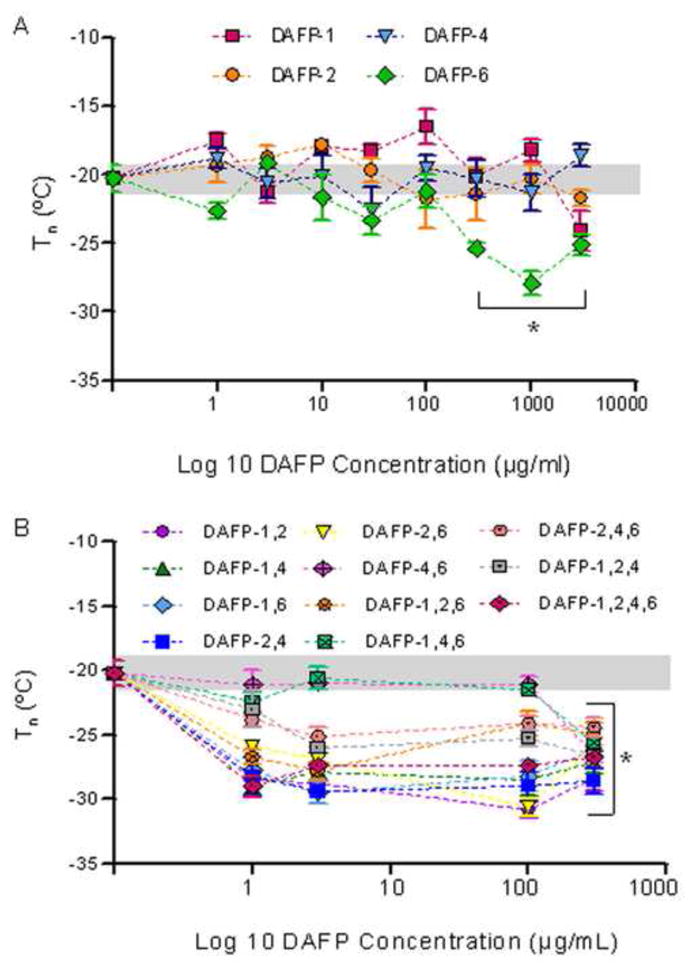
Nucleation temperatures of Unisol solutions containing 1M Me2SO enhanced by A: DAFP types 1, 2, 4, and 6 in single combinations, and B: DAFP types 1, 2, 4, and 6 in multiple combinations compared with DAFP-free 1M Me2SO in Unisol (0-point). Grey band represents one standard error for Me2SO control means without DAFPs. Data are expressed as mean ±SE for at least 3 replicates. *p<0.05 vs. Me2SO control.
Growth morphology of seed ice crystals
As presented in Figure 4, following slow cooling from the melt, initially round seed ice crystals became hexagonal in shape and with time developed into dendritic ice crystals in all solutions. While morphologically the ice crystals were very similar with and without the addition of DAFPs, the difference was that crystal growth in the samples containing DAFPs grew at a significantly lower rate as demonstrated in Figure 4. In addition, nucleation temperatures of the observed solutions were reflective of the ice crystal growth behavior, where solutions containing DAFP-1,2,4,6 showed the slowest seed crystal growth as well as the lowest nucleation temperature. Previous studies by Duman et al demonstrated more distinctive changes in ice crystal morphology than was observed in these experiments (12). However, changes in ice morphology were noted in the presence and absence of DAFPs (Fig. 2), and the differences observed between our experiments and previous studies is likely due to the use of much lower DAFP concentrations, the presence of the cryoprotectant Me2SO, and/or the use of the high solute base solution Unisol. Any of these parameters could affect the ice crystal morphology.
Figure 4.

Seed ice crystal growth in A: 1M Me2SO vs. B: 1 μg/mL DAFP-1,2,4,6 in 1M Me2SO during slow cooling (0.2°C/min) from melt at different time points.
Cryomicroscopic analysis of cells treated with cryoprotectant solutions
The nucleation temperatures of cells in CPA solutions were not statistically different from that of CPA solutions without cells. Also, there was no preferential ice nucleation in association with the cells. Figure 5 shows that spontaneous ice first formed in the cryoprotectant solution at temperatures below −25.9°C while the cells supercooled (Figs. 5B &D) before freezing as indicated by the darkening of the cells (Figs. 5C &E). Cooling rate only affected the nucleation temperature of 1M Me2SO which was significantly lower (p<0.001) during slow cooling (1°C/min) relative to rapid cooling (50 C°/min), whereas the cooling rate had no effect on Unisol- Me2SO -DAFP solutions (Fig. 6). During rapid cooling, cells in solutions containing DAFP combinations had a lower nucleation temperature (p<0.001) relative to single DAFP-2 and Me2SO -only solutions. Basically, cooling rate had no effect on the degree of supercooling when DAFP combinations were used, whereas Me2SO expressed greater supercooling (lower Tn) during slow cooling only when cells were present.
Figure 5.
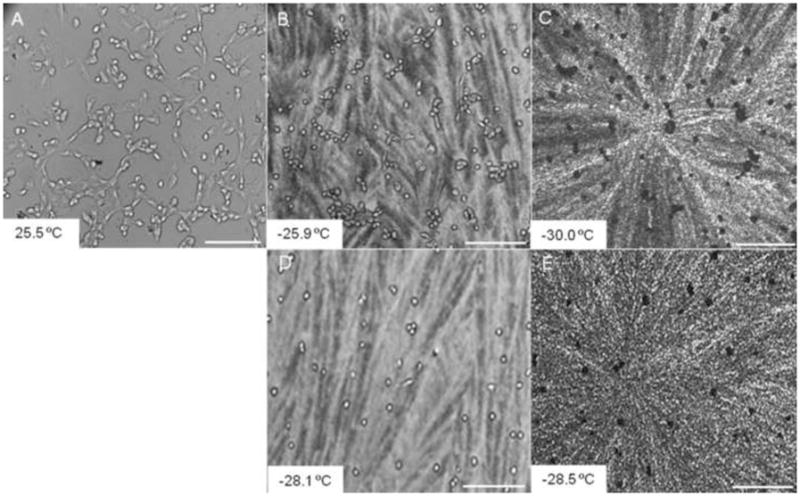
Cryomicroscopic images of A10 cells in 3μg/mL DAFP 1,2 solution. A: During cooling before freezing, B&D: After solution freezing during rapid cooling and slow cooling respectively, and C&E: after cellular freezing during rapid and slow cooling respectively. At both cooling rates, cells supercooled to temperatures below ~ −25.9°C before freezing as observed by the darkening of the cells. The length of the scale bar represents 200μm.
Figure 6.
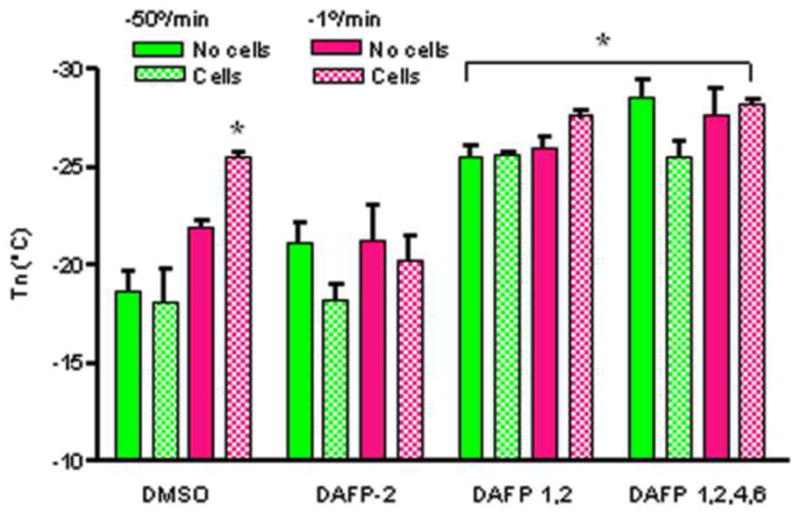
Nucleation temperatures of different CPA solutions with and without cells. *p<0.001 versus the rest of solutions. Data are expressed as mean ±SE for at least 3 replicates.
Viability of cells preserved in cryoprotectant solutions
Concentration ranges (0–3 mg/mL) of each DAFP (1,2,4 and 6 separately) in Unisol and 1M Me2SO were evaluated. (Only data for DAFP-6 is shown in Fig. 7A.) Across the DAFP-6 concentration range, the best post-thaw cell viability was observed at concentrations less than 100 μg/mL while greater concentrations resulted in a deleterious effect on the cells due to cytotoxicity. Cryopreservation with concentrations as low as 1 μg/mL, DAFP-6 (in Unisol and 1M Me2SO) demonstrated significant improvement (p<0.05) in post-thaw cell viability over 1M Me2SO alone (in Unisol) (Fig. 7A). Similar positive results were observed with DAFP-2, while addition of DAFP-4 or DAFP-1 did not result in significant improvement in peak post-thaw cell viability over Me2SO alone (in Unisol) (Fig. 7B).
Discussion
Antifreeze proteins have the unique ability to depress the freezing point of water without significantly affecting the melting point in a process called thermal hysteresis [9]. Overwintering Dendroides canadensis larvae produce potent antifreeze proteins in their hemolymph supplemented with the production of low molecular mass enhancers such as glycerol [13]. Thus, the supercooling point, or nucleation temperature, of this insect in the summer is ~−4°C to −6°C whereas in the winter this temperature is significantly lowered to between −16°C and −26°C [33,24]. In addition, expression of this insect’s DAFP-1 genes in the plant, Arabidopsis thaliana, resulted in a small, yet significant, decrease in its freezing temperature (−13.3°C) relative to that of wild-type even in the presence of extrinsic nucleating agents [25]. The mechanism by which DAFPs depress the freezing point and promote supercooling has been thought to occur by adsorbing onto the surface of ice crystals at preferred growth sites [36,28] likely by means of hydrogen bonding in the case of DAFPs and other beetle AFPs [10, 40], as well as by long range changes in water dynamics resulting from AFP-water interactions [32]. The preferred site for ice crystal growth in pure water is along the axes parallel to the low free surface energy basal plane with little growth on the high free surface energy axis perpendicular to the basal plane (c-axis). This process is reversed when AFPs are present resulting in preferential inhibition of ice growth along the preferred regions forcing the ice to grow along the high energy c-axis. It has been previously shown that AFPs from the spruce budworm, Choristoneura fumiferana, that have an ice-binding motif similar to that of DAFPs bind to both the basal and the prism planes of ice crystals [35]. Consequently, ice crystallization must occur at a lower temperature. This phenomenon was illustrated by the differences in ice morphology observed in different formulations (Fig. 2) as well as in the significantly delayed growth of seed ice crystals during slow cooling from melt (Fig. 4) in the presence of DAFPs. In the absence of DAFPs, freezing profiles had no distinct features indicating that most of the ice growth was circular and along the low energy planes. However, when DAFPs were added to Me2SO in multiple combinations or as individual types in higher concentrations, the ice morphology was changed indicating DAFP binding. This alteration in the process of ice crystal growth consistently resulted in significant suppression of the nucleation temperatures (Fig. 3).
Furthermore, as reported in previous literature, DAFPs were found to enhance one another’s activities, especially in the presence of low molecular weight enhancers [47]. In this study 1M Me2SO was selected as the low molecular weight enhancer compound due to its effectiveness in maintaining A10 cell viability during cryopreservation [4]. In contrast to single DAFPs, which required relatively high concentrations (1–3 mg/mL) to prolong supercooling, most combinations of DAFPs resulted in a significant decrease in the nucleation temperature at concentrations as low as 1μg/mL (Fig. 3). The exception was in the case of combinations of DAFPs 4,6 and DAFPs 1,4,6 which did not delay freezing at concentrations below 300 μg/mL suggesting that binding of DAFP 4 to DAFP 6 is not efficiently enhanced in the presence of Me2SO. It has been previously shown that DAFP-DAFP interactions do not always occur; for instance DAFP-1 was found to effectively bind to DAFP-2 and DAFP-4, but not to DAFP-6 in the presence of glycerol [47].
Given their low temperature advantages, the feasibility of several types of AFPs in improving cryopreservation was previously tested. AFPs from freeze avoiding organisms such as those from winter flounder and the beetle Dendroides canadensis were found to inhibit recrystallization during rewarming [6,7,29,30]. This effect was experimentally validated on several types of cells, including human red blood cells, by which cell survival was significantly enhanced as a result of attenuation of ice recrystallization during the freeze-thawing cycle [6,7,44]. In addition, other studies have shown that some fish AFPs protect cells from cryoinjury by blocking ion channels such as Ca++ and K+ to suppress ion flux across cell membranes [38]. Thus, the incorporation of successful AFP technology in cryopreservation of cells or subzero storage of tissues and organs is dependent on the type and concentration of AFP used as well as the composition of the cell or tissue being cryopreserved.
Cryopreservation of A10 cells with single DAFPs in 1M Me2SO demonstrated that DAFPs can significantly improve post-thaw cell viability compared to conventional cryopreservation by 1M Me2SO alone (Fig. 7). Significantly improved cell viability was observed with DAFP-6 and DAFP-2, but no significant improvement in viability was observed with either DAFP-1 or DAFP-4. Further experiments are planned to test combinations of these DAFPs and other insect-derived AFPs for their ability to improve cell viability after cryopreservation.
In other cell studies, A10 cells were treated with CPA solutions containing DAFPs followed by cooling (slow and rapid) until they were frozen (Figs. 5–6). DAFPs prepared in 1M Me2SO -Unisol were then compared to the DAFP-free Me2SO -Unisol solution. Consistent with the experiment without the presence of cells, the nucleation temperature of solutions containing DAFP combinations was significantly lower than those containing single DAFPs (Fig. 6) and Me2SO during rapid cooling. An additional benefit was observed; cooling rate had no effect on the activity of DAFP combinations, whereas Me2SO showed greater nucleation temperature suppression during slow cooling only (Fig. 6). Therefore, the incorporation of low concentrations of different combinations of DAFPs may enhance cryopreservation of mammalian cells in the presence of Me2SO. Even though DAFP-treated cells eventually froze, they were able to maintain supercooling down to ~29°C during both slow and rapid cooling (Figs. 5–6), a temperature similar to the lowest nucleation temperature observed of the Dendroides canadensis larvae (−26°C) during the winter [33,24]. Thereby, DAFPs were capable of depressing the nucleation temperature (supercooling) of Me2SO solutions by ~9°C. Similarly, it has been previously shown that DAFPs were capable of depressing the freezing point of cells isolated from the midgut of summer collected centipedes, Lithobius forficatus by ~7°C coupled with a significant increase in cell survival [44]. Furthermore, it is possible that incorporation of antifreeze proteins in the matrices of tissue engineered cellular constructs may facilitate retention of post-cryopreservation cell viability, function and matrix integrity.
Conclusions
This study determined the in vitro physical properties of insect-derived DAFP solutions as potential cryoprotectants in combination with Me2SO. DAFPs significantly lowered the nucleation temperature of the conventional 1M Me2SO solution by ~9 °C. Combinations of DAFPs in Me2SO were found to correlate well in vitro with the freeze avoidance ability of overwintering Dendroides canadensis. The same magnitude of reduction occurred in the presence of mammalian cells and improvement in post-thaw cell viability after cryopreservation was also observed in the presence of two types of DAFPs (DAFP-6 and DAFP-2). These findings support the potential of incorporating insect-derived DAFPs in mammalian cell and tissue cryopreservation protocols. Future studies are planned to investigate whether additional DAFP combinations can provide further enhancement to post-thaw viability of A10 cells.
Acknowledgments
Role of Funding Source
This study was funded by U.S. Public Health Grant # R43GM088900-01 and SCLaunch Matching Grant Agreement #2010-129. Funding sources played no role in this study besides financial support.
We thank Dr. John Duman’s research team at the Department of Biological Sciences, University of Notre Dame for processing and providing recombinant insect-derived antifreeze proteins
Statement of Funding
Funding sources had no involvement in the design and execution of this study besides financial support.
Footnotes
All authors have no conflict of interest associated with this publication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amir G, Rubminsky B, Kassif Y, Horowitz L, Smolinsky AK, Lavee J. Preservation of myocyte structure and mitochondrial integrity in subzero cryopreservation of mammalian hearts for transplantation using antifreeze proteins—an electron microscopy study. Eur J Cardiothorac Surg. 2003;24:292–297. doi: 10.1016/s1010-7940(03)00306-3. [DOI] [PubMed] [Google Scholar]
- 2.Block W, Duman JG. The presence of thermal hysteresis producing antifreeze proteins in the Antarctic mite, Alaskozetes antarcticus. J Exp Zool. 1989;250:229–231. [Google Scholar]
- 3.Brockbank KGM, Campbell LH, Greene ED, Brockbank MCG, Duman JG. Lessons learned from nature for preservation of mammalian cells, tissues, and organs. In Vitro Cell Dev Biol– Animal. 2011;47:210–217. doi: 10.1007/s11626-010-9383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell LH, Brockbank KGM. Serum-free solutions for cryopreservation of cells. In Vitro Cellular & Developmental Biology-Animal. 2007;43:269–275. doi: 10.1007/s11626-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 5.Campbell LH, Taylor MJ, Brockbank KGM. U.S. Patent #6,596,531. Two stage method for thawing cryopreserved cells. 2003 Jul;
- 6.Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci. 1992;89:8953–8957. doi: 10.1073/pnas.89.19.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao HM, Davies PL, Carpenter JF. Effect of antifreeze proteins on red blood cell survival during cryopreservation. J Exp Biol. 1996a;199:2071–2076. doi: 10.1242/jeb.199.9.2071. [DOI] [PubMed] [Google Scholar]
- 8.DeVries AL. Glycoproteins as biological antifreeze agents in antarctic fishes. Science. 1971;172(3988):1152–1155. doi: 10.1126/science.172.3988.1152. [DOI] [PubMed] [Google Scholar]
- 9.DeVries AL. Antifreeze glycopeptides and peptides: interactions with ice and water. Meth Enzymol. 1986;127:293–303. doi: 10.1016/0076-6879(86)27024-x. [DOI] [PubMed] [Google Scholar]
- 10.DeVries AL, Cheng C-HC. The role of antifreeze glycopeptides and peptides in the survival of cold-water fishes. In: Somero GN, Osmond CB, Bolis CL, editors. Water and life: comparative analysis of water relationships at the organismic, cellular, and molecular levels. Springer-Verlag; 1992. pp. 303–315. [Google Scholar]
- 11.Duman JG. Subzero temperature tolerance in spiders: The role of thermal hysteresis factors. J Comp Physiol B. 1979;131:347–352. [Google Scholar]
- 12.Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001;63:327–357. doi: 10.1146/annurev.physiol.63.1.327. [DOI] [PubMed] [Google Scholar]
- 13.Duman JG. The inhibition of ice nucleators by insect antifreeze proteins is enhanced by glycerol and citrate. J Comp Physiol B. 2002;172(2):163–168. doi: 10.1007/s00360-001-0239-7. [DOI] [PubMed] [Google Scholar]
- 14.Duman JG. Thermal hysteresis factors in overwintering insects. J Insect Physiol. 1979;25:805–810. [Google Scholar]
- 15.Duman JG, Horwath KL. The role of hemolymph proteins in the cold tolerance of terrestrial arthropods. Ann Rev Physiol. 1983;45:261–270. doi: 10.1146/annurev.ph.45.030183.001401. [DOI] [PubMed] [Google Scholar]
- 16.Duman JG, Olsen TM. Thermal hysteresis activity in bacteria, fungi and primitive plants. Cryobiology. 1993;30:322–328. [Google Scholar]
- 17.Duman JG, Wu DW, Olsen TM, Urrutia M, Tursman D. Thermal hysteresis proteins. Advances in Low Temperature Biology. 1993;2:131–182. [Google Scholar]
- 18.Duman JG, Walters KR, Sformo T, Carrasco MA, Nickell P, Barnes BM. Antifreeze and ice nucleator proteins. In: Denlinger D, Lee RE, editors. Low Temperature Biology of Insects. Cambridge University Press; Cambridge, UK: 2010. pp. 59–90. [Google Scholar]
- 19.Ebbinghaus S, Meister K, Born B, DeVries AL, Gruebele M, Havenith M. Antifreeze glycoprotein activity correlates with long-range protein-water dynamics. J Am Chem Soc. 2010;132(35):12210–12211. doi: 10.1021/ja1051632. [DOI] [PubMed] [Google Scholar]
- 20.Graham LA, Davies PL. Glycine-rich antifreeze proteins from snow fleas. Science. 2005;310:461. doi: 10.1126/science.1115145. [DOI] [PubMed] [Google Scholar]
- 21.Griffith M, Ala P, Yang DSC, Hon W-C, Moffit BA. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiology. 1992;100:593–596. doi: 10.1104/pp.100.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith M, Yaish MW. Antifreeze proteins in overwintering plants: A tale of two activities. Trends in Plant Sciences. 2004;9:399–405. doi: 10.1016/j.tplants.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Hansen TN, Smith KM, Brockbank KGM. Type I antifreeze protein attenuates cell recoveries following cryopreservation. Transplantation Proc. 1993;24(6):3186–3188. [PubMed] [Google Scholar]
- 24.Horwath KL, Duman JG. Yearly variations in the overwintering mechanism of the cold hardy beetle Dendroides canadensis. Physiol. Zool. 1984;57(1):40–45. [Google Scholar]
- 25.Huang T, Nicodemus J, Zarka DG, Thomashow MF, Wisniewski M, Duman JG. Expression of an insect (Dendroides canadensis) antifreeze protein in Arabidopsis thaliana results in a decrease in plant freezing temperature. Plant Mol Biol. 2002;50:333–344. doi: 10.1023/a:1019875922535. [DOI] [PubMed] [Google Scholar]
- 26.Husby JA, Zachariassen KE. Antifreeze agents in the body fluid of winter active insects and spiders. Experientia. 1980;36:963–964. [Google Scholar]
- 27.Knight CA, Wen1 D, Laursen RA. Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology. 1995;32(1):23–34. doi: 10.1006/cryo.1995.1002. [DOI] [PubMed] [Google Scholar]
- 28.Knight CA, Cheng CC, DeVries AL. Adsorption of α-helical antifreeze peptides on specific ice crystal surface planes. Biophys J. 1991;59:409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight C, Duman J. Inhibition of recrystallization of ice by insect thermal hysteresis proteins: A possible cryoprotective role. Cryobiology. 1986;23(3):256–262. [Google Scholar]
- 30.Knight C, DeVries AL, Oolman LD. Fish antifreeze protein and the freezing and recrystallization of ice. Nature. 1984;308(5956):295–296. doi: 10.1038/308295a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee CY, Rubinsky B, Flecher GL. Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo Letters. 1992;13:59–66. [Google Scholar]
- 32.Meister K, Ebbinghaus Y, Xu Y, Duman JG, DeVries AL, Gruebele DM, Havenith M. Long-range protein-water dynamics in hyperactive insect antifreeze proteins. Proceedings of the National Academy of Sciences, US A. 2013;110:1617–1622. doi: 10.1073/pnas.1214911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olsen TM, Duman JG. Maintenance of the supercooled state in overwintering Pyrochroid beetle larvae, Dendroides canadensis: role of hemolymph ice nucleators and antifreeze proteins. J Comp Physiol B. 1997a;167:105–113. [Google Scholar]
- 34.Olsen TM, Duman JG. Maintenance of the supercooled state in the gut fluid of overwintering Pyrochroid beetle larvae, Dendroides canadensis: role of gut ice nucleators and antifreeze proteins. J Comp Physiol B. 1997b;167:114–122. [Google Scholar]
- 35.Petraya N, Marshall CB, Celik Y, Davies PL, Braslavsky Y. Direct visualization of spruce budworm antifreeze protein interacting with ice: Basal plane affinity confers hyperactivity. Biophys J. 2008;95:333–341. doi: 10.1529/biophysj.107.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raymond JA, Wilson PW, DeVries AL. Inhibition of growth on nonbasal planes in ice by fish antifreeze. Proc Natl Acad Sci. 1989;86:881–885. doi: 10.1073/pnas.86.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubinsky B, Arav A, Fletcher GL. Hypothermic protection – a fundamental property of “antifreeze” proteins. Biochem Biophys Res Commun. 1991;180(2):566–571. doi: 10.1016/s0006-291x(05)81102-7. [DOI] [PubMed] [Google Scholar]
- 38.Rubinsky B, Mattioli M, Arav A, Barboni B, Fletcher GL. Inhibition of Ca++ and K+ currents by “antifreeze” proteins. Am J Physiol. 1992;262:R542–545. doi: 10.1152/ajpregu.1992.262.3.R542. [DOI] [PubMed] [Google Scholar]
- 39.Scholander PFL, Van Dam JW, Kanwisher HT, Hammel HT, Gordon MS. Supercooling and osmoregulation in arctic fish. J Cell Comp Physiol. 1957;49:5–24. [Google Scholar]
- 40.Sincheri F, Yang DSC. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature. 1995;375:427–431. doi: 10.1038/375427a0. [DOI] [PubMed] [Google Scholar]
- 41.Soltys KA, Batta AK, Koneru B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J Surg Res. 2001;96:30–34. doi: 10.1006/jsre.2000.6053. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Griffith M, Pasternak JJ, Glick BB. Low temperature growth, freezing survival, and production of antifreeze protein by the plant growth promoting rhizobacterium Pseudomonas sputida GR12-2. Can J Microbiol. 1995;41:776–784. doi: 10.1139/m95-107. [DOI] [PubMed] [Google Scholar]
- 43.Taylor MJ. U.S. Patent: 6,492,103. Assigned to Organ Recovery Systems, Inc; System for organ and tissue preservation and hypothermic blood substitution. 2002 Dec;
- 44.Tursman D, Duman JG. Cryoprotective effects of thermal hysteresis protein on survivorship of frozen gut cells in the freeze-tolerant centipede Lithobius forficatus. J Exp Zool. 1995;272(4):249–257. [Google Scholar]
- 45.Urrutia ME, Duman JG, Knight CA. Plant thermal hysteresis proteins. Biochim Biophys Acta. 1992;1121:199–206. doi: 10.1016/0167-4838(92)90355-h. [DOI] [PubMed] [Google Scholar]
- 46.Venketesh S, Dayananda C. Properties potentials, and prospects of antifreeze proteins. Crit Rev Biotechnol. 2008;28(1):57–82. doi: 10.1080/07388550801891152. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Duman JG. Antifreeze proteins of the beetle Dendroides canadensis enhance one another’s activities. Biochemistry. 2005;44:10305–10312. doi: 10.1021/bi050728y. [DOI] [PubMed] [Google Scholar]